Abstract
Objective:
This meta-analysis evaluates the prognosis value of C-reactive protein to albumin ratio (CAR) in colorectal cancer.
Methods:
Embase, PubMed, and Web of Science were searched. Pooled hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were used as effective values.
Results:
A total of 6 studies with 1942 patients were included in this study. Pooled results revealed that elevated pretreatment CAR was related with poorer overall survival (OS) (HR: 2.09, 95%CI: 1.78–2.45, P < .001) in colorectal cancer.
Conclusion:
Elevated CAR was associated with poor prognosis in colorectal cancer. Thus CAR might be used as a prognostic system and classification of colorectal patients in clinical potential.
Keywords: colorectal cancer, C-reactive protein to albumin ratio, prognosis, meta-analysis
1. Introduction
Colorectal cancer (CRC) is one of the most common types of cancer worldwide.[1] In 2016, an estimated 95,270 new cases of colon cancer and approximately 39,220 cases of rectal cancer will occur in the US.[2] A retrospective cohort study of the SEER CRC registry found that patients with CRC younger than 50 years has been ascending gradually.[3] Therefore, the identification of simple and objective prognostic factors is critical for management and surveillance of CRC patients.
Many factors have been proved, such as inflammation, wound repair, systemic inflammatory response syndrome (SIRS), and cancer have close correlation.[4,5] Inflammation can induce expression of cytokines, such as tumor necrosis factor-α (TNF-α) and multifunctional cytokine interleukin 6 (IL-6) et al, which contribute to cancer cells growth, invasion and metastasis with inhibiting the apoptosis and promoting the DNA damage.[6–9] Therefore, systemic inflammatory markers, such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) et al, have been widely applied as prognostic factors in the field of clinical oncology. Pro-inflammatory cytokines induce expression of variety circulating acute phase proteins, such as albumin and C-reactive protein (CRP), in which C-reactive protein to albumin ratio (CAR) has been validated recently to stratify the outcome of pan-cancer patients.[10–12]
Relationship between CAR and human various cancers, such as lung cancer, hepatocellular carcinoma, esophageal cancer et al, has been reported by many groups, but there is not any comprehensive and quantitative evaluation on its prognostic role in CRC. Therefore, we conducted this meta-analysis to assess the prognostic value of pretreatment CAR based on some available CRC related studies.
2. Materials and methods
This meta-analysis was conducted following Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria.[13] This study does not need ethical approval because it does not include data linked to individual patient information.
2.1. Literature search and study selection
Two investigators independently performed a comprehensive search of eligible studies in Embase, PubMed, and Web of Science to appraise the prognostic value of CAR in CRC. The search strategy which updated to June 2017 included the following search terms: (“C-reactive protein/Albumin ratio” OR “C-reactive protein to Albumin ratio” OR “CRP/Alb ratio”) and (“colorectal neoplasm” OR “colorectal tumor” OR “colorectal carcinoma” OR “colorectal neoplasms” OR “colorectal cancer”). The reference list was also checked for relevant articles.
2.2. Inclusion and exclusion criteria
Studies were included in this meta analysis with the following criteria:
-
1.
patients with CRC were pathological examination confirmed;
-
2.
the pretreatment CAR was measured by serum based methods;
-
3.
correlation of CAR with overall survival (OS) was reported;
-
4.
hazard ratio (HR) for overall survival (OS) was evaluated with multivariate analysis;
-
5.
a distinct cutoff value of CAR was given;
-
6.
publications were written in English.
Exclusion studies were as the following criteria:
-
1.
letters, reviews, nonclinical studies, or case reports;
-
2.
non-CRC;
-
3.
insufficient data for extracting hazard ratio (HR) and 95% confidence internal (CI);
-
4.
studies had repeat analysis or duplicate data.
2.3. Data extraction and methodological quality assessment
Data from all candidate articles were independently evaluated and extracted by two investigators (FW and PL). Articles would be retrieved through full-text review after not being categorized by title and abstract alone. Each disagreement was discussed until the two investigators reached a consensus to ensure the accurate data extraction. The extracted data included the first author, year of publication, country, total number of cases, study style, cutoff value of CAR, age, follow-ups, stages, treatment, and HRs for OS, as well as their 95% confidence intervals (CIs) and P values for the correlation between prognosis and CAR.
Qualities of candidate studies were appraised by the Newcastle-Ottawa Quality Assessment Scale (NOS).[14] The NOS consists of three parameters of quality: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Studies with NOS score no <7 points were defined as high-quality articles[15] and the maximum score is 9 points. Any disagreement was settled by discussion.
2.4. Statistical analysis
Analyses were performed by two statistical tools: STATA version 12.0 (Stata Corp LP, TX, USA) and RevMan software (version 5.3; Te Cochrane Collaboration). HRs and 95% CIs were extracted from each eligible study to calculate the pooled HRs. The heterogeneity of the pooled data was evaluated by using Cochran's Q test and Higgins I-squared statistic. Pheterogeneity < .1 or I2 > 50% defined as significant heterogeneity. The random-effects model (DerSimonian-Laird method)[16] was used to analyze the pooled HRs which had significant heterogeneity; otherwise, the fixed-effects model (Mantel Haenszel method)[17] was used. Publication bias was formally investigated by three methods, the Egger's[18] and Begg's test,[19] and “the trim and fill” method.[20] Subgroup analysis was performed to explore and explain the heterogeneity among the different studies. Sensitivity analysis was used to evaluate the stability of the pooled results using STATA software.
3. Results
As shown in Fig. 1, we retrieved a total of 280 relevant studies from the first searching. The 14 potential studies were selected after screening the titles and abstracts. After reading these articles, we excluded 8 studies (2 studies with survival outcomes unavailable; 6 studies based on complication of surgery, severe side effects of adjuvant chemotherapy and infection, etc). Finally, the 6 eligible studies were included in this meta-analysis.
Figure 1.
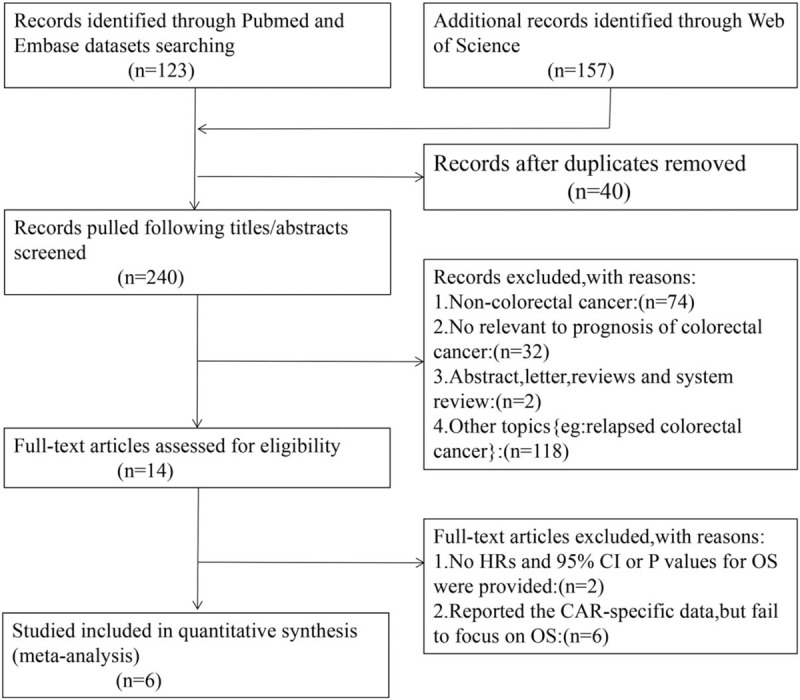
The flow diagram of searching literature for this meta-analysis.
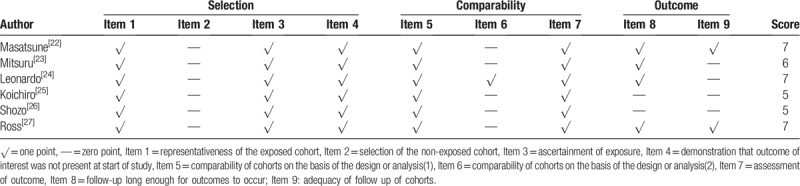
The major characteristics of included studies are shown in Table 1. These 6 studies compromised 1942 patients with CRC. Among them, participants in 4 studies were Asian, 2 studies were Caucasian. All studies were published between 2015 and 2018 and were from Japan (n = 4) or British (n = 2). The study sample sizes ranged from 99 to 801, with the median size of 194. The cut-off values of CAR ranged from 0.038 to 0.22, with the median cut-off value of 0.091. All studies performed a multivariable analysis of OS. Based on different treating methods, the studies included surgical resection, radiotherapy, chemotherapy, and multidisciplinary treatments. All studies were directly extracted HRs and their 95% CIs for OS. The quality of 6 articles was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) (Table 2).
Table 1.
Characteristics of the included studies.
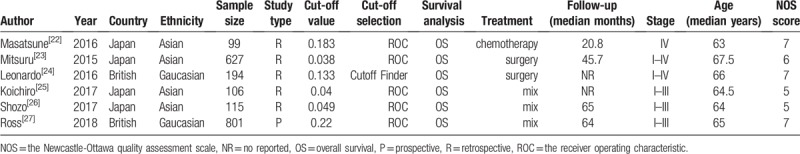
Table 2.
Assessment of study quality.
The pooled results revealed that patients with higher pretreatment CAR, which was higher than cut-off value, had significantly poorer OS than those with low CAR (HR: 2.09, 95% CI: 1.78–2.45, P < .001, Fig. 2) and a fix-effects model was applied. In all eligible studies, 5 studies reported the cut-off value which was gained by the receiver operating characteristic (ROC) curve, and 1 study got the cut-off value based on “Cut Off Finder” as described by Budczies et al.[21] A sensitivity analysis was conducted to determine whether any study would affect the pooled HRs, and the result was negative (Fig. 3). Egger's and Begg's test were used to evaluate the publication bias. The evidence for obvious publication bias for OS was not found, as the P value for Egger's test (Fig. 4) was 0.083, and the P value for Begg's test was 0.26. No missing studies were required to make the filled funnel plots symmetrical after being estimated via the trim-and-fill method (Fig. 5).
Figure 2.
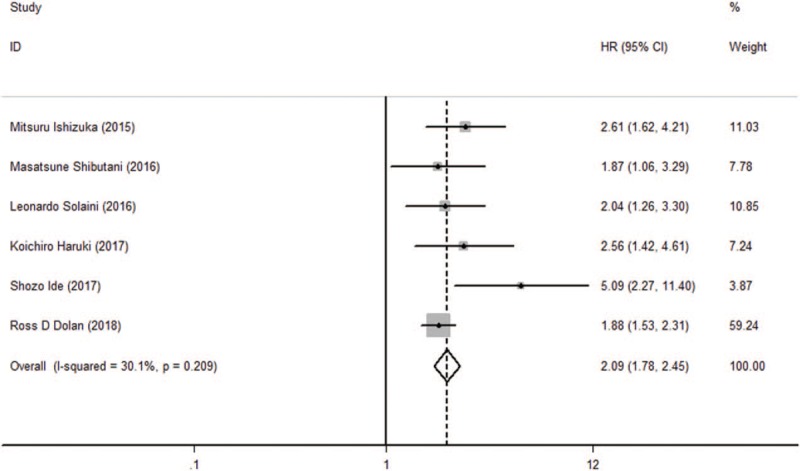
Forest plot of hazard ratio (HR) for the relationship between CAR and overall survival (OS).
Figure 3.
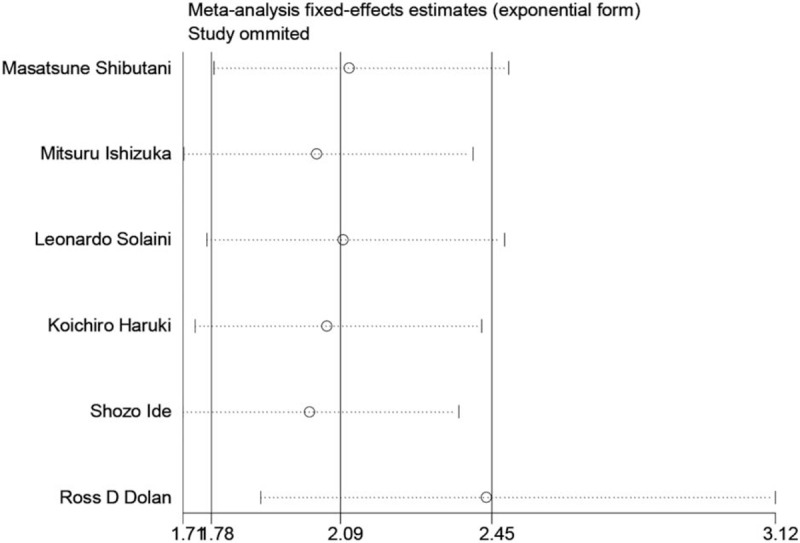
Sensitivity analysis of the relationship between CAR and OS.
Figure 4.
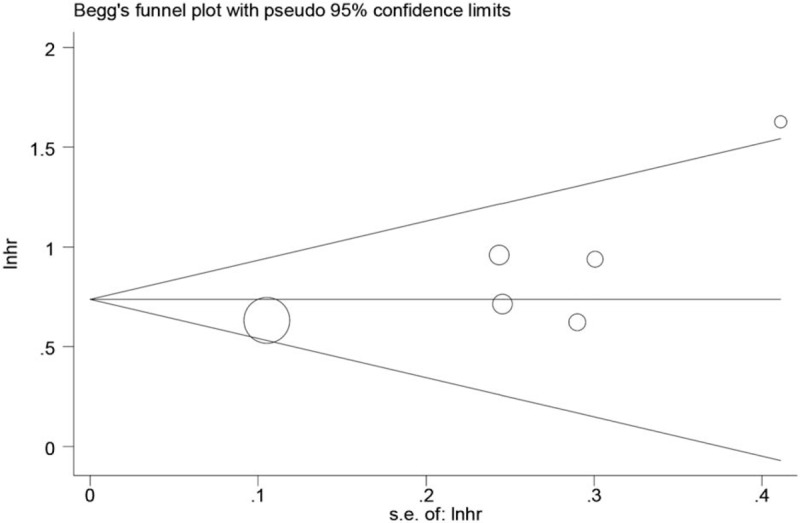
Begg's funnel plot for publication bias test of OS.
Figure 5.
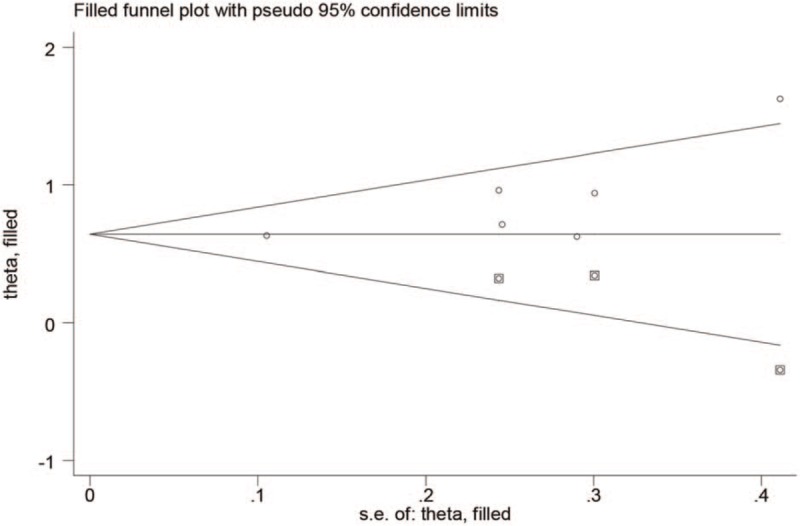
Filled funnel plots for publication bias test of OS.
4. Discussion
We evaluated the correlation of CAR with the prognosis value in CRC patients for the first time using meta-analysis. In this meta-analysis, we combined the outcomes of 1942 subjects from 6 individual studies, revealing that elevated pretreatment CAR was evidently related with poorer OS (HR: 2.09, 95%CI:1.78–2.45, P < .001) in CRC and there was no heterogeneity. Taking together, we hypothesized that CAR should be a potential prognostic biomarker in CRC.
CAR, composed of CRP and serum albumin, was initially proposed to identify acute patients and independently predicted the mortality of sepsis patients.[22,23] Then, there are many studies reported CAR was a good prognostic indicator in patients with various malignancies such as nasopharyngeal cancer, lung cancer.[24–31] CRP is an acute-phase protein synthesized by liver. Elevated serum concentration of CRP has been shown to induce cancer-related production of inflammatory cytokines. Meanwhile serum levels of albumin is the common marker of nutritional status and relate to chronic inflammation which activate cytokines such as IL-1 and TNF-α et al.[8,32] Thus, CRP/albumin (CAR) ratio can effectively reflects potential inflammatory state caused by expansion or invasion in the tumor microenvironment and be used to be an simple and independent prognostic factor.
This meta-analysis has some possible limitations:
-
1.
The number of included studies and subjects was limited which led to insufficient statistical power;
-
2.
The majority of included studies were retrospective which remain bias susceptibility;
-
3.
We performed three methods of the publication bias testing and the results showed no obvious publication bias in this study, while the tests may have false negatives because of the small number of studies included in our study;
-
4.
The cut-off values of CAR remained diverse in these included studies which led to inconsistent outcome threshold;
-
5.
Some databases (e.g., Cochrane Library) were not obtainable for this research screening which led to inadequate data extracted.
In summary, elevated pretreatment CAR was related with poor OS in CRC. Thus CAR might be used as a simple and reliable prognostic indicator which is applied to prognostication and classification of colorectal patients in clinical application. But its prognostic value still needed to be confirmed in large-scale studies.
Acknowledgments
None.
Author contributions
Conceptualization: Pei Li, Feng-sen Li.
Data curation: Pei Li.
Formal analysis: Fan Wang.
Methodology: Pei Li.
Project administration: Fan Wang.
Resources: Fan Wang, Pei Li.
Software: Fan Wang.
Supervision: Feng-sen Li.
Writing – original draft: Fan Wang.
Writing – review & editing: Feng-sen Li.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, HR = hazard ratio.
FW and PL contributed equally to this work.
Conflict of interest information: The authors have no conflicts of interests to declare.
References
- [1].Edwards Brenda K, Noone Anne-Michelle, Mariotto Angela B, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel Rebecca L, Miller Kimberly D, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [3].Bailey Christina E, Hu Chung-Yuan, You Y Nancy, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [5].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [6].Fondevila C, Metges JP, Fuster J, et al. p53 and VEGF expression are independent predictors of tumor recurrence and survival following curative resection of gastric cancer. Br J Cancer 2004;90:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaiswal M, LaRusso NF, Burgart LJ, et al. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000;60:184–90. [PubMed] [Google Scholar]
- [8].Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39:S143–6. [DOI] [PubMed] [Google Scholar]
- [9].Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fc gamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell 2007;12:252–65. [DOI] [PubMed] [Google Scholar]
- [10].Roxburgh Campbell SD, McMillan Donald C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. [DOI] [PubMed] [Google Scholar]
- [11].Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]
- [12].Xu X-L, Yu H-Q, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS ONE 2015;10:e0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McInnes Matthew DF, Moher D, Thombs Brett D, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–96. [DOI] [PubMed] [Google Scholar]
- [14].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [15].Zhang J, Chen L, Zhou R, et al. Pretreatment lymphocyte monocyte ratio predicts long-term outcomes in patients with digestive system tumor: a meta-analysis. Gastroenterol Res Pract 2016;2016:9801063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [20].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [21].Budczies J, Klauschen F, Sinn Bruno V, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012;7:e51862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fairclough E, Cairns E, Hamilton J, et al. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim MH, Ahn JY, Song JE, et al. The C-reactive protein/albumin ratio as an independent predictor of mortality in patients with severe sepsis or septic shock treated with early goal-directed therapy. PLoS ONE 2015;10:e0132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park H-C, Kim M-Y, Kim C-H. C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg 2016;42:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li J-P, Chen S-L, Liu X-M, et al. A novel inflammation-based stage (I Stage) predicts overall survival of patients with nasopharyngeal carcinoma. Int J Mol Sci 2016;17: pii: E1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang Y, Zhou G-Q, Liu X, et al. Exploration and validation of C-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer 2016;7:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yu S-T, Zhou Z, Cai Q, et al. Prognostic value of the C-reactive protein/albumin ratio in patients with laryngeal squamous cell carcinoma. Onco Targets Ther 2017;10:879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang F, Ying L, Jin J, et al. The C-reactive protein/albumin ratio predicts long-term outcomes of patients with operable non-small cell lung cancer. Oncotarget 2017;8:8835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol 2015;8:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017;24:561–8. [DOI] [PubMed] [Google Scholar]
- [32].Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, et al. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol 2007;14:381–9. [DOI] [PubMed] [Google Scholar]


