Abstract
Background:
Statins have key lipid-lowering, anti-inflammatory, and anti-oxidative effects. However, it remains unclear whether statins are beneficial to patients with Parkinson's disease (PD). This study aimed to evaluate the relationship between statins and PD through a systematic review.
Methods:
This study adhered to the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Potentially relevant case-control or cohort studies published before March 2018 were identified by searching the MEDLINE (PubMed), EMBASE (OVID), CENTRAL (Cochrane Library), CNKI, WANGANG, VIP, CBM, CMCC, Clinicaltrials.gov, ProQuest, Opengray, and ISI Proceedings databases and conducting a manual search. Summarized relative risks (RRs) and 95% confidence intervals (CIs) were calculated using a fixed effect model. Sensitivity and subgroup analyses were also performed.
Results:
The meta-analysis included 17 studies (3,845,303 patients; 8 case-control and 9 cohort studies), including 5 articles not cited by other studies. We searched the Chinese database, but unfortunately, no Chinese literature can be included in the study. Briefly, statins could decrease the risk of PD, with a summary OR of 0.92 (95% CI: 0.86–0.99). A sensitivity analysis demonstrated the robustness of the results. Subgroup analyses revealed heterogeneity across the studies in terms of subject race, study type, reporting style, quality, statins type, and time for taking statins.
Conclusion:
Our study provides evidence that statins, especially atorvastatin, can reduce the risk of PD. Different time of statins using has different effects on PD. However, additional randomized controlled trials and observational studies are needed to confirm this conclusion.
Registration Id:
PROSPERO CRD: 42018095580
Keywords: meta-analysis, Parkinson's disease, statins, systematic review
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease.[1] PD not only reduces the quality of life of patients, but also places heavy financial burdens on their families.[2] The pathogenesis of PD remains unclear and may be related to oxidative stress, mitochondrial dysfunction, and nerve inflammation.[3] Our previous studies confirmed the anti-oxidative and anti-neuroinflammatory effects of statins.[4] Therefore, anti-oxidative stress, mitochondrial protection, and neuroinflammatory antagonists may affect the risk of PD.
Mutations and accumulation of α-synuclein can cause dopaminergic neuronal cell death, which has been suggested as a major contributor to PD. Research attention has been directed toward the role of the mevalonate pathway in the incidence of PD.[5] However, statins can reduce damage to dopaminergic neurons by inhibiting the mevalonate pathway.[1] Statins can also ameliorate enzyme complex dysfunction, a key step in the progression of neurodegenerative disorders.
Currently, the relationship between statins and PD remains unclear. Recent studies have indicated the potential abilities of these drugs to attenuate motor and non-motor symptoms and ameliorate the incidence and/or progression of PD.[6–8] Sheng observed a significant reduction in the risk of PD for statin users,[9] while Lin reported that statin use was associated with a lower incidence of PD.[10] By contrast, Huang et al[11] reported that statin use was associated with a higher risk of PD after adjusting for cholesterol levels. To clarify this issue, we performed a meta-analysis to assess the association between statin use and the risk of PD under and the acquired PROSPERO CRD (No. 42018095580).
2. Methods
The ethical approval was not necessary because the research is not involved in human beings or experimental subjects. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies.[12] This study adhered to the guideline of the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA).[13] The protocol registration is listed in the PROSPERO International Prospective Register for Systematic Reviews. The registration number is CRD42018095580
2.1. Eligibility criteria
Eligibility criteria were defined using a participants, interventions, comparisons, outcomes, and study design (PICOS) model.[13] The participants were patients with PD, and the intervention was statin use. Participants were compared with normal controls, and the outcome was the risk of PD. Studies with a case-control or cohort design were included.
2.2. Search strategy
A systematic literature search of the MEDLINE (PubMed), Web of Science, EMBASE (OVID), CENTRAL (Cochrane Library), CNKI, WANFANG, VIP, CBM, CMCC, Clinicaltrials.gov, ProQuest, Opengray, and ISI Proceedings databases was conducted to identify relevant literature. Additional records were identified through a manual search.
2.3. Study selection
Two authors (YJQ and QL) performed a 2-level eligibility assessment by first checking the title and abstract and then checking the full text of each record. Another 2 authors (ZYD and LAR) subsequently checked the included articles. Disagreements were resolved through consultation with 2 additional authors (HJR and WJN).
2.4. Data extraction
Three authors (QL, LAR, and HJR) independently extracted data from tables. The following information was obtained from each of the included studies: surname of the first author, year of publication, study period, country of study, design study (case control or cohort), number of participants and PD cases, description of statins and drugs used, PD diagnostic methods, adjusted variables, estimation of effects (adjusted odds ratios [ORs], relative risks [RRs], or hazard ratios [HRs]), and 95% confidence intervals (CIs). The maximum adjusted effect estimates were extracted in the overall and subgroup analyses.
2.5. Assessment of methodologic quality
The quality of each included study was assessed independently by 3 authors (QL, LAR, and HJR) using the Newcastle Ottawa Scale (NOS). The NOS evaluates 3 areas: cohort study selection, comparability, and case study results (or exposure for controlled studies). The highest possible scores for each area are 4, 2, and 3 stars, respectively, with total scores ranging from 0 to 9 stars. A score of 6 stars indicates medium quality, while scores greater or less than 6 stars are considered high or low quality, respectively. Differences in the analysis were resolved through discussion and consensus.
2.6. Synthesis of the results and analysis
Differences between RR, HR, and OR could be ignored[13] because of the low incidence of PD. Therefore, we calculated the summed RR and 95% CI of combined case-control and cohort studies. We assessed heterogeneity between studies using Cochrane's Q test and I2 statistics. For the Q test, a P value < .10 was considered to indicate significant heterogeneity in the Q test. Additionally, I2 values of 0% to 25%, >25% to 50%, >50% to 75%, and >75% indicated insignificant, low, moderate, and high heterogeneity, respectively.[14] A DerSimonian and Laird random effects model was used to calculate the summary RR in the case of moderate heterogeneity, which allowed the estimation of a different effect size for each meta-analyses. We used the original study results from multivariate models to ensure the most complete adjustments to potential confounders. Publication bias was evaluated using the Begg rank correlation test and Egger linear regression test, and a P value < .10 was considered to indicate statistical significance. In addition, a funnel plot was applied to sensitivity and subgroup analyses. We conducted a subgroup meta-analysis by studying the design types, study areas, adjustment variables, and research quality. We calculated the combined RRs of studies providing these specific data to examine the association between individual (or long-term) statin use and the risk of PD. All analyses were performed using Review Manager Software (version 5.3).
3. Results
3.1. Study selection
Figure 1 depicts a PRISMA diagram of the selection process, which resulted in the final inclusion of 17 studies. The strategy identified 743 records, from which 131 duplicates were removed. Furthermore, the titles and abstracts of 560 studies did not meet our criteria. An additional 35 studies were excluded after a review of the full text for eligibility. Finally, we identified 17 articles for inclusion in our meta-analysis. Unfortunately, no suitable Chinese literature was identified.
Figure 1.
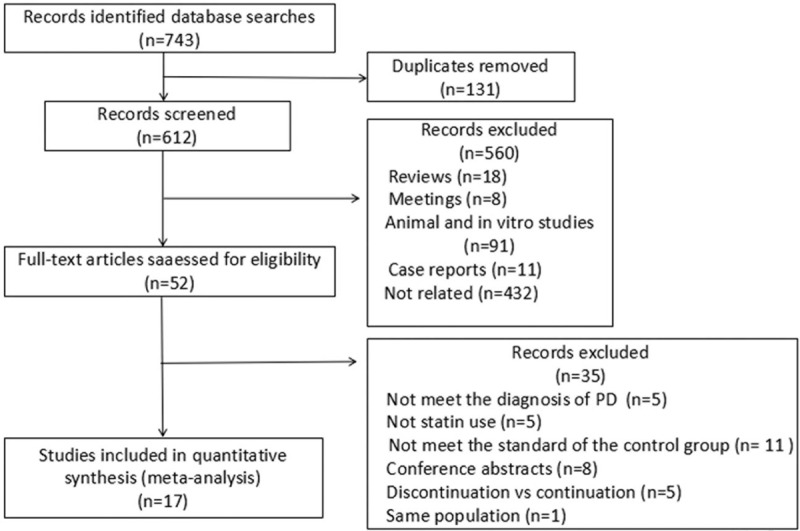
PRISMA diagram of the selection process. PRISMA = Preferred Reported Items for Systematic Reviews and Meta-Analyses.
3.2. Study characteristics
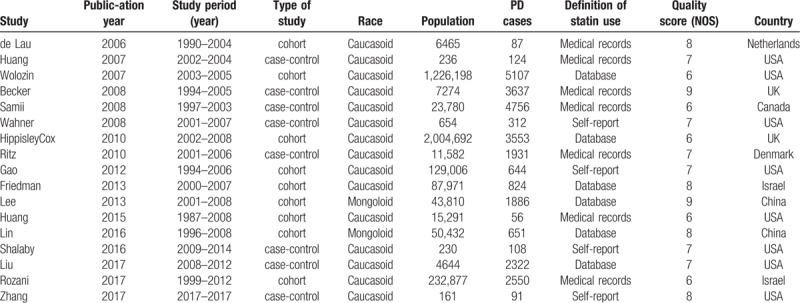
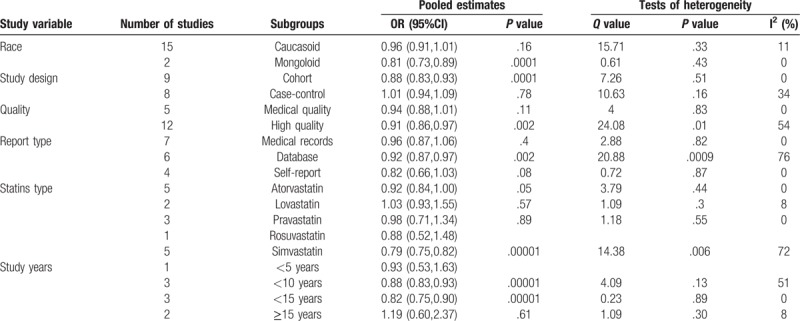
The 17 studies summarized in Table 1 met our inclusion criteria. We identified 9 cohort studies and 8 case-control studies that included a total of 3,845,303 participants and 28,639 incident cases of PD. The studies were published from 1990 to 2017, a span of 21 years.[11] Among the studies, 15 involved Caucasian and 2 involved Asian populations.[10] Six articles were based on datasets, 7 involved medical records, and 4 involved self-reported. Eleven studies were determined to be of high quality, while 6 were of medium quality. We determined a mean quality score of 7.18 for the 17 studies (Table 1).
Table 1.
Main characteristics of the eligible studies.
3.3. PD in patients treated with statins
We combined the 17 studies using a fixed-effects model and obtained a summary OR of 0.92 (95% CI: 0.88–0.97). Moderate homogeneity was detected across the studies (Cochrane Q value = 26.21, P = .0008; I2 = 39%) (Fig. 2).
Figure 2.
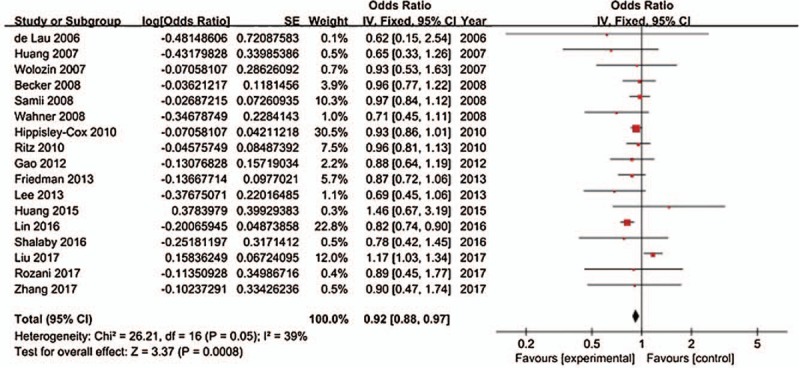
Forest plot in overall analysis.
3.4. Publication bias
The visual interpretation and asymmetry analysis of funnel plots of publications are not very reliable when a small number of studies are included. Figure 3 shows a funnel plot indicating the existence of publication bias. Egger regression test of funnel asymmetry revealed highly significant publication bias (P value < .05).
Figure 3.
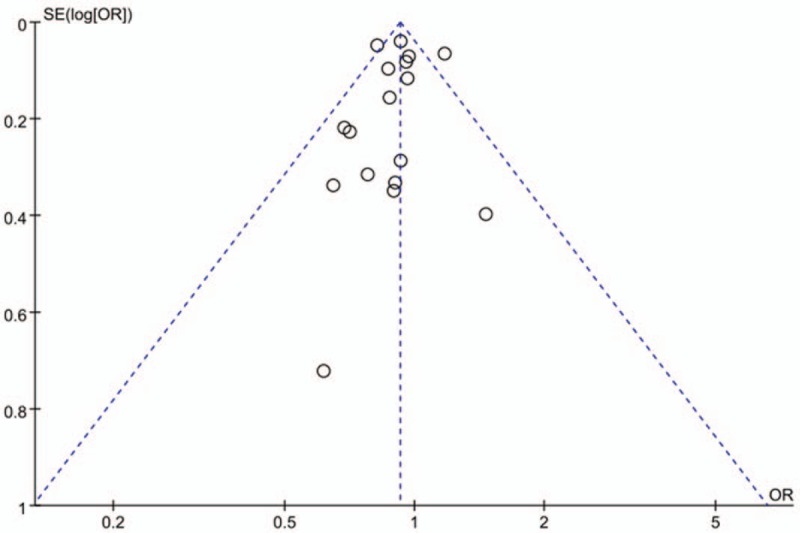
Funnel plot (Publication bias).
3.5. Sensitivity and subgroup analyses
We used sensitivity analyses to test the stability of the outcomes. The pooled ORs were calculated by excluding 1 study at a time in a process that was repeated 17 times. During the elimination process, the removal of 1 article greatly reduced the associated bias (Q value = 12.12, P = .67, I2 = 0%).[15] Similarly, the removal of another document also greatly reduced the risk of bias.[10]
The subgroup analysis revealed heterogeneity among the high-quality studies (Q value = 24.08, P = .01, I2 = 54%), which might have been related to the presence of 2 heterogeneous studies[10,15] in this group. Regarding the report type, studies based on datasets exhibited greater heterogeneity (Q value = 20.88, P = .0009, I2 = 76%). In the analysis according to statin type, only atorvastatin was associated with statistically significant heterogeneity (Q value = 3.78, P = .44, I2 = 0%) (Table 2 and 3).
Table 2.
Subgroup analyses.
4. Discussion
4.1. Quality of the literature
This study included 17 observational studies, including 8 case-control and 9 cohort studies. Of these, 12 and 5 studies were determined to be of high quality and moderate quality, respectively, according to the NOS criteria. In the step of assessment of methodologic quality, the most frequent disagreements between reviewers are the outcome whether the follow-up is sufficient. PD as a chronic disease may not be found in short-term follow-up. However, in the cohort study, the age range of the population included in the different studies was different, and the follow-up time was different. In the study of Wolozin et al[16] is clear that the 2-year follow-up time is not scientific for the incidence of PD. The most frequent reasons were for medium quality compared to high quality is the part of research population selection. All of the studies adjusted potential confounding factors to ensure comparability among groups, and most corrected for age, sex, smoking status, and antipsychotic use.
4.2. Heterogeneity testing
The meta-analysis based on Cochrane Q test and the I2 statistical test included 17 observational studies. The analysis demonstrated a lower level of heterogeneity among the studies. Accordingly, we used a fixed-effects model to perform a statistical analysis. This heterogeneity may be attributable to differences in the types of study designs, locations of the study, basic characteristics of the study populations, methods of statin use, diagnostic criteria, and correction of relevant factors. Therefore, a subgroup analysis was performed to explore the source of heterogeneity.
Two articles with different levels of heterogeneity were based on cholesterol research. Liu et al[15] demonstrated that the use of statins (especially lipophilics) was associated with a higher risk of PD. In contrast to other studies, this study found that the risk of PD was highest during the initial period after statin use (<2.5 years). The results may be related to the limited duration of the study and the pathogenesis of PD. Huang also showed that higher cholesterol predicts lower risk of PD. But in this study, 95 cases had no follow-up results of the 293 cases. The risk of bias cannot be ignored. Therefore, large-scale long-term studies are needed to demonstrate the effects of statins on blood lipids in different periods of time on the risk of PD. Statins may have a potentially beneficial effect on neurodegenerative diseases due to their anti-inflammatory properties; however, their lipid lowering may be detrimental to PD. Yet, there is currently not enough research to prove it. In the subgroup analyses of study years, only 5 to 10 years of follow-up studies were statistically significant, and statins has a protective effect on PD duration. Differences in the efficacy of statins across ethnic groups may be attributable to racial differences between studies [Caucasian: Q value = 15.71, P = .33, I2 = 11%, OR = 0.96 (0.91, 1.01), P = .16 and Asian: Q valve = 0.61, P = .43, I2 = 0%, OR = 0.81 (0.73,0.89), P = .001]. This is consistent with previous reports of differences in the efficacy of statins among different racial groups. Therefore, diversity within the Caucasian race may explain the wider CI in this subgroup. Furthermore, fewer studies of Asian populations may have been conducted. Only 2 such studies were included in our meta-analysis.
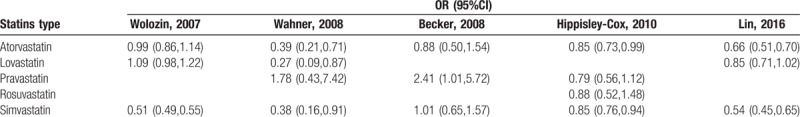
The subgroup analyses of different types of statins identified only atorvastatin as statistically significant [Q value = 3.79, P = .44, I2 = 0%, OR = 0.92 (0.84, 1.00), P = .05]. In other words, atorvastatin exhibits a protective effect. In Statins type analyses (Table 3), Becker study[17] was the source of major heterogeneity, and its study for Atorvastatin, Pravastatin and Simvastatin were not statistically significant. Currently, few studies have evaluated different statins, and those studies have exhibited relatively high heterogeneity. Related studies are needed to further explain the effects of different statins on PD.
Table 3.
Statins type analyses.
4.3. Animal and in vitro experiments
Statins can reduce the risk of PD by counteracting the degeneration of dopaminergic neurons, which may be associated with the ability of statins to directly stimulate sterol regulatory element-binding protein (SREBP) translocation. Similar results were observed in animal experiments and in vitro cellular experiments. SREBP-mediated transcriptional activity may restore the expression of presynaptic dopamine markers and contribute to the neuroprotection of dopaminergic neurons. A previous study by Eriksson indicated a dual role for high cholesterol in PD; specifically, cholesterol acts as both a protector against lysosomal membrane permeabilization and a stimulator of α-synuclein accumulation.[18] Tan also demonstrated that simvastatin could improve the function of the substantia nigra in the LPS-PD model by promoting neuronal repair and regeneration and inhibiting oxidative stress.[19]
4.4. Limitations of the research
This study had some limitations of note. First, all of the results were obtained from observational studies, which are susceptible to various biases. Accordingly, selective, information, confounding, and follow-up biases were unavailable in the cohort and case-control studies. Second, the RR, OR, and HR approximations were all considered ORs and used to calculate combined ORs in this study. Assuming a low disease incidence (usually <5%), the RR is numerically approximately equal to the OR; furthermore, the HR can be considered to include the time factor of the OR. However, the OR still tends to overestimate the RR. Third, the methods used to diagnose PD varied among the included studies, and most cases were diagnosed using medical records. Therefore, the included cases may have included some patients with secondary PD. Fourth, although the results of this study suggest that statin drugs can reduce the risk of PD, consistent doses and courses of treatment have not yet been established. Fifth, the numbers of subgroup analyses and analyses of different types of statins were small, and therefore, the results have a limited reference value. Sixth, all of the included studies’ countries have higher socioeconomic status. It is unable to compare the effects of nutrition on Parkinson's effects in statins. Therefore, it is necessary to include cases in economically underdeveloped areas. Seventh, at present, research on the application time, measurement, and types of statin drugs are still lacking. So, related researches are needed.
The pathogenesis of PD is unclear. It is also unclear how statins can protect PD, which may be related to inhibit the activation of pro-inflammatory molecules and microglia, attenuate the accumulation of α-synuclein, inhibit oxidative stress, regulate immune, increase the expression of neurotrophic factors, and lower cholesterol. Further research is needed to explore which machine make a more important role.
5. Conclusions
Our meta-analysis showed that statins use was associated with a modest reduction in the risk of PD. Atorvastatin has implications for the treatment of PD. Using statins for 10 to 15 years continuously has a protective effect on PD. However, we cannot clarify whether this conclusion is due to a causal effect or to an unmeasured confounding variable. In addition, it is needed that more controlled trials and observational studies about Chinese database to confirm this conclusion.
Author contributions
Conceptualization: Junqiang yan.
Data curation: Jing Tian, Anran Liu, Jiarui Huang, Xiaoyi Lai.
Formal analysis: Anran Liu, Jiannan Wu, Jiarui Huang.
Funding acquisition: Junqiang yan.
Methodology: Jing Tian.
Project administration: Liang Qiao, Mengmeng Shen, Xiaoyi Lai.
Software: Mengmeng Shen.
Supervision: Jiannan Wu.
Writing – original draft: Liang Qiao.
Writing – review & editing: Junqiang yan.
Junqiang yan orcid: 0000-0002-6489-2824.
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle–Ottawa Scale, OR = odds ratio, PD = Parkinson's disease, PRISMA = Preferred Reported Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trials.
This work was supported by the National Natural Science Fund of china (Grant No. U1304809).
The authors have no conflicts of interest to disclose.
References
- [1].Saeedi Saravi SS, Saeedi Saravi SS, Khoshbin K, et al. Current insights into pathogenesis of Parkinson's disease: approach to mevalonate pathway and protective role of statins. Biomed Pharmacother 2017;90:724–30. [DOI] [PubMed] [Google Scholar]
- [2].Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist 2011;17:244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zeng XS, Geng WS, Jia JJ, et al. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci 2018;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yan J, Xu Y, Zhu C, et al. Simvastatin prevents dopaminergic neurodegeneration in experimental parkinsonian models: the association with anti-inflammatory responses. PloS One 2011;6:e20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wood WG, Mΰller WE, Eckert GP. Statins and neuroprotection: basic pharmacology needed. Mol Neurobiol [Review] 2014;50:214–20. [DOI] [PubMed] [Google Scholar]
- [6].Carroll CB, Wyse RKH. Simvastatin as a potential disease-modifying therapy for patients with Parkinson's disease: rationale for clinical trial, and current progress. J Parkinson's Dis 2017;7:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deck BL, Rick J, Xie SX, et al. Statins and cognition in Parkinson's disease. J Parkinson's Dis 2017;7:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saeedi Saravi SS, Saeedi Saravi SS, Arefidoust A, et al. The beneficial effects of HMG-CoA reductase inhibitors in the processes of neurodegeneration. Metab Brain Dis 2017;32:949–65. [DOI] [PubMed] [Google Scholar]
- [9].Sheng Z, Jia X, Kang M. Statin use and risk of Parkinson's disease: a meta-analysis. Behav Brain Res 2016;309:29–34. [DOI] [PubMed] [Google Scholar]
- [10].Lin KD, Yang CY, Lee MY, et al. Statin therapy prevents the onset of Parkinson disease in patients with diabetes. Ann Neurol 2016;80:532–40. [DOI] [PubMed] [Google Scholar]
- [11].Huang X, Alonso A, Guo X, et al. Statins, plasma cholesterol, and risk of Parkinson's disease: a prospective study. Mov Disord 2015;30:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tveden-Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol 2018;123:233–5. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu G, Sterling NW, Kong L, et al. Statins may facilitate Parkinson's disease: Insight gained from a large, national claims database. Mov Disord 2017;32:913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wolozin B, Wang SW, Li NC, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 2007;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Becker C, Jick SS, Meier CR. Use of statins and the risk of Parkinson's disease: a retrospective case-control study in the UK. Drug Saf 2008;31:399–407. [DOI] [PubMed] [Google Scholar]
- [18].Eriksson I, Nath S, Bornefall P, et al. Impact of high cholesterol in a Parkinson's disease model: prevention of lysosomal leakage versus stimulation of alpha-synuclein aggregation. Eur J Cell Biol 2017;96:99–109. [DOI] [PubMed] [Google Scholar]
- [19].Tan W, Xue-bin C, Tian Z, et al. Effects of simvastatin on the expression of inducible nitric oxide synthase and brain-derived neurotrophic factor in a lipopolysaccharide-induced rat model of Parkinson disease. Int J Neurosci 2016;126:278–86. [DOI] [PubMed] [Google Scholar]


