Abstract
Rationale:
Solitary fibrous tumor(SFT) is a rare and distinct clinical entity. There are few descriptions in the literature regarding the PET manifestations of SFTs. Herein,we report a case of multiple malignant SFT with PET/CT imaging findings and PET/Contrast Enhanced CT image findings.
Patient concerns:
A 30-year-old woman presented with a history of a mass in neck increased gradually over 6 months without jaundice or other symptoms of obstruction.Serum laboratory results and tumor markers (AFP, CEA,CA199,CA724,CA153 and CA125)were normal. The whole body PET/CT scan showed lightly or mildly hypermetabolic and inhomogeneous metabolic which was different from other reports, and it was the first report of 18F-FDG-PET/CT findings of multiple malignant SFTs, which were confirmed by positive immunohistochemical staining for CD2-40, CD99, SMA and negative immunohistochemical staining for S100 and CD34.
Diagnoses:
She was diagnosed with multiple malignant solitary fibrous tumors which was confirmed by pathological results and 18F-FDG-PET/CT findings.
Interventions:
The patient didn’t receive any treatment because she was not suitable for surgery and refused any other therapy.
Outcomes:
The patient has been followed up for one year,and she was still alive.
Lessons:
SFTs should be detected early and treated early.It was of high value in the diagnosis and differential diagnosis of SFTs by PET/CT imaging findings, which can not only identify the benign and malignant lesions, but also identify the lesion involved range.
Keywords: fluorodeoxyglucose, PET/CT, solitary fibrous tumors
1. Introduction
Solitary fibrous tumor (SFT) in the pleura was first described by Klemperer and Rabin in 1931.[1] Solitary fibrous tumors are uncommon neoplasms of mesenchymal origin that could be either benign or malignant. Although SFTs most commonly occur in the pleura, numerous extra pleural sites of involvement have been reported. SFTs can be associated with hypoglycemia secondary to production of insulin-like growth factor, osteoarthropathy, arthralgia, and clubbing.[2] Overall, approximately 15% to 20% of SFTs are malignant, and even benign SFTs have indeterminate malignant potential. Therefore, complete resection is the treatment of choice.[3]
There are few descriptions in the literature regarding the PET manifestations of SFTs. While in this case the whole body 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) scan showed inhomogeneous and slightly or mildly hypermetabolism, with a standardized uptake value from 2.4 to 5.2, which was extremely different from other reported cases.[4]
To the best of our knowledge, this was the first reports of 18F-FDG-PET/CT findings of multiple malignant SFTs, which were confirmed by positive immunohistochemical staining for D2–40, CD99, SMA and negative immunohistochemical staining for S100 and CD34.
2. Case report
A 30-year-old woman presented with a history of a mass increased gradually in neck over 6 months. After 2 months, she felt pain gradually in the right chest and left shoulders. No jaundice or other symptoms of obstruction were found. Serum laboratory results and tumor markers (AFP, CEA, CA199, CA724, CA153, and CA125) were also within normal limits. The patient had never suffered from hypoglycemia and the fasting blood glucagon was within normal levels. The ultrasound showed a number of large soft tissue masses which located in the right neck with size 3.5cm × 3.3 cm, the left neck with size 2.3cm × 2.1 cm and size 4.0cm × 4.3 cm, the right supraclavicular fossa with size 4.7cm × 4.5 cm, the right axillary fossa with size 6.2cm × 4.1 cm, and the right retroperitoneal space with size 5.6cm × 4.3 cm, the left retroperitoneal space with size 8.0cm × 6.1 cm.
18F-FDG-PET/CT Scan showed multiple soft tissue masses located in neck, pleura, retroperitoneal space and pelvic cavity, which showed slightly or mildly and inhomogeneous hypermetabolism, with the SUVmax from 2.4 to 5.2 (upper row, A–D). And PET/CT fused images (lower row, E–H) revealed that mildly to strongly FDG-avid masses located in the right posterior cervical muscles with SUVmax 4.5 and size 3.4cm × 3.0cm × 3.3cm (arrow in A), the left posterior cervical muscles with SUVmax 4.6 and size 2.0cm × 2.1cm × 2.2 cm, the posterior side of the left thyroid gland with SUVmax 3.5 and size4.0 cm × 4.3cm × 4.7 cm, the right supraclavicular fossa with SUVmax 3.6 and size 4.8cm × 4.6cm × 4.7 cm, the right axillary fossa with SUVmax 3.0 and size 6.2cm × 4.1cm × 6.3 cm, the right upper thoracic cavity with SUVmax 3.3 and size 9.5cm × 8.7cm × 10.7cm (arrow in B), the right retroperitoneal space with SUVmax 2.4 and size 4.8cm × 5.7cm × 4.5cm (arrows in C), the left retroperitoneal space with SUVmax 3.1 and size 6.3cm × 7.9cm × 6.5 cm, the left part of pelvic cavity with adjacent internal obturator muscle and left pelvic floor fascia involvement with SUVmax 5.2 and size 8.6cm × 5.4cm × 7.6cm (arrow in D).
As for pathological examination, we performed the surgery and obtained the mass from the left neck, which was encapsulated and medium hardness with size about 3.0cm × 3.0cm × 2.5 cm and whitish like color. Immunohistochemical staining showed that D2–40, CD99, SMA were positive and S100, CD34 were negative. The patient refused the operation and biopsy of masses located in other sites, so the pathological results of the masses in pleura, retroperitoneal space and pelvic cavity were unknown. Therefore, the diagnosis of multiple malignant solitary fibrous tumors was considered based on the patient's clinical symptoms, PET/CT results and neck mass pathological results.
Afterwards she refused any kinds of examination and treatment, and was followed up by us using telephone. The patient has been followed up for 1 year, and she was still alive.
The informed written consent was obtained from the patient for publication of this case report and accompanying images.
3. Discussion
SFTs are uncommon neoplasms of mesenchymal origin that can be benign or malignant. It most commonly arises from the pleura, although it can originate in any part of our body. SFT most commonly present during the fifth and sixth decades of life, and there is no significant sex predilection.[5] The majority of reported cases are older than 40 years,[6] but the patient in this case was only 30 years old. She had never suffered from hypoglycemia and high blood glucagon, which was different from the majority of reported cases in which the patients were all found to complain of hypoglycemia.[2] Moreover, PET/CT showed large calcifications in the pelviccavity mass and right pleural effusion, which were uncommon findings in pleural SFTs as others reported[6] (Figs. 1 and 2). Besides, contrast enhancement computed tomography (CECT) showed that all of the masses in this case had obvious heterogeneous enhancement (Fig. 1), which were consistent with other reports.[6] Currently, there were just few literatures about PET imaging of SFTs, Ginat et al[6] have found that benign SFT exhibited low-grade activity at PET and malignant SFTs tended to be strongly hypermetabolism and inhomogeneous, however, this case showed merely slightly or mildly hypermetabolic and inhomogeneous metabolism, with a standardized uptake value from 2.4 to 5.2, which was significant different from other reports.[4] We deduced that the inhomogeneous metabolism of the masses was caused by the different components of the masses, such as protein, coagulative component and Calcifications.
Figure 1.
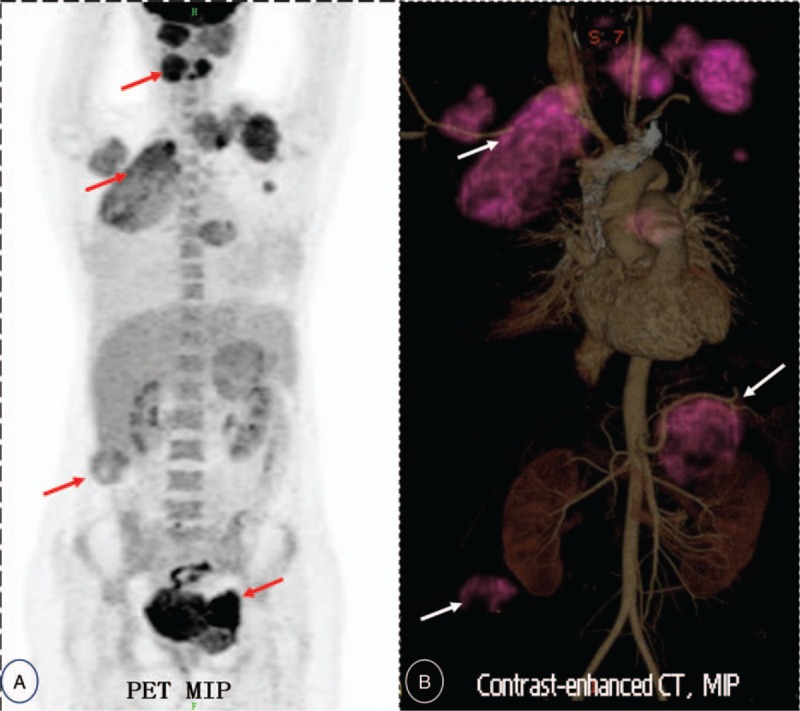
18F-FDG-PET/CT (Maximum intensity projection, MIP) image demonstrating multiple intensely or mildly FDG-avid masses (with maximal SUV from 2.4 to 5.2) in bilateral cervical muscles, bilateral pleura, retroperitoneum space and left pelvic cavity, suggestive of metastatis or multiple malignant disease. (see A, red arrows). MIP (Maximum intensity projection based on contrast-enhanced Computerized Tomography imaging) image showing multiple irregular masse locate in bilateral neck, upper pleural cavity, bilateral perinephric spaces (see B, displayed with purple artificial-color and white arrow). 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, CECT = contrast enhancement computed tomography, SFT = solitary fibrous tumor.
Figure 2.
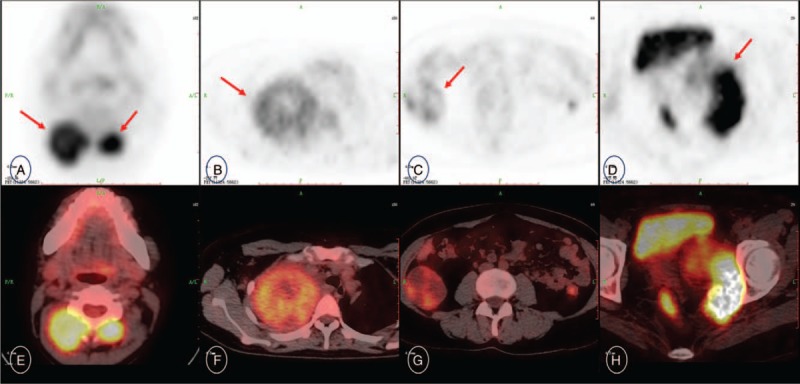
Axial PET (upper row, A–D), and PET/CT fused images (lower row, E–H) revealing mildly to strongly FDG-avid masses locate in bilateral posterior cervical muscles (arrow in A), the right upper thoracic cavity (arrow in B), the right retroperitoneal space (arrow in C), the left part of pelvic cavity with adjacent internal obturator muscle and left pelvic floor fascia involvement (arrow in D). 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, CECT = contrast enhancement computed tomography, SFT = solitary fibrous tumor.
SFTs in the kidneys and retroperitonal space are rare.[7] Limited reports showed that Contrast-enhanced CT may reveal an avidly enhancing well defined mass that contains areas of cystic degeneration. The retroperitoneal SFTs are typically well-circumscribed hypoechoic lesions. In this case, PET/CT scan showed strongly hypermetabolic malignant metastatic SFT in the right retroperitoneal space (Figs. 1 and 2). Histologically, SFTs are composed of spindle cells within a background of collagen stroma, often in a whorled pattern or patternless.[8] These tumors are highly vascularized and have a propensity to undergo myxoid degeneration. The diagnosis was confirmed by characteristic positive immunohistochemical staining for CD34 and negative staining for S-100,[8] neverthless, this case confirmed by positive immunohistochemical staining for D2–40, CD99, SMA and negative immunohistochemical staining for S100 and CD34.
The majority of localized SFTs are usually treated by complete surgical resection. The reported median 10-year overall survival (OS) rates of SFTs after surgery range from 54% to 89%. However, there is no consensus on the treatment of malignant and advanced SFTs. Chemotherapy is not effective for metastatic or unresectable diseases.[9] The local control rate of radiotherapy is 30% to 60% of patients who are not suitable for surgery or refuse surgery.[10] Some anti-angiogenesis drugs have also been used in the treatment of SFTs, which showed some efficacy, but still need to be proved by further and larger sample size prospective studies.[11–12] Cell density, cell atypia, degree of necrosis and mitotic activity are associated with disease-free survival and overall survival of SFT, and high mitotic rate, cell atypia and cell density are significantly correlated with recurrence rate. However, the morphology of SFTs does not fully indicate the prognosis, so it is necessary to evaluate the prognosis of tumors comprehensively, so long-term follow-up is very important.[13]
Author contributions
Software: Jia Gui Su, Xiaohui Oyang.
Writing – original draft: Xiaofei Liu, Xiao Hong Zhou, Hao Yao.
Writing – review & editing: Xiaofei Liu, Wen Hua Zhu, Baoming He.
Footnotes
Abbreviations: 18F-FDG PET/CT = 18F-fluorodeoxyglucose positron emission tomography/computed tomography, CECT = contrast enhancement computed tomography, SFT = solitary fibrous tumor.
XL and WZ are contributed equally to this article and should be considered co-first authors.
The authors report no conflicts of interest.
References
- [1].Klemperer P, Rabin CB. Primary neoplasms of the pleura. Arch Pathol 1931;11:385–412. [Google Scholar]
- [2].Steigen SE, Schaeffer DF, West RB, et al. Expression of insulin-like growth factor 2 in mesenchymal neoplasms. Mod Pathol 2009;22:914–21. [DOI] [PubMed] [Google Scholar]
- [3].Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control 2006;13:264–9. [DOI] [PubMed] [Google Scholar]
- [4].Fukui T, Kawaguchi Y, Kawamoto T, et al. Solitary fibrous tumor arising in extremity: a report of two cases with 201-thallium scintigraphic and PET findings. Cancer Ther 2008;6B:1017–22. [Google Scholar]
- [5].Cardillo G, Facciolo F, Cavazzana AO, et al. Localized (solitary) fibrous tumors of the pleura: an analysis of 55 patients. Ann Thorac Surg 2000;70:1808–12. [DOI] [PubMed] [Google Scholar]
- [6].Daniel T. Ginat Aqiba Bokhari Shweta Bhatt Vikram Dogra. Imaging features of solitary fibrous tumors. AJR 2011;196:487–95. [DOI] [PubMed] [Google Scholar]
- [7].Nagasako Y, Misawa K, Kohashi S, et al. Solitary fibrous tumor in the retroperitoneum. J Am Coll Surg 2004;198:322–3. [DOI] [PubMed] [Google Scholar]
- [8].Ali SZ, Hoon V, Hoda S, et al. Solitary fibrous tumor: a cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer 1997;81:116–21. [DOI] [PubMed] [Google Scholar]
- [9].Park MS, Ravi V, Conley A, et al. The role of chemotherapy in advanced solitary fibrous tumors: a retrospective analysis. Clin Sarcoma Res 2013;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang H, Lucas DR, Pass HI, et al. Disseminated malignant solitary fibrous tumor of the pleura. Pathol Int 2004;54:111–5. [DOI] [PubMed] [Google Scholar]
- [11].Graaf WTAVD, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- [12].Khalifa J, Ouali M, Chaltiel L, et al. Efficacy of trabectedin in malignant solitary fibrous tumors: a retrospective analysis from the French Sarcoma Group. BMC Cancer 2015;15:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pasquali S, Gronchi A, Strauss D, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: a multi-centre prognostic study. Eur J Surg Oncol 2016;42:1064–70. [DOI] [PubMed] [Google Scholar]


