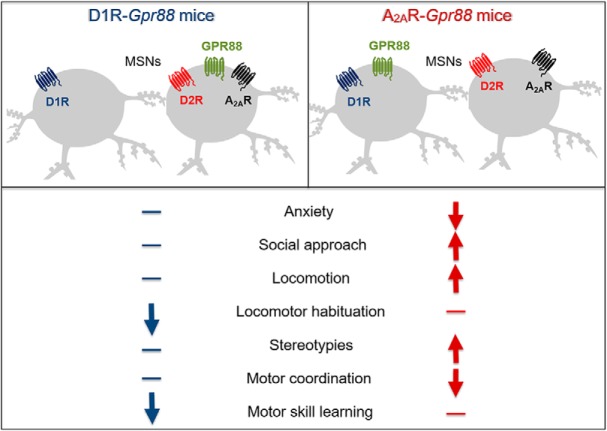
Visual Abstract
Keywords: anxiety, locomotion, medium spiny neuron, motor coordination, orphan GPCR, striatum
Abstract
The orphan receptor GPR88 is highly expressed in D1 receptor (D1R)- and D2R-medium spiny neurons (MSNs) and has been associated to striatum-dependent functions in rodents. The total deletion of Gpr88 in mice was shown to decrease anxiety-like behaviors, increase stereotypies and locomotion, and impair motor coordination and motor learning. Knowing the opposing role of D1R- and D2R-MSNs, we here investigated the respective roles of GPR88 in the two MSN subtypes for these behaviors. To do so, we compared effects of a conditional Gpr88 gene knock-out (KO) in D1R-MSNs (D1R-Gpr88 mice) or D2R-MSNs (A2AR-Gpr88 mice) with effects of the total Gpr88 KO (CMV-Gpr88 mice). Overall, most phenotypes of CMV-Gpr88 mice were recapitulated in A2AR-Gpr88 mice, including reduced marble burying, increased social interactions, increased locomotor activity and stereotypies in the open field, and reduced motor coordination in the rotarod. Exceptions were the reduced habituation to the open field and reduced motor skill learning, which were observed in CMV-Gpr88 and D1R-Gpr88 mice, but not in A2AR-Gpr88 mice. D1R-Gpr88 mice otherwise showed no other phenotype in this study. Our data together show that GPR88 modulates the function of both D1R- and D2R-MSNs, and that GPR88 activity in these two neuron populations has very different and dissociable impacts on behavior. We suggest that GPR88 in D2R-MSNs shapes defensive and social behavior and contributes in maintaining the inhibition of basal ganglia outputs to control locomotion, stereotypies and motor coordination, while GPR88 in D1R-MSNs promotes novelty habituation and motor learning.
Significance Statement
GPR88, an orphan G-protein-coupled receptor (GPCR), has been implicated in the regulation of striatum-dependent behaviors. In the striatum, GPR88 is most abundant in both medium spiny neurons (MSNs)-expressing dopamine D1 and D2 receptors. We compared effects of a conditional Gpr88 gene knock-out (KO) in D1 receptor (D1R)-MSNs or D2R-MSNs with effects of the total Gpr88 deletion. Our data suggest that GPR88 in D2R-MSNs shapes defensive and social behavior and contributes in maintaining the inhibition of basal ganglia outputs to control locomotion, stereotypies and motor coordination, while GPR88 in D1R-MSNs promotes novelty habituation and motor learning. Gpr88 therefore plays very distinct roles in modulating D1R-type and D2R-type neurons function and the related behaviors.
Introduction
Among brain orphan G-protein-coupled receptors (GPCRs), GPR88 shows highest and almost restricted expression in the striatum, a key region in motor control, cognitive functions and motivational processes (Liljeholm and O'Doherty, 2012; Quintana et al., 2012; Ehrlich et al., 2018). Homozygous deleterious mutation of Gpr88 in humans was linked to a familial developmental disorder characterized by a childhood chorea (hyperkinetic movement disorder), learning disabilities and marked speech retardation (Alkufri et al., 2016). Previous reports have shown that mice lacking Gpr88 present hyperlocomotion, increased stereotypies, motor coordination and motor learning deficits (Logue et al., 2009; Quintana et al., 2012; Meirsman et al., 2016b). The total Gpr88 gene deletion in mice also induced failure to habituate to an open field or automated home-cage environment and decreased anxiety-like behaviors (Meirsman et al., 2016b; Maroteaux et al., 2018). Additionally, AAV-mediated re-expression of GPR88 in the dorsal striatum [caudate putamen (CPu)] restored the locomotor hyperactivity and motor learning deficits in knock-out (KO) animals, thus providing a direct link between GPR88 loss in the dorsal striatum and the locomotor phenotype of KO mice (Quintana et al., 2012; Meirsman et al., 2016b).
Within the striatum, GPR88 is expressed in the majority of medium spiny neurons (MSNs) of both the direct [co-expressing dopamine D1 receptors (D1Rs) and substance P, D1R-MSNs] and indirect (co-expressing dopamine D2Rs, adenosine A2A receptor (A2AR) and Enkephalin, D2R-MSNs] pathways (Quintana et al., 2012). Converging evidence support the opposing influence of these two MSNs populations in motor output systems and motivated behavior. For example, optogenetic depolarization of D2R-MSNs decreased locomotor initiation (Kravitz et al., 2010), while ablation or disruption of these neurons increased motor activity (Durieux et al., 2009, 2012; Bateup et al., 2010). In contrast, optical stimulation of D1R-MSNs increased locomotion whereas disruption or ablation of these neurons had the opposite effect (Kravitz et al., 2010; Durieux et al., 2012). Also, cell-specific neuron ablation using an inducible diphtheria toxin receptor (DTR)-mediated cell targeting strategy further suggests a differential role of D2R- and D1R-MSNs in acquisition and expression of motor skill learning (Durieux et al., 2012). Ablation of D2R-MSNs neurons delayed the acquisition of a rotarod task but had no effect in a previously acquired motor skill. Contrarily, ablation of D1R-MSNs neurons impaired motor skill learning regardless of the training extension and also disrupted performance of a previously learned motor sequence (Durieux et al., 2012). Further, in recent years, research on MSNs subtypes function has revealed that these two neuronal populations differentially regulate not only motor behaviors but also responses to rewarding and aversive stimuli: while optogenetic activation of the D1R-MSNs was shown to increase reinforcement, activation of D2R-MSNs induced transient punishment and depressive-like behavior (Kravitz et al., 2012; Hikida et al., 2013; Francis et al., 2015).
Despite the established overall function of striatal GPR88 in brain functions and deficits (in humans and mice), no study to date has directly compared the specific role of GPR88 in D1R-and D2R-MSNs. A conditional KO mouse line for GPR88 in D2R-MSNs was developed in a previous study, using a A2AR-Cre driver line (A2AR-Gpr88 mice), and mutant mice showed hyperactive behavior, decreased anxiety-like behaviors and increased locomotor response to dopaminergic agonists (Meirsman et al., 2016a, 2017). In this study, we have generated conditional Gpr88 KO for D1R-MSNs (D1R-Gpr88 mice), and compared behavioral responses of D1R-Gpr88 with those of A2AR-Gpr88 mice and total KO (CMV-Gpr88) mice. Results show that GPR88 in D1R neurons regulates locomotor habituation to novel environments and motor skill learning. In contrast, GPR88 in D2R, but not in D1R neurons, control defensive burying and social approach, and also regulate levels of locomotion, stereotypies and initial motor coordination.
Materials and Methods
Subjects
Mice (male and female) aged 9–15 weeks where bred in house and grouped-house three to five animals per cage. Animals where maintained on a 12/12 h light/dark cycle at controlled temperature (22 ± 1°C). Food and water were available ad libitum throughout all experiments.
Generation of mutant mice
Gpr88-floxed mice, total Gpr88 KO (CMV-Gpr88) and A2AR-Gpr88 mice were produced as previously described (Meirsman et al., 2016a,b). To generate CMV-Gpr88, Gpr88-floxed mice (C57BL/6 background) were crossed with CMV-Cre mice (50%-C57BL/6J; 50%-129/sv) expressing Cre recombinase under the cytomegalovirus promoter. To generate a conditional KO of Gpr88 in D2R-MSNs (A2AR-Gpr88) or D1R-MSNs (D1R-Gpr88) Adora2a-Cre (Durieux et al., 2009) and Drd1a-Cre (gensat.org; congenic on C57BL/6J) mice were, respectively, crossed with Gpr88-floxed mice (Meirsman et al., 2016b). First generation animals expressing the Cre under the control of A2AR or D1R promotor (Gpr88A2AR-Cre/+ and Gpr88 D1R-Cre/+) were crossed a second time to eliminate the wild-type Gpr88 gene. We therefore generated 3 mouse lines with different mixed genetic background.
For all experiments, and considering the different genetic background, A2AR-Gpr88 and D1R-Gpr88 mice were compared to their Gpr88-floxed littermates (A2AR-CTL and D1R-CTL, respectively) and CMV-Gpr88 mice were compared to their wild-type controls (CMV-CTL). Baseline responses may therefore slightly differ when comparing the three mouse colonies.
Tissue preparation and fluorescent in situ hybridization
RNAscope was used as previously described (Meirsman et al., 2016a). Mice (n = 3 D1R-CTL; n = 3 D1R-Gpr88) were killed by cervical dislocation and fresh brains were extracted and embedded in optimal cutting temperature (OCT) medium (Thermo Scientific) frozen and kept at –80°C. Frozen brains were coronally sliced into 20-µm serial sections by using cryostat (CM3050 Leica), placed in superfrost slides (Thermo Scientific) and kept at –80°C until processing. In situ hybridizations were performed using the RNAscope Multiplex Fluorescent Assay. GPR88 and D1R probes were alternatively coupled to FITC or TRITC while D2R probes were coupled with Cy5.
Relative expression of D1R and D2R mRNA in GPR88-positive cells
Image acquisition was performed using the slide scanner Olympus VS120 (Olympus Corporation). Regions of interest (ROIs) were selected using Olyvia software (Olympus) and saved as PNG files. Three brain regions where analyzed: rostral CPu (from 1.42 to 0.98 mm from bregma), caudal CPu (from 0.98 to –0.58 mm from bregma), and nucleus accumbens (Nacc; from 1.42 to 1.10 mm from bregma).
For the CPu (rostral and caudal), at least four ROIs were selected: two for the dorso-lateral striatum (DLS) and two for dorso-median striatum (DMS). Counting was balanced between right and left hemispheres. To evaluate expression of D1R and D2R mRNA in GPR88-expressing cells, counting was performed manually using the FIJI (ImageJ) cell counter. First, cells expressing GPR88 mRNA were marked and counted (±55 cells/ROI in D1R-CTL mice; ±21 cells/ROI in D1R-Gpr88 mice). For each GPR88-positive cell, co-expression of D1R or D2R was verified and counted separately. Relative co-expression (GPR88/D1R or GPR88/D2R) is represented as a percentage of total GPR88-positive cells counted [(number of GPR88-expressing cells co-expressing D1R or D2R × 100)/total number of GPR88-expressing cells]. Statistical analysis where realized with percentages of each ROI calculated using excel. Given the lack of difference in GPR88 expression between lateral and medial CPu, relative percentage of each was pooled for graphical representation and statistical analysis.
[S35]-GTPγS binding assay
[S35]-GTPγS assays were performed on membrane preparations as described in previous report (Pradhan et al., 2009). To perform [S35]-GTPγS assays on whole striatum mice were killed by cervical dislocation and both striatum were rapidly manually removed, frozen in dry ice, and stored at –80°C until use. Two (CMV-Gpr88 and CMV-CTL) and three (D1R-Gpr88 and D1R-CTL) membrane preparations were used. Each membrane preparation was generated using striatum from three animals (males and females). Results are expressed by meaning measures from the three-membrane preparation. All assays were performed on membrane preparations. Membranes were prepared by homogenizing the tissue in ice-cold 0.25 M sucrose solution 10 vol (ml/g wet weight of tissue). Samples were then centrifuged at 2500 × g for 10 min. Supernatants were collected and diluted 10 times in buffer containing 50 mM TrisHCl (pH 7.4), 3 mM MgCl2, 100 mM NaCl, and 0.2 mM EGTA, following which they were centrifuged at 23,000 × g for 30 min. The pellets were homogenized in 800-μl ice-cold sucrose solution (0.32 M) and kept at –80°C. For each [35S]GTPγS binding assay 2 μg of protein per well was used. Samples were incubated with and without ligands, for 1 h at 25°C in assay buffer containing 30 mM GDP and 0.1 nM [35S]GTPγS. Bound radioactivity was quantified using a liquid scintillation counter. Bmax and Kd values were calculated. Non-specific binding was defined as binding in the presence of 10 μM GTPγS and binding in the absence of agonist was defined as the basal binding.
Gene expression analysis
Mice were killed by cervical dislocation. Brains structures (Nacc n = 8 D1R-CTL; n = 7 D1R-Gpr88 and CPu n = 9 D1R-CTL; n = 9 D1R-Gpr88, hippocampus: n = 9 D1R-CTL; n = 7 D1R-Gpr88 and amygdala: n = 6 D1R-CTL; n = 7 D1R-Gpr88) from D1R-Gpr88 and controls were quickly dissected out, frozen on dry ice and stored at −80°C until used. RNA was isolated using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. cDNA was synthetized using the first-strand Superscript II kit (Invitrogen, Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed in triplicates on a LightCycler 480 RT- PCR (Roche) and SyberGreen masterMix (Roche). Thermal cycling parameters were 1 min at 95°C followed by 40 amplification cycles of 15 s at 95°C, 15 s at 60°C, and 30 s at 72°C. Relative expression ratios were normalized to the level of actin and the 2−ΔΔCt method was applied to assess differential expression level of GPR88.
Behavioral experiments
For all behavioral measures, mice from different mouse lines and genotypes were tested in a random order and data were analyzed blind to genotype. However, to avoid order-related variability between mouse lines and genotypes, mouse lines were stratified so that each session included both genotypes from all lines. One mouse cohort (N = 8 CMV-CTL, N = 10 CMV-Gpr88; N = 10 A2AR-Gpr88, N = 10 A2AR-CTL; N = 10 D1R-Gpr88, N = 14 D1R-CTL) was used to measure marble burying and social interaction. Independent cohorts of mice (N = 21 CMV-CTL, N = 21 CMV-Gpr88; N = 10 A2AR-Gpr88, N = 17 A2AR-CTL; N = 13 D1R-Gpr88, N = 12 D1R-CTL) underwent 5 d of open field locomotion followed by a 48-h resting period before 7 d of rotarod motor skill learning.
Marble burying
Defensive burying was measured as previously described (Meirsman et al., 2016b) using the marble burying test conducted with 20 small glass marbles (15 mm) evenly spaced in a transparent single cage (21 × 11 × 17 cm) over 4-cm sawdust bedding. The cage was covered by a plastic lid in a room illuminated at 40 Lux. Mice were left in the cage for 10 min, and the number of marbles buried more than half in sawdust was counted.
Social interaction test
Social interaction was assessed, as previously described (Meirsman et al., 2016a), in an open field (50 × 50 cm) dimly lit (<10 Lux) using naive wild-type mice of the same age and weight as interactors. On the first day, all mice were individually placed in the open field arena and left for a 10-min period of habituation. The next day, mice were placed in the open field arena with a wild-type naive interactor and a 10-min session was recorder. Nose contacts were measured manually using video recordings. If an interactor failed to engage in any interaction data from the respective mice were exclude from analysis.
Open field locomotion
To assess basal locomotion and habituation to novel environment mice were placed in a dimly lit open field (Accuscan Instruments) for 30-min daily session. The experiment lasted 5 d, and mice were placed in the same open field for all sessions tested. Open field was cleaned with water and 70% ethanol between trials. Total distance traveled, stereotypy counts, and durations were automatically recorded.
Rotarod
The first day, mice were placed on the rod (30-mm plastic roller, Panlab Harvard) at constant speed of 4 rpm. until achieving 90 s without falling from the rod (habituation; data not shown). On the six next consecutive days, mice were placed on the rod accelerating from 4 to 40 rpm in 5 min and the remaining at maximum speed for the next 5 min for four trials every day. Light intensity in the room was inferior to 10 Lux. Mice rested a minimum of 1 min between trials to avoid fatigue and exhaustion. When mice hang on the rod instead of running, they were left for one complete turn but the timer was stopped if the mice engaged in a second consecutive turn. Animals were scored for their latency to fall (in seconds) in each trial. Mean values of four daily trials were used for statistical analysis.
Statistics
For in situ hybridization cell counting and GPR88 agonist-induced binding assay data were analyzed using two-way ANOVA followed by Sidak’s and Tukey’s multiple comparisons, respectively. Repeated measures (RM) two-way ANOVA was used to analyze global open field and rotarod results with genotypes as the between-subject factor and time as the RM. One-way ANOVA was used for open field habituation analysis (days 1 and 5), first and last rotarod session analysis. Method of contrasts was used to compare day 1 and day 6 performance on the rotarod. Stereotypies, marble burying and social interaction contacts were analyzed using t test (unpaired with Welch’s correction). All statistical analyses were realized using GraphPad Prism 7 (GraphPad Software, Inc) and the accepted level of significance was p < 0.05. All the statistical methods are summarized in Table 1.
Table 1.
Detailed statistical analysis
| ANOVA | t test | ||||||
|---|---|---|---|---|---|---|---|
| Assay | Mouse line | Number | Figure | Genotype effect | Cell type/time/treatment | Interaction | |
| RT-qPCR | D1R-Gpr88 | N = 9 D1R-CTL; N = 9 D1R-Gpr88 | 1A (CPu) | t(16) = 3.01, p = 0.008 | |||
| N = 8 D1R-CTL; N = 7 D1R-Gpr88 | 1A (Nacc) | t(13) = 4.19, p = 0.001 | |||||
| N = 9 D1R-CTL; N = 7 D1R-Gpr88 | 1A (Hipp) | t(14) = 2.7, p = 0.017 | |||||
| N = 6 D1R-CTL; N = 7 D1R-Gpr88 | 1A (Amy) | t(11) = 0.53, p = 0. 6 | |||||
| [35S]-GTPgS binding | CMV-Gpr88; D1R-Gpr88 | N = 3 D1R-CTL, D1R-Gpr88, CMV-CTL, CMV-Gpr88 | 1B | F(3,66) = 185.2; p < 0.0001 | F(10,66) = 95.64; p < 0.0001 | F(30,66) = 23.19; p < 0.0001 | |
| In situ hybridization/cell counting | D1R-Gpr88 | N = 3 D1R-CTL, N = 3 D1R-Gpr88 | 2B (CPu) | F(1,134) = 0.4164; p = 0.5198 | F(1,134) = 957.2; p < 0.0001 | F(1,134) = 387.8; p < 0.0001 | |
| 2B (Nacc) | F(1,36) = 0.2597; p = 0.6134 | F(1,36) = 204.3; p < 0.0001 | F(1,36) = 97.83; p < 0.0001 | ||||
| Marble burying | CMV-Gpr88 | N = 9 CMV-CTL, N = 10 CMV-Gpr88 | 3A, left | t(17) = 2.03, p = 0.059 | |||
| D1R-Gpr88 | N = 14 D1R-CTL, N = 10 D1R-Gpr88 | 3B, left | t(22) = 1.002, p = 0.33 | ||||
| A2AR-Gpr88 | N = 10 A2AR-CTL, N = 10 A2AR-Gpr88 | 3C, left | t(18) = 4.01, p < 0.001 | ||||
| Nose contact in social interaction | CMV-Gpr88 | N = 8 CMV-CTL, N = 8 CMV-Gpr88 | 3D, right | t(14) = 2.88, p = 0.012 | |||
| D1R-Gpr88 | N = 14 D1R-CTL, N = 8 D1R-Gpr88 | 3E, right | t(20) = 2.57, p = 0.018 | ||||
| A2AR-Gpr88 | N = 10 A2AR-CTL, N = 10 A2AR-Gpr88 | 3F, right | t(18) = 2.06, p = 0.01 | ||||
| Open field (all sessions) | CMV-Gpr88 | N = 21 CMV-CTL, N = 21 CMV-Gpr88 | 4A, left | F(1,40) = 4.357; p = 0.0425 | F(4,180) = 4.419; p = 0.002 | F(4,180) = 7.189; p < 0.0001 | |
| D1R-Gpr88 | N = 13 D1R-CTL, N = 12 D1R-Gpr88 | 4B, left | F(1,23) = 1.106; p = 0.3038 | F(4,92) = 31.03; p < 0.0001 | F(4,92) = 11.82; p < 0.0001 | ||
| A2AR-Gpr88 | N = 17 A2AR-CTL, N = 10 A2AR-Gpr88 | 4C, left | F(1,25) = 8.004; p = 0.0091 | F(4,100) = 43.28; p < 0.0001 | F(4,100) = 3.939; p = 0.0052 | ||
| Open field (sessions 1 and 5) | CMV-Gpr88 | N = 21 CMV-CTL, N = 21 CMV-Gpr88 | 4A, right | F(1,80) = 4.93; p = 0.0292 | F(1,80) = 1.25;p = 0.2679 | F(1,80) = 8.94; p = 0.0037 | |
| D1R-Gpr88 | N = 13 D1R-CTL, N = 12 D1R-Gpr88 | 4B, right | F(1,46) = 0.78; p = 0.3811 | F(1,46) = 26.75;p < 0.0001 | F(1,46) = 11.01; p = 0.0018 | ||
| A2AR-Gpr88 | N = 17 A2AR-CTL, N = 10 A2AR-Gpr88 | 4C, right | F(1,50) = 8.17; p = 0.0062 | F(1,50) = 18.71; p < 0.0001 | F(1,50) = 0.1479; p = 0.7021 | ||
| Stereotypies | CMV-Gpr88 | N = 21 CMV-CTL, N = 21 CMV-Gpr88 | 5A | Score: t(40) = 2.228; p = 0.0316Time: t(40) = 2.818; p = 0.0075 | |||
| D1R-Gpr88 | N = 13 D1R-CTL, N = 12 D1R-Gpr88 | 5B | Score: t(23) = 1.156; p = 0.2594Time: t(23) = 0.7174; p = 0.4803 | ||||
| A2AR-Gpr88 | N = 17 A2AR-CTL, N = 10 A2AR-Gpr88 | 5C | Score: t(25) = 2.291; p = 0.0307Time: t(25) = 2.317; p = 0.0290 | ||||
| Rotarod (all sessions) | CMV-Gpr88 | N = 21 CMV-CTL, N = 21 CMV-Gpr88 | 6A, left | F(1,40) = 17.73; p = 0.0001 | F(23,920) = 13.49; p < 0.0001 | F(23,920) = 3.159; p < 0.0001 | |
| D1R-Gpr88 | N = 13 D1R-CTL, N = 12 D1R-Gpr88 | 6B, left | F(1,23) = 8.759; p = 0.0070 | F(23,529) = 10.09; p < 0.0001 | F(23,529) = 7.607; p < 0.0001 | ||
| A2AR-Gpr88 | N = 17 A2AR-CTL, N = 10 A2AR-Gpr88 | 6C, left | F(1,25) = 8.008; p = 0.0091 | F(23,575) = 13.74; p < 0.0001 | F(23,575) = 1.017; p = 0.4403 | ||
| Rotarod (sessions 1 and 6) | CMV-Gpr88 | N = 21 CMV-CTL, N = 21 CMV-Gpr88 | 6A, right | F(1,80) = 32.62; p < 0.0001 | F(1,80) = 17.67 p < 0.0001 | F(1,80) = 4.517; p = 0.0367 | |
| D1R-Gpr88 | N = 13 D1R-CTL, N = 12 D1R-Gpr88 | 6B, right | F(1,46) = 13.62; p = 0.0006 | F(1,46) = 11.15; p = 0.0017 | F(1,46) = 7.643; p = 0.0082 | ||
| A2AR-Gpr88 | N = 17 A2AR-CTL, N = 10 A2AR-Gpr88 | 6C, right | F(1,50) = 8.067; p = 0.0065 | F(1,50) = 16.31; p = 0.0002 | F(1,50) = 1.299; p = 0.2598 | ||
Results
D1R-Gpr88 mice show Gpr88 mRNA deletion in D1R-expressing neurons
To conditionally delete Gpr88 exon 2 in cells expressing D1R, mice carrying two LoxP sites flanking the second exon of the Gpr88 gene (Meirsman et al., 2016b) were crossed with mice expressing the Cre recombinase under the control of the Drd1a gene promoter (Gensat). We first tested whether GPR88 transcript and protein are reduced in the striatum. We quantified Gpr88 mRNA levels by RT-qPCR for CPu and Nacc from D1R-Gpr88 and their control littermates. As shown in Figure 1A, Gpr88 expression was significantly decreased in striatal regions of conditional KO compared to controls (CPu: t(16) = 3.01, p = 0.008; Nacc: t(13) = 4.19, p = 0.001; Table 1). Testing Gpr88 mRNA levels in the hippocampus and amygdala showed a milder but significant reduction in hippocampus but not amygdala (Hipp: t(14) = 2.7, p = 0.017; Amy t(11) = 0.53, p = 0.6), indicating that GPR88 KO may also have occurred in some extrastriatal regions containing D1R-type neurons (see Discussion). There was no significant difference in D1R expression levels across genotypes (data not shown). To establish whether reduced mRNA level translates into lower protein level, we tested GPR88 signaling in the striatum. To this aim, we performed GPR88 agonist-induced [S35]-GTPγS binding assays (Fig. 1B) with membranes prepared from whole striatum (CPu and Nacc) of D1R-Gpr88 mice and their controls, as well as with total KO CMV-Gpr88 mice (negative control) and their wild-type control mice (positive control). Two-way RM ANOVA revealed a significant genotype effect (F(3,66) = 185.2, p < 0.0001) and interaction effect (F(30,66) = 23.19, p < 0.0001). Post hoc analysis (Tukey’s multiple comparisons) revealed significant differences (p < 0.0001) between D1R-Gpr88 mice (118.4 ± 1.17%) and their control littermates (209.5 ± 2.47%) as well as between D1R-Gpr88 mice and CMV-Gpr88 mice (95.61 ± 1.72%; p < 0.0001). This result confirms that the Drd1a-Cre-driven conditional Gpr88 gene deletion produced a significant reduction of GPR88 expression. Importantly, deletion of GPR88 does not affect the function of D1R. Indeed, locomotor response to D1R agonist SKF 81297 is comparable in D1R-Gpr88 mice and their corresponding controls (data not shown).
Figure 1.
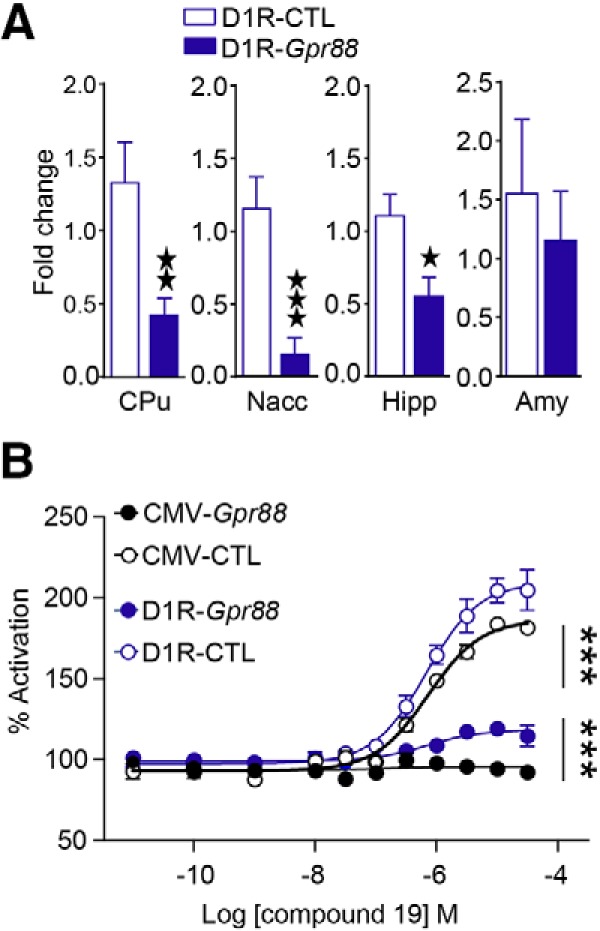
GPR88 agonist-induced activation and mRNA levels in D1R-Gpr88 mice. We measured levels of Gpr88 mRNA in D1R-CTL and D1R-Gpr88 mice (A) and show a significant reduction of GPR88 expression in the CPu, Nacc, hippocampus (Hipp), and amygdala (Amy). We also performed GPR88-mediated [35S]-GTPγS assay (B) and show that protein activation was totally and partially abolished in the striatum of CMV-Gpr88 and D1R-Gpr88 mice, respectively. Two (CMV-Gpr88 and control mice) and three (D1R-Gpr88 and control mice) membrane preparations were used per genotype. Data are presented as mean ± SEM. A, CPu: n = 9 D1R-CTL; n = 9 D1R-Gpr88; Nacc: n = 8 D1R-CTL; n = 7 D1R-Gpr88; Hipp: n = 9 D1R-CTL; n = 7 D1R-Gpr88; Amy: n = 6 D1R-CTL; n = 7 D1R-Gpr88; two black stars p < 0.01; three black stars p < 0.001 (Welch’s t test). B, n = 3 D1R-CTL; n = 3 D1R-Gpr88; n = 2 CMV-Gpr88 and n = 2 CMV-CTL. Three text stars p < 0.001 Tukey’s multiple comparisons of D1R-CTL or CMV-CTL versus D1R-Gpr88 and CMV-Gpr88 versus D1R-Gpr88.
We then tested whether the genetic deletion was specific to D1R MSNs, using in situ hybridization. As depicted in Figure 2A, we first demonstrate that, in control mice cells expressing Gpr88 mRNA colocalize with both Drd1a (left panel), and Drd2 mRNA-expressing cells (right panel). In D1R-Gpr88 mice, however, cells expressing Gpr88 do not colocalize with Drd1a-expressing cells (left panel), but still colocalize with Drd2-expressing cells (right panel). Quantitative analysis (Fig. 2B) in the CPu and Nacc confirmed that, in control animals, Gpr88 mRNA is found in both Drd1a-positive (CPu: 43.96 ± 1.54% and Nacc: 45.13 ± 3.57%) and Drd2-positive (CPu: 62.11 ± 1.95% and Nacc: 59.03 ± 4.47%) cells whereas in D1R-Gpr88 mice the great majority of cells expressing Gpr88 are found in Drd2-expressing cells (CPu: 92.83 ± 1.45% and Nacc: 91.88 ± 2.48%) with significantly reduced number of cells co-expressing Drd1a mRNA (CPu: 11.16 ± 1.43% and Nacc: 15.50 ± 2.18%; CPu; genotype: F(1,134) = 0.42; p = 0.52; cell type: F(1,134) = 957.2; p < 0.0001; interaction: F(1,134) = 387.8, p < 0.0001; Nacc; genotype: F(1,36) = 0,26, p = 0,6134; cell type: F(1,36) = 204,3, p < 0,0001; interaction: F(1,36) = 97.83, p < 0.0001; n = 3/genotype), indicating that the Gpr88 deletion had occurred mostly in D1R-type MSNs.
Figure 2.
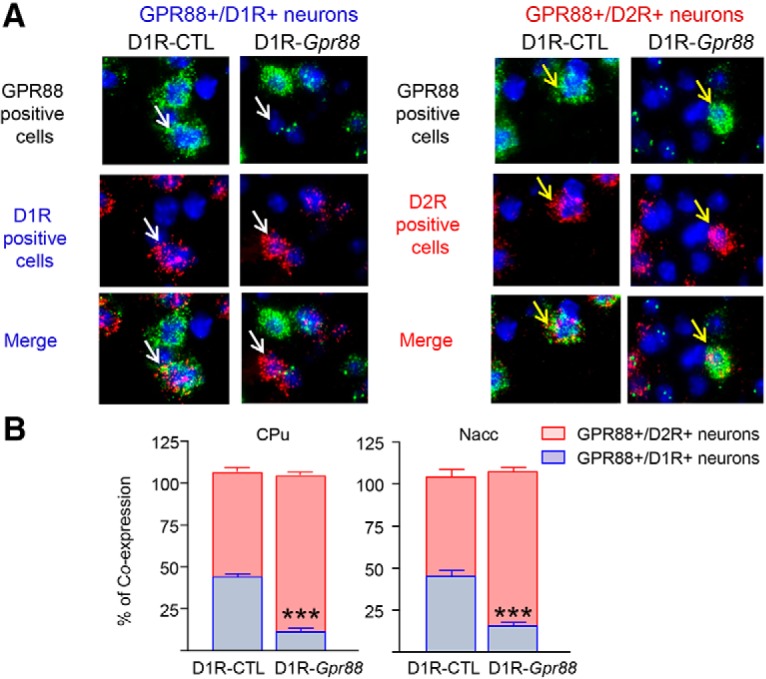
Molecular characterization of conditional D1R-Gpr88 mice. Gpr88, Drd1a and Drd2 mRNA expression in the CPu of D1R-CTL (left panels) and D1R-Gpr88 (right panels) mice using triple fluorescent in situ hybridization (A). Gpr88 is labeled in green (FITC), Drd1a (left panels) in red (TRITC) and Drd2 (right panels) in red (Cy5). In D1R-CTL animals, Gpr88 mRNA colocalizes with both Drd2 and Drd1a mRNA. In contrast, Drd2 but not Drd1a colocalize with Gpr88 mRNA in D1R-Gpr88 mice. White and yellow arrows indicate examples of Drd1a-positive and Drd2-positive cells, respectively. DAPI staining (blue) was used to label all cells nuclei. Quantification of Gpr88/Drd2 (red) and Gpr88/Drd1a (blue) mRNA co-expression in the CPu and Nacc (B) of D1R-Gpr88 and control mice (n = 3/genotype). Colocalization of Gpr88 and Drd1a mRNA was significantly decreased in the CPu and Nacc of D1R-Gpr88 mice compared to control littermates (Sidak’s multiple comparison; p < 0.0001). Percentage of co-expression was calculated based on the total number of Gpr88-positive cells counted [(number Gpr88-expressing cells co-expressing Drd1a or Drd2 × 100)/total number of Gpr88-expressing cells]. Data are presented as mean ± SEM. B, n = 3 D1R-CTL; n = 3 D1R-Gpr88. Text stars: three stars p < 0.001 (Sidak’s multiple comparison of Gpr88/Drd1a co-expression between genotypes).
A2AR-Gpr88 but not D1R-Gpr88 mice show altered defensive burying and social approach
The deletion of Gpr88 specifically in D2R-neurons is sufficient to decrease anxiety-like behaviors and increase social approach (Meirsman et al., 2016a). Here, we investigated whether deletion of Gpr88 in D1R-neurons also modifies anxiety-related and/or social behaviors. As depicted in Figure 3A–C, using the defensive burying paradigm we first confirmed that CMV-Gpr88 (t(17) = 2.03, p = 0.059; n = 9–10) buried less marbles than their control littermates. D1R-Gpr88 mice showed equal numbers of buried marbles compared to D1R-CTL mice (t(22) = 1.002, p = 0.33; n = 10–14), whereas A2AR-Gpr88 mice, like CMV-Gpr88, showed reduced number of buried marbles (t(18) = 4.01, p < 0.001; n = 10/genotype). Also, in the presence of a naive, wild-type, congener (Fig. 3D–F), CMV-Gpr88 (t(14) = 2.88, p = 0.012; n = 8/genotype), and A2AR-Gpr88 mice (t(18) = 2.06, p = 0.01; n = 10/genotype) showed increased numbers of nose contacts but D1R-Gpr88 mice displayed similar numbers of contacts than their control littermates (t(20) = 2.57, p = 0.018; n = 10–14).
Figure 3.
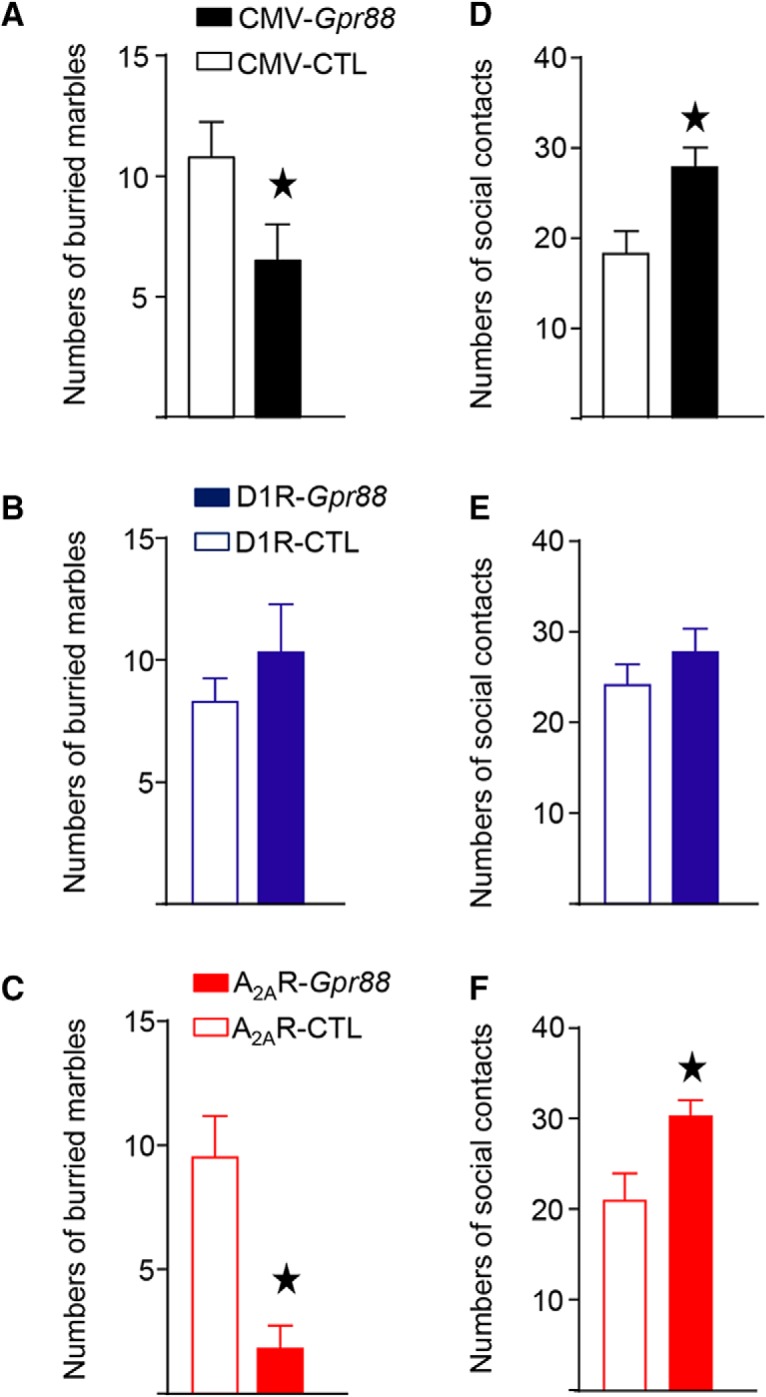
CMV-Gpr88 and A2AR-Gpr88 but not D1R-Gpr88 mice show altered defensive burying and social behavior. When placed in the presence of 20 marbles CMV-Gpr88 (A) and A2AR-Gpr88 (C) mice buried less marbles than control animals. D1R-Gpr88 mice (B) show similar numbers of buried marbles compared to control animals. To test social behaviors all mice where left in the presence of a naive wild-type congener and nose contact was counted. Once again, both CMV-Gpr88 (D) and A2AR-Gpr88 (F) mice but not D1R-Gpr88 mice (E) showed increased number of nose contacts compared to their littermates. Data are represented as mean ± SEM. A, D, n = 8 CMV-CTL, n = 10 CMV-Gpr88. B, E, N = 14 D1R-CTL, N = 10 D1R-Gpr88. C, F, n = 10 A2AR-CTL; n = 10 A2AR-Gpr88. Black stars: one star p < 0.05 (Welch’s t test).
The present results confirm previous findings (Meirsman et al., 2016a) that Gpr88 deletion in D2R-neurons is sufficient to recapitulate emotional and social phenotypes observed in CMV-Gpr88 mice and reveal that deletion of Gpr88 in D1R-neurons does not alter neither defensive marble burying or social approach.
A2AR-Gpr88 mice show hyperlocomotion, whereas D1R-Gpr88 mice show lack of habituation in a novel environment
Previous studies showed that mice lacking Gpr88 display increased spontaneous locomotor activity as well as lack of habituation to a novel environment (Quintana et al., 2012; Meirsman et al., 2016b; Maroteaux et al., 2018). Deletion of Gpr88 in D2R expressing neurons was further shown sufficient to recapitulate the hyperlocomotion phenotype observed in CMV-Gpr88 mice (Meirsman et al., 2016a). Here, we tested whether GPR88 in D1R and D2R MSNs play a differential role in the regulation of locomotor and exploratory behavior. To do this, CMV-Gpr88, A2AR-Gpr88, and D1R-Gpr88 mice and their corresponding controls were individually placed in a dimly lit open field chambers during five successive daily 30-min sessions. Analysis of total locomotion confirmed a significantly increased locomotor activity for CMV-Gpr88 mice (two-way RM ANOVA; genotype: F(1,40) = 5.98, p = 0.0189; day: F(4,180) = 4.42; p = 0.002; interaction: F(4,180) = 7.19; p < 0.0001; n = 21/genotype; Fig. 4A, left panel). Further, while control animals decreased their general locomotion between the first and last session (see Fig. 4A, right panel), CMV-Gpr88 mice showed rather increased locomotion in the last compared to the first session (two-way ANOVA; genotype: F(1,80) = 4.93, p = 0.029; day: F(1,80) = 1.25, p = 0.27; interaction: F(1,80) = 8.94, p = 0.0037). D1R-Gpr88 mice (Fig. 4B, left panel) presented similar levels of general locomotor activity when compared to their littermates (two-way RM ANOVA; genotype: F(1,23) = 1.11, p = 0.30; day: F(4,92) = 31.03; p < 0.0001; interaction: F(4,92) = 11.82; p < 0.0001; n = 12–13) but, similar to CMV-Gpr88 mice, showed lack of locomotor habituation to the open field environment (two-way ANOVA; genotype: F(1,46) = 0.78, p = 0.38; day: F(1,46) = 26.75, p < 0.0001; interaction: F(1,46) = 11.01, p = 0.0018; Fig. 4B, right panel). Similar to CMV-Gpr88, A2AR-Gpr88 mice (Fig. 4C, left panel) significantly increased their locomotion when compared to control littermates (two-way RM ANOVA; genotype: F(1,25) = 8.0, p = 0.009; day: F(4,100) = 43.28; p < 0.0001; interaction: F(4,100) = 3.94; p = 0.005; n = 10–17). These mice, however, showed equal locomotor habituation profile than their littermates with decreased locomotion in the last compared to the first open field session (right panel; two-way ANOVA; genotype: F(1,50) = 8.17, p = 0.006; day: F(1,50) = 18.71, p < 0.0001; interaction: F(1,50) = 0.15, p = 0.70).
Figure 4.
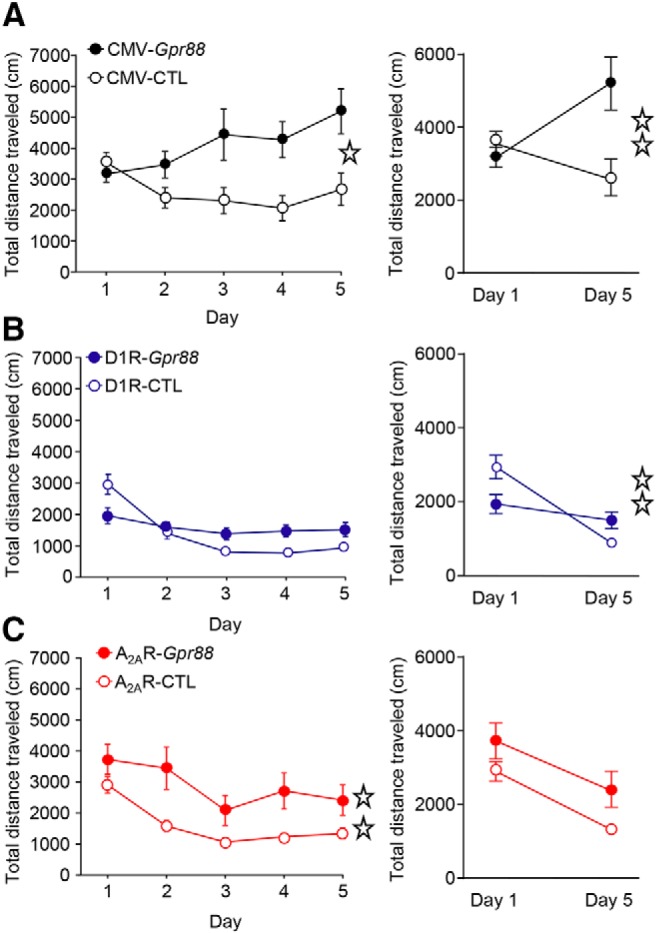
Locomotor activity is increased in A2AR-Gpr88 mice whereas D1R-Gpr88 mice show lack of locomotor habituation. When placed individually in a dimly lit open field for 30-min daily sessions during 5 d, both CMV-Gpr88 (A) and A2AR-Gpr88 (C) but not D1R-Gpr88 (B) mice traveled a longer distance then their control littermates. D1R-Gpr88 mice, however, present similar total locomotion when compared to their control littermates (B). When comparing locomotion between the first (1) and last session (5), CMV-Gpr88 mice, in contrast to CMV-CTL, traveled a longer distance in the last compared to the first day. In contrast to their control littermates, D1R-Gpr88 mice show similar locomotion in the first and last open field session. Regardless of their hyperlocomotion, A2AR-Gpr88 mice habituated to the open field presenting decreased overall locomotion in the last test session. Line graphs show the distance traveled (cm) in 5-min bins over a 30-min session. Bar graphs show the average total distance traveled (cm) over the 30-min sessions period. Data are represented as mean ± SEM. A, n = 21 CMV-CTL; n = 21 CMV-Gpr88. B, N = 13 D1R-CTL, N = 12 D1R-Gpr88. C, n = 17 A2AR-CTL; n = 10 A2AR-Gpr88. Open stars: one star p < 0.05; two stars p < 0.01 (RM two-way ANOVA).
These results first confirm that deletion of Gpr88 increases general locomotion and simultaneously abolishes locomotor habituation to a novel environment. Further, our results suggest that deletion of D1R-Gpr88 does not impact general locomotion but abolishes locomotor habituation to a novel environment. In contrast, deletion of Gpr88 in D2R-MSNs increases locomotor activity without altering habituation to a novel environment.
A2AR-Gpr88 but not D1R-Gpr88 mice show increased stereotypies in the open field
Previous studies indicate increased repetitive motor behaviors or stereotypies (Logue et al., 2009; Meirsman et al., 2016b), as well as increased perseverative behavior (Maroteaux et al., 2018) in CMV-Gpr88 mice. To examine whether this phenotype results from Gpr88 deletion in D1R- and/or D2R-MSNs, we analyzed stereotypies scores in the first open field session (30 min). Results indicate that both CMV-Gpr88 (Fig. 5A) and A2AR-Gpr88 mice (Fig. 5C) presented higher stereotypies score (t(40) = 2.23; p = 0.031; n = 21/genotype and t(25) = 2.29; p = 0.031; n = 10–17, respectively) and increased stereotypy time (t(40) = 2.82; p = 0.007; and t(25) = 2.32; p = 0.029, respectively). On the contrary, D1R-Gpr88 mice (Fig. 5B) presented no altered stereotyped behavior when compared to control animals.
Figure 5.
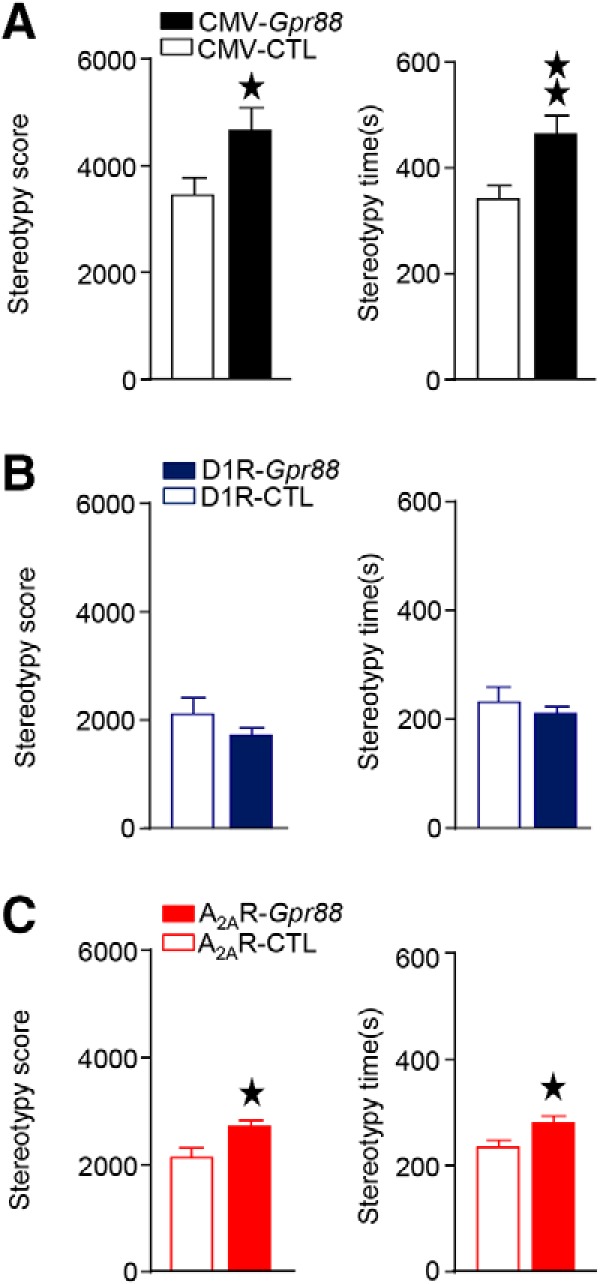
CMV-Gpr88 and A2AR-Gpr88 gene deletion increases stereotypies. When placed in an open field for 30 min (day 1), CMV-Gpr88 (A) and A2AR-Gpr88 (C) present increased number and duration of stereotypies. D1R-Gpr88 mice (B), however, show no difference in the number or time spent in stereotypies when compared to their control littermates. Data are represented as mean ± SEM. A, n = 21 CMV-CTL; n = 21 CMV-Gpr88. B, N = 13 D1R-CTL, N = 12 D1R-Gpr88. C, n = 17 A2AR-CTL; n = 10 A2AR-Gpr88. black stars: one star p < 0.05; two stars p < 0.01 (Student’s t test).
These results show that GPR88 in D2R-expressing but not D1R-expressing neurons regulates motor stereotypies.
A2AR-Gpr88 mice show impaired motor coordination, whereas D1R-Gpr88 mice show lack of motor skill learning
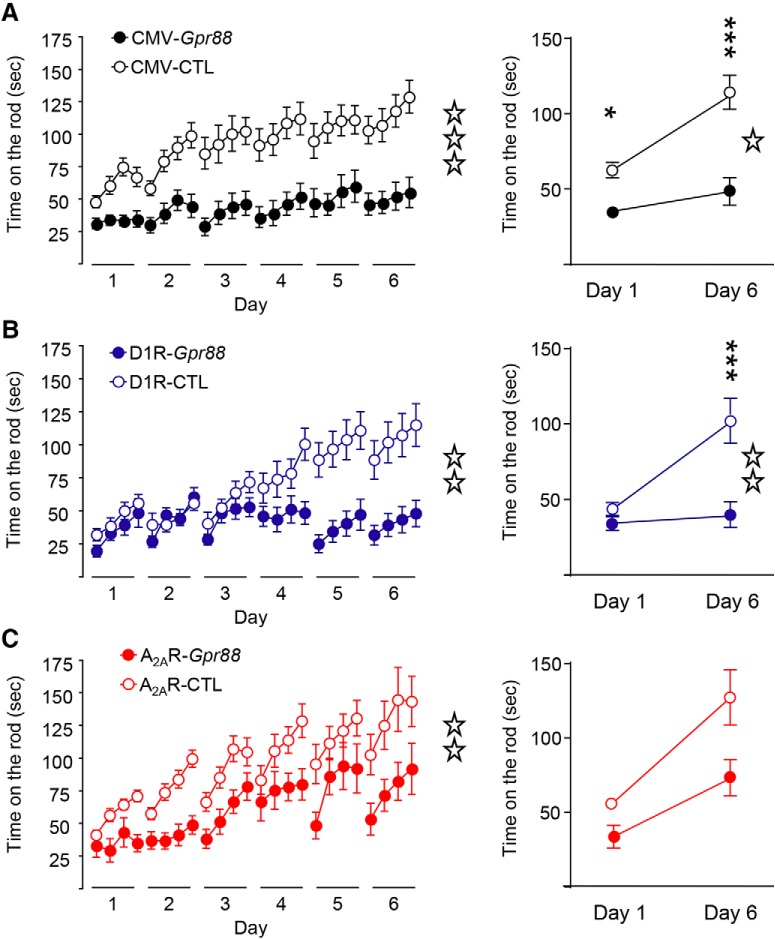
CMV-Gpr88 mice have been previously shown to present initial motor coordination deficits coupled with abolished motor skill learning throughout the rotarod tasks (Quintana et al., 2012; Meirsman et al., 2016b). We therefore compared motor coordination and motor skill learning performances of CMV-, D1R-and A2AR-Gpr88 mice by testing them in a rotating rod for six consecutive daily sessions. As depicted in Figure 6A, left panel, two-way RM ANOVA confirmed an impaired motor coordination for CMV-Gpr88 mice (genotype: F(1,40) = 17.73, p < 0.0001; day: F(23,920) = 13.49, p < 0.0001; n = 21/genotype) as well as a significant genotype × time effect (F(23,920) = 3.16; p < 0.0001) confirming the lack of motor skill learning as previously published (Quintana et al., 2012; Meirsman et al., 2016b). In fact, CMV-Gpr88 mice show decreased motor coordination in day 1 and maintained poor performance until the end of the task (day 6; right panel; two-way ANOVA; genotype: F(1,80) = 32.62, p < 0.0001, day: F(1,80) = 17.67, p < 0.0001; interaction: F(1,80) = 4.52, p = 0.0367). Post hoc analysis revealed significant differences between CMV-Gpr88 mice and control animals during day 1 (p < 0.05) and 6 (p < 0.0001). In addition, only control animals showed an improved motor performance between days 1 and 6 (p < 0.0001). Similarly, D1R-Gpr88 mice (Fig. 6B, left panel) presented significantly decreased motor learning performance (two-way RM ANOVA; genotype: F(1,23) = 8.76, p = 0.007; day: F(23,529) = 10.09, p < 0.0001; n = 12–13) and significant genotype × time effect (F(23,529) = 7.61; p < 0.0001). Despite similar motor coordination than their control littermates on day 1, D1R-Gpr88 mice failed to learn the task presenting decreased time on the rod on day 6 compared to their littermates (right panel; two-way ANOVA; genotype: F(1,46) = 13.62, p = 0.0006; day: F(1,46) = 11.15, p = 0.0017; interaction: F(1,46) = 7.64, p = 0.008). Post hoc analysis revealed significant differences between D1R-Gpr88 mice and control animals only during day 6 (p < 0.0001). Moreover, only control animals showed an improved motor performance between days 1 and 6 (p < 0.001). A2AR-Gpr88 mice (Fig. 6C, left panel) on the other hand also presented significantly decreased global performance (two-way RM ANOVA; genotype: F(1,25) = 8.01, p = 0.0091; day: F(23,575) = 13.74; p < 0.0001; interaction: F(23,575) = 1.02; p = 0.44; n = 10–17) but show motor learning skills over days of experiment. In fact, when comparing day 1 and day 6 (right panel), two-way ANOVA showed significant genotype (F(1,50) = 8.07, p = 0.0065) and day (F(1,50) = 16.31, p = 0.0002) effect but not interaction: F(1,50) = 1.29, p = 0.2598). Subsequent analyses using the method of contrasts showed that both genotypes improved their performance in the accelerating rotarod between day 1 and day 6 (A2AR-CTL: p = 0.001; A2AR-Gpr88 p = 0.018).
Figure 6.
Motor coordination deficits in A2AR-Gpr88 mice and motor skill learning deficits in D1R-Gpr88 KO mice. Mice where tested on a rotating rod for four daily trials lasting 6 d. Overall (left panel), CMV-Gpr88 (A), D1R-Gpr88 (B), and A2AR-Gpr88 (C) mice show decreased latency to fall form the rod. When selectively analyzing the first and last training session (right panel), we observe that CMV-Gpr88 and A2AR-Gpr88 mice presented motor coordination deficits as soon as the first session which is not present in D1R-Gpr88. In the last session, however, CMV-Gpr88 and D1R-Gpr88 both present a significantly decreased time on the rod when compared to control mice, whereas A2AR-Gpr88 mice present no significant difference in the time spent on the rod when compared to their littermates. Data are represented as mean ± SEM. A, n = 21 CMV-CTL; n = 21 CMV-Gpr88. B, N = 13 D1R-CTL, N = 12 D1R-Gpr88. C, n = 17 A2AR-CTL; n = 10 A2AR-Gpr88. Open stars: one star p < 0.05; two stars p < 0.01; three stars p < 0.001 (RM two-way ANOVA). Text stars: three stars p < 0.001; one star p < 0.05 (Sidak’s multiple comparison).
These data first confirm that lack of GPR88 abolishes both motor coordination and motor skill learning and further shows that the deletion of A2AR-Gpr88 alters initial motor coordination while preserving motor skill learning, whereas D1R-Gpr88 deletion selectively impairs motor skill learning.
Discussion
Results from the comparison of total versus conditional mouse lines are summarized in Table 2. In sum, data from marble burying and social interaction tests reveal a D2R cell-specific function of GPR88 in anxiety-related and social behavior (De Boer and Koolhaas, 2003), as modifications are detected in CMV-Gpr88 and A2AR-Gpr88 KO, but not D1R-Gpr88 KO, mice. With regards to open field results, we observe differential roles of GPR88 in D1R- and D2R-MSNs, suggesting that GPR88 in D1R-MSNs has no role on general locomotion or stereotypies but regulates locomotor habituation to a novel environment, whereas deletion of this receptor in D2R-MSNs increases spontaneous locomotion and stereotypies while preserving locomotor habituation. In the rotarod also, we show differential roles of GPR88 in D1R- and D2R-MSNs, indicating that GPR88 in D1R-MSNs contributes to motor skill learning, whereas the receptor in D2R-MSNs contributes to motor coordination but not learning in the task. Overall therefore, our study demonstrates that GPR88 modulates the function of both D1R- and D2R-MSNs and that GPR88 activity in these two neuron populations has very different and dissociable impacts on behavior.
Table 2.
Summary of behavioral phenotypes observed in CMV-Gpr88, D1R-Gpr88, and A2AR-Gpr88 mice
| CMV-Gpr88 | D1R-Gpr88 | A2AR-Gpr88 | ||
|---|---|---|---|---|
| Marble burying | ↓ | ↔ | ↓ | |
| Social interaction | ↑ | ↔ | ↑ | |
| Open field | Locomotion | ↑ | ↔ | ↑ |
| Habituation | ↓ | ↓ | ↔ | |
| Stereotypies | ↑ | ↔ | ↑ | |
| Rotarod | Motor coordination | ↓ | ↔ | ↓ |
| Motor skill learning | ↓ | ↓ | ↔ | |
We find that specific deletion of Gpr88 in D2R-MSNs, but not in D1R-MSNs, decreases anxiety-like behavior as shown by reduced defensive burying activity (Borsini et al., 2002; De Boer and Koolhaas, 2003; Meirsman et al., 2016a). This result is in line with data showing that blocking D2R-MSNs activity disrupts avoidance behavior and aversive learning (Hikida et al., 2013). We also extend previous data (Meirsman et al., 2016a) by showing that Gpr88 deletion in D2R but not D1R-neurons increases social approach. Reports have shown that dopamine signaling through D1Rs is necessary for mediating pro-social behavior (Gunaydin et al., 2014). Also, it was shown that while D1R-MSNs display reduced mEPSC frequency after chronic social defeat, optical stimulation of D1R-MSNs was sufficient to reverse social avoidance induced by social defeat stress (Francis et al., 2015; Francis and Lobo, 2017). Therefore, although baseline social approach is not affected by D1R-Gpr88 deletion, different results may be obtained after chronic social stress. Knowing that inducible ablation of D1R-MSNs also reduces anxiety behaviors in mice (Révy et al., 2014), our result suggest that D1R-MSNs and D1R‘s-dependent anxiety and social behaviors was not affected by D1R-Gpr88 ablation. However, to fully understand GPR88 function in affective and social behaviors future studies should compare responses of D1R-Gpr88 and A2AR -Gpr88 mice in reward and aversive learning paradigms and investigate the role of this orphan receptor in stress-induced social avoidance.
We then show a differential effect of Gpr88 gene KO in D1R- or D2R-MSNs on general locomotion, with hyperlocomotor activity observed after D2R-Gpr88 deletion only. Converging data show that disruption of D2R-MSNs activity results in hyperlocomotor behavior (Durieux et al., 2012; Révy et al., 2014) while ablation of D1R-MSNs decreases locomotion (Durieux et al., 2012; Révy et al., 2014). Therefore, the increased locomotion observed in CMV-Gpr88 and A2AR-Gpr88 mice could simply result from decreased D2R-MSNs driven inhibition of locomotor output. Although deletion of Gpr88 in D1R-neurons did not alter overall locomotion throughout the five sessions, D1R-Gpr88 mice displayed decreased acute locomotor activity during the first open field session which would suggests impaired D1R-MSNs activity. Overall, locomotion results suggest that lack A2AR-Gpr88 mimics D2R-MSNs ablation (Durieux et al., 2009, 2012; Bateup et al., 2010; Révy et al., 2014). The question of how Gpr88 cell-specific deletion affects MSNs firing activity and basal ganglia output remains open, and future electrophysiological studies should measure basal ganglia output in D2R-Gpr88 and D1R-Gpr88 mice.
Another interesting locomotor phenotype in the open field is the lack of intersession habituation to the environment selectively observed in D1R-Gpr88 mice. Open field habituation is described as an adaptive process in which rodents decrease their locomotion with increasing exposure to the same environment and is taken as an index of memory (Tomaz et al., 1990; Cerbone and Sadile, 1994). A previous study showed that total deletion of Gpr88 improved spatial learning and memory tasks performances, thus suggesting that the non-habituating phenotype is not linked to spatial memory functions (Meirsman et al., 2016b). Surprisingly, our results contrast with the lack of open field habituation previously observed after ablation of D2R-MSNs (but not D1R-MSNs; Durieux et al., 2012). Therefore, in opposite to locomotion results, deletion of GPR88 in D1R-MSNs matches results obtained after D2R-MSNs ablation, suggesting either MSNs cross talk or alteration of a common network shaping locomotor habituation. In fact, data show (Sanguedo et al., 2016) that locomotor habituation to novel environments is accompanied by activation of striatal and extra-striatal regions such as amygdala and frontal cortex. Accordingly, CMV-Gpr88 mice have been shown to have altered transcriptional profiles in these structures where both GPR88 and D1R are expressed (Meirsman et al., 2016b). Most importantly, recent studies using CMV-Gpr88 mice have shown impaired multisensory processing (Ehrlich et al., 2018) and sensorimotor gating (Meirsman et al., 2017) that, coupled with altered sensorimotor and cortico-striatal functional connectivity (Arefin et al., 2017), suggest a role of this receptor in the integration and processing of sensory information. Interestingly, it has also been suggested that modifications of the striato-cortical circuitry may underlie the hyperactivity observed in CMV-Gpr88 mice (Arefin et al., 2017). As such, future studies measuring functional connectivity in D2R-Gpr88 and D1R-Gpr88 mice will elucidate how cell-specific deletion of Gpr88 reshape brain connectome leading to persistent changes in behavior.
Finally, the open field observations also reveal that A2AR-Gpr88 but not D1R-Gpr88 mice present increased number of stereotypies in the open field. Animal and clinical data indicate that dysregulation of cortico-striato-thalamo-cortical circuitry are associated with stereotypies (Lewis and Kim, 2009). Further, one study linked decreased D2R-MSNs activity with enhances stereotypies (Tanimura et al., 2010, 2011) and a recent report indicates that increasing D2R-MSNs activity is sufficient to rescue repetitive behaviors observed in a genetic model of autism (Wang et al., 2017). The increased stereotypies of A2AR-Gpr88 mice may therefore result from diminished D2R-MSNs inhibitory projection. As for locomotion result, the electrophysiological impact of Gpr88 specific deletion should be assessed in future studies. On the other hand, stereotypies have been linked to dopaminergic overstimulation (Katherine, 2018), which could also cause the phenotype observed in A2AR-Gpr88 mice. In fact, we have previously reported altered DA levels in the CPu and midbrain nuclei of CMV-Gpr88 mice, and future studies should verify DA levels in conditional Gpr88 KO mice.
As for the open field experiments, rotarod testing also reveals differential D1R- versus D2R-MSNs activities of GPR88. Mutants lacking Gpr88 in D1R-neurons present similar initial rotarod performance than control animals but show absence of motor skill learning throughout 6 d of task. On the contrary, mice lacking Gpr88 in D2R-neurons show decreased latency to fall in the first day but learned the task and increased their motor performances across days. Interestingly, as for the locomotor phenotype, results are comparable to those obtained after inducible ablation of D1R-MSNs and D2R-MSNs (Durieux et al., 2012). Worth noting, previous reports indicate that Gpr88 deletion does not alter striatal cell population or cytoarchitectural organization (Logue et al., 2009; Quintana et al., 2012) but increased levels of striatal pDARPP-32 Thr-34 and the ratio of pDARPP-32 Thr-34/DARPP-32 suggesting compromised MSNs functioning (Logue et al., 2009). Also, mRNA levels of genes encoding neurotransmitter receptors as well as GPCRs activation were found altered in the striatum of CMV-Gpr88 mice (Quintana et al., 2012; Meirsman et al., 2016b). In particular, Gpr88 deletion increased mu opioid and delta opioid receptors activation in the striatum. These receptors are known to activate Gi/o pathways, and could therefore contribute to increase MSNs hyperpolarization in Gpr88 mutant mice (Le Merrer et al., 2013; Pellissier et al., 2018). Interestingly, a previous report showed that chronic administration of DOR antagonist (naltrindole) in CMV-Gpr88 mice reversed their initial motor coordination impairment suggesting that increased DOR activity may underlie the deficit observed in A2AR-Gpr88. Future studies pharmacologically tackling receptors known to interact with GPR88 will elucidate MSNs-specific GPR88 interactions with other receptors.
In conclusion, the present study demonstrates dissociable roles of GPR88 at the level of MSNs. While GPR88 in D2R-MSNs regulates levels of anxiety, social behavior, stereotypies, locomotion, and motor coordination, this receptor in D1R-MSNs does not seem to impact affective behaviors but regulates habituation to novelty and motor skill learning. It is important to note that in the present study deletion of Gpr88 is not exclusively striatal. Thus, a new approach to restrict D1R-Gpr88 deletion to the striatum will determine if extra-striatal structures are involved in the phenotypes observed in mutants lacking Gpr88 in D1R-neurons. In addition, cellular mechanisms underlying phenotypes observed in this study remain to be clarified. Interesting to note, behavioral analyses show that both the total ablation of D2R MSNs (Durieux et al., 2012) and the deletion of Gpr88 in D2R-neurons (our study) reduce motor coordination and induces hyperlocomotion, suggesting that GPR88 activity normally stimulates D2R-MSNs. This is counterintuitive, as GPR88 has been proposed to be an inhibitory GPCR (Jin et al., 2018). Also, Quintana et al. (2012) have previously shown that total GPR88 ablation reduced tonic GABA current and enhanced glutamatergic signaling in MSNs. They also showed that deletion of Gpr88 similarly affect the response to cortical excitatory input or the tonic GABA currents in D1R or D2R MSNs. We may, however, consider a strong differential effect of selective versus total deletion of GPR88 on MSNs intrinsic electrical properties. Therefore, electrophysiological studies using cell-specific Gpr88 deletion and also the precise anatomic localization of the receptor at presynaptic or postsynaptic levels should help clarifying how GPR88 modulates D1R- and D2R-MSNs activities. In addition, deficient long distance communication between brain structures observed in CMV-Gpr88 mice (Arefin et al., 2017) may explain some of the present results and upcoming studies should compare respective functional connectivity alterations in the two conditional Gpr88 KO mouse lines. Hence, further dissection of D1R versus D2R specific GPR88 activities is essential to explore the full potential of this receptor as a target for affective and motor disorders.
Acknowledgments
Acknowledgements: We thank Professor Bruno Giros for kindly offer the Drd1a-Cre mice. We also thank the staff at the animal facility of the Neurophenotyping Center at the Douglas Research Center (Montréal, Canada) as well as Aude Villemain, Eujin Kim, Annie Salesse, Karine Lachapelle, and Aimee Lee Luco for animal care and genotyping.
Synthesis
Reviewing Editor: Carmen Sandi, Swiss Federal Institute of Technology
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Deveroux Ferguson.
The two reviewers and editor consider that this manuscript reports a well-designed and performed study, with sound results and discussion. In previous studies, the orphan receptor GPR88 had been described to regulate striatal medium spiny neuron activity and to participate in several striatum-dependent and -independent behaviors but its contribution in a cell type-specific manner in dopamine receptor D1R- or D2R- MSNs was elusive. Here, using Gpr88KO and D1- or D2-Gpr88KO mice, the authors provide novel data on the role of Gpr88 in dopamine receptor specific cells. Overall, the manuscript is well written. This informative and comprehensive characterization of the behavioral contribution of specific subsets of Gpr88-expressing cells, pave the way for future studies on underlying molecular mechanisms. We only have a few minor points to raise to the authors:
- The authors emphasize the striatal nature of their results. However, although GPR88 is highly enriched in the striatum, its expression is also abundant in other areas (such as cortex, some subcortical nuclei and brainstem). Similarly, Drd1a is also expressed in cortex, brainstem and other nuclei (i.e hypothalamus). Hence, to convincingly demonstrate a striatal origin of the behavioral alterations described, authors should show either that GPR88 expression is not affected in other regions (i.e. cortex/brainstem) in D1R-Gpr88 mice or, alternatively, to perform an approach to restrict D1R-Gpr88 deletion to the striatum. In case the authors have good reasons not to add these analyses, we urge them to include a clear limitation for the interpretation of their data in the Discussion section.
- In light of their results, the authors suggest that GPR88 ablation in D2R-expressing MSN equates to D2-MSN inactivation, pointing at a stimulatory role for GPR88 in D2R-expressing MSNs. The authors discuss that this would be in apparent conflict with the suggested inhibitory role for GPR88 as described by Jin et al (2018) and that detailed electrophysiological studies are required to assess D1R- and D2R-MSN function in the context of GPR88 deficiency. While this conclusion is sound, the authors should take into consideration that it has been shown how global GPR88 deficiency leads to reduced GABAergic signaling and enhanced glutamatergic signaling in MSNs, leading to overall increased MSN activity (Quintana et al., 2012). Therefore, we urge the authors to acknowledge this point in the Discussion section, even though these studies did not study individual D1R- and D2R- responses, which may be underlying the observed (potential) discrepancies.
- The authors conclude that only GPR88 in D2-MSNs are related to effects on social behavior. Actually, both MSN subtypes have distinct roles in social avoidance behaviors in the chronic social defeat model (Francis and Lobo, 2017, Biol Psychiatry 81:645). We urge the authors to explain/discuss the social behavior results deeper.
Figures:
- In the Figure 1, signal intensity and number of D1R positive cells of D1R-Gpr88 (Fig.1F) are relatively lower than control groups. It is recommended that the authors use better representative images for the D1R-Gpr88.
- Figure 1B seems to be mislabeled (CMV-CTL should respond while CMV-Gpr88 should show no response).
- In Figure 2, GPR88 positive cells in D1R-Gpr88 mice seem to have higher intensity than in CTL mice. Do the authors know if there is a compensatory increase in Gpr88 expression in non-D1R Grp88-expressing cells? Along the same lines, do authors have data on behavioral response to repeated D1R activation (i.e. sensitization)?
- In Fig. 5 (as in several other figures for other parameters), the baselines of stereotypic behavior are clearly different between CMV (Fig. 5A) and cell-type specific KO lines (Fig. 5B & C). This point of inter-experimental variability should be mentioned and discussed briefly in the manuscript.
- Are efficiencies of primers used in the qRTPCR around 100% (80-120%)? That would be a mandatory requirement to use de ddCT method. Otherwise, the use of a standard curve is required.
References
- Alkufri F, Shaag A, Abu-Libdeh B, Elpeleg O (2016) Deleterious mutation in GPR88 is associated with chorea, speech delay, and learning disabilities. Neurol Genet 2:e64. 10.1212/NXG.0000000000000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefin TM, Mechling AE, Meirsman AC, Bienert T, Hübner NS, Lee HL, Ben Hamida S, Ehrlich A, Roquet D, Hennig J, von Elverfeldt D, Kieffer BL, Harsan LA (2017) Remodeling of sensorimotor brain connectivity in Gpr88-deficient mice. Brain Connect 7:526–540. 10.1089/brain.2017.0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA 107:14845–14850. 10.1073/pnas.1009874107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D (2002) Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 163:121–141. 10.1007/s00213-002-1155-6 [DOI] [PubMed] [Google Scholar]
- Cerbone A, Sadile AG (1994) Behavioral habituation to spatial novelty: interference and noninterference studies. Neurosci Biobehav Rev 18:497–518. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM (2003) Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol 463:145–161. 10.1016/s0014-2999(03)01278-0 [DOI] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d'Exaerde A (2009) D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci 12:393–395. 10.1038/nn.2286 [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A (2012) Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J 31:640–653. 10.1038/emboj.2011.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich AT, Semache M, Bailly J, Wojcik S, Arefin TM, Colley C, Le Gouill C, Gross F, Lukasheva V, Hogue M, Darcq E, Harsan LA, Bouvier M, Kieffer BL (2018) Mapping GPR88-Venus illuminates a novel role for GPR88 in sensory processing. Brain Struct Funct 223:1275–1296. 10.1007/s00429-017-1547-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK (2017) Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol Psychiatry 81:645–653. 10.1016/j.biopsych.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iñiguez SD, O'Donnell P, Kravitz A, Lobo MK (2015) Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77:212–222. 10.1016/j.biopsych.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K (2014) Natural neural projection dynamics underlying social behavior. Cell 157:1535–1551. 10.1016/j.cell.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, Nakanishi S (2013) Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci USA 110:342–347. 10.1073/pnas.1220358110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Decker AM, Makhijani VH, Besheer J, Darcq E, Kieffer BL, Maitra R (2018) Discovery of a potent, selective, and brain-penetrant small molecule that activates the orphan receptor GPR88 and reduces alcohol intake. J Med Chem 61:6748–6758. 10.1021/acs.jmedchem.8b00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katherine M (2018) Stereotypic movement disorders. Semin Pediatr Neurol 25:19–24. 10.1016/j.spen.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622–626. 10.1038/nature09159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 15:816–818. 10.1038/nn.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL (2013) Impaired hippocampus-dependent and facilitated striatum-dependent behaviors in mice lacking the delta opioid receptor. Neuropsychopharmacology 38:1050–1059. 10.1038/npp.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Kim SJ (2009) The pathophysiology of restricted repetitive behavior. J Neurodev Disord 1:114–132. 10.1007/s11689-009-9019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, O'Doherty JP (2012) Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci 16:467–475. 10.1016/j.tics.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Grauer SM, Paulsen J, Graf R, Taylor N, Sung MA, Zhang L, Hughes Z, Pulito VL, Liu F, Rosenzweig-Lipson S, Brandon NJ, Marquis KL, Bates B, Pausch M (2009) The orphan GPCR, GPR88, modulates function of the striatal dopamine system: a possible therapeutic target for psychiatric disorders? Mol Cell Neurosci 42:438–447. 10.1016/j.mcn.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Maroteaux G, Arefin TM, Harsan LA, Darcq E, Ben Hamida S, Kieffer BL (2018) Lack of anticipatory behavior in Gpr88 knockout mice showed by automatized home cage phenotyping. Genes Brain Behav 17:e12473. 10.1111/gbb.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirsman AC, Robe A, de Kerchove d'Exaerde A, Kieffer BL (2016a) GPR88 in A2AR neurons enhances anxiety-like behaviors. eNeuro 3 10.1523/ENEURO.0202-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirsman AC, Le Merrer J, Pellissier LP, Diaz J, Clesse D, Kieffer BL, Becker JA (2016b) Mice lacking GPR88 show motor deficit, improved spatial learning, and low anxiety reversed by delta opioid antagonist. Biol Psychiatry 79:917–927. 10.1016/j.biopsych.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirsman AC, de Kerchove d'Exaerde A, Kieffer BL, Ouagazzal AM (2017) GPR88 in A2A receptor-expressing neurons modulates locomotor response to dopamine agonists but not sensorimotor gating. Eur J Neurosci 46:2026–2034. 10.1111/ejn.13646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier LP, Pujol CN, Becker JAJ, Le Merrer J (2018) Delta opioid receptors: learning and motivation. Handb Exp Pharmacol 247:227–260. 10.1007/164_2016_89 [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Massotte D, Gavériaux-Ruff C, Kieffer BL (2009) In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One 4:e5425. 10.1371/journal.pone.0005425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Sanz E, Wang W, Storey GP, Güler AD, Wanat MJ, Roller BA, La Torre A, Amieux PS, McKnight GS, Bamford NS, Palmiter RD (2012) Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci 15:1547–1555. 10.1038/nn.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révy D, Jaouen F, Salin P, Melon C, Chabbert D, Tafi E, Concetta L, Langa F, Amalric M, Kerkerian-Le Goff L, Marie H, Beurrier C (2014) Cellular and behavioral outcomes of dorsal striatonigral neuron ablation: new insights into striatal functions. Neuropsychopharmacology 39:2662–2672. 10.1038/npp.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguedo FV, Dias CV, Dias FR, Samuels RI, Carey RJ, Carrera MP (2016) Reciprocal activation/inactivation of ERK in the amygdala and frontal cortex is correlated with the degree of novelty of an open-field environment. Psychopharmacology (Berl) 233:841–850. 10.1007/s00213-015-4163-z [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH (2010) Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Brain Res 210:116–122. 10.1016/j.bbr.2010.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, King MA, Williams DK, Lewis MH (2011) Development of repetitive behavior in a mouse model: roles of indirect and striosomal basal ganglia pathways. Int J Dev Neurosci 29:461–467. 10.1016/j.ijdevneu.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz C, Aguiar MS, Nogueira PJ (1990) Facilitation of memory by peripheral administration of substance P and naloxone using avoidance and habituation learning tasks. Neurosci Biobehav Rev 14:447–453. [DOI] [PubMed] [Google Scholar]
- Wang W, Li C, Chen Q, van der Goes MS, Hawrot J, Yao AY, Gao X, Lu C, Zang Y, Zhang Q, Lyman K, Wang D, Guo B, Wu S, Gerfen CR, Fu Z, Feng G (2017) Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J Clin Invest 127:1978–1990. 10.1172/JCI87997 [DOI] [PMC free article] [PubMed] [Google Scholar]