Supplemental Digital Content is available in the text
Keywords: FRNS, immunosuppressant, multiple-treatments meta-analysis, pediatrics, SDNS
Abstract
Introduction:
A network meta-analysis was conducted to regard the effects of available immunosuppressive medications in pediatric frequently-relapsing nephrotic syndrome (FRNS) and steroid-dependent nephrotic syndrome (SDNS).
Methods:
We reviewed systematically 26 randomized controlled trials (1311 patients) that compared any of the following immunosuppressive agents to placebo/nontreatment (P/NT) or another drug for FRNS/SDNS treatment in children.
Results:
The main outcomes were efficacy and acceptability. At the 6-month, cyclophosphamide, chlorambucil, levamisole, and rituximab had better efficacy than P/NT (odds ratio [OR]: 0.09, 0.03, 0.28, and 0.07, respectively); cyclophosphamide was significantly more effective than azathioprine and chlorambucil. At 12 months, cyclophosphamide, chlorambucil, cyclosporine, levamisole, and rituximab had better efficacy than P/NT (0.10, 0.03, 0.10, 0.23, and 0.07, respectively); Chlorambucil were found to be more efficacious than levamisole and MMF (0.12 and 0.09, respectively). At 24 months, cyclophosphamide, chlorambucil, and levamisole had better efficacy than P/NT (0.09, 0.04, and 0.03, respectively); cyclophosphamide had better efficacy than cyclosporine and vincristine (0.17 and 0.39, respectively).
Conclusion:
No significant differences in acceptability were found. Our results suggest that cyclophosphamide may be preferred initially in children with FRSN/SDNS, chlorambucil, and rituximab may be acceptable medications for patients with FRSN/SDNS. Long-term follow-up trials focused on gonadal toxicity and limitation of maximum dosage of cyclophosphamide should been carried out.
1. Introduction
In most of cases, clinical remission of pediatric idiopathic nephrotic syndrome (INS) can be reached with corticoid (e.g., prednisolone) therapy.[1] However, 80% of children treated for PNS suffer from edema and proteinuria recurrence, and up to 50% of these children go on to develop frequently relapsing nephrotic syndrome (FRNS) or steroid-dependent nephrotic syndrome (SDNS) during corticosteroid dose reduction or within a few weeks after steroid withdrawal.[2,3] It has been suggested that immunosuppressive medications may help extend the duration of remission in PNS patients, particularly during the corticosteroid withdrawal process.[4]
Some kinds of new hypotoxic immunosuppressive agents have been presented in recent decades for the intervention of pediatric FRNS/SDNS recovering from INS.[5] But, these emerging agents may be lesser efficacious for prolonged remission when corticoid withdrawal than conventional immunosuppressant medications. Consequently, there is still no consensus on which immunosuppressive agents are most effective for pediatric FRNS/SDNS. Factors that may be related to treating efficiency have been identified by conventional meta analyses estimating the efficiency of emerging immunosuppressive drugs and system reviews demonstrated different efficacy among non-steroidal immunosuppressive agents.[6–9] Nevertheless, conclusions of previous evidences are inconsistent because indirect comparisons could not be conducted. Furthermore, the degree to which effectiveness and acceptability differentiates across available FRNS/SDNS medications is not clear.[6,7,10,11]
In view of the above, a network meta-analysis[12] is reported in which comparisons of eight non-steroidal immunosuppressive medications were made with regard to effectiveness and acceptability in pediatric FRNS/SDNS. The purpose of this study was to defined a better pediatric FRNS/SDNS therapeutic regimen.
2. Methods
2.1. Trials identification
Prepared for this multiple-treatments meta-analysis, a study protocol had been drafted and published on the prospero website (CRD 42016048032). There are no ethical conflicts involved in the article. A literature search for relevant studies was performed in Medline (from 1950 to March 2019), the Central (Cochrane Central Register of Controlled Trials, Issue 3, 2019), and Embase (1974 to March 2019) using the following terms: “alkylating agents,” “immunosuppressive agents,” “cyclosporine,” “azathioprine” or “mycophenolic acid,” “cyclophosphamide,” “tacrolimus,” “chlorambucil,” “levamisole,” “rituximab”; and “nephrotic syndrome,” “nephrosis lipoid,” “focal segment glomerulosclerosis,” “glomerulonephritis membranoproliferative,” “minimal change nephrotic syndrome,” “membranoproliferative glomerulonephritis,” “IgM nephrothay.” We restricted the search results to papers reporting clinical trials on children. In addition, we screened the reference lists of eligible articles and correlative reviews and searched ClinicalTrials.gov for ongoing studies to identify other potentially germane studies.
2.2. Selection criteria
We included randomized controlled trials compared with any of the following interventions and in which children with FRNS/SDNS were the subjects: cyclosporine, azathioprine, MMF, tacrolimus, chlorambucil, cyclophosphamide, levamisole, and rituximab. The experience group might be compared to a P/NT and/or another agent. FRNS was defined as at least two recurrences within 6 months or at least four recurrences within 12 months of an initial remission. SDNS was defined as two consecutive relapses during corticosteroid reduction or within 14 days of corticosteroid withdrawal.[13]
We excluded trials involving patients experiencing their first bout of steroid sensitive nephrotic syndrome, steroid resistant nephrotic syndrome, congenital nephrotic syndrome, and other renal/ systemic forms of nephrotic syndrome. We also excluded trials in which only abstracts were published with no available data available from other ways. Language restrictions were not applied.
2.3. Outcome measures
The primary efficacy outcome was relapse rate and the primary acceptability outcomes were dropout rate and adverse effects. We extracted primary outcome data for the following timepoints (±1 month): 6 months, 12 months, 24 months, and the final follow-up examination reported. We defined relapse as urinalysis results of ≥3+ (300 mg/dL) protein level in early morning urine samples for three consecutive days.[13] We defined adverse effects (acceptability outcome) as sequelae happening in the initial 6 ± 1 months post-treatment period. Dropout for any reason (acceptability outcome) included patients who withdrew before the end of the study follow-up period.
2.4. Extraction of data and assessment of bias risk
Two researcher (LT and SL) evaluated references and abstracts, reviewed quality of trials and rated the integrity of abstracted data respectively. Emails were sent to the writer of papers with inadequate data who were required to provide supplemental material. We used Cochrane risk of bias tool to assessed the quality of methodology and bias risk.
2.5. Statistics analysis
First, traditional pairwise comparisons of random-effects were conducted using the metan command with Knapp–Hartung method,[14] from which odds ratios (ORs) with 95% confidence intervals (CIs) were reported. We used the Haldane method to add 0.5 to each arm when trial reported a zero event. We performed conventional meta-analyses with the DerSimonian-Laird random effects model. We used the I2 statistic as heterogeneity index.
Secondly, we performed network meta-analyses using the network suite based on a frequentist framework.[15] Based on the assumption that all treatment-contrasts had the same heterogeneity variance, we performed the network meta-analysis with a multivariable meta-analysis of random-effects using mvmeta command. Netleague command was used to report relative effects of treatment for all pairwise comparisons obtained by the multiple-treatments meta-analysis. We considered that P < .05 was significant. For population difference magnitude, a plausible range was looked at. The network rank option was used to estimate the probability which agent might be the most, second most, third most, etc efficacious intervention. We determined the surface under the cumulative ranking curve (SUCRA)[16] as an evaluation of the ranking probability for each medication to obtain a treatment hierarchy. We ranked the agents’ acceptability using the same method. Consistency within the networks was assessed between direct and indirect comparison using interaction model of the design-by-treatment.[17] We applied a loop-specific approach to check local inconsistency in network meta-analysis models when information was adequately similar among combined data. For a specific comparison, we calculated inconsistency factor with 95% CIs between indirect and direct evaluations as a method of within-loop inconsistency.[18] The definition of inconsistency was disagreement between indirect and direct comparison (95% CIs excluding 0). We conducted all data analyses in Stata 14.0 (Stata Corp, College Station, TX).
3. Results
3.1. Characteristics of study and network of evidence
Total number of 10,464 possibly correlative articles was included identified by literature searches, which identified 7236 unique qualified studies. We excluded 7174 reports during the review process based on our eligibility criteria. Ultimately, 26 studies reported from 1970 to 2018 were selected for inclusion in ultimate analysis. The 26 eligible trials included 1311 participants who were randomly assigned to a treatment group or placebo/nontreatment (P/NT) group. The summary of study screening process is shown in Fig. 1.
Figure 1.
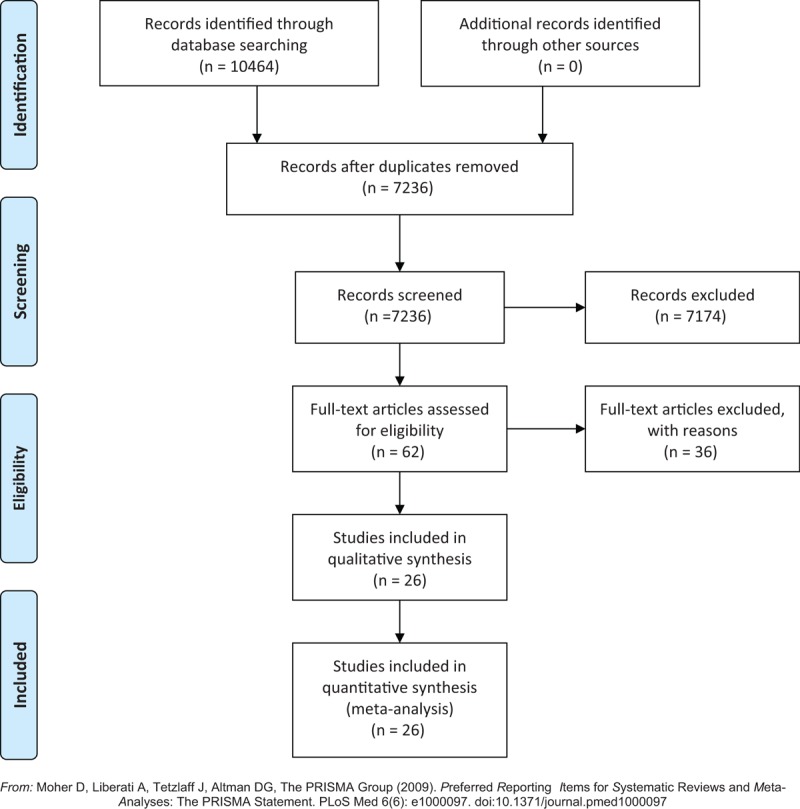
Study selection process.
3.2. Characteristics of study
Table 1 summarizes the characteristics of 26 included studies.[3,19–43] In brief, study time of duration varied from 6 to 24 months and the age of included participants ranged from 1 to 17 years old. Most (71%) of the individuals were male. Data from 1311 participants were processed in ours study. The average sample number was 50 participants each group with range from 20 to 149. Majority (25/26; 96%) of the trials were two-arms (one experimental medication and one P/NT); one study had three arms (two experimental medications and one P/NT).[28] On the quality of trials, 23% of the studies were patient-blinded, 27% were outcome-blinded, 69% were allocation-concealed, and 15% were incomplete outcome. In general, low risk of bias was showed in the included studies (see Fig. 2 and Fig. 3).
Table 1.
Descriptive characteristics of studies included in the meta-analysis.

Figure 2.

Risk of bias graph.
Figure 3.
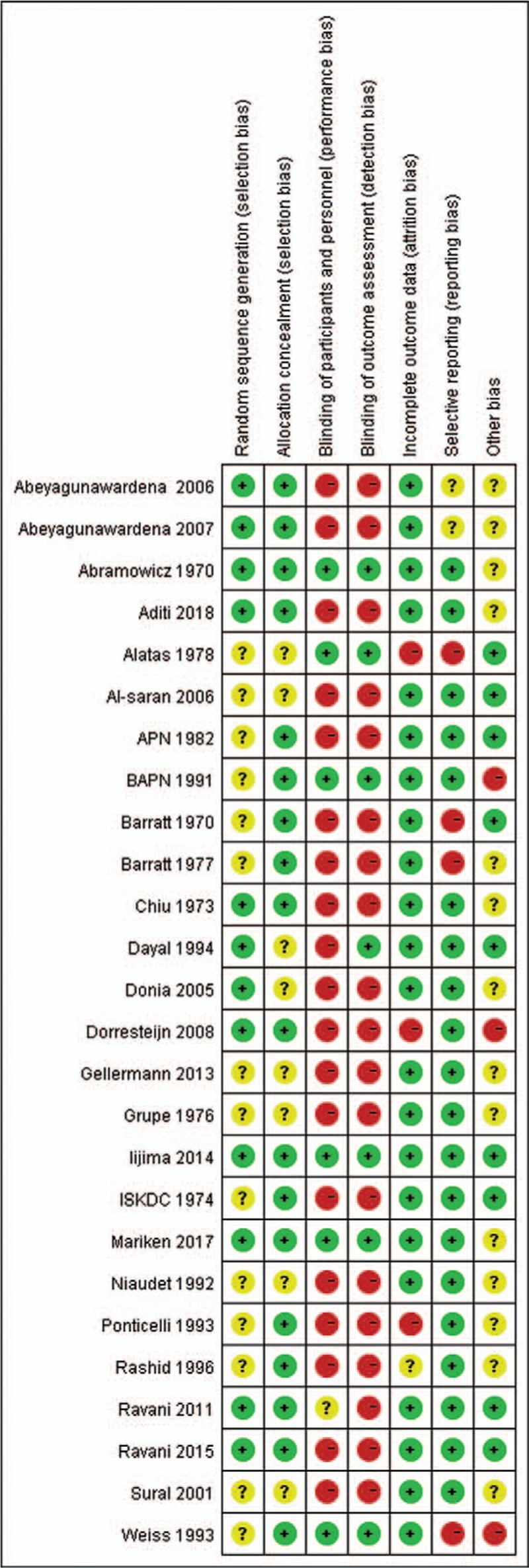
Risk of bias summary.
3.3. Network of evidence
Our efficacy and acceptability analyses at the 6-, 12-, and 24-month follow-up time points included 566 participants in 14 studies of total six drugs (Fig. 4A), 1008 participants in 21 studies of total eight drugs (Fig. 4B), and 318 participants in 8 studies of total six drugs (Fig. 4C), respectively. Altogether, the following eight drugs were analyzed compared with P/NT in the presenting analysis: cyclophosphamide (7 trials), cyclosporine (5 trials), azathioprine (2 trials), chlorambucil (4 trials), levamisole (10 trials), rituximab (3 trials), vincristine (1 trials), and mycophenolate mofetil (3 trials).
Figure 4.

Network of eligible efficacy and acceptability comparisons. The thickness of the lines reflects the number of studies being compared, and node size reflects the number of randomized individuals.
3.4. Direct pairwise pooled-analyses of single-drugs
Table 2 shows the efficacy and acceptability analysis results from single immunosuppressive agents at 6, 12, and 24 months follow up as obtained by conventional meta-analyses. Cyclophosphamide, chlorambucil, and rituximab were found to be associated with a significantly better efficacy (reduced relapse rate) compared with P/NT at both the 6- and 12-month follow-up time points (Table 2, Fig. S3). Additionally, chlorambucil had better efficacy than cyclosporine, cyclosporine had better efficacy than MMF, and levamisole better than P/NT at 12-month follow-up time points (Table 2, Fig. S3). At the 24-month follow-up time point, cyclophosphamide was more efficacious than cyclosporine and P/NT and chlorambucil was more efficacious than cyclosporine (Table 2).
Table 1 (Continued).
Descriptive characteristics of studies included in the meta-analysis.
Table 2.
Efficacy and acceptability in meta-analyses of direct comparisons between each pair of immunosuppressive medications.

There were no significant differences in acceptability between any of the eight experimental drugs versus P/NT nor between one another. But, it is noteworthy that most of the 95% CIs obtained from the comparisons were reflective of high or no heterogeneity as result of a small amount of included trials in direct comparisons; in general, there was moderate heterogeneity.
3.5. Network meta-analysis of single-drugs
Our network meta-analysis results for immunosuppressive medications, active comparators, and P/NT are presented in Fig. 5 presents network meta-analysis outcomes for immunosuppressive agents and P/NT. At the 6-month follow-up time point (Fig. 5A), cyclophosphamide, chlorambucil, levamisole, and rituximab had significant associations with relapse reduction compared with P/NT. Chlorambucil was associated with reduced relapse rates compared with azathioprine, while cyclophosphamide versus azathioprine, cyclophosphamide versus levamisole and chlorambucil versus levamisole were noted with the 95% CI for OR slightly more than 1. At the 12-month follow-up time point (Fig. 5B), cyclophosphamide, chlorambucil, cyclosporine, levamisole, and rituximab were associated with reduced relapse rates compared with P/NT. Chlorambucil were found to be more efficacious than levamisole and MMF while cyclophosphamide was not found to be more efficacious than levamisole with 95% CIs for ORs slightly more than 1. Meanwhile, chlorambucil was found to be more efficacious than vincristine. At the 24-month follow-up time point (Fig. 5C), cyclophosphamide, chlorambucil, and levamisole were associated with reduced relapse rates compared with P/NT, cyclophosphamide was more efficacious than cyclosporine. Cyclophosphamide and chlorambucil was not more efficacious than vincristine with 95% CIs for ORs slightly more than 1. No significant differences in acceptability were found.
Figure 5.
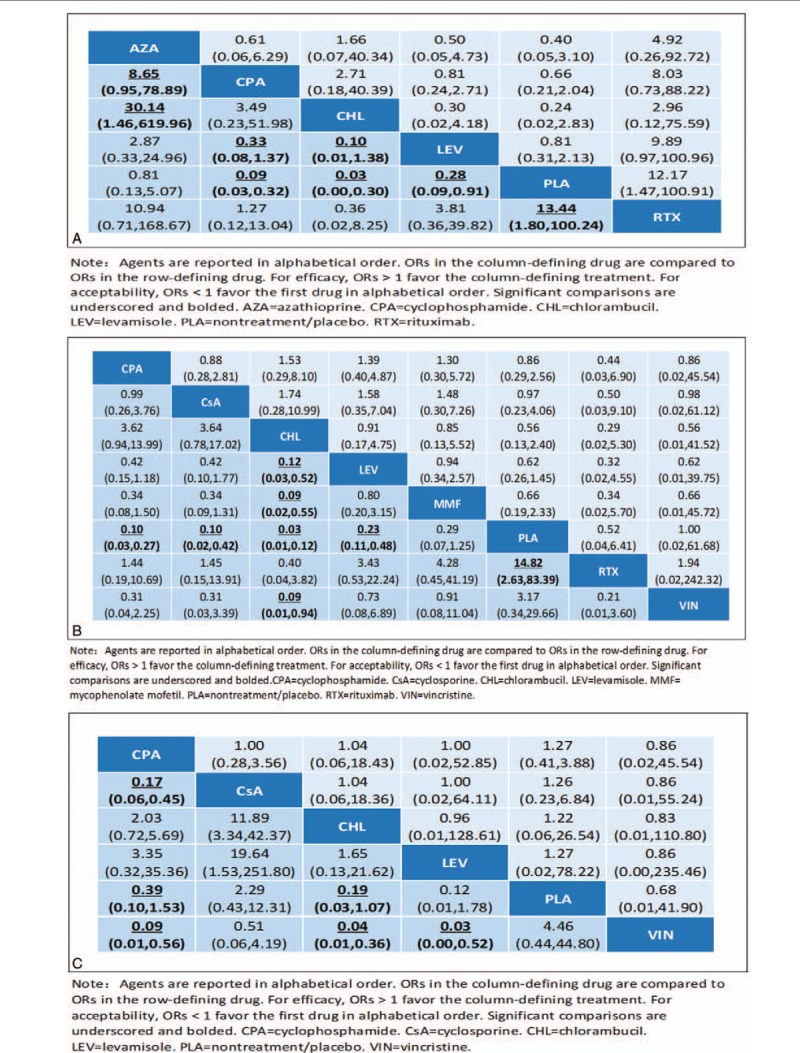
Efficacy and acceptability of agents at 6-month (A), 12-month (B), and 24-month (C) follow-up time points. Agents are reported in alphabetical order. ORs in the column-defining drug are compared to ORs in the row-defining drug. For efficacy, ORs > 1 favor the column-defining treatment. For acceptability, ORs < 1 favor the first drug in alphabetical order. Significant comparisons are underscored and bolded. AZA = azathioprine, CHL= chlorambucil, CPA = cyclophosphamide, CsA = cyclosporine, LEV = levamisole, MMF = mycophenolate mofetil, PLA= nontreatment/placebo, RTX = rituximab.
3.6. Medications ranking
The relative rankings of efficacy and acceptability of the drugs evolved over time. At the 6-month follow-up time point, chlorambucil, rituximab, and cyclophosphamide were among the most efficacious treatments, while P/NT, levamisole, and azathioprine were better tolerated than the remaining immunosuppressive medications (Fig. 6A). The accumulative chances of efficacy for the testing agents at 6 months were: chlorambucil (68.7%), rituximab (21.2%), cyclophosphamide (9.9%), azathioprine (0.1%), levamisole (0.1%), and nontreatment/placebo (0%). The cumulative acceptability rates for the examined medications were: nontreatment/placebo (37.0%), levamisole (23.5%), azathioprine (15.1%), cyclophosphamide (13.0%), chlorambucil (10.7%), and rituximab (0.7%).
Figure 6.

Efficacy (real line) and acceptability (dashed line) rankings at 6 months (A), 12 months (B), and 24-month (C) follow-up time points. Ranking reflects the probability of being the best, second best etc agent among the eight tested medications.
At 12 months (Fig. 6B), chlorambucil, rituximab, cyclophosphamide, and cyclosporine were the most efficacious treatments, and rituximab, levamisole, nontreatment/placebo, and vincristine were better tolerated than the other medications. The accumulative chances of being the most efficacious agent were: chlorambucil (74.8%), rituximab (18.7%), cyclosporine (3.7%), MMF (0.1%), vincristine (1.2%), cyclophosphamide (1.5%), levamisole (0.1%), and nontreatment/placebo (0%). The probabilities for being the most acceptable at 12 months were: rituximab (43.1%), vincristine (31.3%), levamisole (1.0%), cyclosporine (11.1%), MMF (3.6%), nontreatment/placebo (4.7%), chlorambucil (3.1%), and cyclophosphamide (2.1%).
At 24 months (Fig. 6C), levamisole, chlorambucil, and cyclophosphamide were the most efficacious treatments, while vincristine, levamisole, and chlorambucil were the best tolerated. The accumulative chances of the most efficacious agent at 24 months were: levamisole (64.0%), chlorambucil (33.5%), cyclophosphamide (1.6%), vincristine (0.9%), cyclosporine (0%), and nontreatment/placebo (0%). The probabilities for being the most acceptable at 24 months were: vincristine (32.8%), levamisole (36.8%), chlorambucil (21.5%), cyclosporine (7.8%), nontreatment/placebo (5.6%), and cyclophosphamide (5.5%).
Cluster ranking (Fig. S1) indicated that chlorambucil, cyclophosphamide, and rituximab may have the best efficacy and acceptability profiles, relative to the other examined drugs, at 6 months. Cluster ranking indicated that chlorambucil, cyclosporine, cyclophosphamide, and rituximab may have the best efficacy and acceptability profiles, relative to the other examined drugs, at 12 months. And, finally, cluster ranking indicated that cyclophosphamide, levamisole, and chlorambucil may have the best efficacy and acceptability profiles at 24 months.
3.7. Inconsistency and publication bias
Figure S2 shows low inconsistency of indirect and direct comparisons of rates of relapse. It was consistent in the most of loops, with the 95% CIs (contained 0) illustrating similar evaluations of effects between indirect and direct comparisons. Therefore, the results of network meta-analysis were robust. No asymmetry evidence was showed in comparison adjusted funnel plots for 12 months efficacy (Fig. S4).
4. Discussion
Our analysis included 24 trials, with 1062 participants who were randomly allocated to either one of eight immunosuppressive agent groups or P/NT group. Chlorambucil, rituximab, and cyclophosphamide were more efficacious than azathioprine, levamisole, and P/NT at all examined time points. However, P/NT, levamisole, and azathioprine were better tolerated than cyclophosphamide, chlorambucil, and rituximab at 6 months. Chlorambucil, rituximab, cyclophosphamide, and cyclosporine may control recurrence better than MMF, vincristine, levamisole, and nontreatment/placebo, whereas rituximab, vincristine, levamisole, and MMF appear to be tolerated better than cyclosporine, nontreatment/placebo, chlorambucil, and cyclophosphamide at 12 months. At 24 months, levamisole, chlorambucil, and cyclophosphamide were more efficacious than vincristine, cyclosporine, and nontreatment/placebo, while vincristine, levamisole, and chlorambucil were better tolerated than cyclosporine, nontreatment/placebo, and cyclophosphamide. Our results, which illustrate different efficacy amongst the tested agents may provide useful information useful for the selection of immunosuppressive medications for FRSN/SDNS treatment in pediatric patients. Although prior research has produced apparently favorable efficacy and safety results for rituximab in pediatric FRSN/SDNS patients compared with other immunosuppressive agents, sufficient power was lacking to yield clinically significant differences in treatment effects.[6]
A clinical significance of our findings is that chlorambucil, rituximab, and cyclophosphamide should be considered as preferred immunosuppressive medications for FRSN/SDNS in children due to their more effectiveness and overall good, although not preferable, acceptability. Among these three drugs, cyclophosphamide is more affordable than rituximab in most countries. However, without a formal cost-effectiveness analysis, this recommendation cannot be made unequivocally. Conversely, azathioprine, vincristine, and levamisole were less favorable options for FRSN/SDNS in terms of efficacy. Azathioprine and vincristine were also not ranked high for acceptability among the presently examined immunosuppressive medications. Hence, the present findings suggest that azathioprine, vincristine, and levamisole should not be first-line treatments for FRSN/SDNS.
Existing traditional head-to-head meta-analyses of the effectiveness of immunosuppressive agents for pediatric FRSN/SDNS were not conclusive owing to limited information of treating effects and failures to provide evidence of relative effect of eligible agents. But all, a published systematic review, reported by Nanthiya et al, demonstrated that 8-week courses of cyclophosphamide or chlorambucil and extended-term courses of cyclosporine and levamisole can reduce relapse risk in children with SSNS compared with corticosteroids.[7] The present research provides much necessary direct comparisons among immunosuppressive drugs in patients of primary nephrotic syndrome.
Rituximab, a monoclonal anti-CD-20 antibody, represents a new treatment strategy of B cell apoptosis induction.[20] Some studies have reported that rituximab treatment prolonged clinical remission and decreased glucocorticoid dose levels in children with FRSN/SDNS.[19,20] Clinical guidelines for pediatric idiopathic nephrotic syndrome recommend the immunosuppressive agents cyclosporine and cyclophosphamide (recommendation grade A) as well as mizoribine (recommendation grade C2), while suggesting rituximab only for refractory disease (recommendation grade C).[5]
The most problematic adverse secondary effect of cyclosporine is chronic renal toxicity, an rising risk for which has be related to cyclosporine treatment for more than 2 years.[44] The rates of associated side effect of immunosuppressive medications reported may be underestimated because the initial trials were designed primarily not to estimate adverse effect. In addition, because we included studies with combined corticoid treatment, related results of immunosuppressive medications should not be considered unrelated of possible corticoid effects. Moreover, our findings cannot be generalized to children who suffer from steroid-resistant nephrotic syndrome because we excluded studies with that patients.
The findings of our meta-analysis should be applied to duration of <2 years. Practice efficacy and acceptability >2 years might be quite different from results obtained within 2 years.[45] In addition, the quality of the initial trials may limit the quality of this review. Most eligible trials in this study reported insufficient information on randomization and allocation concealment, which may have an affect on the total validity of the data.[46] About 65% of the included trials had high performance bias and detection bias. The small sample-sizes and small number of the eligible trials might also be considered for the generalizability of findings. Lastly, all of the eligible trials did not address long-term fertility-related adverse effect of alkylating-agent.
In conclusion, on the basis of all obtainable indirect and direct evidences, our analyses suggest that cyclophosphamide may be preferred initially in children with FRSN/SDNS, chlorambucil, and rituximab may be acceptable medications for patients with FRSN/SDNS. Moreover, long-term follow-up trials focused on gonadal toxicity and limitation of maximum dosage of cyclophosphamide should be carried out. Additional evidences about the safety and efficacy of rituximab in children with FRSN/SDNS are also needed.
Author contributions
Conceptualization: Liping Tan, Qiu Li.
Investigation: Shaojun Li, Haiping Yang, Qing Zou.
Methodology: Shaojun Li, Haiping Yang.
Resources: Junli Wan.
Software: Junli Wan.
Writing – review & editing: Liping Tan, Shaojun Li, Qiu Li.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, FRNS = frequently-relapsing nephrotic syndrome, INS = idiopathic nephrotic syndrome, MMF = mycophenolate mofetil, OR = odds ratio, P/NT = placebo/nontreatment, SDNS = steroid-dependent nephrotic syndrome, SUCRA = surface under the cumulative ranking curve.
Funding: This research was supported by the projects of basic and frontier research, Chongqing Science and Technology Commission (Fund number: cstc2014jcyjA10032).
Contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission. LT and QL conceived and designed the study. SL and LT wrote the protocol. HY designed and implemented the search strategies. LT and QZ selected studies, assessed validity, and extracted data. JW entered and analyzed the data. All authors interpreted the data, prepared the full review and contributed to its revision, interpretation of results, and approval.
The authors declare that they have no conflict of interests.
Supplemental Digital Content is available for this article.
References
- [1].Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child 1982;57:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 1997;8:769–76. [DOI] [PubMed] [Google Scholar]
- [3].Listed N. Effect of cytotoxic drugs in frequently relapsing nephrotic syndrome with and without steroid dependence. N Engl J Med 1982;306:451–4. [DOI] [PubMed] [Google Scholar]
- [4].Habashy D, Hodson E, Craig J. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev 2004;Cd003594. [DOI] [PubMed] [Google Scholar]
- [5].Kaku Y, Ohtsuka Y, Komatsu Y, et al. Clinical practice guideline for pediatric idiopathic nephrotic syndrome 2013: general therapy. Clin Exp Nephrol 2015;19:34. [DOI] [PubMed] [Google Scholar]
- [6].Zhao Z, Liao G, Li Y, Zhou S, Zou H. The efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome: a meta-analysis. Sci Rep 2015;5:8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Datab System Rev 2013;10:CD002290. [DOI] [PubMed] [Google Scholar]
- [8].Hodson EM, Willis NS, Craig JC. Non-corticosteroid treatment for nephrotic syndrome in children. The Cochrane Library; 2008. [DOI] [PubMed] [Google Scholar]
- [9].Sun Q, Shen Y. A meta-analysis on the effect of cyclophosphamide in treatment of nephrotic syndrome in children. Zhonghua Er Ke Za Zhi Chin J Pediatr 2006;44:199–201. [PubMed] [Google Scholar]
- [10].Metz DK, Kausman JY. Childhood nephrotic syndrome in the 21st century: what's new? J Paediatr Child Health 2014;51:497–504. [DOI] [PubMed] [Google Scholar]
- [11].Peco-Antić A. Management of idiopathic nephrotic syndrome in childhood. Srp Arh Celok Lek 2004;132:352–9. [DOI] [PubMed] [Google Scholar]
- [12].Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- [13].Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of, diagnosis. A report of the International Study of Kidney Disease in, Children. Kidney Int 1978;13:159–65. [DOI] [PubMed] [Google Scholar]
- [14].Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J 2008;8:493–519. [Google Scholar]
- [15].White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J 2011;11:255–70. [Google Scholar]
- [16].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [17].Jackson D, Barrett JK, Rice S, et al. A design-bytreatment interaction model for network meta-analysis with random inconsistency effects. Stat Med 2014;33:3639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol Jasn 2015;26:2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Iijima K, Sako M, Nozu K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014;384:1273. [DOI] [PubMed] [Google Scholar]
- [21].Gellermann J, Weber L, Pape L, et al. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol 2013;24:1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 2011;6:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, et al. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 2008;23:2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abeyagunawardena A. Intravenous pulsed cyclophophamide versus vincristine therapy in steroid dependant nephrotic syndrome: a randomised controlled trial. Paper presented at Pediatric Nephrology; 2007. [Google Scholar]
- [25].Al-Saran K, Mirza K, Al-Ghanam G, Abdelkarim M. Experience with levamisole in frequently relapsing, steroid-dependent nephrotic syndrome. Pediatr Nephrol 2006;21:201–5. [DOI] [PubMed] [Google Scholar]
- [26].Abeyagunawardena A, Trompeter R. Efficacy of Levamisole as a single agent in maintaining remission in steroid dependant nephrotic syndrome. Pediatr Nephrol 2006;21:1503. [DOI] [PubMed] [Google Scholar]
- [27].Donia AF, Ammar HM, El-Agroudy EB, Moustafa EH, Sobh AK. Long-term results of two unconventional agents in steroid-dependent nephrotic children. Pediatr Nephrol 2005;20:1420–5. [DOI] [PubMed] [Google Scholar]
- [28].Sural S, Pahari D, Mitra K, Bhattacharya S, Mondal S, Taraphder A. Efficacy of levamisole compared to cyclophosphamide and steroid in frequently relapsing (FR) minimal change nephrotic syndrome (MCNS). J Am Soc Nephrol 2001;12:126A. [Google Scholar]
- [29].Rashid HU, Ahmed S, Fatima N, Khanam A. Levamisole in the treatment of steroid dependent or frequent relapsing nephrotic syndrome in children 1996. [Google Scholar]
- [30].Dayal U, Dayal AK, Shastry JC, Raghupathy P. Use of levamisole in maintaining remission in steroid-sensitive nephrotic syndrome in children 1994;66:408–12. [DOI] [PubMed] [Google Scholar]
- [31].Weiss R. Randomized double-blind, placebo (p) controlled trial of levamisole (l) for children (ch) with frequently relapsing/steroid dependant (fr/sd) nephrotic syndrome (ns). [abstract]; 1993. [Google Scholar]
- [32].Ponticelli C, Edefonti A, Ghio L, et al. Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol Dial Transplant 1993;8:1326–32. [PubMed] [Google Scholar]
- [33].Niaudet P. Comparison of cyclosporin and chlorambucil in the treatment of steroid-dependent idiopathic nephrotic syndrome: a multicentre randomized controlled trial. French Soc Paediatr Nephrol Pediatr Nephrol 1992;6:1–3. [DOI] [PubMed] [Google Scholar]
- [34].Nephrology BAFP. Levamisole for corticosteroid-dependent nephrotic syndrome in childhood. Lancet 1991;337:1555. [PubMed] [Google Scholar]
- [35].Alatas H, Wirya IG, Tambunan T, et al. Controlled trial of chlorambucil in frequently relapsing nephrotic syndrome in children (a preliminary report). J Med Assoc Thai 1978;61Suppl 1:222–8. [PubMed] [Google Scholar]
- [36].Barratt TM, Cameron JS, Chantler C, et al. Controlled trial of azathioprine in treatment of steroid-responsive nephrotic syndrome of childhood. Arch Dis Child 1977;52:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grupe WE, Makker SP, Ingelfinger JR. Chlorambucil treatment of frequently relapsing nephrotic syndrome. N Engl J Med 1976;295:746–9. [DOI] [PubMed] [Google Scholar]
- [38].Lancet T. Prospective controlled trial of cyclophosphamide therapy in children with the nephrotic syndrome. Lancet 1974;304:423–7. [PubMed] [Google Scholar]
- [39].Chiu J, Mclaine PN, Drummond KN. A controlled prospective study of cyclophosphamide in relapsing, corticosteroid-responsive, minimal-lesion nephrotic syndrome in childhood. J Pediatr 1973;82:607–13. [DOI] [PubMed] [Google Scholar]
- [40].Barratt TM, Soothill JF. Controlled trial of cyclophosphamide in steroid-sensitive relapsing nephrotic syndrome of childhood. Lancet 1970;2:479–82. [DOI] [PubMed] [Google Scholar]
- [41].Abramowicz M, Barnett HL, Edelmann CM JJr, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1:959–61. [DOI] [PubMed] [Google Scholar]
- [42].Sinha A, Puraswani M, Kalaivani M, et al. Efficacy and safety of mycophenolate mofetil versus levamisole in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Kidney Int 2019;95:210–8. [DOI] [PubMed] [Google Scholar]
- [43].Gruppen MP, Bouts AH, Jansen-van der Weide MC, et al. A randomized clinical trial indicates that levamisole increases the time to relapse in children with steroid-sensitive idiopathic nephrotic syndrome. Kidney Int 2018;93:510–8. [DOI] [PubMed] [Google Scholar]
- [44].Melocoton TL, Kamil ES, Cohen AH, et al. Long-term cyclosporine A treatment of steroid-resistant and steroid-dependent nephrotic syndrome. Am J Kidney Dis 1991;18:583–8. [DOI] [PubMed] [Google Scholar]
- [45].Latta K, Von SC, Ehrich JH. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 2001;16:271–82. [DOI] [PubMed] [Google Scholar]
- [46].Karin H-M, Peter J, Christoph J, et al. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 2002;287:2801–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


