Abstract
Background:
We performed the meta-analysis to evaluate the overall safety of programmed cell death-1 (PD-1) or ligand 1 (PD-L1) inhibitor treatment for lung cancer patients.
Method:
Randomized controlled trials were collected according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Risk ratio (RR) of PD-1/PD-L1 inhibitor treatment-related death, treatment-related adverse events, any serious events, and any events leading to discontinuation were all taken into account for the final evaluation.
Results:
Fourteen studies were collected for the meta-analysis. The RR of treatment-related death for PD-1/PD-L1 was significantly lower than that of the control group (RR = 0.37, 95% confidence interval, CI: [0.21, 0.66]). Similar analysis results could also be seen for the RR of treatment-related adverse events and adverse events leading to discontinuation. When PD-1/PD-L1 was combined with chemotherapy, it increased the RR of adverse events leading to discontinuation (RR = 1.68, 95% CI: [1.22, 3.32]). The RR of overall treatment-related adverse events was lower in nivolumab (PD-1) than that of the control group (nivolumab + ipilimumab) (RR = 0.77, 95% CI: [0.65, 0.90]). Similar analysis results could also be seen in the RR of treatment-related adverse events for grade 3 to 5 and adverse events leading to discontinuation.
Conclusion:
Compared with chemotherapy, RR of the treatment-related deaths associated with PD-1/PD-L1 inhibitor was significantly lower than that of the chemotherapy group, while it did not increase the RR when they were combined with chemotherapy or other drugs. When PD-1/PD-L1 was combined with chemotherapy, it increased the RR of adverse events leading to discontinuation.
Keywords: lung cancer, meta-analysis, programmed cell death-1/programmed cell death ligand 1, safety
1. Introduction
In recent years, more and more clinical trial results showed that programmed cell death-1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors had satisfactory clinical efficacy for lung cancer patients, especially for non-small cell lung cancer (NSCLC), whether it was monotherapy or combined with chemotherapy.[1–14] The expression of PD-L1 is common in NSCLC patients, and the interaction of PD-1 with PD-L1 and PD-L2 ligands inhibits T-cell activation and promotes tumor immune escape.[15,16] The toxic effects associated with PD-1/PD-L1 inhibitors may affect any organ and result from the activation of autoreactive T cells, thereby damage the host tissue and even jeopardize the patient's life.[1–14] So, it was necessary for more and more clinicians to pay their attentions to the drug toxicities caused by PD-1/PD-L1, especially for life-threatening side effects.
In recent years, a large number of meta-analysis on PD-1/PD-L1 safety and toxic side effects have been published.[17–22] Due to the fact that there were too few incorporation data or insufficient subgroup analysis in the previous meta, the conclusions obtained were not accurate enough. As the completion of 6 large clinical trials of PD-1/PD-L1 related to lung cancer in 2018,[1–6] we believed that we could get a new and more accurate conclusion of the PD-1/PD-L1 safety assessment. So, we designed the meta-analysis to evaluate the overall safety of PD-1 or PD-L1 inhibitor treatment for lung cancer patients.
2. Methods
This systematic review and meta-analysis was put into practice according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.[23]
2.1. Types of enrolled studies
In order to meet the preliminary inclusion criteria, the study must report randomized clinical trials or observational studies to investigate the efficacy and side effects of PD-1/PD-L1 monotherapy or combination therapy for lung cancer patients. The reported results of the included studies must include at least 1 of the following information: treatment-related death, treatment-related adverse events, any serious events, or any events leading to discontinuation. Review articles, commentaries, editorials, protocols, case series, or case reports would be firstly excluded from the inclusion criteria. If the enrolled study met the above requirements, but the control group was a placebo rather than an antitumor drug, the study would also be excluded from the final comprehensive analysis.
2.2. Search strategy
Original articles, related to results of prospective clinical trials of PD1/PD-L1 inhibitor regimens for lung cancer patients, were verified by a PubMed search. The date was defined from January 22, 2013 to February 28, 2019. Key words were displayed just as the followings: “lung cancer,” “NSCLC,” “SCLC,” “non-small cell lung cancer,” “small cell lung cancer,” “PD1/PD-L1,” “nivolumab,” “BMS-963558,” “pembrolizumab,” “MK-3475,” “atezolizumab,” “MPDL3280A,” “Avelumab,” “Durvalumab,” “safety,” and “toxicity”. Studies were limited in human beings, shown in full text, abstract, or poster form. Four members of our team (Z.Z., S.Z., Y.Z., and Q.Z.) were appointed to identify their eligibility independently. References from review articles, editorials, and included studies were reviewed and cross-referenced to check completeness. If no useful information was collected, we would try to get in touch with the corresponding author for more information, or the study would be precluded from the final meta-analysis. The characteristics of enrolled studies, including first author, year of publication, journal of article publication, drug name, treatment regimen, study design, phase, number of patients, number of PD-1/PD-L1-related death, and baseline demographic characteristics were collected and would be displayed in a table.
2.3. Assessment of study quality and publication bias
Risk of bias was evaluated by the Cochrane Collaboration tool for assessing risk of bias in randomized trials.[24] The publication bias was evaluated by Funnel plot and Egger's test.[25,26] Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting would be assessed by 4 members of our team independently and shown in a figure together. Finally, the corresponding author of the article would combine all the results to make the final decision.
2.4. Outcome and exposure of interest
The primary data was incidence rate of PD-1/PD-L1 treatment-related death. Incidence of PD-1/PD-L1 inhibitor treatment-related adverse events, any serious events, any events leading to discontinuation were also taken into account for comprehensive evaluation.
2.5. Assessment of heterogeneity and statistical analysis
Newcastle–Ottawa[27] scale, proposed by the Cochrane Collaboration, was used to evaluate the quality of study. Cochrane's Q statistic and the I2 statistic were used for accessing the heterogeneity among studies just as proposed by Higgins et al,[28] while Harbord test was taken to check publication bias for all enrolled studies. Heterogeneity was considered low, moderate or high for I2 values < 25%, 25% to 50%, and >50%, respectively. Risk ratio (RR), and 95% confidence interval (CI) would be calculated by random effect for the heterogeneity inherent in the data.[29]P < .05 was considered to be statistically significant for all the results of meta-analysis. Statistical tests were all two-sided. Meta-analysis was performed by Review Manager (version 5.3, Nordic Cochrane Center: Copenhagen, Denmark). We divided all the data into subgroup by drug type (PD-1 or PD-L1 inhibitor) and treatment regimens. We performed a number of subgroup analysis to assess the potential association between PD-1/PD-L1 inhibitor and chemotherapy in overall safety evaluation.
3. Results
3.1. Literature search results
After preliminary literature reading and screening, a total of 126 articles were deemed to meet our preliminary screening criteria. We read and reviewed the abstracts of the documents and found that 14 of them met our final inclusion criteria and were taken to be in the meta-analysis.[1–14] The flow diagram of the meta-analysis is displayed in Figure 1, while the risk of bias summary is shown in Figure 2. All the included studies had a control group.
Figure 1.
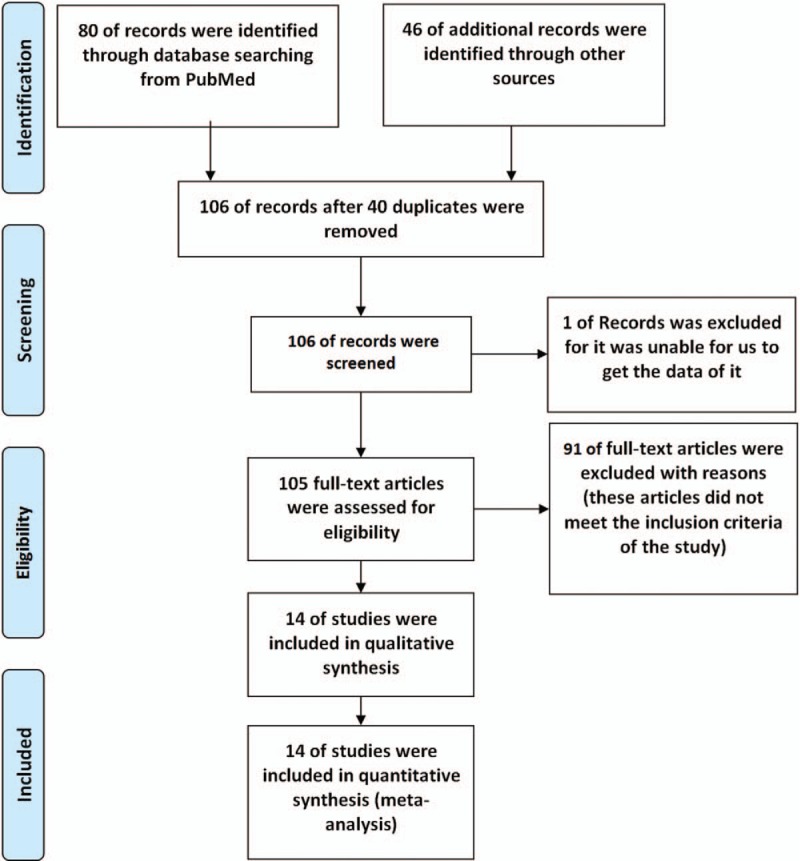
Study flow diagram of inclusion.
Figure 2.
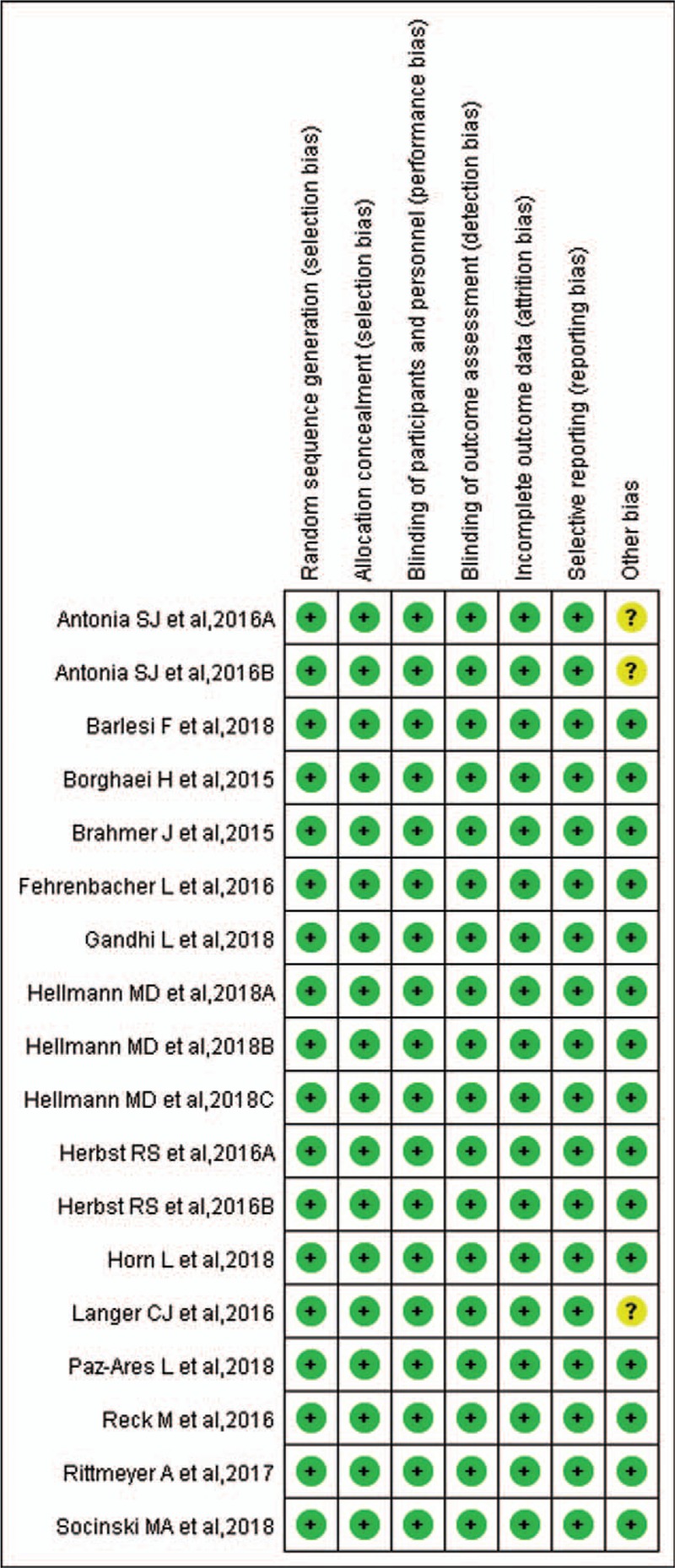
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
3.2. Characteristics of identified trials
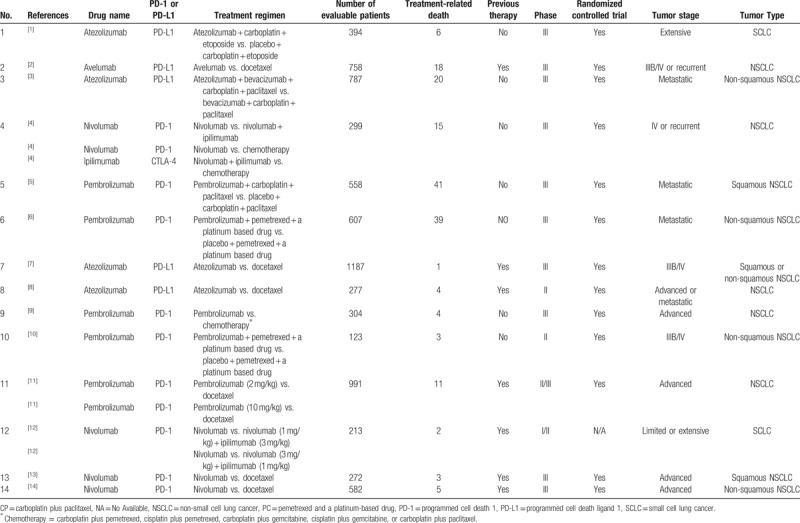
The basic characteristics of 14 enrolled studies are shown in Table 1. They were divided into 3 groups according to the treatment regimens. The specific grouping schemes were listed as follows: group A (PD-1/PD-L1 + chemotherapy vs. chemotherapy),[1,3,5,6,10] group B (PD-1/PD-L1 vs. chemotherapy),[2,4,7–9,11,13,14] and group C (PD-1/PD-L1 vs. PD-1/PD-L1 + CTLA-4).[4,12] Among all the clinical trials, 10 were phase III clinical trials,[1–7,9,13,14] 2 were phase II clinical trials,[8,10] 1 was phase I/II clinical trial,[12] and 1 was phase II/III clinical trial.[11] A total of 7352 participants were included in the clinical trials, of which 172 were reported to be related to treatment-related deaths. Among all the enrolled clinical trials, 12 were related to NSCLC,[2–11,13,14] and 2 were just related to small cell lung cancer (SCLC).[1,12] Seven trials (1 SCLC and 6 NSCLC) had received other antitumor treatments before receiving PD1/PD-L1 medication,[2,7,8,11–14] while PD-1/PD-L1 treatment regimen was prescribed as the first-line therapy for the other 7 trials.[1,3–6,9,10] The drugs used in 9 clinical trials were PD-1 inhibitors,[4–6,9–14] while they were PD-L1 inhibitors in the other 5 clinical trials.[1–3,7,9]
Table 1.
Basic characteristics of the included studies.
3.3. Risk of bias
Newcastle–Ottawa scale was used to evaluate study quality and risk of bias in enrolled studies. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias) would be assessed by 4 members of our team independently and shown in Figure 2. Publication bias, evaluated by Harbord-Egger test, could be seen in funnel plot (Figs. 3–5).
Figure 3.
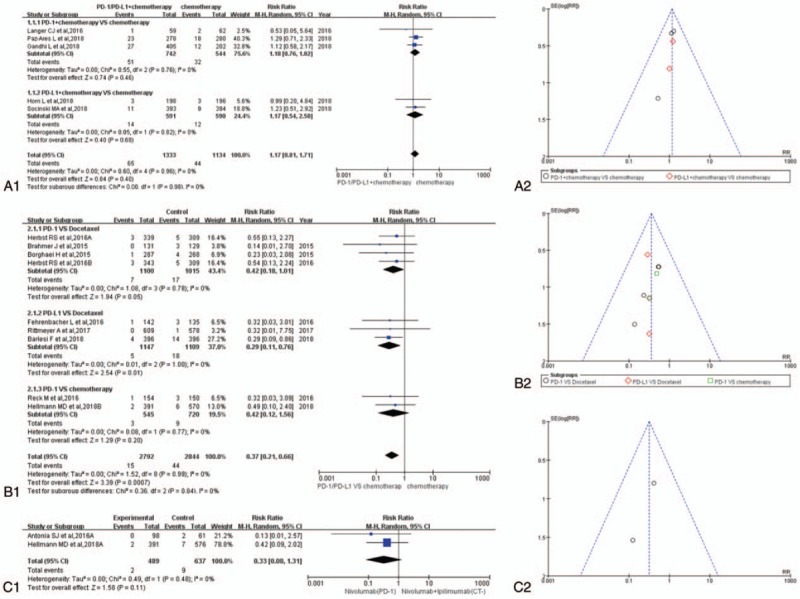
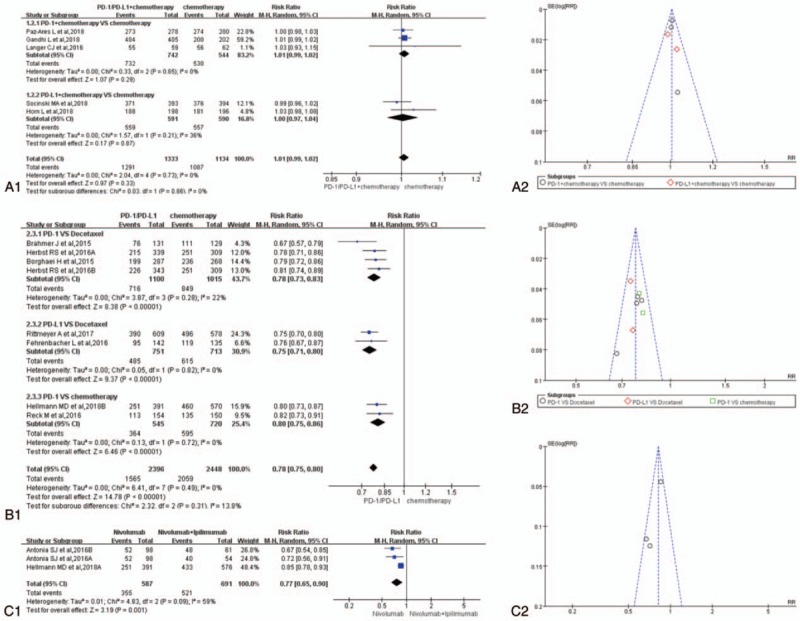
Forest plots and funnel plots for the risk ratio of overall treatment-related adverse events. (A1) Forest plots for the risk ratio of overall treatment-related adverse events (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (A2) Funnel plots for the risk ratio of overall treatment-related adverse events (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (B1) Forest plots for the risk ratio of overall treatment-related adverse events (PD-1/PD-L1 vs. chemotherapy). (B2) Funnel plots for the risk ratio of overall treatment-related adverse events (PD-1/PD-L1 vs. chemotherapy). (C1) Forest plots for the risk ratio of overall treatment-related adverse events (nivolumab vs. nivolumab + ipilimumab). (C2) Funnel plots for the risk ratio of overall treatment-related adverse events (nivolumab vs. nivolumab + ipilimumab). PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1.
Figure 5.
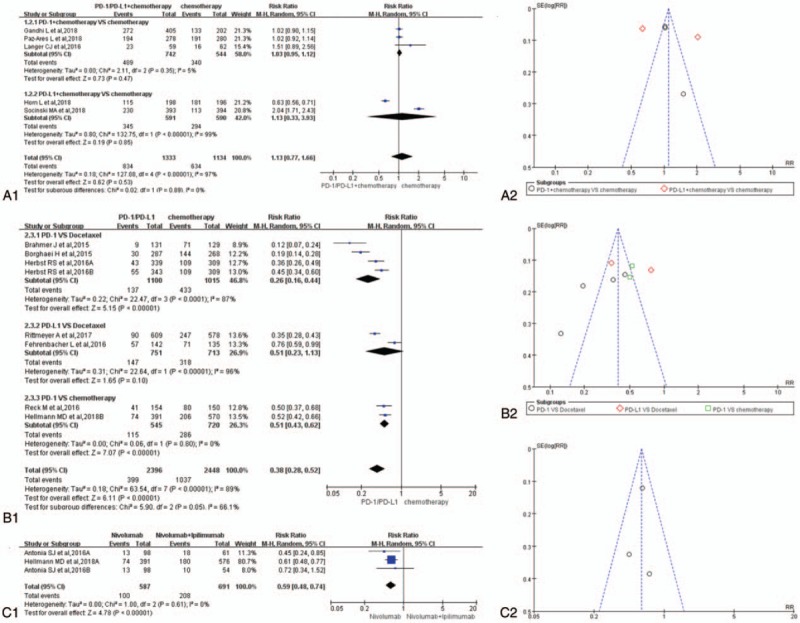
Forest plots and funnel plots for the risk ratio of adverse events leading to discontinuation. (A1) Forest plots for the risk ratio of adverse events leading to discontinuation (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (A2) Funnel plots for the risk ratio of adverse events leading to discontinuation (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (B1) Forest plots for the risk ratio of adverse events leading to discontinuation (PD-1/PD-L1 vs. chemotherapy). (B2) Funnel plots for the risk ratio of adverse events leading to discontinuation (PD-1/PD-L1 vs. chemotherapy). (C1) Forest plots for the risk ratio of adverse events leading to discontinuation (nivolumab vs. nivolumab + ipilimumab). (C2) Funnel plots for the risk ratio of adverse events leading to discontinuation (nivolumab vs. nivolumab + ipilimumab). PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1.
3.4. RR of treatment-related death
The RR of treatment-related death for group A is shown in Figure 6A (RR = 1.17, 95% CI: [0.81, 1.71], I2 = 0%, Z = 0.84 [P = .40]),[1,3,5,6,10] while the result of group C is shown in Figure 6C (RR = 0.33, 95% CI: [0.08, 1.31], I2 = 0%, Z = 1.58 [P = .11]).[4,12] The results of group A and group C were of no statistical significance. Unlike group A and group C, the results of group B showed that the incidence risk of drug-related deaths for PD-1/PD-L1 inhibitors was significantly lower than that of the control group (RR = 0.37, 95% CI: [0.21, 0.66], I2 = 0%, Z = 3.39 [P = .0007]; Fig. 6B).[2,4,7–9,11,13,14] Subgroup analysis of group B showed the same trend (Fig. 6B1). Funnel plots of them are provided in Figure 6A2, B2, and C2. No heterogeneity was found among them (I2 = 0%).
Figure 6.
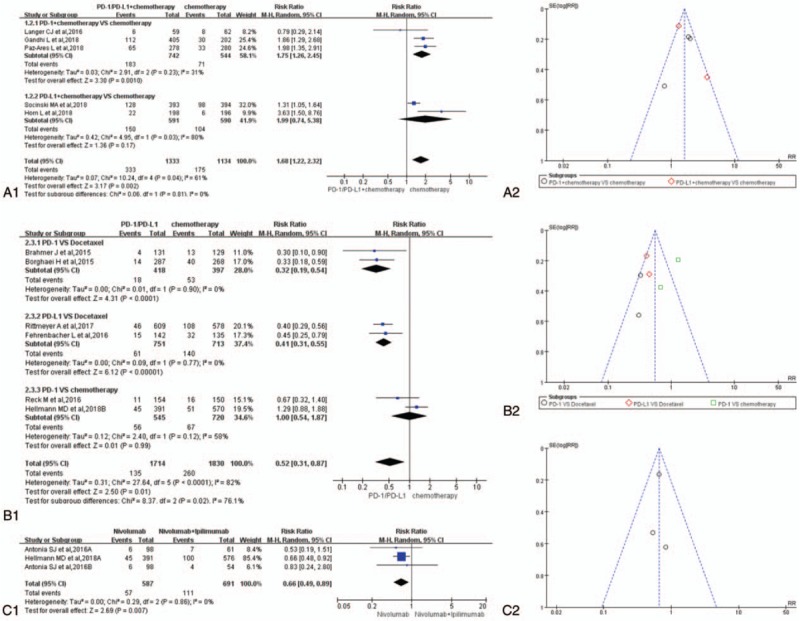
Forest plots and funnel plots for the risk ratio of treatment-related death. (A1) Forest plots for the risk ratio of treatment-related death (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (A2) Funnel plots for the risk ratio of treatment-related death (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (B1) Forest plots for the risk ratio of treatment-related death (PD-1/PD-L1 vs. chemotherapy). (B2) Funnel plots for the risk ratio of treatment-related death (PD-1/PD-L1 vs. chemotherapy). (C1) Forest plots for the risk ratio of treatment-related death (nivolumab vs. nivolumab + ipilimumab). (C2) Funnel plots for the risk ratio of treatment-related death (nivolumab vs. nivolumab + ipilimumab). PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1.
3.5. RR of overall treatment-related adverse events
Fourteen studies were taken into account for the meta-analysis of overall treatment-related adverse events.[1–14] There was no statistically significant difference in the analysis results of group A for overall treatment-related adverse events between the experimental group and the control group (RR = 1.01, 95% CI: [0.99, 1.02], I2 = 0%, Z = 0.97 [P = .33]; Fig. 3A1).[1,3,5,6,10] The results of the meta-analysis for group B were gathered at the bottom of Figure 3B1. Different from the above results of group A, the RR of PD-1/PD-L1 inhibitor group was significantly lower than that of the control group, furthermore the difference was statistically significant (RR = 0.78, 95% CI: [0.75, 0.80], I2 = 0%, Z = 14.78 [P < .00001]; Fig. 3B1).[2,4,7–9,11,13,14] Similar to the results of group B, the meta-analysis of group C was displayed in (RR = 0.77, 95% CI: [0.65, 0.90], I2 = 59%, Z = 3.19 [P = .001]) Figure 3C1. The funnel plots of all the enrolled 14 studies were shown in Figure 3A2, B2, and C2 independently according to the group type. Heterogeneity could be seen in (I2 = 39%) Figure 3C1.[4,12]
3.6. RR of treatment-related adverse events for grade 3 to 5
Thirteen studies were taken into account for evaluation of treatment-related adverse events for grade 3 to 5.[1,3–14] There was no statistically significant difference in the analysis results of group A for overall treatment-related adverse events between the experimental group and the control group (RR = 1.13, 95% CI: [0.77, 1.66], I2 = 97%, Z = 0.62 [P < .00001]; Fig. 4A1).[1,3,5,6,10] The results of the meta-analysis for group B were gathered at the bottom of Figure 4B1. Different from the above results of group A, the RR of PD-1/PD-L1 inhibitor group was significantly lower than that of the control group, furthermore the difference was statistically significant (RR = 0.38, 95% CI: [0.28, 0.52], I2 = 89%, Z = 6.11 [P < .00001]; Fig. 4B1).[4,7–9,11,13,14] Similar to the results of group B, the meta-analysis of group C was displayed in (RR = 0.59, 95% CI: [0.48, 0.74], I2 = 0%, Z = 4.78 [P < .00001]) Figure 4C1. The funnel plots of all the enrolled 14 studies were shown in Figure 4A2, B2, and C2 independently according to the group type. Publication bias could be seen in Figure 4A2 and B2. Obvious heterogeneity could be seen in Figure 4A1 and B1.
Figure 4.
Forest plots and funnel plots for the risk ratio of treatment-related adverse events for grade 3 to 5. (A1) Forest plots for the risk ratio of treatment-related adverse events for grade 3 to 5 (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (A2) Funnel plots for the risk ratio of treatment-related adverse events for grade 3 to 5 (PD-1/PD-L1 + chemotherapy vs. chemotherapy). (B1) Forest plots for the risk ratio of treatment-related adverse events for grade 3 to 5 (PD-1/PD-L1 vs. chemotherapy). (B2) Funnel plots for the risk ratio of treatment related-adverse events for grade 3 to 5 (PD-1/PD-L1 vs. chemotherapy). (C1) Forest plots for the risk ratio of treatment-related adverse events for grade 3 to 5 (nivolumab vs. nivolumab + ipilimumab). (C2) Funnel plots for the risk ratio of overall treatment-related adverse events (nivolumab vs. nivolumab + ipilimumab). PD-1 = programmed cell death 1, PD-L1 = programmed cell death ligand 1.
3.7. RR of adverse events leading to discontinuation
Thirteen studies, reported with the data of adverse events leading to discontinuation, were taken into account for the meta-analysis.[1,3–14] The data analysis of group A showed that the RR of the experimental group was significantly higher than that of the control group (RR = 1.68, 95% CI: [1.22, 3.32], I2 = 61%, Z = 3.17 [P = .002]; Fig. 5A1),[1,3,5,6,10] while the opposite meta-analysis results of group B and group C were shown in (RR = 0.52, 95% CI: [0.31, 0.87], I2 = 82%, Z = 2.50 [P = .01]) Figures 5B1 and (RR = 0.66, 95% CI: [0.49, 0.89], I2 = 0%, Z = 2.69 [P = .007]) C1.[4,7–9,12–14] The funnel plots of all the enrolled 13 studies were shown in Figure 5A2, B2, and C2 independently according to the group type. Publication bias could be seen in Figure 5B2.[4,7–9,13,14] Heterogeneity could be seen in Figure 5A1 (I2 = 61%) and B1 (I2 = 58%).[1,3–10,13,14]
4. Discussion
Treatment regimens, including targeted therapies for those with oncogenic alterations, anti-programmed death 1 (PD-1) mono-therapy for those with programmed death ligand 1 (PD-L1) expression on at least 50% of tumor cells,[9] anti-PD-1 plus platinum-doublet chemotherapy for those with non-squamous cancer,[6] and anti-PD-L1 antibody regardless of PD-L1 expression were recommended for metastatic NSCLC. PD-1 and PD-L1 inhibitors have shown promising efficacy and acceptable safety profiles for lung cancer patients, especially for NSCLC.[2–11,13,14,30,31] While PD-1/PD-L1 inhibitors had achieved satisfactory clinical efficacy, new toxic side effects had been reported and some of them were fatal.[1–6] As the completion of 6 large clinical trials of PD-1/PD-L1 related to lung cancer patients in 2018,[1–6] we had the opportunity to collect their data and conducted a comprehensive analysis for evaluating the safety of PD-1/PD-L1 inhibitors. We believed that we could get a new and more accurate conclusion of the PD-1/PD-L1 safety assessment.
All enrolled studies were deemed to be of high quality. The quality evaluation results were summarized in Figure 2. It meant that the results of our analysis originated from these high-quality data were much more credible.[1–14] Compared with chemotherapy, the mortality associated with PD-1/PD-L1 treatment was significantly lower than that of the chemotherapy group, and the difference was statistically significant (Fig. 6B1).[2,4,7–9,11,13,14] Whether combined with chemotherapy (Fig. 6A1) or other types of immune inhibitors (Fig. 6C1), it did not increase the patient's treatment-related mortality.[1,3–6,10,12] Similar analysis results could also be seen for the RR of overall treatment-related adverse events (Fig. 3A1, B1, and C1). Heterogeneity could be seen in Figure 3C1 (I2 = 39%).[4,12] The study data of group C could not be taken into further subgroup analysis to clarify the source of heterogeneity, so the heterogeneity was considered to be derived from included data themselves.[4,12]
The RR of PD-1/PD-L1-related adverse events for grade 3 to 5 was significantly lower than that of the control group, furthermore the difference was statistically significant (RR = 0.38, 95% CI: [0.28, 0.52], I2 = 89%, Z = 6.11 [P < .00001]; Fig. 4B1).[4,7–9,11,13,14] Similar to the results of group B, the meta-analysis of group C was displayed in (RR = 0.59, 95% CI: [0.48, 0.74], I2 = 0%, Z = 4.78 [P < .00001]) Figure 4C1. Different from the former ones (Figs. 3 and 6), publication bias could be seen in Figure 4A2 and B2. Obvious heterogeneity could be seen in Figure 4A1 and B1. Subgroup analysis revealed that the heterogeneity in group A was mainly derived from 2 studies in the PD-L1 inhibitor group.[1,3] A subgroup analysis of group B showed that the heterogeneity was mainly derived from 2 subgroups (PD-1 vs. docetaxel) and (PD-L1 vs. docetaxel) (Fig. 4B1).[4,7–9,11,13,14]
For adverse events leading to discontinuation,[1,3–14] the data analysis of group A showed that the RR of the experimental group was significantly higher than that of the control group (RR = 1.68, 95% CI: [1.22, 3.32], I2 = 61%, Z = 3.17 [P = .002]; Fig. 5A1),[1,3,5,6,10] while the opposite meta-analysis results of group B and group C were shown in (RR = 0.52, 95% CI: [0.31, 0.87], I2 = 82%, Z = 2.50 [P = .01]) Figure 5B1 and (RR = 0.66, 95% CI: [0.49, 0.89], I2 = 0%, Z = 2.69 [P = .007]) C1.[4,7–9,12–14] It meat that PD-1/PD-L1 plus chemotherapy would increase the RR of adverse events leading to discontinuation, which were different from the former analysis results (Figs. 3, 4, 6). Publication bias could be seen in Figure 5B2.[4,7–9,13,14] Heterogeneity could be seen in (I2 = 61%) Figure 5A1 and (I2 = 58%) B1.[1,3–10,13,14] We performed a subgroup analysis of group A and the results showed that the heterogeneity was mainly from the PD-L1 subgroup (PD-L1 + chemotherapy vs. chemotherapy).[1,3] The similar subgroup analysis showed that the heterogeneity of group B was mainly from the PD-1 subgroup (PD-1 vs. chemotherapy).[4,9]
After a comprehensive evaluation of all enrolled studies, we found that PD-1/PD-L1 inhibitors were excellent antitumor drugs with good safety and low incidence of adverse events in patients with lung cancer.
5. Conclusions
Compared with chemotherapy, RR of the treatment-related deaths associated with PD-1/PD-L1 inhibitor was significantly lower than that of the chemotherapy group, while it did not increase the RR when they were combined with chemotherapy or other drugs. When PD-1/PD-L1 was combined with chemotherapy, it only increased the RR of adverse events leading to discontinuation.
Author contributions
Conceptualization: Shuisheng Zhang, Yuan Tian.
Data curation: Heli Shang, Alei Feng, Shuisheng Zhang, Yantao Mao, Kun Liu, Yuan Tian.
Formal analysis: Zewen Zhang, Shuisheng Zhang, Yantao Mao.
Investigation: Xiaowei Yang.
Resources: Yantao Mao, Qingshan Zhu, Kun Liu.
Software: Zewen Zhang, Kun Liu.
Supervision: Heli Shang, Yi Zhao, Qingshan Zhu, Kun Liu, Yuan Tian.
Validation: Zewen Zhang, Xiaowei Yang, Yi Zhao.
Writing – original draft: Yuan Tian.
Writing – review & editing: Alei Feng, Xiaowei Yang, Yi Zhao, Qingshan Zhu, Yuan Tian.
Yuan Tian orcid: 0000-0002-2296-1246.
Footnotes
Abbreviations: CI = confidence interval, NSCLC = non-small cell lung cancer, PD-1 = programmed cell death-1, PD-L1 = programmed cell death ligand 1, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratio, SCLC = small cell cancer.
HS, ZZ, AF and XY contributed equally to this work.
This study belongs to the type of data analysis and rearrangement, and does not involve human or animal related ethical issues.
The authors have no conflicts of interest to disclose.
References
- [1].Horn L, Mansfield AS, Szczęsna A, et al. IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- [2].Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468–79. [DOI] [PubMed] [Google Scholar]
- [3].Socinski MA, Jotte RM, Cappuzzo F, et al. IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- [4].Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Paz-Ares L, Luft A, Vicente D, et al. KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. [DOI] [PubMed] [Google Scholar]
- [6].Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- [7].Rittmeyer A, Barlesi F, Waterkamp D, et al. OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fehrenbacher L, Spira A, Ballinger M, et al. POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837–46. [DOI] [PubMed] [Google Scholar]
- [9].Reck M, Rodríguez-Abreu D, Robinson AG, et al. KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- [10].Langer CJ, Gadgeel SM, Borghaei H, et al. KEYNOTE-021 investigators. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- [12].Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. [DOI] [PubMed] [Google Scholar]
- [13].Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012;98:751–5. [DOI] [PubMed] [Google Scholar]
- [16].Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Khunger M, Jain P, Rakshit S, et al. Safety and efficacy of PD-1/PD-L1 inhibitors in treatment-naive and chemotherapy-refractory patients with non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer 2018;19:e335–48. [DOI] [PubMed] [Google Scholar]
- [18].Xu X, Huang Z, Zheng L, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer. Int J Cancer 2018;142:2344–54. [DOI] [PubMed] [Google Scholar]
- [19].Chen S, Hu B, Li H. A meta-analysis of nivolumab for the treatment of advanced non-small-cell lung cancer. Onco Targets Ther 2018;11:7691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou Y, Chen C, Zhang X, et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer 2018;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016;7:73068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou GW, Xiong Y, Chen S, et al. Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: a meta-analysis of randomized clinical trials. Medicine (Baltimore) 2016;95:e4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [26].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2009. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed July 6, 2012 [Google Scholar]
- [28].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [30].Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016;21:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu SV, Camidge DR, Gettinger SN, et al. Atezolizumab (atezo) plus platinum-based chemotherapy (chemo) in non-small cell lung cancer (NSCLC): update from a phase Ib study. J Clin Oncol 2017;35Suppl 15:9092–19092. [Google Scholar]


