Abstract
Rationale:
Hürthle cell adenoma (HCA) of the thyroid is a rare thyroid tumor, and there are limited studies on the contrast-enhanced computed tomography (CT).
Patient concerns:
We report the case of a 63-year-old woman with gradual enlargement of the thyroid over 10 years.
Diagnoses:
Preoperative contrast-enhanced CT revealed typical lesion characteristics of HCA, confirmed by postoperative pathology.
Interventions:
Left thyroidectomy and partial right thyroidectomy were performed on the patient after general anesthesia.
Outcomes:
At follow-up of 12 months after surgery, the patient was in good health without recurrence.
Lessons:
The typical imaging features of HCA on contrast-enhanced CT are helpful for the early diagnosis of thyroid eosinophilic adenoma. This will provide an important basis for the preoperative diagnosis and treatment strategy of HCA of the thyroid in future.
Keywords: contrast-enhanced CT, Hürthle cell adenoma, thyroid
1. Introduction
Hürthle cell adenoma (HCA) of the thyroid, namely thyroid eosinophilic adenoma, is a rare thyroid tumor[1] that is generally benign and often requires surgery, but with a good postoperative prognosis. Due to its extremely low incidence, lack of special clinical manifestations, and lack of typical imaging features, preoperative diagnosis of HCA is very difficult. However, HCA of the thyroid can be diagnosed by postoperative pathological findings[2] due to its unique histological features. To our knowledge, very few articles have reported on the manifestations of HCA of the thyroid on contrast-enhanced computed tomography (CT). Here, we report a case with typical lesion characteristics of HCA of the thyroid on contrast-enhanced CT that was confirmed by postoperative pathology.
2. Case report
A 63-year-old woman presented with increasing neck swelling over the past 10 years. She showed no symptoms of palpitation or hidrosis, pain or pressure, hoarseness, or fatigue and had no family history of thyroid cancer. She appeared very healthy, with no features of thyrotoxicity or hypothyroidism on examination. Her pulse was normal, and her blood pressure was moderately high. Her neck swelling showed a cystic and firm mass with a medium texture and a clear border that moved up and down with her swallowing. The swelling measured approximately 8 × 6 cm in size in the left lobe of the thyroid gland, and a cystic and firm mass with the same property measuring approximately 1.0 × 1.5 cm in size was felt in the right lobe of the thyroid gland. No regional lymph nodes were palpated, and significant tracheal deviation was observed to the right. Laboratory indices showed a low thyroid-stimulating hormone (TSH) level. Other biochemical indexes were normal.
Thyroid color Doppler ultrasonography (Fig. 1) showed that both lobes were significantly enlarged, the surface was not smooth, and the left lobe was more pronounced. The normal tissue of the left lobe was replaced by a hypoechoic mass of approximately 8.1 cm × 5.6 cm that was visible at the border of the hypoechoic mass and comprised multiple nodules, with a small number of cystic echoes. Sixty-four spiral contrast-enhanced CT (GE Medical Systems LightSpeed VCT, Hino-Shi, Tokyo, Japan) was used. The CT scanning parameters were as follows: for 64 detector rows, a beam collimation of 40 mm, a pitch of 0.984, a slice thickness of 2.5 mm, reconstruction intervals of 2.5 mm, a tube voltage of 120 kV, and a tube current of 150 mA. Contrast-enhanced CT scans were performed with patient in a supine position, scanning range from basis cranii to aortic arch level. The patient received intravenous contrast agent iopromide (300 mgI/mL) 80 mL as a bolus at the rate of 3 mL/s, and the CT images were obtained during arterial and venous phases at 25 and 60 seconds after contrast material injection, respectively. The results showed that the patient had a huge heterogeneously enhancing mass (8.5 cm × 7.5 cm) in the left lobe of the thyroid. But the enhancement degree of the mass was lower than thyroid gland and adjacent blood vessels. There was no enhanced necrotic area in the center. Radiation-like, low-density scarring was seen in the lesion (Fig. 2A). Intact capsule enhancement was observed around the lesion, the venous lesions continued to strengthen, and the trachea was compressed to the right side (Fig. 2B). After general anesthesia, left thyroidectomy, and partial right thyroidectomy, the postoperative pathological results revealed left thyroid eosinophilic adenoma and a right thyroid nodular goiter (Fig. 3). Microscopically, the tumor capsule was intact, the tumor cells comprised numerous eosinophils and were arranged in a cord, beam, or interconnected network, showing a papillary structure, which meet the diagnostic criteria of HCA of the thyroid.[3,4] The patient was discharged 1 week after surgery. She was followed up for 12 months and was in good health without recurrence. Written informed consent was obtained from the patient for publication of the case details and the images.
Figure 1.
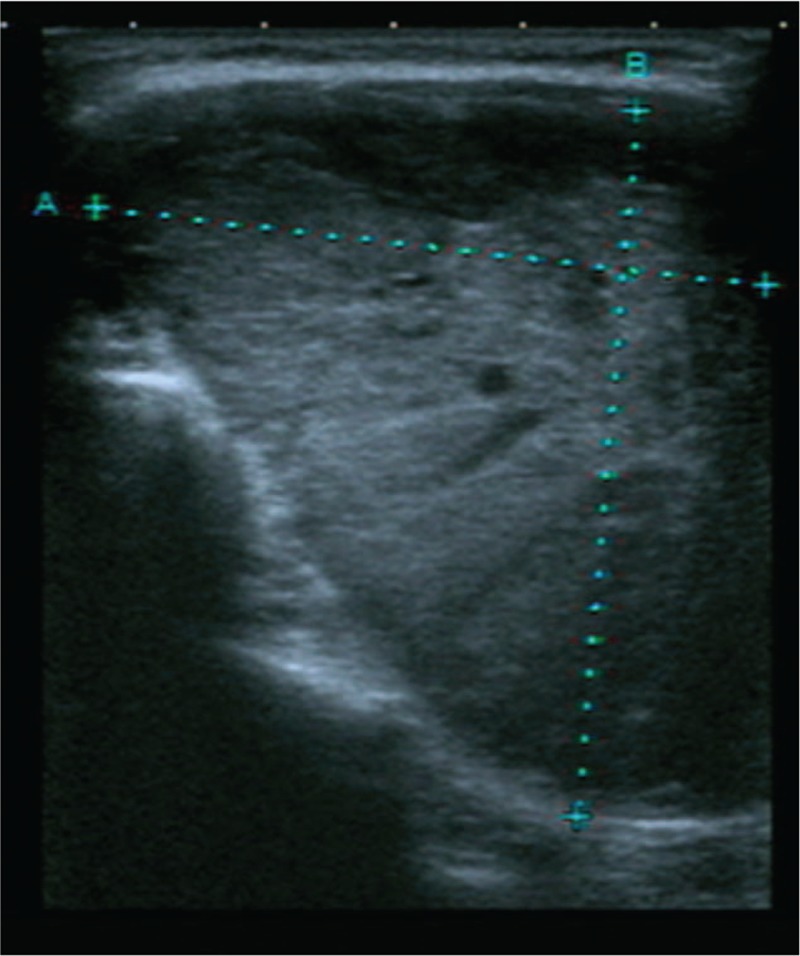
Color Doppler ultrasound shows a hypoechoic massive mass about 8.1 cm × 5.6 cm in the left thyroid gland.
Figure 2.
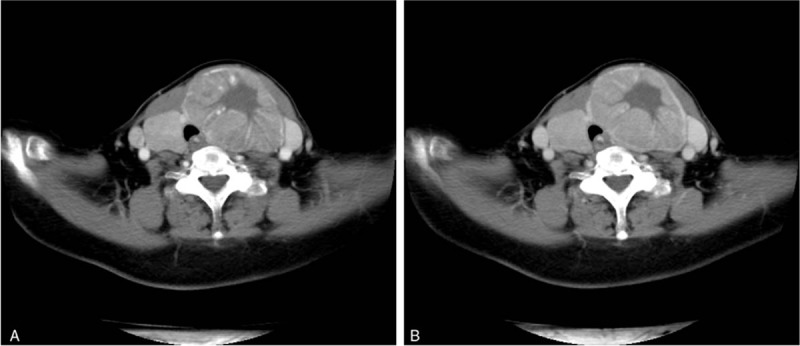
CT-enhanced scan of arterial phase (A) and venous phase (B) of the mass showed no enhanced necrotic area in the center and radiation-like, low-density scarring in the lesion and intact capsule enhancement. CT = computed tomography.
Figure 3.
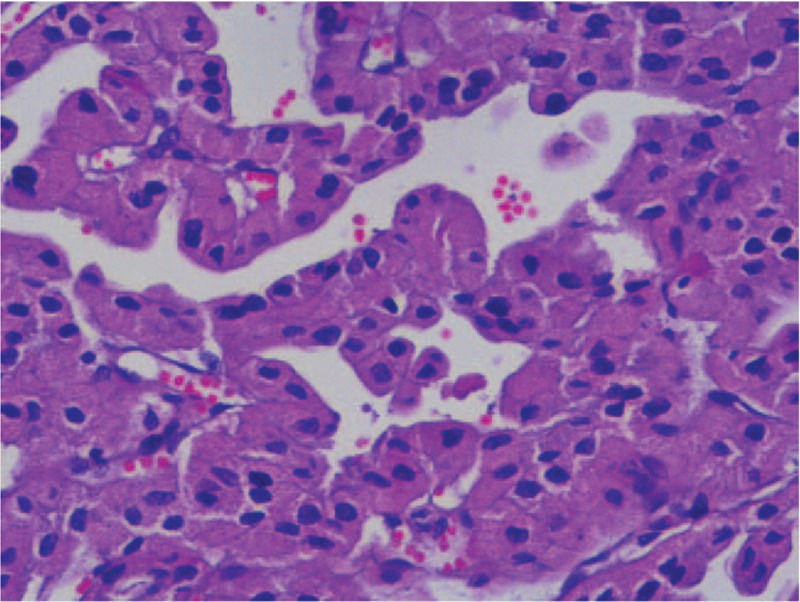
Pathological results of the thyroid mass showed HCA consisted of numerous eosinophils. HCA = Hürthle cell adenoma.
3. Discussion
The domestic and worldwide incidence of HCA of the thyroid is extremely low, accounting for less than 5% of all thyroid tumors.[5] HCA of the thyroid lacks typical clinical manifestations and usually occurs in women older than 40 years. It mostly presents as a painless lump in the neck that could move with swallowing. Thyroid function tests are generally normal or TSH levels can be slightly elevated. Preoperative diagnosis of thyroid eosinophilic adenoma is often difficult due to atypical performance of various imaging examinations. The misdiagnosis rate of HCA of the thyroid is high because it is easily confused with nodular goiter and other thyroid tumors. The benign and malignant status of thyroid eosinophilic adenoma is unclear due to its malignant tendency, but most reports indicate that it is benign.[6]
The tumor cells of Hürthle cell neoplasms (HCNs) can be trabecular or small follicles and they can form glial calcification, containing thick colloid, a focal nipple structure. Numerous methods have been tried to identify benign and malignant disease of HCNs,[7–9] such as cytological morphometry, immunohistochemical feature, and gene rearrangements. But preoperative clinical, cytological, and genetic studies have not reliably discriminated between benign and malignant lesions of HCNs. Therefore, histopathology remains the gold standard for diagnosis of HCA. The Hürthle cell is characterized cytologically as a large cell with abundant eosinophilic, granular cytoplasm and a large hyperchromatic nucleus with a prominent nucleolus.[10] The histopathological diagnostic criteria for HCA of the thyroid are as follows: thyroid tumor cells consist entirely or predominantly (>75%) of follicular cells, characterized by eosinophils[3] and the thyroid tumor tissue has an intact capsule and no vascular invasion.[4]
Color ultrasonography, fine-needle aspiration cytology, contrast-enhanced CT, positron emission tomography–CT (PET–CT), and postoperative pathological examination are generally used to diagnose thyroid eosinophilic adenoma. Because color Doppler ultrasonography shows no characteristic performance, color Doppler physicians generally lack the diagnostic experience of thyroid eosinophilic adenoma, and the current accumulated data are inadequate. Therefore, it is difficult to use color Doppler ultrasonography to clearly diagnose thyroid eosinophilic adenoma. Fine-needle aspiration cytology is simple, safe, and reliable and the diagnostic accuracy is high. But it is impossible to judge whether capsule or vascular invasion is present.[3] In addition, HCNs tend to undergo spontaneous necrosis or show extensive degenerative necrosis following fine-needle aspiration biopsy. In these situations, viable lesional cells are often not present, and it is difficult to fully evaluate the lesion pathologically.[11] Fine-needle aspiration biopsy cannot reliably diagnose neoplasia versus hyperplasia and carcinoma versus adenoma.[12] Although PET–CT images can detect the area of abnormal radiopharmaceutical uptake corresponding to HCAs[13] and improve its diagnostic rate, it is an unpopular modality because of its high price and radiation damage. Pathological examination is a gold standard; however, it often produces hysteresis in actual selection. By contrast, contrast-enhanced CT can make a definitive diagnosis based on the typical features of their imaging features, thus providing a diagnostic basis for early surgical treatment.
Currently, the research of HCA mostly focuses on the kidney. The typical features of renal HCA included the central stellate scar or central scar[14] in contrast-enhanced CT. However, preoperative contrast-enhanced CT examination is very rare in the diagnosis of thyroid eosinophilic adenoma. The patient presented here showed relatively typical imaging features of thyroid eosinophilic adenoma. The thyroid gland was obviously enhanced due to its blood supply after the contrast agent was injected. New capillaries decrease and/or tumor cells, necrotic, or fibrous components increase in the lesions when the thyroid gland has space-occupying lesions and CT enhancement showed that the lesions were lower than the thyroid tissue, which is the pathological basis of contrast-enhanced CT scan. Contrast-enhanced CT imaging of the patient showed lesion expansive growth and clear boundary, intact lesion capsule, radiation-like low-density scarring in the center of the lesion, enhanced surrounding area, and no abnormal enlargement of lymph nodes in the bilateral neck, which indicated the lesions are benign tumor. In addition, it was consistent with the growth characteristics of a benign tumor for her history of more than 10 years. Finally, left thyroidectomy was performed and postoperative pathology confirmed the diagnosis of HCA, which was in line with the diagnosis revealed by contrast-enhanced CT examination.
The case of thyroid eosinophilic adenoma we reported has typical features, which was presented with intact lesion capsule, lower enhancement degree of lesion than thyroid tissue, radiation-like low-density scarring in the center of the lesion, and enhanced surrounding area in contrast-enhanced CT. These features could differentiate from HCC of thyroid and thyroid cancer in contrast-enhanced CT. HCC of thyroid lesion has the same imaging features in contrast-enhanced CT except incomplete lesion capsule. However, thyroid cancer usually has blurred boundary, irregular shape, discontinuous capsule, inhomogeneous enhancement in contrast-enhanced CT, accompanied by abnormal enlargement of lymph nodes in the neck.[15] Radiation-like low-density scarring in the center of the lesion helps to differentiate HCA from other thyroid benign tumors.
Some authors considered that at least 80% of HCNs are benign (especially when studies have included HCA nodules). However, others considered all HCNs were malignant.[11] The latter finding was proposed because the initial studies on Hürthle cell tumors showed that even lesions that were initially diagnosed as benign behaved in a malignant fashion.[16] Therefore, surgical treatment is preferred. The surgical procedure should be based on the size of the tumor, preoperative examination, and intraoperative frozen results. Lobectomy was recommended for benign HCA due to its good prognosis,[6] while total thyroidectomy should be performed for malignant thyroid eosinophilic adenocarcinoma (HCC).[17]
In summary, we report the typical imaging features on contrast-enhanced CT that are helpful for the early diagnosis of thyroid eosinophilic adenoma. This will provide an important basis for the preoperative diagnosis and treatment strategy of thyroid eosinophilic adenoma in future.
Author contributions
Conceptualization: Dayong Deng.
Data curation: Xi Chen.
Software: Hongru Wang.
Writing – original draft: Dayong Deng.
Writing – review & editing: Haidi Wu.
Footnotes
Abbreviations: CT = computed tomography, HCA = Hürthle cell adenoma, HCC = thyroid eosinophilic adenocarcinoma, HCNs = Hürthle cell neoplasms, PET–CT = positron emission tomography–computed tomography, TSH = thyroid-stimulating hormone.
The authors declare no conflict of interest.
References
- [1].Okere PC, Olusina DB, Enyinnah MO. Hurthle cell tumor of the thyroid gland: report of a rare case and review of literature. Niger J Clin Pract 2014;17:375–7. [DOI] [PubMed] [Google Scholar]
- [2].Cannon J. The significance of hurthle cells in thyroid disease. Oncologist 2011;16:1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kochummen E, Tong S, Umpaichitra V, et al. A unique case of bilateral Hurthle cell adenoma in an adolescent. Horm Res Paediatr 2017;87:136–42. [DOI] [PubMed] [Google Scholar]
- [4].Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol 2008;36:425–37. [DOI] [PubMed] [Google Scholar]
- [5].Stojadinovic A, Hoos A, Ghossein RA, et al. Hurthle cell carcinoma: a 60-year experience. Ann Surg Oncol 2002;9:197–203. [DOI] [PubMed] [Google Scholar]
- [6].Bremer AA, Feldman BJ, Iezza G, et al. Report of a hurthle cell neoplasm in a peripubertal girl. Thyroid 2007;17:175–8. [DOI] [PubMed] [Google Scholar]
- [7].Tai P, Korzeniowski M, Sadikov E, et al. Issues in managing Hurthle cell carcinoma of thyroid: a case report. Cureus 2017;9:e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gonzalez JL, Wang HH, Ducatman BS. Fine-needle aspiration of Hurthle cell lesions. A cytomorphologic approach to diagnosis. Am J Clin Pathol 1993;100:231–5. [DOI] [PubMed] [Google Scholar]
- [9].Cheung CC, Ezzat S, Ramyar L, et al. Molecular basis off Hurthle cell papillary thyroid carcinoma. J Clin Endocrinol Metab 2000;85:878–82. [DOI] [PubMed] [Google Scholar]
- [10].Maximo V, Lima J, Prazeres H, et al. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr Relat Cancer 2012;19:R131–47. [DOI] [PubMed] [Google Scholar]
- [11].Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Arch Pathol Lab Med 2008;132:1241–50. [DOI] [PubMed] [Google Scholar]
- [12].Shawky M, Sakr M. Hurthle cell lesion: controversies, challenges, and debates. Indian J Surg 2016;78:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paone G, Treglia G, Bongiovanni M, et al. Incidental detection of Hurthle cell adenoma by 18F-choline PET/CT scan in a patient with prostate cancer. Rev Esp Med Nucl Imagen Mol 2013;32:340–1. [DOI] [PubMed] [Google Scholar]
- [14].Giambelluca D, Pellegrino S, Midiri M, et al. The “central stellate scar” sign in renal oncocytoma. Abdom Radiol (NY) 2019;44:1942–3. [DOI] [PubMed] [Google Scholar]
- [15].Ben HY. Diagnosis and differential diagnosis of thyroid carcinoma by multi-slice spiral CT. Chin J Med Imaging 2011;19:749–52. [Google Scholar]
- [16].Grant CS, Barr D, Goellner JR, et al. Benign Hurthle cell tumors of the thyroid: a diagnosis to be trusted? World J Surg 1988;12:488–95. [DOI] [PubMed] [Google Scholar]
- [17].Desai A, Peterson SE, Raiser F, et al. Hurthle cell adenoma diagnosed by core needle biopsy in a male patient. J Am Osteopath Assoc 2000;100:232–3. [DOI] [PubMed] [Google Scholar]


