Abstract
Rationale:
Pseudo progression is a noted phenomenon of immune checkpoint inhibitors therapy, which has been defined as a response after an initial enlargement of the tumor followed by tumor reduction. In July 2017, the Food and Drug Administration granted accelerated approval of nivolumab for the treatment of metastatic colorectal cancer patients whose tumor harbors deficient mismatch repair.
Patient concerns and diagnosis:
We present a patient who received nivolumab for heterogeneity of right-sided metastatic colon carcinoma.
Intervention:
The patient was treated with nivolumab combined with chemotherapy.
Outcome:
The computed tomography showed mass lesion in the left lobe of liver remained stable while metastasis tumors under envelop of liver were exacerbated after 6 cycles of nivolumab combined with chemotherapy, and later regressed.
Lessons:
The status of mismatch repair in primary tumor and metastatic liver carcinoma is contradictory but using nivolumab demonstrated encouraging efficacy. This is the first case of pseudo progression undergoing immunotherapy for heterogeneity of right-sided metastatic colon carcinoma.
Keywords: heterogeneity, metastatic colon carcinoma, mismatch repair, nivolumab, pseudo progression
1. Introduction
Pseudo progression, the most focused unconventional response during the therapy of immune checkpoint inhibitor, is a phenomenon characterized by initial tumor enlargement and delayed regression during immune checkpoint inhibitors therapy and reported mostly in non-small-lung-cancer and melanoma.[1–3] Herein, we report a case with pseudo progression in right-sided metastatic colon carcinoma during nivolumab therapy. In the meantime, we found the status of mismatch repair in primary tumor and metastatic liver carcinoma is contradictory, but using nivolumab demonstrated encouraging efficacy in this patient. Deficient mismatch repair (dMMR) in colorectal carcinoma (mCRC) hepatic metastases is only 5%, the heterogeneity like this patient which deficient mismatch repair in metastasis samples and proficient mismatch repair (pMMR) in primary tumor occupied <5%.
2. Case report
A 78-year-old woman presented with anemia and intestinal obstruction since March 2018. Due to recto sigmoid junctions extremely curved, she failed to undergo colonoscopy examination. An enhanced computed tomography (CT) scan revealed multiplicity of lesions in lower abdomen, the diameter of the largest mass reached 3.8 cm. Then ultrasonography-guided percutaneous liver biopsy was performed, according to the immunohistochemistry technique,[4,5] clinical stage IV mCRC was demonstrated. Finally, the Positron Emission Tomography-Computed Tomography (PET-CT) is applied to prove the diagnose. The mismatch repair state of liver biopsy was microsatellite instable assessed by immunohistochemistry technique, showed that MSH6, MSH2(>75%), PMS2(–), MLH(–), CK20(+), CDX2(+), judged as dMMR almost 4% in cases with mCRC.[6,7] However, after 2 cycles nivolumab therapy, the tissues of electronic endoscope biopsy and whole blood cell test analyzed by a full-genome scan revealed that it can be regard as microsatellite stabilization, wild-type BRAF, and KRAS mutation and tumor mutational burden was low (5.4 mutations/Mb). The malignancy had been revealed in March 2018 by systemic multiple metastases including liver, lung, and abdominal pelvic metastasis. She was in poor condition, with an Eastern Cooperative Oncology Group performance status score of 2. Therefore a therapy with nivolumab at a dose of 3 mg/kg, every 3 weeks was administered as the first-line therapy in March 2018.[8,9] After 2 cycles from March to April, her significant obstruction and worse performance status improved along with severe exfoliative rash and controllable bone marrow suppression (I degree). Follow-up CT revealed the effusion of chest, abdomen, and pelvis were reduced, pulmonary and liver metastasis was in stable status. For guarantee patient safety, we decided to reduce nivolumab at 100 mg every 3 weeks coupled with S1 of reduced dose (40 mg bid PO d1–14 q21d) from May to July (4 cycles). Her health condition got increasingly better, however, CT showed mass lesion in the left lobe of liver remained stable (Fig. 1) while metastasis tumors under envelop of liver were exacerbated (Fig. 2). Her performance status increasingly improved and the abnormal serum tumor marker levels gradually decreased including carcinoembryonic antigen, cytokeratin 19-fragments, carbohydrate antigen 199, carbohydrate antigen 125 (Figs. 3 and 4). Therefore, we diagnosed the patient with pseudo progression. Nivolumab at a dose of 3 mg/kg, once every 3 weeks plus SOX (S1 40 mg bid po d1–14 q21d, oxaliplatin 100 mg qd iv q21d) were administered from August to November (5 cycles). As expected, the next 2 cycles of therapy led to a significantly partial response. Follow-up is ongoing to date (November 2018), all the lesions were improved by continuous nivolumab therapy without severe adverse events.
Figure 1.
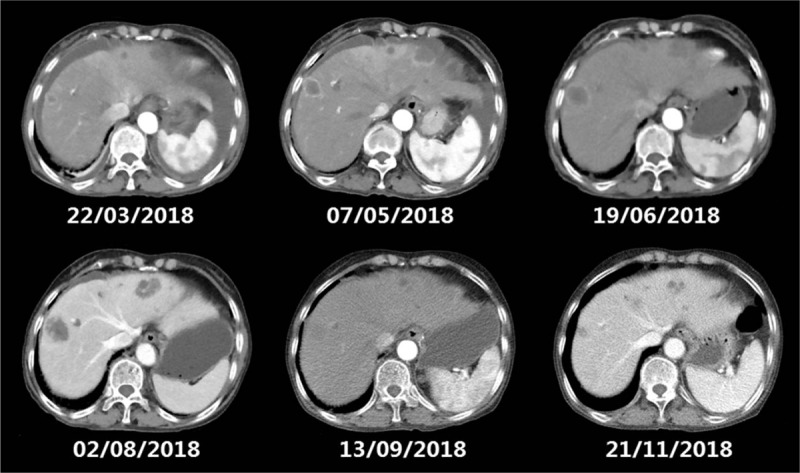
The CT scan shows a lesion tumors under envelop of liver were gradually exacerbated after 2 and 4 courses of nivolumab plus S1 respectively (from February to July), and shrinking after nivolumab combined with SOX (from August to November). CT = computed tomography.
Figure 2.
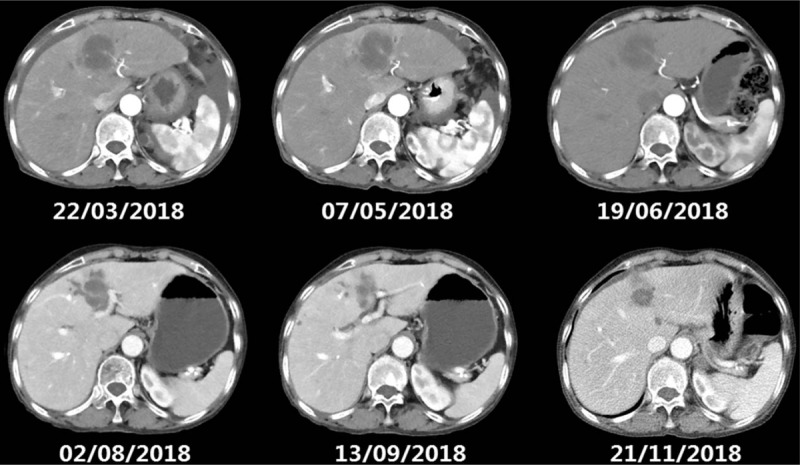
The following CT scan shows mass lesion in the left lobe of liver remained stable (from February to July) and then reduced. CT = computed tomography.
Figure 3.
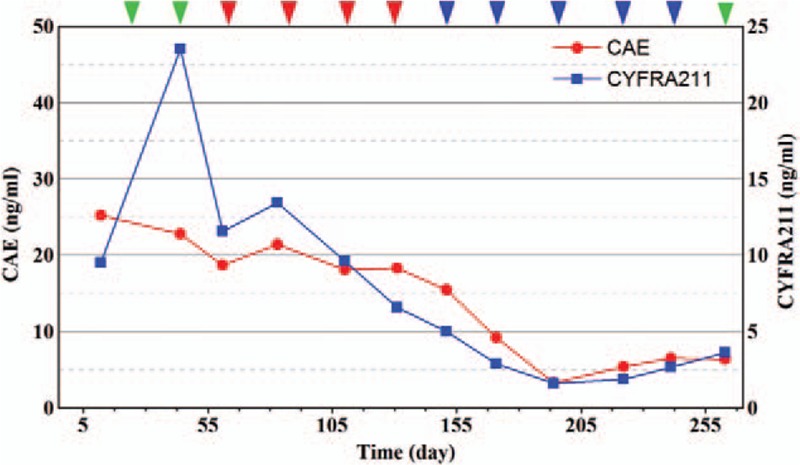
The level of carcinoembryonic antigen and cytokeratin-19 fragments. After nivolumab combined with chemotherapy is administered, the serum tumor marker level continues to reduce. Nivolumab was administered (green arrows); nivolumab combined with S1 was given (red arrows); nivolumab combined with SOX was given (blue arrows).
Figure 4.
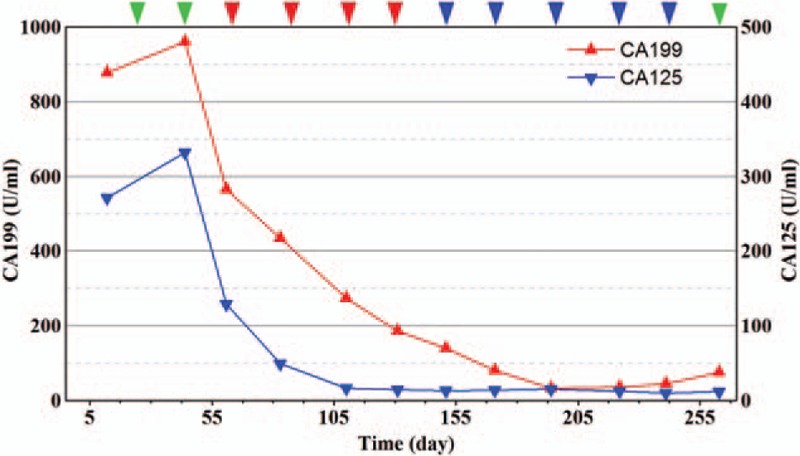
The serum tumor marker levels of carbohydrate antigen 199 and carbohydrate antigen 125. After nivolumab combined with chemotherapy is administered, the serum tumor marker level continues to reduce. Nivolumab was administered (green arrows); nivolumab combined with S1 was given (red arrows); nivolumab combined with SOX was given (blue arrows).
3. Discussion
Compared with patients with pMMR and mCRC, those with dMMR and mCRC always benefit less from conventional chemotherapy (median overall survival 13–6 vs 16–8 months).[10] When it comes to mCRC patients aged above 75, especially in bad condition, either received monotherapy with 5-FU or standard chemotherapy have no significant benefit.[11,12] The objective response rate of S1 was 34%. As a consequence, S1 is rarely used as monotherapy as the first line.[13] The effective control condition was mostly attributed to nivolumab. After 6 courses of nivolumab therapy, CT showed metastasis tumors under envelop of liver were exacerbated. It was meaningful to explore the phenomena to explain the dissociation of tumor exacerbation from her performance status improved and the abnormal serum tumor marker levels decreased. For the sake of security, we added with oxaliplatin, which approximately achieved a better objective response rate of 40% as the first line in mCRC.[13] A good response was observed in our patient after combination SOX with nivolumab. In this regard, the effect was achieved by nivolumab, or a possible synergy between chemotherapy and nivolumab. Pseudo progression cannot be ruled out when the metastasis tumors under envelop of liver were exacerbated. And the later shrinkage of the tumor may also attribute to the addition of oxaliplatin, which stimulate tumor-specific immune responses.[14] Pseudo progression is a noted phenomenon of immune checkpoint inhibitors therapy, which has been defined as a response after an initial enlargement of the tumor followed by tumor reduction. Though the mechanisms of pseudo progression is considered either continued tumor growth until a sufficient immune response occurs or an infiltration of immune cells to the tumor burden. How to distinguish pseudo progression from the first sign of tumor progression is an increasingly vital problem for patients. Serum tumor marker levels decreased may have a correlation with clinical response, regarding as an indicator for pseudo progression, especially carcinoembryonic antigen reduction. Or the early control of effusion maybe proposed as an indicator for pseudo progression.[8,15]
For management of the patients with mCRC, the European Society for Medical Oncology guidelines recommend that gene testing be performed in tissues from other metastatic sites can be accepted, such as liver metastases and lymph node metastases, if a primary tumor is not available.[16] The contradictory results of this patient have been reported about the status of mismatch repair in primary tumor and its corresponding of liver metastasis. As we know, colorectal cancers are highly heterogeneous tumors at the intratumoral and intertumoral genetic levels.[17,18] A survey showed that immune checkpoint inhibitor for the treatment of patients with pMMR in colorectal carcinoma has no response in 20 weeks immune-related objective response rate, at the 2015 American Society of Clinical Oncology (ASCO) Annual Meeting. It has been clarified that the therapeutic effect of the immune checkpoint inhibitor is related to microsatellite instability or dMMR.[19,20] The occurrence rate like this patient with microsatellite instability high in liver metastases and microsatellite instability in primary tumor is a rare condition occupied 3.6% in mCRC. When we encounter the primary tumor belongs to pMMR and other metastatic site is contradictory. Which one should we use as the standard? Nivolumab demonstrated encouraging efficacy in this patient.[11] However, heterogeneity in tumors like mCRC would affect the response of therapies could be a well-recognized as a challenge.
Author contributions
Resources: Wei Zhen.
Writing – review & editing: Jing Wang, Xindan Kang.
Xindan Kang orcid: 0000-0002-4715-3130.
Footnotes
Abbreviations: ASCO = American Society of Clinical Oncology, CT = computed tomography, dMMR = deficient mismatch repair, mCRC = metastatic colorectal carcinoma, PET-CT = Positron Emission Tomography-Computed Tomography, pMMR = proficient mismatch repair.
Patient consent: Informed written consent was obtained from the patient for publication of this case report and accompanying images.
The authors have no conflicts of interest to disclose.
References
- [1].Chiou VL, Burotto M. Pseudo progression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and IrecisTcriteria. Eur J Cancer 2018;88:38–47. [DOI] [PubMed] [Google Scholar]
- [3].Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudo progression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy: a multicenter retrospective cohort study. J Thorac Oncol 2019;14:468–74. [DOI] [PubMed] [Google Scholar]
- [4].Overman MJ, Mcdermott R, Leach JL, et al. Nivolumabu in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (Check Mate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trabelsi M, Farah F, Blel A, et al. Prognostic values of detecting MSI phenotypes in colorectal carcinoma by immunohistochemical method compared to molecular investigation. Tunis Med 2017;95:229–36. [PubMed] [Google Scholar]
- [6].Shia J, Stadler Z, Weiser MR, et al. Immunohistochemical staining for DNA mismatch repair proteins in intestinal tract carcinoma: how reliable are biopsy samples? Am J Surg Pathol 2011;35:447–54. [DOI] [PubMed] [Google Scholar]
- [7].Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol 2014;25:1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sarshekeh AM, Overman MJ, Kopetz S. Nivolumab in the treatment of microsatellite instability high metastatic colorectal cancer. Future Oncol 2018;14:1869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sorbye H, Cvancarova M, Qvortrup C, et al. Age-dependent improvement in median and long-term survival in unselected population-based Nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol 2013;24:2354–60. [DOI] [PubMed] [Google Scholar]
- [12].Landre T, Uzzan B, Nicolas P, et al. Doublet chemotherapy vs. single-agent therapy with 5FU in elderly patients with metastatic colorectal cancer. a meta-analysis. Int J Colorectal Dis 2015;30:1305–10. [DOI] [PubMed] [Google Scholar]
- [13].Winther SB, Österlund P, Berglund A, et al. Randomized study comparing full dose monotherapy (S-1 followed by irinotecan) and reduced dose combination therapy (S-1/oxaliplatin followed by S-1/irinotecan) as initial therapy for older patients with metastatic colorectal cancer: NORDIC 9. BMC Cancer 2017;17:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215–33. [DOI] [PubMed] [Google Scholar]
- [15].Guihong L, Tao C, Ronghui L, et al. Well-controlled pleural effusion indicated pseudo progression after immunotherapy in lung cancer: a case report. Thorac Cancer 2018;9:1190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cutsem EV, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. [DOI] [PubMed] [Google Scholar]
- [17].Del Carmen S, Sayagués JM, Bengoechea O, et al. Spatio-temporal tumor heterogeneity in metastatic CRC tumors: a mutational-based approach. Oncotarget 2018;9:34279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baldus SE, Schaefer KL, Engers R, et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal aden carcinomas and their corresponding metastases. Clin Cancer Res 2010;16:790–9. [DOI] [PubMed] [Google Scholar]
- [19].Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site — when a biomarker defines the indication. N Engl J Med 2017;377:1409–12. [DOI] [PubMed] [Google Scholar]


