Abstract
Background:
Number of studies have been performed to investigate the relationship between the CYP1A1 rs4646903 polymorphism and male infertility risk, but the sample size was small and the results were conflicting. A meta-analysis was performed to assess these associations.
Methods:
A systematic search was conducted to identify all relevant studies from Medline, Web of science, Embase, China biology medical literature database (CBM), China National Knowledge Infrastructure (CNKI), WanFang and Weipu (VIP) databases up to June 30, 2018. The odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to assess the strength of associations. All of the statistical analyses were conducted using Revman 5.3 and Stata 14.0.
Results:
Ten studies involved 3028 cases and 3258 controls. Overall, significant association was observed between the CYP1A1 rs4646903 polymorphism and male infertility (C vs T: OR = 1.42, 95%CI = 1.14–1.76; CC vs TT: OR = 2.13, 95%CI = 1.36–3.34; CC vs CT+TT: OR = 1.96, 95%CI = 1.30–2.95; CC+CT vs TT: OR = 1.51, 95%CI = 1.16–1.97). In subgroup analysis by ethnic group, a statistically significant association was observed in Asians (C vs T: OR = 1.59, 95%CI = 1.22–2.08), but not in Non-Asians (C vs T: OR = 1.01, 95%CI = 0.79–1.30). Additionally, none of the individual studies significantly affected the association between CYP1A1 rs4646903 polymorphism and male infertility, according to sensitivity analysis.
Conclusion:
Our meta-analysis supports that the CYP1A1 rs4646903 polymorphism might contribute to individual susceptibility to male infertility in Asians.
Keywords: CYP1A1, male infertility, meta-analysis, polymorphism
1. Introduction
Infertility is the inability of a couple to conceive pregnancy after 1 year of unprotected, regular sexual intercourse and affects approximately 10% to 20% of couples.[1,2] Among them, approximately 50% of the cases are associated with male factors.[3] It is thought that lifestyle factors and environmental factors such as cigarette, smoking, and exposure to certain chemicals, are potential causes of male infertility.[4,5] In addition, genetic factors, such as chromosomal aberrations, gene mutations, and polymorphisms, can also be associated with male infertility.[6–8] Several studies suggest the CYP1A1 gene as a candidate gene for male infertility.[9,10]
Cytochrome P4501A1 gene, located on chromosome 15q22-q24, is 5987-bp long and encodes a 512 amino acid protein.[11,12] CYP1A1, a member of the CYP1 family, involved in the metabolism of a vast number of xenobiotics and endogenous substrates.[13] The disturbed testicular expression of CYP1A1 is responsible for producing hydroxyestradiols and/or methoxyestradiols, and the increased intratesticular hydroxyestradiols and methoxyestradiols concentrations could elicit an impaired Sertoli cell function, this could ultimately lead to male infertility.[14] Several single-nucleotide polymorphisms (SNPs) of Cytochrome P4501A1 gene have been identified, including T3801C, T3205C, A2455G.[13] Among them, P4501A1 MspI (3798 T > C; CYP1A1∗2A; rs4646903) polymorphism is one of the most-studied single-nucleotide polymorphism (SNP). Recently, many genetic studies have investigated the association between the CYP1A1 rs4646903 polymorphism and the risk of male infertility. However, these studies have relatively sample size and the results remain controversial. In 2013, a meta-analysis based on 6 case-control studies including 1060 cases and 1225 controls was conducted, the authors concluded that the CYP1A1 rs4646903 polymorphism is capable of causing male infertility susceptibility in Asians, but not in Caucasians.[15] In 2014, a meta-analysis conducted by Luo et al[16] demonstrated that the CYP1A1 rs4646903 polymorphism was associated with male infertility in overall population, however, no association was observed between CYP1A1 rs4646903 polymorphism and male infertility in both Asians and Caucasians. In addition, several novel related studies of male infertility risk have since been published. Therefore, in the present study, we performed an updated meta-analysis based on 10 studies of the CYP1A1 rs4646903 polymorphism (3028 cases and 3258 controls) to elucidate the relationship between the CYP1A1 rs4646903 polymorphism and male infertility risk.
2. Methods
2.1. Search strategy
To assess the complete evidence of an association between the CYP1A1 rs4646903 polymorphism and male infertility, we performed the present comprehensive meta-analysis of published studies. Medline, Web of science, Embase, CBM, CNKI, WanFang, and VIP databases were searched for relevant articles up to June 30, 2018. The following search terms and keywords were used: “CYP1A1 gene” or “Cytochrome P450 1A1 gene,” and “SNP” or “polymorphism” or “mutation” or “variant” and “male infertility”. References of all relevant studies were reviewed to obtain additional references. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.2. Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria: case-control study; full text of the article was available; available data to estimate an odds ratio (OR) with 95% confidence interval (CI); the genetypes in cases and controls were available. Exclusion criteria included: studies not concerning association between the CYP1A1 rs4646903 polymorphism and male infertility risk; repeated or overlapping publications; and animal studies, review articles, meta-analysis, and conference abstracts or editorial articles.
2.3. Quality assessment and data extraction
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of included studies by 2 authors.[17] This scale assesses the quality of case-control studies included 3 areas: selection, comparability, and exposure. A star rating system was used to judge methodological quality. Scores range from 0 stars (worst) to 9 stars (best), and studies with a score ≥7 were defined as high quality. Discrepant opinions were resolved by discussion and consensus. Two investigators extracted the data using a standardized data extraction form independently. Discrepancies were resolved by discussion with a third investigator. The following information was extracted from each study: first author, year of publication, sample size, geographical location, genotype frequencies of CYP1A1.
2.4. Statistical analysis
Odds ratios (ORs) with 95% CIs were used to assess the strength of association between CYP1A1 rs4646903 polymorphism and male infertility risk. The pooled ORs were performed for CYP1A1 rs4646903 polymorphism under the allele comparison model (C vs T), additive model (CC vs TT), recessive model (CC vs CT+TT), and dominant model (CC+CT vs TT), respectively. The significance of the pooled OR was analyzed by the Z test, and P < .05 was considered statistically significant. The Chi-square-based Q-test and I2 statistics were used to calculated heterogeneity among included studies. The P > .05 for Q test or I2 < 50% indicated a statistically significant degree of heterogeneity among studies. In contrast, the random-effects model was used. All statistical analyses were performed by using Revman 5.3 and Stata 14.0. Publication bias was investigated with the funnel plot, Begg test, and Egger test. Sensitivity analysis was conducted to assess the stability of the results by sequentially omitted individual studies.
3. Results
3.1. Description of included studies
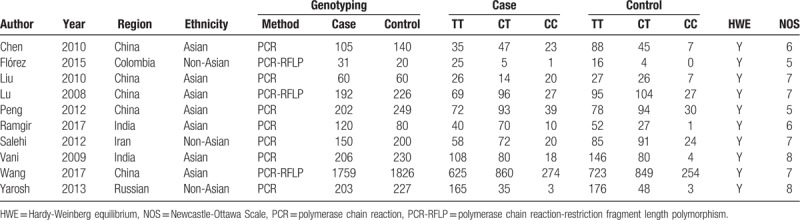
A total of 131 studies were retrieved from online databases. Of these, after the first screening, 121 studies were excluded based on inclusion and exclusion criteria. Finally, 10 studies including 3028 cases and 3258 controls were included in this meta-analysis.[9,17–25] A detailed flow chart of the study selection is shown in Fig. 1. The years of publication ranged from 2008 to 2017, and there were 7 studies of Asian descendants and 3 studies of Non-Asian descendants. The Hardy-Weinberg test (HWE) was performed on all of the included studies, and the results showed that the CYP1A1 rs4646903 gene genotype frequencies of all 10 studies were in HWE in the controls (Table 1).
Figure 1.
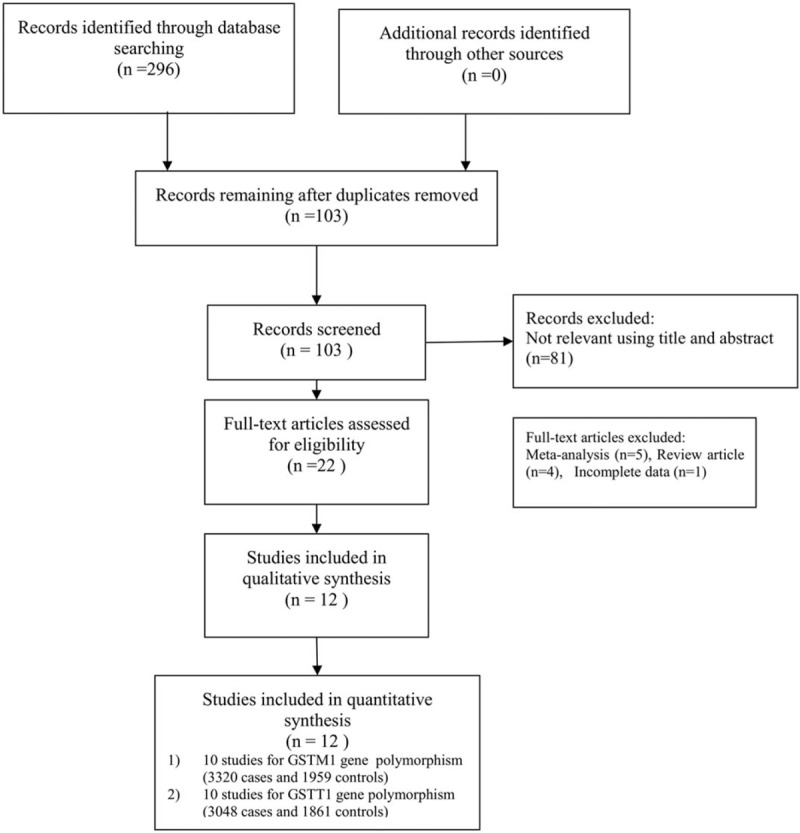
Flowchart showing the study selection.
Table 1.
Characteristics of the studies included in the meta-analysis and their genotype distributions of the CYP1A1 rs4646903 polymorphism.
3.2. Meta-analysis of CYP1A1 rs4646903 polymorphism in male infertility susceptibility
Ten studies involving a total of 6286 individuals evaluated the influence of the CYP1A1 gene variant rs4646903 polymorphism on the risk of male infertility. Figures 2–5 show the meta-analysis results for the allele model, additive model, recessive model, and dominant model, for which the I2 value was 78%, 69%, 67%, and 75%, respectively. Thus, the random effect model was used to synthesize the data. Overall, pooled risk estimates indicated that CYP1A1 rs4646903 polymorphism was associated with an increased risk of male infertility (C vs T: OR = 1.42, 95%CI = 1.14–1.76; CC vs TT: OR = 2.13, 95%CI = 1.36–3.34; CC vs CT+TT: OR = 1.96, 95%CI = 1.30–2.95; CC+CT vs TT: OR = 1.51, 95%CI = 1.16–1.97).
Figure 2.
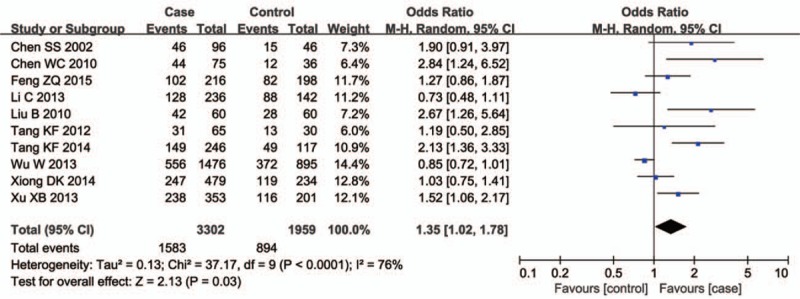
Forest plot of the studies assessing the association between CYP1A1 rs4646903 polymorphism and male infertility. (Allelic model: C vs T).
Figure 5.
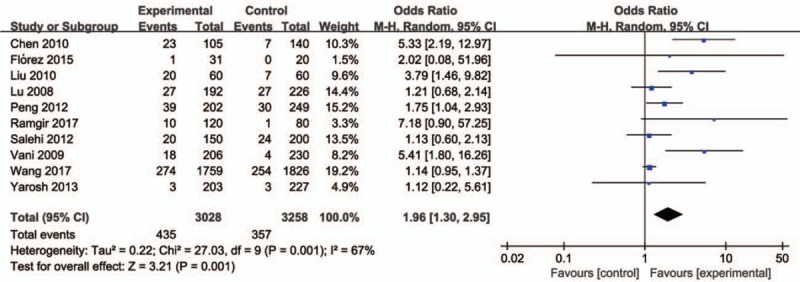
Forest plot of the studies assessing the association between CYP1A1 rs4646903 polymorphism and male infertility. (Recessive model: CC vs CT+TT).
Figure 3.
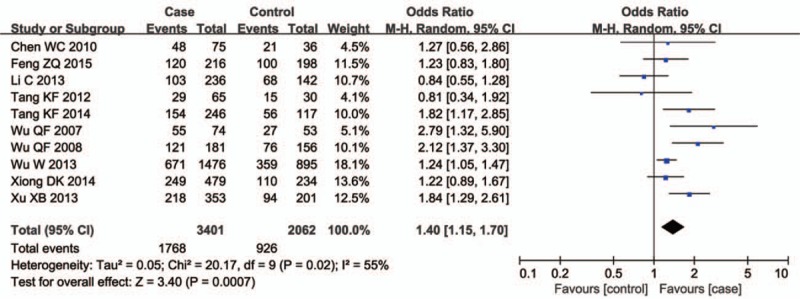
Forest plot of the studies assessing the association between CYP1A1 rs4646903 polymorphism and male infertility. (Additive model: CC vs TT).
Figure 4.
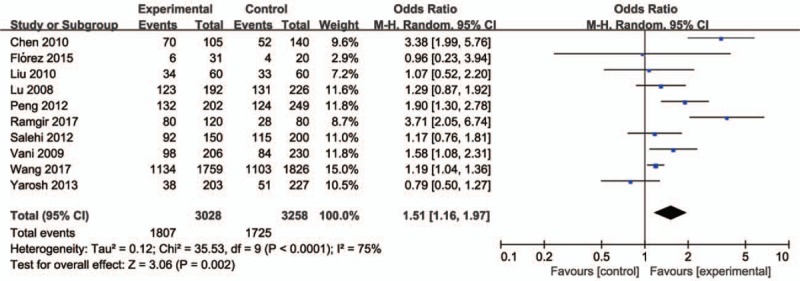
Forest plot of the studies assessing the association between CYP1A1 rs4646903 polymorphism and male infertility. (Dominant model: CC+CT vs TT).
Subgroup analysis based on ethnicity indicated that the CYP1A1 rs4646903 polymorphism was associated with increased susceptibility to male infertility in Asians (C vs T: OR = 1.59, 95%CI = 1.22–2.08; CC vs TT: OR = 2.58, 95%CI = 1.46–4.56; CC vs CT+TT: OR = 2.33, 95%CI = 1.38–3.91; CC+CT vs TT: OR = 1.58, 95%CI = 1.11–2.26), however, no association was found between the CYP1A1 rs4646903 polymorphism and male infertility risk in Non-Asians (C vs T: OR = 1.01, 95%CI = 0.79–1.30; CC vs TT: OR = 1.22, 95%CI = 0.66–2.25; CC vs CT+TT: OR = 1.15, 95%CI = 0.64–2.05; CC+CT vs TT: OR = 0.98, 95%CI = 0.72–1.34) (Table 2).
Table 2.
Meta-analysis of the association of CYP1A1 rs4646903 polymorphism with male infertility.

3.3. Publication bias and sensitivity
There was no evidence of obvious asymmetry in the funnel plots (Fig. 6). The Begg test and Egger test were also performed to access publication bias in the articles included in this meta-analysis. No significant publication bias was observed under all the genetic models, as shown in Fig. 6 and Table 3. Sensitivity analyses were conducted to calculate pooled ORs by omitting one study each time. Results showed that no individual study influenced overall pooled ORs (Fig. 7), indicating that the results of this meta-analysis are relatively stable.
Figure 6.
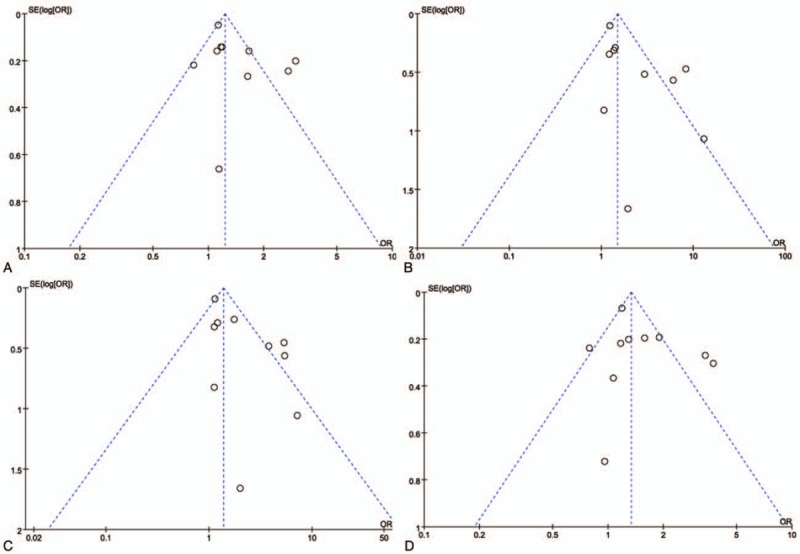
Funnel plot for the CYP1A1 rs4646903 polymorphism and male infertility risk. (A. Allelic model: C vs T; B. additive model: CC vs TT; C. dominant model: CC+CT vs TT; D. recessive model: CC vs CT+TT).
Table 3.
Publication bias test for CYP1A1 rs4646903 polymorphism.
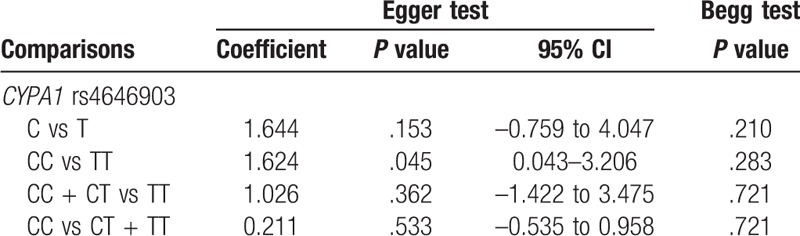
Figure 7.
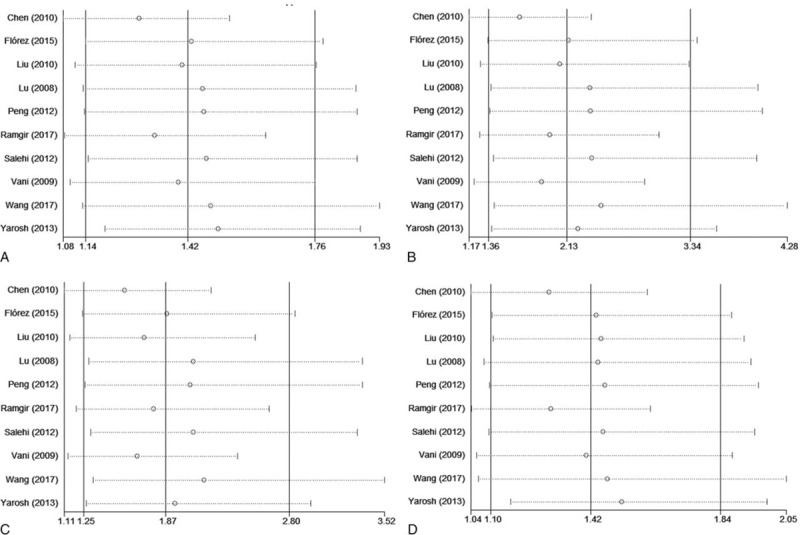
Sensitivity analysis diagram for each study used to assess the relative risk estimates for the CYP1A1 rs4646903 polymorphism and male infertility in all of the included studies. (A. Allelic model: C vs T; B. additive model: CC vs TT; C. dominant model: CC+CT vs TT; D. recessive model: CC vs CT+TT).
4. Discussion
In the present study, significant associations with the risk of male infertility were detected for the CYP1A1 rs4646903 polymorphism. Further subgroup analyses based on ethnicity of study subjects revealed that genetic variations of CYP1A1 gene rs4646903 may contribute to susceptibility to male infertility in Asians, but not in Non-Asians.
Cytochrome P4501A1, an important phase I enzyme, plays an essential role in the metabolism of polycyclic aromatic hydrocarbons (PAHs).[21,26] PAHs are able to form DNA adducts once it has been activated to DNA reactive metabolites.[27] DNA adducts could be considered as a sign of severe DNA damage in sperm cells, which played an essential role in meiotic division during spermatogenesis and associated with male infertility.[18,28] It has been suggested that the SNPs of the CYP1A1 gene could determine the activity and/or inducibility of CYP1A1.[19,29] Recent studies have revealed that CYP1A1 rs4646903 polymorphism was associated with an increased risk of male infertility, but the results of these studies were inconsistent. Lu et al[17] reported that no significant association was detected between CYP1A1∗2A polymorphism and male infertility in Chinese population. Similarly, a case-control study conducted by Yarosh et al[20] revealed that CYP1A1∗2A polymorphism do not contribute to male infertility of Colombian Caribbean men. However, Flórez et al[21] reported that CC genotype of CYP1A1 is associated in the pathogenesis of male infertility in Indian population. The difference between the studies could arise from race, geography, or genetic background of the study population. Subsequently, two meta-analysis, included 6 studies involving 1060 cases and 1225 controls, comprehensively evaluated the associations between CYP1A1 rs4646903 polymorphism and male infertility risk, but there results were still inconsistent.[14,15] This inconsistent result may arise from different search strategies, and the 2 meta-analyses do not include all relevant studies.
In the present study, 10 studies including 3028 cases and 3258 controls were included, the overall results showed that CYP1A1 rs4646903 polymorphism could increase the risk of male infertility (C vs T: OR = 1.42, 95%CI = 1.14–1.76; CC vs TT: OR = 2.13, 95%CI = 1.36–3.34; CC vs CT+TT: OR = 1.96, 95%CI = 1.30–2.95; CC+CT vs TT: OR = 1.51, 95%CI = 1.16–1.97). It reveals that individuals with the variant C allele may have a higher risk for male infertility than those carrying T homozygote. Nevertheless, in the subgroup analysis of ethnicity, we found that CYP1A1 rs4646903 polymorphism had an effect on increase in the male infertility risk in Asians, while the susceptibility to male infertility was not observed in Non-Asian population. However, only 3 studies reported the relationship between the CYP1A1 rs4646903 polymorphism and male infertility risk in Non-Asians and 5 studies for Asian population were included in the present meta-analysis. The sample size was small, thus, studies with larger sample sizes are needed to further investigate the potential relationships of CYP1A1 rs4646903 polymorphism with male infertility risk.
When interpreting the results of the current study, there are still several limitations should be considered. First, only 10 studies were incorporated in the meta-analysis, the sample size of included published articles was small. Second, subgroup analyses such as by infertility type and source of control group were not performed, due to the lack of information. Third, the effects of gene–gene and gene–environment interactions on male infertility susceptibility were not estimated, as the studies enrolled lacked of information. Finally, our results were based on unadjusted estimates, due to the lack of data of smoking, age, and other environmental exposure factors.
5. Conclusions
This meta-analysis result suggests that the CYP1A1 rs4646903 polymorphism may increase the risk of male infertility, especially in Asians. However, large sample size, well-designed, and population-based studies are necessary to comprehensively verify the association between CYP1A1 gene variant rs4646903 polymorphism and male infertility risk.
Author contributions
Conceptualization: Qin Zhang, DeHong Cao.
Data curation: DeHong Cao.
Formal analysis: DongLiang Lu.
Funding acquisition: Qiang Wei.
Methodology: ZhengJu Ren.
Software: Qin Zhang, ZhengJu Ren, Peng Xu.
Supervision: DongLiang Lu, LiangRen Liu, Qiang Wei.
Writing – original draft: DeHong Cao, ZhengJu Ren.
Writing – review & editing: Qin Zhang, DongLiang Lu, LiangRen Liu, Peng Xu.
Uncited Reference
[30].
Footnotes
Abbreviations: CBM = China biology medical literature database, CI = confidence interval, CIs = confidence intervals, CNKI = China National Knowledge Infrastructure, HWE = Hardy-Weinberg equilibrium, NOS = Newcastle-Ottawa Scale, OR = odds ratio, ORs = odds ratios, SNP = single-nucleotide polymorphism, SNPs = single-nucleotide polymorphisms, VIP = Weipu.
DC and ZR have contributed equally to this work and should be considered co-first authors.
The study was supported by National key research and development program of China (SQ2017YFSF090096), National Natural Science Foundation of China (81770756), and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan (ZY2016104).
DC, ZR, LL, PX, and QZ declare that they have no conflicts of interest.
References
- [1].Kamath MS, Bhattacharya S. Demographics of infertility and management of unexplained infertility. Best Pract Res Clin Obstet Gynaecol 2012;26:729–38. [DOI] [PubMed] [Google Scholar]
- [2].Gurunath S, Pandian Z, Anderson RA, et al. Defining infertility-a systematic review of prevalence studies. Hum Reprod Update 2011;17:575–88. [DOI] [PubMed] [Google Scholar]
- [3].Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrin 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harlev A, Agarwal A, Gunes SO, et al. Smoking and male infertility: an evidence-based review. World J Mens Health 2015;33:143–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen MJ, Tang R, Fu GB, et al. Association of exposure to phenols and idiopathic male infertility. J Hazard Mater 2013;250:115–21. [DOI] [PubMed] [Google Scholar]
- [6].Shamsi MB, Kumar R, Malhotra N, et al. Chromosomal aberrations, Yq microdeletion, and sperm DNA fragmentation in infertile men opting for assisted reproduction. Mol Reprod Dev 2012;79:637–50. [DOI] [PubMed] [Google Scholar]
- [7].Hildebrand MS, Avenarius MR, Fellous M, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet 2010;18:1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ren ZJ, Ren PW, Yang B, et al. TheSPO11-C631T gene polymorphism and male infertility risk: a meta-analysis. Ren Fail 2017;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Salehi Z, Gholizadeh L, Vaziri H, et al. Analysis of GSTM1, GSTT1, and CYP1A1 in idiopathic male infertility. Reprod Sci 2012;19:81–5. [DOI] [PubMed] [Google Scholar]
- [10].Fritsche E, Schuppe HC, Dohr O, et al. Increased frequencies of cytochrome P4501A1 polymorphisms in infertile men. Andrologia 1998;30:125–8. [DOI] [PubMed] [Google Scholar]
- [11].Sultana S, Kolla VK, Peddireddy V, et al. Association of CYP1A1 gene polymorphism with ischemic stroke in South Indian population. Transl Stroke Res 2011;2:26–32. [DOI] [PubMed] [Google Scholar]
- [12].Hussein AG, Pasha HF, El-Shahat HM, et al. CYP1A1 gene polymorphisms and smoking status as modifier factors for lung cancer risk. Gene 2014;541:26–30. [DOI] [PubMed] [Google Scholar]
- [13].Sergentanis TN, Economopoulos KP. Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 2010;122:459–69. [DOI] [PubMed] [Google Scholar]
- [14].Parada-Bustamante A, Molina C, Valencia C, et al. Disturbed testicular expression of the estrogen-metabolizing enzymes CYP1A1 and COMT in infertile men with primary spermatogenic failure: possible negative implications on Sertoli cells. Andrology 2017;5:486–94. [DOI] [PubMed] [Google Scholar]
- [15].Fang JZ, Wang SQ, Wang HN, et al. The Cytochrome P4501A1 gene polymorphisms and idiopathic male infertility risk: a meta-analysis. Gene 2014;535:93–6. [DOI] [PubMed] [Google Scholar]
- [16].Luo HQ, Li HJ, Yao N, et al. Association between 3801T > C polymorphism of CYP1A1 and idiopathic male infertility risk: a systematic review and meta-analysis. PLoS One 2014;9:e86649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [18].Lu N, Wu B, Xia Y, et al. Polymorphisms in CYP1A1 gene are associated with male infertility in a Chinese population. Int J Androl 2008;31:527–33. [DOI] [PubMed] [Google Scholar]
- [19].Ramgir SS, Sekar N, Jindam D, VGA Association of CYP1A1∗2A polymorphism with idiopathic non-obstructive Azoospermia in a South Indian cohort. Int J Fertil Steril 2017;11:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yarosh SL, Kokhtenko EV, Starodubova NI, et al. Smoking status modifies the relation between CYP1A1∗2C gene polymorphism and idiopathic male infertility: the importance of gene–environment interaction analysis for genetic studies of the disease. Reprod Sci 2013;20:1302–7. [DOI] [PubMed] [Google Scholar]
- [21].Flórez GC, Gamarra RE, Prieto LV, et al. CYP1A1∗2A polymorphism and infertility in Colombian Caribbean male subjects. Salud Uninorte Barranquilla 2015;31:1–9. [Google Scholar]
- [22].Vani GT, Mukesh N, Siva Prasad B, et al. Association of CYP1A1∗2A polymorphism with male infertility in Indian population. Clin Chim Acta 2009;410:43–7. [DOI] [PubMed] [Google Scholar]
- [23].Wang T, Hu T, Zhen JK, et al. Association of MTHFR, NFKB1, NFKBIA, DAZL and CYP1A1 gene polymorphisms with risk of idiopathic male infertility in a Han Chinese population. Int J Clin Exp Pathol 2017;10:7640–9. [PMC free article] [PubMed] [Google Scholar]
- [24].Chen W, Kang X, Huang Z, et al. CYP1A1 gene polymorphism in oligozoospermic infertilie patients of zhuang population in Guangxi area. Guang Dong Med J 2010;31:1669–71. [Google Scholar]
- [25].Central South University, Liu B, XW Genetic Susceptibility to CYP1A1 and GSTM1 Polymorphisms and Smoking With Malformed Sperm. 2010. [Google Scholar]
- [26].Peng L, Wang G, Jiao HY, et al. Association between CYP1A1 rs4646903 polymorphism and male infertility in Ningxia area. J Ningxia Med Univ 2012;34:208–10. [Google Scholar]
- [27].Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 2005;206:73–93. [DOI] [PubMed] [Google Scholar]
- [28].Tarantini A, Maitre A, Lefebvre E, et al. Polycyclic aromatic hydrocarbons in binary mixtures modulate the efficiency of benzo[a]pyrene to form DNA adducts in human cells. Toxicology 2011;279:36–44. [DOI] [PubMed] [Google Scholar]
- [29].Gunes S, Al-Sadaan M, Agarwal A, et al. DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online 2015;31:309–19. [DOI] [PubMed] [Google Scholar]
- [30].Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103–41. [DOI] [PubMed] [Google Scholar]


