Abstract
Multiple sclerosis (MS) is a chronic demyelinating autoimmune disorder affecting the central nervous system and targets the myelin sheaths around nerves. Local problem: Medical advances have enabled patients to lead a better quality of life (QoL) than before. However, because of its chronicity and unpredictability, it remains a very challenging disease for both patients and their families, as it involves the continued use of medication to slow down progression. The aim of this study is to assess drug adherence in patients with MS. In particular, we will examine how the way drugs are administered (oral or injective) affects compliance with therapy, including the correlation with coping strategies and the QoL of each patient.
We enrolled 88 patients with MS, divided into 2 groups according to therapy (injective or oral). The Morisky Medication Adherence scale was administered to evaluate adherence to treatment, the MS QoL 54 to estimate mental and physical health, and Brief coping orientation to problems experienced Inventory for coping strategies.
The results showed that in both groups the patients showed a good therapeutic alliance and trust in treatment. In particular, a correlation has been found between therapeutic adherence, adaptive coping strategies, and mental health when drug therapy is administered by injection. In conclusion, this result suggests that for patients receiving injection treatment to have greater adherence to therapy, appropriate coping strategies and good mental health must be developed in order for patients receiving injection therapy to have greater adherence to therapy; they need to develop appropriate coping strategies and good mental health to address this mode of administration successfully.
Keywords: coping strategies, multiple sclerosis, quality of life, therapeutic adherence
1. Introduction
Multiple sclerosis (MS) is a chronic demyelinating autoimmune disorder affecting the central nervous system and targets the myelin sheaths around nerves, leading to inflammation, myelin loss, and axonal destruction.[1] It is estimated to affect 2.5 million people worldwide and is the most common cause of neurological disability among people aged between 20 and 50 years,[2] resulting in a greater loss of productivity and quality of life (QoL) compared with other diseases.[3]
Patients’ behavior toward treatment is called adherence or compliance. MS has several clinical forms. This manifests itself in episodes (relapses or relapses) that alter neurological functions. The relapses are followed by partial or total functional recovery and a period of relative stability (remission) until the next episode.[4] Unfortunately, there is no treatment for MS, even if there are several pharmacological strategies that are efficacious in reducing relapse frequency in MS, preventing new lesion formation on brain magnetic resonance imaging (MRI), to modify the course of the disease, manage symptoms, and improve motor function.[5] However, the experiences of many patients show poor adherence to therapy. This has a negative impact on morbidity and mortality of patients, as well as on the overall cost of patient care. Published rates of adherence range from 28% to 88%.[5]
Portaccio and Amato[6] have shown that there is a high rate of drug disruption, particularly of injectable drugs.
Some specific features of MS may be related to therapeutic adhesion, including long periods of disease remission, lack of predictability of the disease, inadequate knowledge of the disease or its treatments, fear of needles, side effects of therapies, low self-efficacy,[7] and cognitive deficits including low cognitive reserve.[8] Furthermore, patients may not readily perceive the benefits of such costly, inconvenient, and at times painful treatments since disease-modifying therapies (DMTs) are prescribed to prevent relatively uncommon but disruptive relapse events and disability progression which occurs over years.[9] From a methodological point of view, therefore, it is essential to use statistical tools and coding flows in which to process data in as much detail and precision as possible.[10] It is also important to consider all aspects of biopsychosocial information to find intervention strategies to improve the care service and QoL of patients.[11]
Patient participation in medication brings many benefits, including reduced rates for first aid visits, hospital admissions, and absence from work. These factors can significantly influence not only the results of the treatment itself but can also influence both their QoL and mental health (depression and anxiety).[12]
A key instrument for adaptation to the needs of chronic diseases is called coping strategies, a dynamic process that takes into consideration changing characteristics of the stressor over time. The physiological impact of stress is different for each individual. It would be desirable to find an efficient stress monitoring system that assesses both the physiological and psychological impact of stress by translating these assessments into accurate quantitative metrics.[13] Actually, in recent years, there has been an increasing interest to study this process; above all McCabe and McKern[14] observed that coping strategies were important predictors of QoL in MS.
Through the appraisal process, patients evaluate the stressor (the personal consequence of MS to their lives) and select the coping strategy that is most effective in reducing or removing it. Moreover, certain types of coping may lead to better adjustments.[15]
The objective of our observational study is to assess the level of therapeutic compliance in patients with MS. We aimed to examine how the mode of administration of drug can correlate with therapeutic compliance and whether it is influenced by the coping strategies and QoL of the patient. Although several studies have been conducted on the therapeutic adherence in patients with MS, no one has shown a possible correlation with psychological variables such as the coping strategies present in the individual and his QoL. In addition, demographic variables and the overall cognitive profile were considered. Our hypothesis assumes that subjects with better coping strategies may have greater adherence to therapy and a better QoL independently froms cognitive impairment.
2. Materials and methods
2.1. Study population
We examined a series of clinically definite MS patients during a period of 1year. Inclusion criteria for the study were: a neurologist-confirmed diagnosis of definite MS according to the prevailing criteria; 16 years old over; capability and disposition to provide informed consent and to complete the study questionnaires. Specifically, for this study, participants were included if the use of drugs for sclerosis was reported during the study period. Institutional ethics approval was obtained and participants provided informed consent.
2.2. Assessment
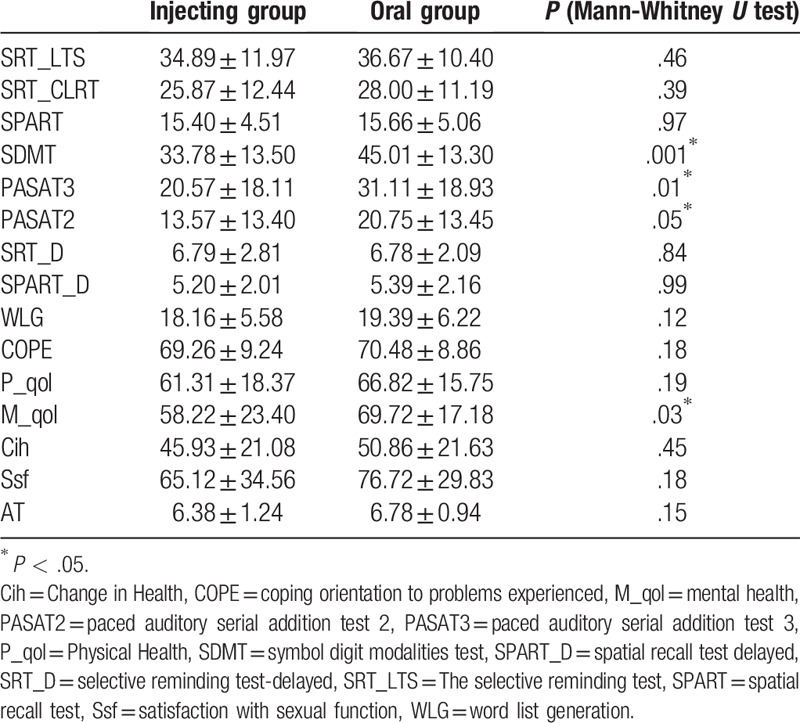
All patients were submitted to clinical and behavioral scales such as, the Italian version of the Morisky Medication Adherence Scale (MMAS-8),[16] the Quality of Life-54 (MSQOL-54),[17] the Brief coping orientation to problems experienced (COPE) Inventory[18], and the Brief Repeatable Battery of Neuropsychological Tests (BRB-N).[18] The MMAS-8 is an 8-item self-report measure that evaluates the adherence to therapy. The “Total Score" ranges from 0 to 8; high scores indicates presence of adherence to treatment. The MSQOL-54 is a 54-item self-report questionnaire that evaluates mental and physical health of the patient. Previous findings indicate that the Italian version of the MSQOL-54 is a reliable and valid measure of the QoL in patients with MS.[19] The Brief COPE Inventory is a 28-item self-report questionnaire that evaluates the frequency of use of different coping strategies. Higher scores indicate that a particular coping strategy is more frequently used. BRB-N is a neuropsychological battery sensitive to the cognitive deficits that typically characterize MS. For all the BRB-N tasks, higher scores mean better performance (Table 1).
Table 1.
Score neuropsychology and psychology tests.
2.3. Statistical analysis
Analyses were performed using an open source R3.0 software package. A 95% confidence level was set with a 5% alpha error. Statistical significance was set at P < .05. Descriptive statistic of sociodemographic characteristic, followed by the mean, standard deviation of the 2 groups was conducted. Normal distribution of the data was evaluated using the Shapiro-Wilk normality test. The Mann-Whitney U test was performed to compare the 2 groups (intergroup analysis), whereas correlations between the therapeutic adherence and clinical variables were computed by Spearman coefficient for intragroups analysis.
3. Results
3.1. Sample characteristics
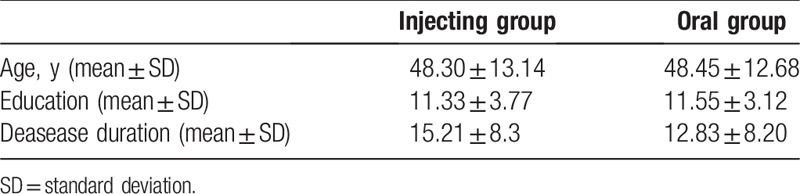
In total, a total of 88 patients were enrolled and divided into 2 groups (injecting or oral) according to drug therapy: injecting group, 44 patients with age 48.30 ± 13.14; the oral group, 44 patients with age 48.45 ± 12.68 years. The groups were characterized by comparable demographic characteristics. The demographic or clinical variables of the 2 groups are summarized in Table 2.
Table 2.
Sociodemographic and clinical characteristics of groups.
3.2. Intergroup and correlation analysis
In intergroup analysis, we observed significant differences between the two groups in symbol digit modalities test (P = .001), paced auditory serial addition test 3 (P = .01), paced auditory serial addition test 2 (P = .05), and mental health (P = .03). No significant differences were found for the other clinical scales. For correlation analysis, in injecting group a significant correlation was highlighted between therapeutic adherence and COPE (r = 0.45, P = .002) and therapeutic adherence and mental health (r = 0.34, P = .03) (Fig. 1A and B). No significant correlation in the oral group was found.
Figure 1.
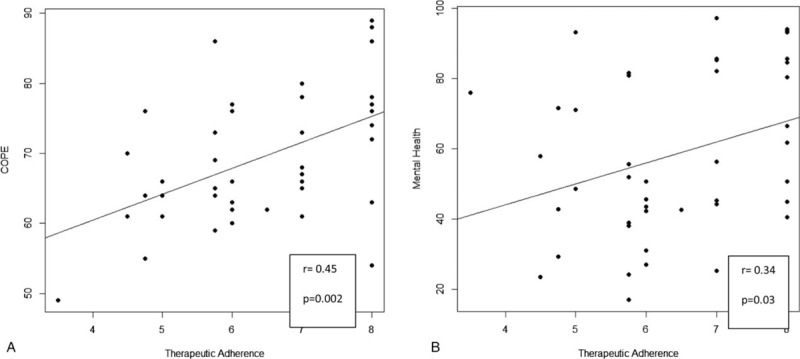
Spearman correlation analysis. (A) Significant correlation between therapeutic adherence and COPE. (B) Significant correlation between therapeutic adherence and mental health. COPE = coping orientation to problems experienced.
4. Discussion
It is a challenge to maintain adherence therapies during the long term. Adherence to therapy is a key factor in the success of treatment.[20] In many cases, the treatment of chronic diseases questions patients’ self-management abilities and motivational resources by requiring medication, following a diet and changing individual lifestyles.[21] This also applies to standard immunomodulating therapies for MS, which require careful maintenance of (self-)injection programs and sometimes tolerance of unwanted side effects. As with other chronic conditions, a substantial proportion of patients do not adhere to treatment for at least a certain period.[22]
Current evidence indicates that a progressive course of MS, higher disability, lower self-efficacy, lower motivation for change, and lower perceived benefits suggests that DMTs may not be adhered to in MS.[23] Achieving treatment goals in MS requires strict adherence to treatment programs.[24]
This study analyzed persistence and adherence to oral medications compared to injectable DMTs in patients with MS. Our study identified specific features that influenced the likelihood of adhesion, including some modifiable attributes. These characteristics can put a person at risk of nonadherence or be useful indicators of future nonadherence. Two different groups were examined for the type of drug administration used. The results showed that therapeutic adherence is similar in both groups, indeed almost all our patients show a willingness to treat. It was important that coping strategies and mental health are correlated with therapeutic adherence in the group of subjects who practice injective drug therapy. This suggests that for injection patients to have greater adherence and endurance over long periods of time, they need to develop adequate strategies to cope with the type of administration and good mental health. On the contrary, oral users probably do not need specific coping strategies as the therapy is more accepted, easier to administer, and more like other pharmacological therapies. This is confirmed by the literature, which shows that there is greater therapeutic adherence to orally administered drugs.
For example, Bergvall et al,[25] in most studies, have shown that persistence and adherence to oral medication are higher than that of injectable and infusible DMTs.[26] This is probably because of both a perceived lack of efficacy and with greater side effects.[27] However, if patients develop good coping strategies and greater mental stability there is no difference in therapeutic adherence. Understanding coping strategies before treatment may allow a personalized support during long-term management of the disease and may improve adherence to treatment. Therefore, evaluating coping strategies is important in MS, especially if it is possible to identify the variables associated with anxiety or depression and to develop targeted psychotherapeutic measures that can provide long-term resistance to depression.[28] Actually, some studies have shown a strong link between adherence to treatment and the emotional functioning of patients with MS. Almost 63% of patients with MS and with current mood or anxiety disorder showed variable or poor adherence throughout the study. These patients were almost 5 times more likely than patients with MS without a psychiatric diagnosis to have problems with adherence to DMTs. Variable/poor adherents also approved more anxiety and mood symptoms by self-reporting than patients with adequate adherence during the study. Poor adherence was associated with memory defaults, anxiety, depression, neuroticism, and reduced consciousness. The results highlight the importance of conducting in-depth psychiatric and neuropsychological evaluation when patients have problems taking medications as prescribed.[28] For this reason, it is very important to promote adherence, given that it has been shown to be associated with better clinical outcomes, with less use of healthcare resources, with a lower incidence of hospitalization in MS, and, most of all, with a better QoL in terms of health.[29] Tan et al[30,31] found that patients who were adherent had significantly lower rates of relapse than nonadherent patients.
4.1. Limitations and conclusion
Our results have demonstrated the importance of developing effective coping strategies to reduce psychological distress and improve therapeutic adherence when administering the drug by injection. Despite the functional limitation imposed by the disease, greater attention to psychological aspects might be significant in ensuring a greater state of well-being. For this reason, it would be useful to replicate this study on a larger group of patients considering further psychological variables, the start of the drug, other methods of administration, and any relapses of the disease.
Author contributions
Conceptualization: Francesco Corallo.
Data curation: Lilla Bonanno.
Investigation: Francesco Corallo, Marcella Di Cara.
Resources: Marcella Di Cara, Francesco Corallo, Viviana Lo Buono, Giuseppe Venuti.
Supervision: Silvia Marino.
Validation: Silvia Marino, Carmela Rifici, Edoardo Sessa, GianGaetano D’Aleo.
Visualization: Carmela Rifici, Giangaetano D’Aleo, Edoardo Sessa, Silvia Marino, Placido Bramanti.
Footnotes
Abbreviations: BRB-N = brief repeatable battery of neuropsychological tests, MMAS-8 = Morisky Medication Adherence Scale, MRI = magnetic resonance imaging, MS = multiple sclerosis, QoL = quality of life, RRMS = relapsing-remitting multiple sclerosis.
Ethical Approval: The study protocol was approved by the Local Ethics Committee according to Declaration of Helsinki
Informed Consent: Informed written consent was obtained from the patient for publication of this case report and accompanying images
The authors report no conflicts of interest.
References
- [1].Koutsouraki E, Costa V, Baloyannis S. Epidemiology of multiple sclerosis in Europe: a review. Int Rev Psychiatry 2010;22:2–13. [DOI] [PubMed] [Google Scholar]
- [2].OptumRx. Multiple sclerosis: background, new developments, key strategies. 2014. Available at: https://cdnaem.optum.com/content/dam/optum3/optum/en/resources/whiteapers/M53018_G_MS_Insight_Report_ORx_ FINAL.pdf Accessed September 24, 2016. [Google Scholar]
- [3].Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs 2010;70:1677–91. [DOI] [PubMed] [Google Scholar]
- [4].National Multiple Sclerosis Society. Understanding Multiple Sclerosis. Available at: http://calmain.nationalmssociety.org/site/PageNavigator/CAL_Exercise_MS1_Treated Accessed March 21, 2016. [Google Scholar]
- [5].Menzin J, Caon C, Nichols C, et al. A narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013;191 suppl A:S24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Portaccio E, Amato MP. Improving compliance with interferon-beta therapy in patients with multiple sclerosis. CNS Drugs 2009;23:453–62. [DOI] [PubMed] [Google Scholar]
- [7].Caon C, Saunders C, Smrtka J, et al. Injectable disease-modifying therapy for relapsing-remitting multiple sclerosis: a review of adherence data. J Neurosci Nurs 2010;425 suppl:S5–9. [DOI] [PubMed] [Google Scholar]
- [8].Nunnari D, De Cola MC, Costa A, et al. Exploring cognitive reserve in multiple sclerosis: new findings from a cross-sectional study. J Clin Exp Neuropsychol 2016;38:1158–67. [DOI] [PubMed] [Google Scholar]
- [9].Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 2010;30:89–100. [DOI] [PubMed] [Google Scholar]
- [10].Wang Y, Liu H, Zheng W, et al. Multi-objective workflow scheduling with Deep-Q-network-based multi-agent reinforcement learning. IEEE Access 2019;7:39974–82. [Google Scholar]
- [11].Han J, Ji X, Hu X, et al. Representing and retrieving video shots in human-centric brain imaging space. IEEE Trans Image Process 2013;22:2723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011;28:51–61. [DOI] [PubMed] [Google Scholar]
- [13].Wu W, Pirbhulal S, Zhang H, et al. Quantitative assessment for self-tracking of acute stress based on triangulation principle in a wearable sensor system. IEEE J Biomed Health Inform 2019;23:703–13. [DOI] [PubMed] [Google Scholar]
- [14].McCabe MP, McKern S. Quality of life and multiple sclerosis: comparison between people with multiple sclerosis and people from the general population. J Clin Psychol Med Settings 2002;9:287–95. [Google Scholar]
- [15].Arnett PA, Higginson CI, Voss WD, et al. Relationship between coping, cognitive dysfunction and depression in multiple sclerosis. Clin Neuropsychol 2002;16:341–55. [DOI] [PubMed] [Google Scholar]
- [16].Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [17].Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res 1995;4:187–206. [DOI] [PubMed] [Google Scholar]
- [18].Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997;4:92–100. [DOI] [PubMed] [Google Scholar]
- [19].Rao SM. Cognitive function in patients with multiple sclerosis: impairment and treatment. Int J MS Care 2004;6:9–22. [Google Scholar]
- [20].World Health Organization. Adherence to long-term therapies: evidence for action, 2003. Available at: http://www.who.int/chp/knowledge/publications/adherence_report_fin.pdf?ua=1 [Google Scholar]
- [21].Rotheram-Borus MJ, Ingram BL, Swendeman D, et al. Adoption of self-management interventions for prevention and care. Prim Care 2012;39:649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Henze T, Rieckmann P, Toyka KV. Symptomatic treatment of multiple sclerosis. Eur Neurol 2006;56:78–105. [DOI] [PubMed] [Google Scholar]
- [23].Saiz A, Mora S, Blanco J. Treatment compliance with first line disease-modifying therapies in patients with multiple sclerosis. COMPLIANCE Study. Neurología (English Edition) 2015;30:214–22. [DOI] [PubMed] [Google Scholar]
- [24].Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 2015;3:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ 2014;17:696–707. [DOI] [PubMed] [Google Scholar]
- [26].Clerico M, Barbero P, Contessa G, et al. Adherence to interferon-beta treatment and results of therapy switching. J Neurol Sci 2007;259:104–8. [DOI] [PubMed] [Google Scholar]
- [27].Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler 2012;18:932–46. [DOI] [PubMed] [Google Scholar]
- [28].Solari A, Filippini G, Mendozzi L. Validation of Italian multiple sclerosis quality of life 54 questionnaire. J Neurol Neurosurg Psychiatry 1999;67:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bruce JM, Hancock LM, Arnett P, et al. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med 2010;33:219–27. [DOI] [PubMed] [Google Scholar]
- [30].Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization (Ed) 2003. [Google Scholar]
- [31].Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 1. Clinical course and disability. Brain 1989;112(pt 1):133–46. [DOI] [PubMed] [Google Scholar]


