Supplemental Digital Content is available in the text
Keywords: KLT injection, meta-analysis, pancreatic cancer, radiochemotherapy, traditional Chinese medicine
Abstract
Background:
Kanglaite (KLT) injection, a kind of Chinese medicine, is considered a promising complementary therapeutic option for malignant cancer treatment. This study aimed to systematically investigate the efficacy and safety of the combination of KLT injection and radiochemotherapy for the treatment of advanced pancreatic cancer (PC).
Methods:
Studies were identified by searching Cochrane Library, Web of Science, PubMed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biological Medicine Database (CBM), Wanfang database and Chinese Scientific Journal Database (VIP) before October 2018. The primary reported outcomes including efficacy, quality of life (QoL), and adverse events were systematically evaluated.
Results:
Data from 16 trials with 960 patients with advanced PC were included. Compared with radiochemotherapy alone, the combination of KLT injection and radiochemotherapy significantly improved the 1-year overall survival (OS, odds ratio [OR] = 2.58 95% CI: 1.12–5.93 P = .03), overall response (ORR, OR = 2.16 95% CI: 1.58–2.94 P <.00001) and disease control rates (DCR, OR = 2.50 95% CI: 1.84–3.38 P <.00001). The QoL of patients, who received a combination of radiochemotherapy and KLT injection, also improved compared with radiochemotherapy treatment alone as indicated by the increased quality of life improved rate (QIR, OR = 3.68 95%CI: 2.36–5.75 P <.00001), pain relief rate (PRR, OR = 3.70 95% CI: 2.23–6.14 P <.00001) and weight gain rate (WGR, OR = 3.69 95% CI: 2.22–6.13 P <.00001). Adverse events related to radiochemotherapy including gastrointestinal side effects, nephrotoxicity, leukopenia, thrombocytopenia, and myelosuppression were alleviated (P <.05) when KLT was injected to patients with PC.
Conclusions:
Evidence from the Meta-analysis suggested that the combinational treatment of radiochemotherapy and KLT injection is more effective in advanced PC treatment than radiochemotherapy alone. Additionally, the combination therapy improved QoL of the patients. KLT injection can alleviate the adverse effects associated with the radiochemotherapy.
1. Introduction
Pancreatic cancer (PC) represents the seventh leading cause of cancer-related deaths and caused 432,242 deaths worldwide in 2018.[1,2] Currently, the incidence of PC has significantly increased, with about 460,000 new cases every year.[1,2] China is at a high-risk; PC-related deaths in China account for about 20% in the world.[3] PC is a fatal disease with high mortality and poor prognosis. The median overall survival (OS) of patients with advanced PC is 4 to 6 months and the 5-year OS rate <10%.[4,5] Despite the improvements in the past decades, the therapeutic effect of current conventional treatments, including radiotherapy and chemotherapy, for advanced PC remains unsatisfactory.[4,5] Therefore, effective treatment strategies are required.
Traditional Chinese medicine has an extensive history and has been widely used as an effective adjuvant drug for cancer treatment.[6,7] Kanglaite (KLT) injection is an extract from Coix lacryma-jobi seed whose main active ingredient is a triglyceride containing 4 types of fatty acids (Fig. 1).[5,8–10] In 1997, KLT injection was formally approved by the Ministry of Health of China for the treatment of malignancies such as PC, lung cancer, gastric cancer, and breast cancer.[5,11–13] Over millions cancer patients in more than 2000 hospitals in China have been treated with KLT.[5] Moreover, KLT has shown good efficacy in the US and is also the first traditional Chinese medicine approved by the US Food and Drug Administration (FDA) to carry out clinical trials in the United States.[14] Researches showed that KLT could effectively reverse multiple-drug resistance in cancer cells and enhance the sensitivity of tumor cells to chemotherapeutic drugs.[10,14–16] Many in vitro studies have shown that KLT can block the G2/M transition and reduce mitotic divisions, thereby suppressing tumor cell proliferation. KLT also inhibits the invasion of cancer cells and migration induced by tumor necrosis factor α.[17–20] In addition, it can induce cancer cell apoptosis through the activation of proapoptotic factors, such as p53, Fas, and caspase-3.[15,21] Several studies indicated that radiochemotherapy combined with KLT injection is more effective for the treatment of advanced PC than radiochemotherapy alone.[5,22] Despite intensive clinical studies, the efficacy and safety of the combination of KLT injection and radiochemotherapy have not been systematically evaluated. In this study, we conducted a meta-analysis to investigate the efficacy and safety of the combination of KLT injection and radiochemotherapy compared with radiochemotherapy alone in advanced PC (Fig. 1). This could provide a basis for the design of future clinical trials.
Figure 1.
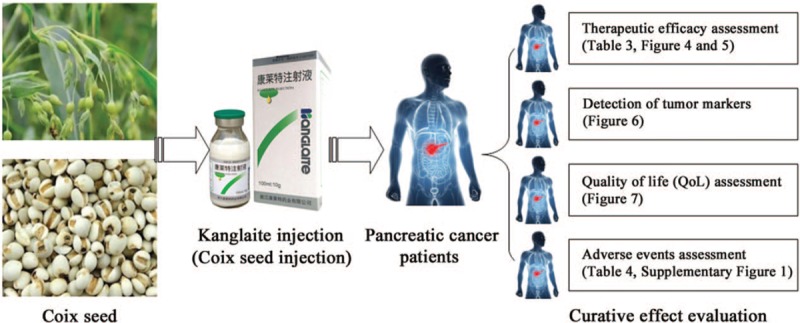
Work flow of present study.
2. Materials and methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines and Cochrane Handbook. The ethical approval and patient consent are not required because this study was a meta-analysis.
2.1. Search strategy and selection criteria
Original articles were searched across 8 electronic databases, including Cochrane Library, Web of Science, PubMed, Embase, China National Knowledge Infrastructure (CNKI), Chinese Biological Medicine Database (CBM), Wanfang database and Chinese Scientific Journal Database (VIP) before October 2018. The search terms were: “Kanglaite injection” or “coix seed injection” combined with “pancreatic carcinoma” or “pancreatic cancer.”
The inclusion criteria were:
-
(1)
controlled trials conducted with patients with advanced PC;
-
(2)
studies comparing the clinical outcomes of radiochemotherapy and KLT injection adjuvant therapy (experimental group) with radiochemotherapy alone (control group); and
-
(3)
studies that included >30 patients with PC.
The exclusion criteria were:
-
(1)
non-contrast articles, case studies, and review articles; and
-
(2)
patients with mixed malignancies.
2.2. Data extraction and quality assessment
Data were extracted by 2 investigators (Liu JL and Yu LB) independently; any disagreement was adjudicated by a third reviewer (Ding W). The following data were extracted:
-
(1)
name of the first author;
-
(2)
year of publication;
-
(3)
study location;
-
(4)
tumor stage;
-
(5)
Karnofsky Performance Score (KPS);
-
(6)
Eastern°Cooperative°Oncology°Group (ECOG) Score;
-
(7)
number of cases;
-
(8)
age of the patients;
-
(9)
study parameters;
-
(10)
therapeutic regimens;
-
(11)
enrollment period; and
-
(12)
dosage of KLT injection.
The trial quality was evaluated according to Cochrane Handbook.[23]
2.3. Outcome definitions
Clinical responses included treatment efficacy, quality of life (QoL), and adverse events. Treatment efficacy was assessed in terms of the OS rates (defined as the length of time from the start of treatment to death from any cause), complete response (CR) rates, partial response (PR) rates, stable disease (SD) rates, progressive disease (PD) rates, overall response rate (ORR, ORR = CR + PR), and disease control rate (DCR, DCR = CR + PR + SD). QoL was evaluated using quality of life improved rate (QIR), pain relief rate (PRR), and weight gain rate (WGR). Adverse events including gastrointestinal adverse effects, hepatotoxicity, nephrotoxicity, neurotoxicity, leucopenia, thrombocytopenia, anemia, myelosuppression, nausea, vomiting, diarrhea, rash, weak, fatigue, and anorexia were assessed.
2.4. Statistical analysis
RevMan 5.3 (Nordic Cochran Centre, Copenhagen, Denmark) and Stata 13.0 (Stata Corp., College Station, TX) software were used for statistical analyses. Cochrane's Q-test and I2 statistics were used to assess heterogeneity between studies; if P >.1 or I2 <50%, the fixed effects model was used for the meta-analysis; otherwise, the random-effects model was used according to the DerSimonian and Laird method.[24] The presence of publication bias was investigated using the Egger test and funnel plots. A 2-tailed P value <.05 was considered statistically significant. Treatment effects were mainly represented by odds ratio (OR) with a 95% confidence interval (CI). Sensitivity analysis was conducted to evaluate the impact of different therapeutic regimens, KLT injection dosages, sample sizes, and type of research on the clinical efficacy of the combination of KLT injection and radiochemotherapy.
3. Results
3.1. Search results
A total of 237 publications were identified after the initial search. Of these, 172 publications were excluded due to duplication. After title and abstract review, 38 articles were further excluded because they did not include clinical trials (n = 31) or were unrelated studies (n = 7). Of the remaining 27 publications, studies that did not include a control group (n = 3), meta-analyses (n = 3), and studies with insufficient data (n = 5) were excluded. Finally, 16 trials[5,22,25–38] involving 960 patients with advanced PC were included in the final analysis (Fig. 2).
Figure 2.
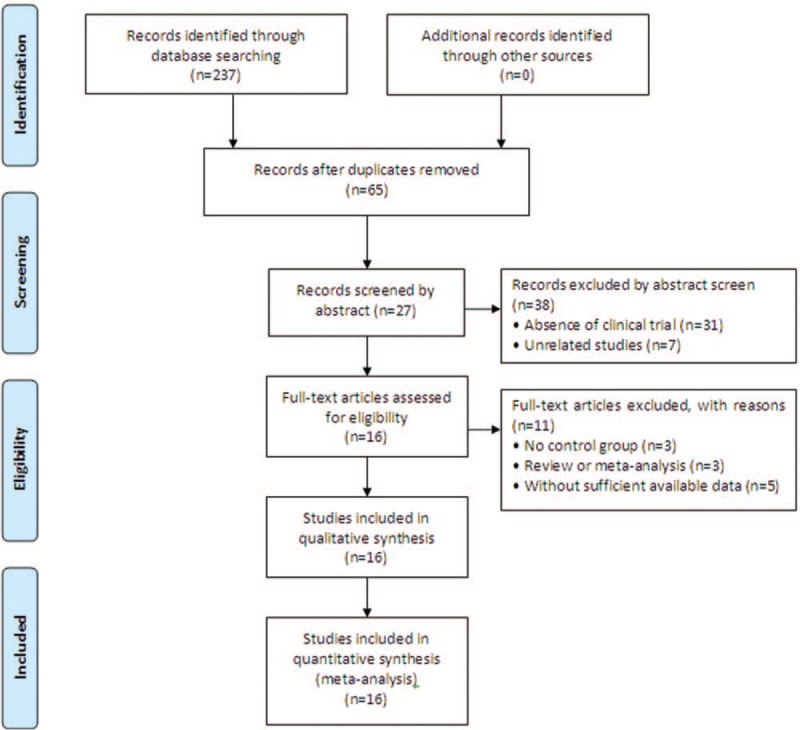
Study selection process for the meta-analysis.
3.2. Patient characteristics
Most of the included studies were performed in different medical centers in China;[22,25–38] 1 trial was conducted in the US.[5] In total, 494 patients with advanced PC were treated with radiochemotherapy in combination with KLT injection adjuvant therapy, while 466 patients were treated with radiochemotherapy alone. Study and patient characteristics are shown in Table 1 and Table 2.
Table 1.
Clinical information from the eligible trials in the meta-analysis.
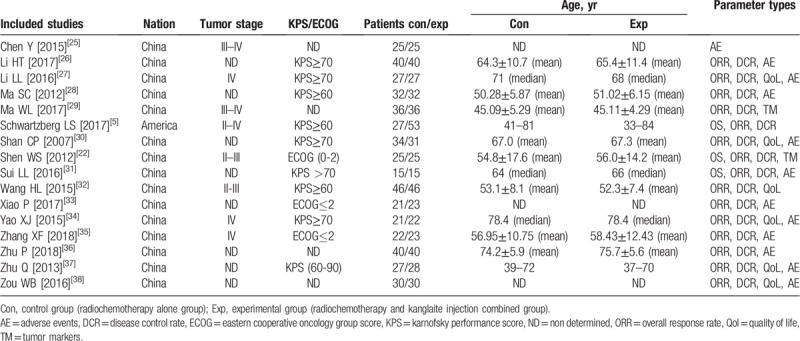
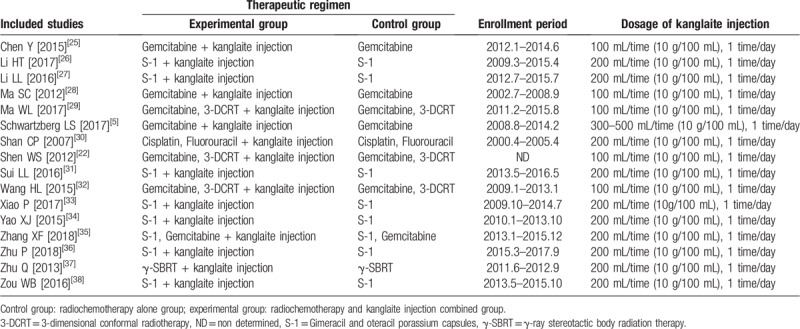
Table 2.
Information of kanglaite injection combined with radiochemotherapy.
3.3. Quality assessment
The assessment of the risk of bias is shown in Figure 3. Fifteen studies[5,22,25–35,37,38] were determined as low risk and the remaining 1 study[36] was not a true randomized controlled trial. Most included trials did not provide a clear description of performance and detection risks. One open-label study[5] was considered as high performance and detection risk. The attrition risks of the selected studies were low; 1 study[22] was categorized as high risk due to the absence of follow-up and the risk of 2 studies[25,32] was considered unclear owing to selective reporting.
Figure 3.
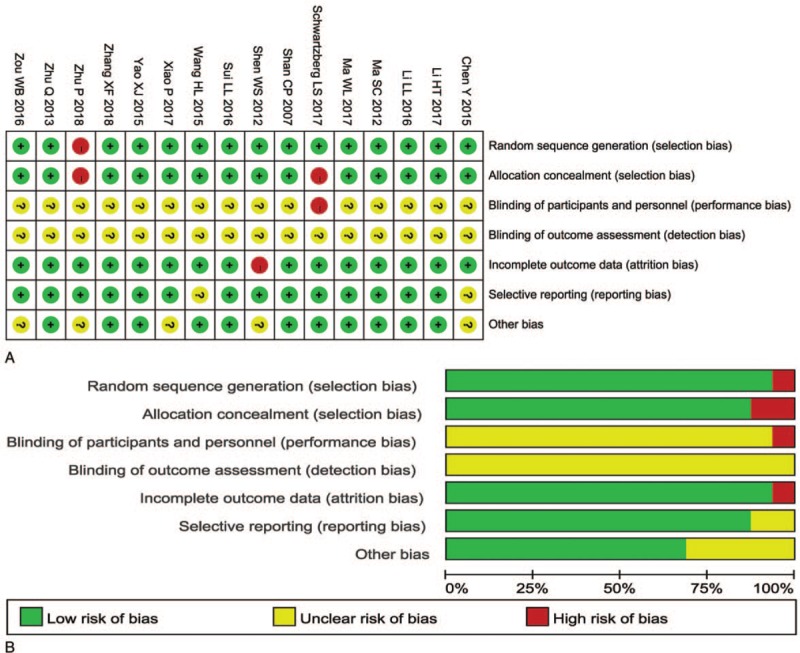
(A) Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. (B) Risk of bias graph: review of authors’ judgments about each risk of bias item presented as percentages across all included studies. Note: Each color represents a different level of bias: red for high-risk, green for low-risk, and yellow for unclear-risk of bias.
3.4. Efficacy assessments
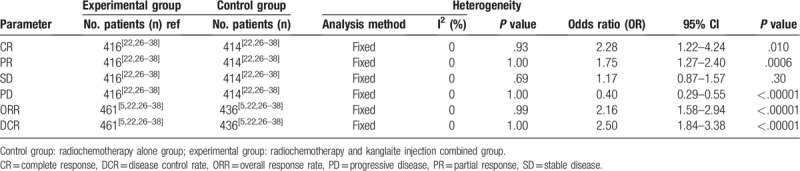
As shown in Figures 4 and 5, and Table 3, the pooled results showed that patients who underwent combination therapy had significantly improved 1-year OS (OR = 2.58, 95% CI = 1.12–5.93, P = .03), CR (OR = 2.28, 95% CI = 1.22–4.24, P = .010), PR (OR = 1.75, 95% CI = 1.27–2.40, P = .0006), ORR (OR = 2.16, 95% CI = 1.58–2.94, P <.00001) and DCR (OR = 2.50, 95% CI = 1.84–3.38, P <.00001) and significantly decreased PD (OR = 0.40, 95% CI = 0.29–0.55, P <.00001) compared with those who received radiochemotherapy alone. In contrast, SD and 2-years OS rates of patients who received combination therapy were not significantly different from patients those who received radiochemotherapy alone (SD: OR = 1.17, 95% CI = 0.87–1.57, P = .30; 2 years OS: OR = 1.59, 95% CI = 0.49–5.15, P = .44). Fixed-effect models were used to analyze OR rate because of low heterogeneity.
Figure 4.
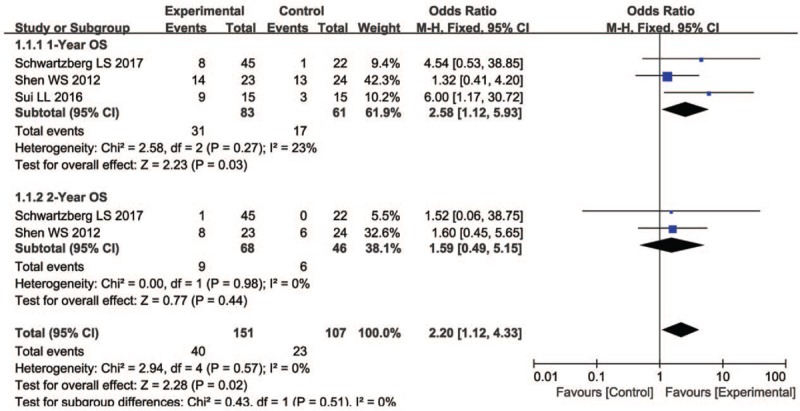
Forest plot of the comparison of one-year (A) and 2-years (B) overall survival (OS) between the experimental and control group. Control group, chemotherapy alone group; Experimental group, KLT injection and radiochemotherapy combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used. KLT = kanglaite injection.
Figure 5.
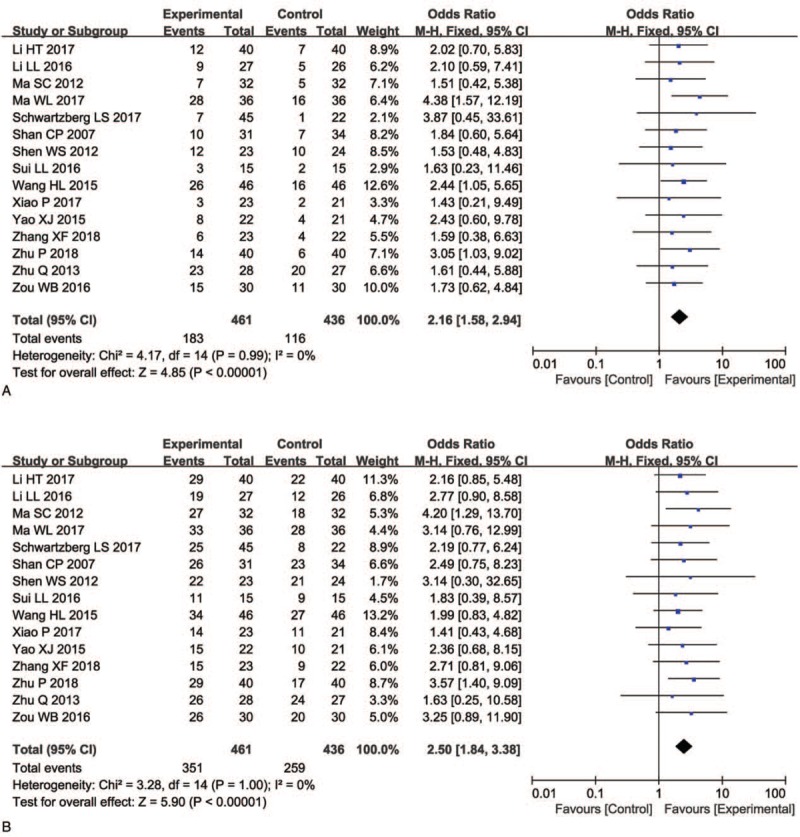
Forest plot of the comparison of overall response rate (ORR, A) and disease control rate (DCR, B) between the experimental and control group. Control group, radiochemotherapy alone group; Experimental group, KLT injection and radiochemotherapy combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Table 3.
Comparison of CR, PR, SD, PD, ORR, and DCR between the experimental and control group.
3.5. Detection of tumor markers
Two clinical trials[22,29] evaluated tumor markers in patients with PC patient between the 2 groups. As shown in Figure 6, the levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) were significantly decreased after the combination therapy compared with radiochemotherapy alone (CEA, OR = –4.49, CI = –6.57 to –2.40, P <.0001; CA199, OR = –103.05, CI = –127.42 to –78.67, P <.00001).
Figure 6.
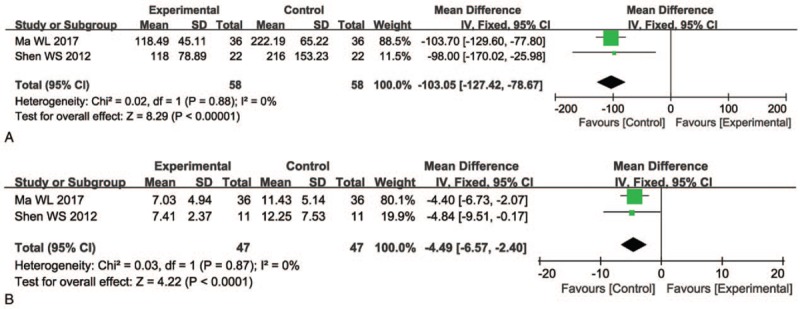
Forest plot of the comparison of carcinoembryonic antigen (CEA, A) and carbohydrate antigen 199 (CA199, B) between the experimental and control group. Control group, radiochemotherapy alone group; Experimental group, KLT injection and radiochemotherapy combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
3.6. QoL assessment
We found that QoL of patients who received combination treatment was significantly better than that of the control group, indicated by significantly increased QIR, PRR, and WGR (Fig. 7, QIR: OR = 3.68, 95% CI = 2.36–5.75, P <.00001; PRR: OR = 3.70, 95% CI = 2.23–6.14, P <.00001; WGR: OR = 3.69, 95% CI = 2.22–6.13, P <.00001).
Figure 7.
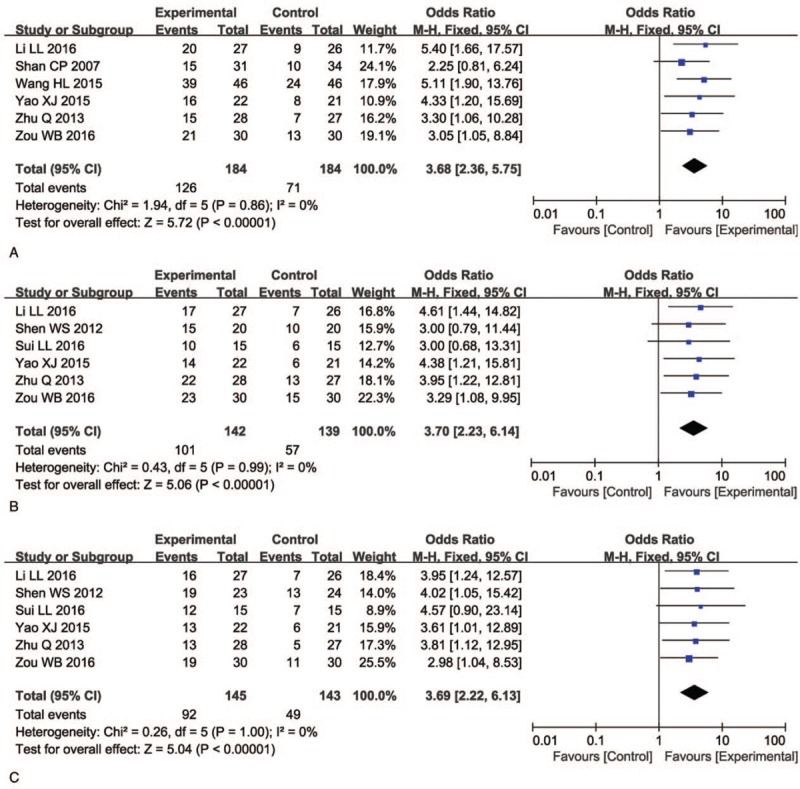
Forest plot of the comparison of quality of life improved rate (QIR, A), pain relief rate (PRR, B) and weight gain rate (WGR, C) between the experimental and control group. Control group, radiochemotherapy alone group; Experimental group, KLT injection and radiochemotherapy combined group. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
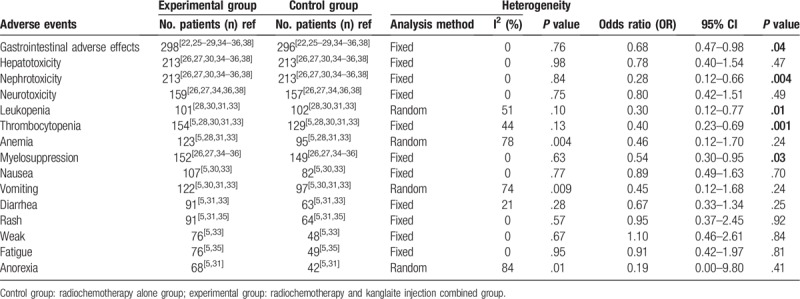
3.7. Adverse events assessment
As shown in Table 4 and Supplementary Figure 1, patients treated with a combination of KLT injection and radiochemotherapy displayed lower incidences of gastrointestinal side effects (OR = 0.68, 95% CI = 0.47–0.98, P = .04), nephrotoxicity (OR = 0.28, 95% CI = 0.12–0.66, P = .004), leucopenia (OR = 0.30, 95% CI = 0.12–0.77, P = .01), thrombocytopenia (OR = 0.40, 95% CI = 0.23–0.69, P = .001), and myelosuppression (OR = 0.54, 95% CI = 0.30–0.95, P = .03) compared with those treated with radiochemotherapy alone. In contrast, hepatotoxicity (OR = 0.78, 95% CI = 0.40–1.54, P = .47), neurotoxicity (OR = 0.80, 95% CI = 0.42–1.51, P = .49), anemia (OR = 0.46, 95% CI = 0.12–1.70, P = .24), nausea (OR = 0.89, 95% CI = 0.49–1.63, P = .70), vomiting (OR = 0.45, 95% CI = 0.12–1.68, P = .24), diarrhea (OR = 0.67, 95% CI = 0.33–1.34, P = .25), rash (OR = 0.95, 95% CI = 0.37–2.45, P = .92), weak (OR = 1.10, 95% CI = 0.46–2.61, P = .84), fatigue (OR = 0.91, 95% CI = 0.42–1.97, P = .81), and anorexia (OR = 0.19, 95% CI = 0.00–9.80, P = .41) did not differ significantly between the 2 groups.
Table 4.
Comparison of adverse events between the experimental and control group.
3.8. Publication bias
Publication bias was assessed visually by funnel plots and quantified using Egger's test and Begg regression test. As shown in Figure 8, no significant publication bias for ORR (Begg = 0.533; Egger = 0.395) and DCR (Begg = 0.843; Egger = 0.981) was observed in these analyses, which confirmed the reliability of our primary outcomes.
Figure 8.
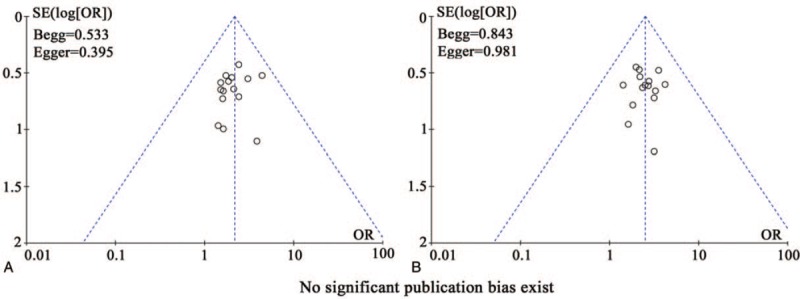
Funnel plot of overall response rate (ORR, A), disease control rate (DCR, B).
3.9. Sensitivity analysis
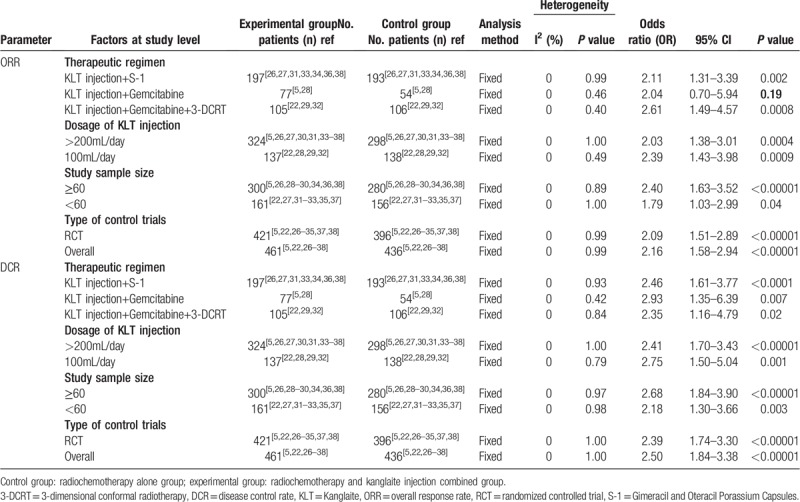
We conducted a subgroup analysis to explore the source of heterogeneity in ORR and DCR with respect to therapeutic regimen, KLT injection dose, sample size, and type of study. As shown in Table 5, there was no significant difference between different doses of KLT injection, sample size, and types of studies. Moreover, in terms of ORR index, KLT injection combined with S-1/Gemcitabine + 3-dimensional conformal radiotherapy regimen was found to be more effective for PC treatment.
Table 5.
Subgroup analyses of ORR and DCR between the experimental and control group.
4. Discussion
The limitations of the current radiochemotherapeutic treatment for malignancies include drug resistance and toxic side effects. Clinicians have been exploring complementary and alternative treatments to improve survival time or QoL of patients and to reduce side effects caused by radiochemotherapy.[39–41] Traditional Chinese medicine, particularly KLT injection, has been used as an adjuvant therapy for decades. Several studies have been reported that the addition of KLT injection could be beneficial to patients with advanced PC. Even though there was statistical analysis of published clinical trials, the exact therapeutic effects were still not systematically evaluated because of small sample size and different protocols used in the studies. Therefore, in this study, we conducted a wide range of online search and applied strict inclusion and exclusion criteria to derive a clear and systematic conclusion.
Our meta-analysis revealed that a combination of radiochemotherapy and KLT injection was more effective for the treatment of PC compared with radiochemotherapy alone. Patients treated with combined therapy exhibited significantly prolonged 1-year OS, broadly increased ORR and DCR (P <.05), and significantly improved QoL. Specific molecular markers including CEA and CA199 are commonly used to predict the recurrence, metastasis, and prognosis of PC after treatment.[42,43] Our analysis showed that both CEA and CA199 were differentially decreased after radiochemotherapy and KLT injection combination treatment. These results indicated that using KLT injection could improve the curative effects of radiochemotherapy for advanced PC.
Our analysis showed that some of the adverse events caused by radiochemotherapy, including gastrointestinal side effects, nephrotoxicity, leukopenia, thrombocytopenia, and myelosuppression, were alleviated with KLT injection combination therapy. Therefore, KLT injection may be a well-tolerated treatment for PC and can effectively alleviate partial adverse events associated with radiochemotherapy.
The analysis on therapeutic effects may be influenced by several factors. In our study, no difference was found between different drug forms of KLT injection, sample size, and type of research types. In terms of ORR, KLT injection combined with S-1/Gemcitabine+ 3-dimensional conformal radiotherapy regimen was more effective for PC treatment. However, studies on the impact of these factors on the curative effect of KLT injection adjuvant therapy remain insufficient and further investigations are required.
There are some limitations in this study. First, there was significant heterogeneity among the included trials, which may be due to variation in tumor stage and age of the patients, year of publication, and duration of treatment. However, based on currently available literature, there are insufficient data to perform further statistical analysis to evaluate the correlation. Second, as an important Chinese herbal injection, KLT was mainly used in China; this may lead to an unavoidable regional bias and subsequently, influence the clinical application of KLT injection worldwide. Third, smoking history and other diseases may have an impact on CA 199.[44] It is advisable to collect the smoking history and past medical history of the patients to strengthen the rationale. However, our data were extracted from publications where this information was not sufficiently provided. Therefore, based on currently available literature, there are insufficient data to perform a statistical analysis to evaluate the correlation. We will keep paying close attention to this concern in our later studies. Finally, as the sources of our data were published articles instead of raw records of clinical trials, analytical bias could exist.
5. Conclusion
In summary, this meta-analysis indicated that the combination of KLT injection and radiochemotherapy was effective in treating patients with advanced PC. Clinical application of KLT injection not only improved the therapeutic effects of radiochemotherapy but also alleviated the side effects caused by radiochemotherapy. However, the long-term efficacy of KLT injection-mediate adjuvant therapy for advanced PC requires further investigation through clinical trials.
Author contributions
Conceptualization: Wei Ding.
Formal analysis: Jianling Liu, Lingbo Yu.
Investigation: Jianling Liu, Lingbo Yu, Wei Ding.
Methodology: Jianling Liu, Lingbo Yu.
Project administration: Wei Ding.
Supervision: Wei Ding.
Validation: Jianling Liu, Lingbo Yu, Wei Ding.
Visualization: Jianling Liu, Lingbo Yu, Wei Ding.
Writing – original draft: Jianling Liu, Lingbo Yu.
Writing – review & editing: Wei Ding.
Supplementary Material
Footnotes
Abbreviations: CA199 = carbohydrate antigen 199, CEA = carcinoembryonic antigen, CI = confidence interval, CR = complete response rates, DCR = disease control rate, KLT = kanglaite, OR = odds ratio, ORR = overall response rate, OS = overall survival, PC = pancreatic cancer, PD = progressive disease, PR = partial response rates, PRR = pain relief rate, QIR = quality of life improved rate, QoL = quality of life, SD = stable disease, WGR = weight gain rate.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [4].Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J 2017;23:333–42. [DOI] [PubMed] [Google Scholar]
- [5].Schwartzberg LS, Arena FP, Bienvenu BJ, et al. A Randomized, Open-Label, Safety and Exploratory Efficacy Study of Kanglaite Injection (KLTi) plus Gemcitabine versus Gemcitabine in Patients with Advanced Pancreatic Cancer. J Cancer 2017;8:1872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 2010;4:297–307. [PubMed] [Google Scholar]
- [7].Qi F, Zhao L, Zhou A, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends 2015;9:16–34. [DOI] [PubMed] [Google Scholar]
- [8].Liu X, Xu F, Wang G, et al. Kanglaite injection plus chemotherapy versus chemotherapy alone for non-small cell lung cancer patients: a systematic review and meta-analysis. Curr Ther Res Clin Exp 2008;69:381–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cao N, Ma X, Guo Z, et al. Oral kanglaite injection (KLTI) attenuates the lung cancer-promoting effect of high-fat diet (HFD)-induced obesity. Oncotarget 2016;7:61093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang C, Hou A, Yu C, et al. Kanglaite reverses multidrug resistance of HCC by inducing apoptosis and cell cycle arrest via PI3K/AKT pathway. Onco Targets Ther 2018;11:983–96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [11].Liu X, Yang Q, Xi Y, et al. Kanglaite injection combined with chemotherapy versus chemotherapy alone in the treatment of advanced non-small cell lung carcinoma. J Cancer Res Ther 2014;10:46–51. [DOI] [PubMed] [Google Scholar]
- [12].Zhan YP, Huang XE, Cao J, et al. Clinical safety and efficacy of Kanglaite(R) (Coix Seed Oil) injection combined with chemotherapy in treating patients with gastric cancer. Asian Pac J Cancer Prev 2012;13:5319–21. [DOI] [PubMed] [Google Scholar]
- [13].Guo HY, Cai Y, Yang XM, et al. Randomized phase II trial on mitomycin-C/cisplatin +/- KLT in heavily pretreated advanced breast cancer. Am J Chin Med 2008;36:665–74. [DOI] [PubMed] [Google Scholar]
- [14].Zhang XW, Liu L, Zhang XZ, et al. Kanglaite inhibits the expression of drug resistance genes through suppressing PVT1 in cisplatin-resistant gastric cancer cells. Exp Ther Med 2017;14:1789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Y, Zhang C, Zhang S, et al. Kanglaite sensitizes colorectal cancer cells to Taxol via NF-kappaBeta inhibition and connexin 43 upregulation. Sci Rep 2017;7:1280DOI: 10.1038/s41598-017-01480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang JC, Tian JH, Ge L, et al. Which is the best Chinese herb injection based on the FOLFOX regimen for gastric cancer? A network meta- analysis of randomized controlled trials. Asian Pac J Cancer Prev 2014;15:4795–800. [DOI] [PubMed] [Google Scholar]
- [17].Shi G, Zheng X, Zhang S, et al. Kanglaite inhibits EMT caused by TNF-alpha via NF-kappaBeta inhibition in colorectal cancer cells. Oncotarget 2018;9:6771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu Y, Li CS, Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: a minireview of progress between Kanglaite injection and related genes. J Exp Clin Cancer Res 2008;27:31DOI: 10.1186/1756-9966-27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Woo JH, Li D, Wilsbach K, et al. Coix seed extract, a commonly used treatment for cancer in China, inhibits NFkappaB and protein kinase C signaling. Cancer Biol Ther 2007;6:2005–11. [DOI] [PubMed] [Google Scholar]
- [20].Pan P, Wu Y, Guo ZY, et al. Antitumor activity and immunomodulatory effects of the intraperitoneal administration of Kanglaite in vivo in Lewis lung carcinoma. J Ethnopharmacol 2012;143:680–5. [DOI] [PubMed] [Google Scholar]
- [21].Lu Y, Wu LQ, Dong Q, et al. Experimental study on the effect of Kang-Lai-Te induced apoptosis of human hepatoma carcinoma cell HepG2. Hepatobiliary Pancreat Dis Int 2009;8:267–72. [PubMed] [Google Scholar]
- [22].Shen WS, Shu ZQ, Deng LC, et al. Curative effect of 3D-CRT combined with gemcitabine concurrently with addition of Kanglaite injection in treatment of locally advanced pancreatic carcinoma. Chin J Integr Tradit West Med 2012;32:902–5. [PubMed] [Google Scholar]
- [23].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [24].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Y. Safety and effectiveness of Kanglaite injection combined with gemcitabine on advanced pancreatic cancer. Clin J Chin Med 2015;7:24–5. [Google Scholar]
- [26].Li HT, Wang MM, Liu HJ, et al. Therapeutic effect of S-1 combined with Kanglaite injection in the treatment of advanced pancreatic cancer. Modern J Integr Tradit Chin West Med 2017;26:4063–5. [Google Scholar]
- [27].Li LL, Chen J, Zhang DR, et al. Efficacy of tegafur gimeracil oteracil potassium capsule combined with kanglaite injection for advanced pancreatic carcinoma. Modern Chin Doctor 2016;54:67–9. [Google Scholar]
- [28].Ma SC, Zhao D, Hou XM, et al. Combination treatment with gemcitabine chemotherapy and Kanglaite injection on advanced pancreatic cancer. J Modern Oncol 2012;20:1415–7. [Google Scholar]
- [29].Ma WL, Yan YF, Wang XP. Effect of Kanglaite injection on immune target in patients with advanced pancreatic cancer. Pract J Cancer 2017;32:1863–6. [Google Scholar]
- [30].Shan CP, Yang XQ, Cui W, et al. Clinical observation of Kanglaite injection combined with low dose chemotherapy in the treatment of advanced pancreatic cancer. Tradit Chin Drug Res Pharmacol 2007;18:72–3. [Google Scholar]
- [31].Sui LL, Zheng XF, Ding WN, et al. Second-line treatment of advanced pancreatic cancer: a clinical study of Kanglaite injection combined with S-1. China Health Care Nutrit 2016;26:80–1. [Google Scholar]
- [32].Wang HL, Zhang Y, Wang JT, et al. Clinical research on Kanglaite injection concurrent adjuvant radio-chemotherapy treatment of locally advanced pancreatic cancer. J Chin Med 2015;30:1242–3. [Google Scholar]
- [33].Xiao P, Bai H, Li M, et al. Effect of second-line treatment with S-1 and Kanglaite injection for patients with advanced pancreatic cancer. J Basic Clin Oncol 2017;30:289–91. [Google Scholar]
- [34].Yao XJ. Clinical observation of S-1 combined with kanglaite injection in the treatment of senile advanced pancreatic cancer. J Basic Clin Oncol 2015;28:350–2. [Google Scholar]
- [35].Zhang XF, Qiao CX, Chen XF, et al. Clinical trial of Kanglaite injection combined with gemcitabine injection and tegafur-gimeracil- oteracil potassium capsule in the treatment of advanced pancreatic cancer. Chin J Clin Pharmacol 2018;34:111–3. [Google Scholar]
- [36].Zhu P. Clinical observation of tegafur gimeracil oteracil potassium capsule combined with Kanglaite injection for the Treatment of senile advanced pancreatic cancer. China Pract Med 2018;13:101–2. [Google Scholar]
- [37].Zhu Q, Kang JB, Nie Q, et al. Kanglaite injection combined with γ-ray stereotactic body radiation therapy for 28 cases with locally advanced pancreatic cancer. China Cancer 2013;22:931–4. [Google Scholar]
- [38].Zou WB, Dong YY, Liu Y, et al. Clinical observation on tegafur gimeracil oteracil potassium capsules combined with Kanglaite injection in palliative treatment of advanced pancreatic carcinoma. China Health Stand Manag 2016;7:136–8. [Google Scholar]
- [39].Huang X, Qin J, Lu S. Kanglaite stimulates anticancer immune responses and inhibits HepG2 cell transplantationinduced tumor growth. Mol Med Rep 2014;10:2153–9. [DOI] [PubMed] [Google Scholar]
- [40].Zhang P, Meng X, Tang X, et al. The effect of a coix seed oil injection on cancer pain relief. Support Care Cancer 2019;27:461–5. [DOI] [PubMed] [Google Scholar]
- [41].Guo N, Miao Y, Sun M. Transcatheter hepatic arterial chemoembolization plus cinobufotalin injection adjuvant therapy for advanced hepatocellular carcinoma: a meta-analysis of 27 trials involving 2,079 patients. Onco Targets Ther 2018;11:8835–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chang JC, Kundranda M. Novel Diagnostic and Predictive Biomarkers in Pancreatic Adenocarcinoma. Int J Mol Sci 2017;18:667DOI: 10.3390/ijms18030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are biomarkers for the efficacy and prognosis of cryoablation in pancreatic cancer patients. Cell Biochem Biophys 2015;71:1287–91. [DOI] [PubMed] [Google Scholar]
- [44].Kawai S, Suzuki K, Nishio K, et al. Hamajima. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int J Cancer 2008;123:2880–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


