Abstract
Iron status, body mass index (BMI) and blood pressure (BP) are all important health indicators. In this study, ferritin and transferrin saturation levels and their correlations with BMI and BP were investigated in first-time and regular male blood donors in Taiwan. Serum ferritin and transferrin saturation values represented iron status of blood donors. Serum ferritin, serum iron, and total iron binding capacity (TIBC) were determined by chemiluminescent immunoassay sandwich method, timed-endpoint method, and turbidimetric method, respectively. Transferrin saturation was calculated as 100× serum iron/TIBC. Statistical analyses included 2-sample t test, chi-square test, Pearson correlation coefficient, and multiple linear regression. Comparisons of ferritin and transferrin saturation mean values with BMI, age, systolic blood pressure (SBP), diastolic blood pressure (DBP), and occupation were conducted. A total of 111 first-time donors and 1249 regular blood donors participated in this study. The ferritin and transferrin saturation mean values of regular male blood donors were lower than those of first-time male blood donors, but remained within the safe range. BMI was positively correlated with serum log ferritin, but not with transferrin saturation value in first-time and regular blood donors. First-time donors with BMI ≥24 kg/m2 and aged more than 40 years demonstrated 1.37-fold higher serum ferritin on average. Among regular donors, significant effects of BMI ≥24 kg/m2 and age >40 years were observed with 1.25- and 1.18-fold higher serum ferritin levels, respectively. First-time donors with SBP ≥120/DBP ≥80, ≥120/<80, and <120/≥80 mm Hg had on average 1.65-, 1.54-, and 2.59-fold higher serum ferritin levels than those with normal BP. Ferritin level was higher in BMI ≥24 kg/m2 subgroup than in BMI <24 kg/m2 subgroup among first time and regular male donors, but no difference was found in transferrin saturation values.
Abnormal SBP/DBP was associated with increased ferritin level only in first-time male blood donors.
Keywords: blood pressure (BP); body mass index (BMI); ferritin, transferrin saturation; male blood donors
1. Introduction
The blood donation rate in Taiwan is about 7.5%, which is relatively high when compared with other parts of the world. In addition, 86.12% of donors are repeat donors. Males donate blood more frequently than females. In many countries, blood donation rates range from 0.3 to 56 per 1000 persons[1] and the rate of repeat blood donation is about 84%.[2] In the Netherlands; however, the rate of successful repeat donations is about 95%.[3] There are different selection criteria for male blood donors in different countries. For example, hemoglobin (Hb) value of at least 13.0 g/dL is the criterion for male blood donors in Taiwan. In the UK, the minimum predonation Hb value is 13.5 g/dL for males. Such criterion is intended to prevent collection of blood from donors with anemia. For every 450 mL of whole blood donated there is a loss of approximately 200 mg of iron. Hb value does not ensure that the donor has an adequate store of iron; however. Serum ferritin level indicates total iron stores, while transferrin saturation value reflects iron transportation, which decreases before anemia develops. In this study, ferritin and transferrin saturation were used to evaluate iron status. Frequent blood donors have been shown to be at risk of iron deficiency (ID).[4,5] Iron status in blood donors is an important issue, as iron participates in a variety of metabolic processes and is essential to oxygen transport, deoxyribonucleic acid (DNA) synthesis and electron transport.
Many studies have reported an association between iron status and body mass index (BMI) in adults, as well as children and adolescents. Based on the findings of a previous study, obesity is significantly associated with ID on meta-analysis.[6] From the American National Health and Nutrition Examination Survey III, among children from transition countries (Morocco and India), prevalence of ID increases as BMI increases from normal weight to obesity.[7,8] Studies on adult populations have shown conflicting results for the link between high BMI and ID.[9–11]
It has been reported that serum ferritin and transferrin saturation levels are higher among males with hypertension than among a corresponding group of females.[12] However, the relationship between blood pressure (BP) and iron status has not been well established.
The relationships between serum ferritin level and BMI and serum ferritin level and BP are poorly described in healthy men eligible to donate blood. Ferritin is an acute-phase protein that plays a major role in storing iron in the human body. No relationship has been reported between BP and ferritin turn-over rate in nondonor volunteer and long-term blood donors.[13] The association between BP and ferritin level varies among subjects.
Therefore, the objective of this study is to investigate the iron status in first-time and regular male blood donors in Taiwan. In this study, BMI was stratified into 18.5≤ BMI <24 kg/m2 and >24 kg/m2. The associations of BMI with serum ferritin and transferrin saturation levels in first-time and regular male blood donors in Taiwan were investigated. The associations of normal BP (SBP <120/DBP <80 mm Hg) and abnormal SBP/DBP with serum ferritin and transferrin saturation levels were also analyzed in the same subjects.
2. Methods
2.1. Study design and participants
All of the participants in this study were eligible to donate blood and were recruited by the Taichung Blood Center, located in central Taiwan. The population of the Taichung Blood Center's service area is about 4.5 million with approximately 350,000 blood donations received each year. The criteria for blood donors are in accordance with Taiwan's Ministry of Health and Welfare guidelines. In this study, we stratified BMI and BP and examined the ferritin and transferrin saturation values in different subgroups to understand the relationships among these variables in first-time and regular male blood donors.
Donor selection was based on blood donor registration form, which included demographic information such as height, weight, date of birth and occupation. The health status questionnaire included present and past medical/surgical history, recent infections, and drug taking history. Interview regarding lifestyle and habits and a limited physical examination were also conducted. Those taking aspirin or anti-inflammatory drugs were not allowed to donate blood. Moreover, these drugs can influence ferritin level. Other exclusion criteria for donating blood included alcoholism, bleeding disorders, acute or chronic inflammatory conditions such as systemic lupus erythematosus, ankylosing spondylitis or any acute illness or chronic infection. As females may be affected by menstrual blood loss and child-bearing, we only recruited male blood donors aged between 17 and 65. In Taiwan, the legal adult age is defined as 20. Therefore, in this study, we enrolled participants aged 20 to 65. BP was defined as systolic value of 90 to 160 mm Hg and diastolic value of 50 to 95 mm Hg. Hb value of 13 g/dL or higher and weight of 50 kg or more were required for males to provide whole blood donation. There are 2 volumes of whole blood donations, 250 mL and 500 mL. The intervals between blood donations are 2 months for 250 mL and 3 months for 500 mL. The maximum amount of whole blood donation, hereinafter referred to as maximum donation, by male donors is 1500 mL per annum, adjusted by date of birth. This criterion was established to prevent imbalance in blood donation.
2.2. Enrollment
First-time donors were defined as those who had never donated blood. Regular donors were defined as those giving the maximum donation over 1 to 5 years.
In this study, 111 first-time donors were recruited on site. Regular donors mainly visited fixed donation sites and received invitations by mail. Of the 3289 regular blood donors invited by mail, 1249 agreed to enroll in the study. All participants reviewed and signed an informed consent form. Ethics approval was obtained from the Ethical Review Board of the Taiwan Blood Services Foundation (PM-102-TC-119, PM-104-TC-148).
2.3. Stratifications of BMI, BP, and occupation
BMI is defined as body mass divided by the square of body height, and is universally expressed in units of kg/m2. Based on BMI, participants were stratified into 2 groups, normal weight (18.5≤ BMI <24 kg/m2) and overweight >24 kg/m2. This definition followed that of the Health Promotion Administration, Ministry of Health and Welfare in Taiwan. SBP lower than 120 mm Hg and DBP lower than 80 mm Hg were defined as normal BP based on the guidelines of the American Heart Association. In this study, we stratified participants into 2 groups, SBP lower than 120 mm Hg and SBP equal to or higher than 120 mm Hg. For DBP, participants were also stratified into 2 groups: lower than 80 mm Hg and equal to or higher than 80 mm Hg.
Middle-age was defined as 40 to 65 years. Participants were stratified by age into less than 40 years and greater than or equal to 40 years.
Participants had different occupations such as civil servant, teacher, student, businessman, technician, and laborer. Laborers tend to do more physical work than those in other occupational categories. For this reason, we stratified participants into laborer and nonlaborer groups.
2.4. Laboratory testing
Before donation and enrollment, Hb screening was carried out using copper sulfate. Copper sulfate specific gravity of 1.054 corresponds to Hb concentration of 13 g/dL. The donor was retested and permitted to donate when one of the measurements was within the acceptable limit, if BP was observed to exceed the acceptable limit upon initial measurement.
A 6-mL venous blood sample was collected from the diversion for measurement of serum ferritin, serum iron, and TIBC. After collection of blood into SST test tube, it was centrifuged at 2000×G for 10 minutes. Clear serum was separated and stored in Eppendorf at −80°C until assayed. Serum iron (ug/dL) was analyzed by timed-endpoint method (kit Ref 467910 supplied by Beckman Coulter, Inc, 250 S. Kraemer Blvd., Brea, CA 92821). Serum TIBC (ug/dL) was calculated as serum transferrin using the formula TIBC = serum transferrin × 1.4. Serum transferrin (mg/dL) was determined by turbidimetric method (kit Ref 467942 supplied by Beckman Coulter, Inc). Serum ferritin (ng/mL) was determined by chemiluminescent immunoassay sandwich method (kit Ref 33020 supplied by Beckman Coulter, Inc). Transferrin saturation was calculated as 100× serum iron/TIBC. From the results of a previous study, a ferritin level of less than 12 ng/mL indicates absent iron stores.[7,14] Moreover, a ferritin level of less than 25 ng/mL represents low ferritin.[8,15] In our study, safe levels were defined as serum ferritin level higher than 23.9 ng/mL (male ferritin reference interval: 23.9–336.2 ng/mL) and transferrin saturation higher than 20%, respectively.
2.5. Statistical analysis
Demographic and clinical characteristics of study subjects including age, BMI, SBP, DBP, occupation, ferritin, serum iron, TIBC, and transferrin saturation were examined, with continuous variables reported as mean ± standard deviation and categorical variables reported as number and percentage. The 2-sample t test for continuous variables and the chi-square test for categorical variables was used for bivariate analysis. As distribution of ferritin levels was skewed to the right, natural log-transformation was used to normalize data for analysis. Pearson correlation coefficient was applied to determine the strength of relationships between 2 continuous variables. Furthermore, linear regression analysis with and without (crude model) adjustment was used to examine whether BMI is associated with serum ferritin on natural log-transformation scale. Backward elimination procedure for selected predictors reached significance at 0.05. Estimated coefficient of regression model (β), standard error, and exponential transformation for estimated coefficient were reported. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, NC). A P-value less than .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics of blood donors
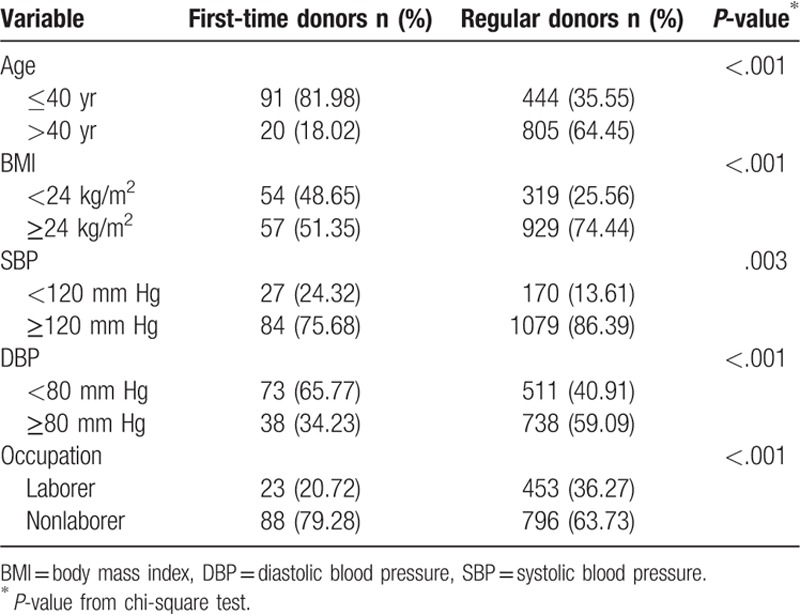
A total of 1360 male blood donors, including 111 first-time and 1249 regular blood donors, were enrolled in this study. The average age was 30.8 ± 10.7 years for first-time blood donors and 44.9 ± 10.4 years for regular blood donors (Table 1). Compared with first-time blood donors, regular blood donors had higher mean BMI (26.1 vs 24.6 kg/m2), SBP (134.4 vs 128.4 mm Hg), DBP (80.8 vs 75.4 mm Hg), and TIBC (406.6 vs 346.3 μg/dL). However, regular donors had lower serum ferritin (35.7 vs 217.9 ng/mL), serum iron (104.6 vs 125.6 μg/dL), and transferrin saturation (26.2% vs 36.5%) levels. Moreover, higher proportions of regular donors had abnormal SBP and DBP and were overweight when compared with first-time donors (SBP ≥120 mm Hg: 86.39% vs 75.68%; DBP ≥80 mm Hg: 59.09% vs 34.23%; BMI ≥24 kg/m2: 74.44% vs 51.35%) (Table 2). These differences between first-time and regular donors were all statistically significant.
Table 1.
Mean biomarkers of the study subjects.
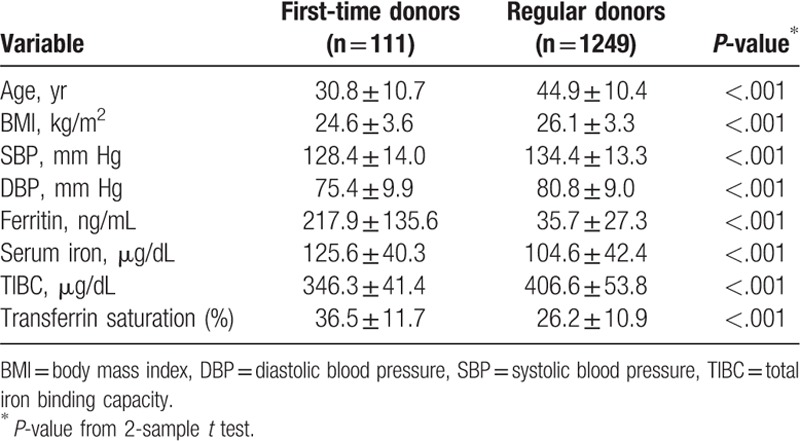
Table 2.
Characteristics of study subjects.
3.2. Relationship between BMI and serum ferritin
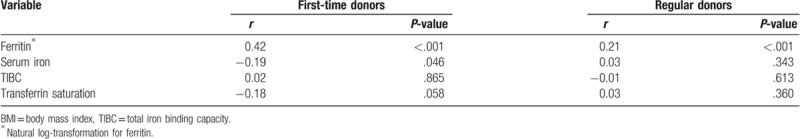
Two scatter plots of BMI and serum ferritin for first-time and regular blood donors are shown in Figure 1. Pearson correlation coefficients for BMI and ferritin on natural log-transformation scale indicated moderately positive correlation for first-time blood donors (r = 0.42, P < .001) and weakly positive correlation for regular blood donors (r = 0.21, <.001) (Table 3).
Figure 1.
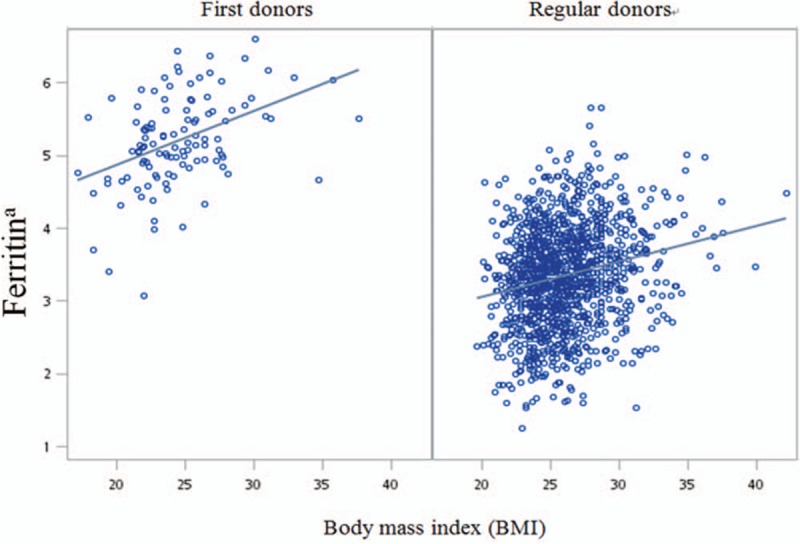
Correlations between BMI and ferritina in first-time and regular blood donors on 2-way scatter diagram. a: natural log-transformation. BMI = body mass index.
Table 3.
Pearson correlation coefficients for comparisons of BMI and biomarkers.
3.3. Univariate and multiple variate analyses of ferritin level associated with BMI, BP, and age
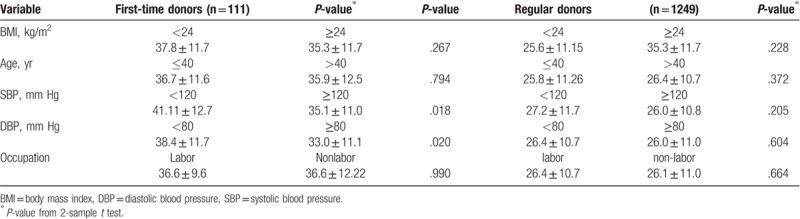
Mean serum ferritin levels were compared among BMI, age, SBP, DBP, and occupation subgroups, as shown in Table 4. Mean ferritin level was significantly higher in BMI ≥24 kg/m2 group than in BMI <24 kg/m2 group in first-time and regular blood donors, 264.8 ± 152.1 versus 168.3 ± 94.2 ng/mL, 37.9 ± 29.2 versus 29.1 ± 19.2 ng/mL, respectively. There were no significant differences in transferrin saturation among the BMI subgroups for first-time or regular blood donors (Table 5).
Table 4.
Comparisons of mean ferritin levels with BMI, age, SBP, DBP, and occupation.
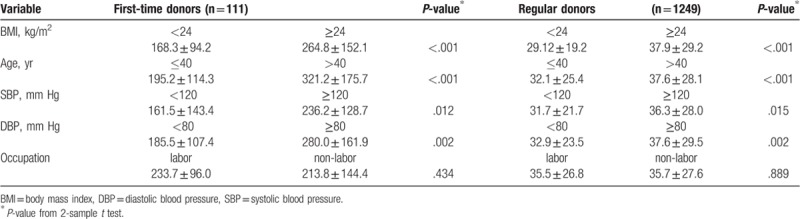
Table 5.
Comparisons of mean transferrin saturation levels with BMI, age, SBP, DBP, and occupation.
Linear regression analysis was performed to examine the relationships of log ferritin with BMI, age, and BP (Table 6). Multiple regressions of serum ferritin with natural log-transformation scale using backward elimination procedure showed that serum ferritin is associated with BMI, age, and BP in first-time blood donors. Compared to first-time donors with BMI <24 kg/m2 or aged ≤40 years old, first-time donors with BMI ≥24 kg/m2 or aged more than 40 years had an average 1.37-fold higher serum ferritin level. Among regular blood donors, there were significant differences in ferritin levels associated with BMI and age. Moreover, significant effects of BMI ≥24 kg/m2 and age >40 years were observed with 1.25- and 1.18-fold higher levels of serum ferritin, respectively, in regular blood donors.
Table 6.
Fold of ferritin∗ associated with BMI, age, BP, and occupation of first-time and regular blood donors using simple linear regression and multiple linear regression.
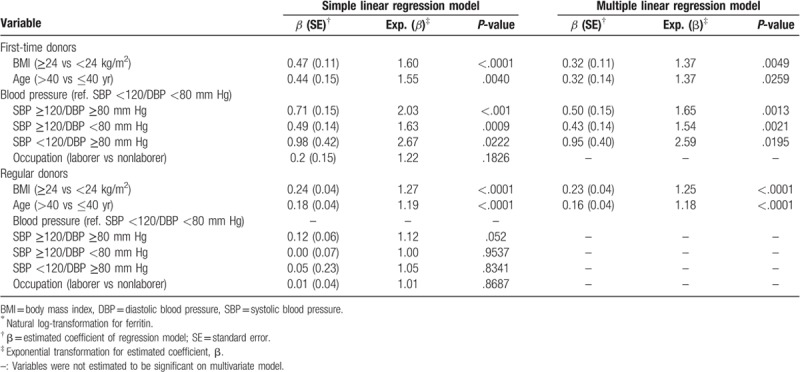
First-time donors with SBP ≥120/DBP ≥80, ≥120/<80, and <120/≥80 mm Hg had 1.65-, 1.54-, and 2.59-fold higher on average serum ferritin levels than those with normal BP of <120/<80 mm Hg, respectively. Serum ferritin was not found to be associated with BP in regular blood donors.
In addition, we examined the association between BMI and transferrin saturation using multiple regression with backward elimination procedure. We found no significant association between BMI and transferrin saturation among first-time or regular blood donors (data not shown).
4. Discussion
Three important independent effects of blood donors were described in this study: iron status, BMI, and BP.
Regular male blood donors had a lower ferritin level than first-time male donors, but this level was still within the safe range. From the results of a previous study,[16] among first-time donors, mean ferritin value is 43 ng/mL and drops to 29 ng/mL at the fifth donation. Median values are stable, ranging between 20 and 30 ng/mL among those donating blood for over 14 years. This indicates that risk of ID does not increase in high-frequency donors. From the results of this study, the mean ferritin levels were 217 ng/mL and 35.66 ng/mL in first-time and regular donors, respectively, which were higher than the values reported in O’Meara et al's study. This discrepancy may be due to exclusion of female donors. Lower reference ranges for Hb and ferritin have been reported for women of reproductive age when compared with equivalent-aged males.[17] In addition, as iron status is significantly influenced by diet, differences in diet may also have led to this discrepancy. Our data showed that ID risk does not increase among regular donors. We also found that maintaining and improving health are related to blood donation behavior. Repeat donors may pay more attention to their diet and lifestyle than lapsed donors. Moreover, donors must maintain their iron stores, otherwise, they may not be eligible to donate blood. Donors with perceived good health status tend to donate blood more often than donors with perceived poor health status.[18]
In this study, BMI and serum log ferritin showed moderately positive correlation for first-time blood donors (r = 0.42, P < .001) and weakly positive correlation for regular blood donors (r = 0.21, <.001). However, there was no significant correlation between transferrin saturation and BMI. Our findings are inconsistent with those of a previous study[19] in which BMI had a strongly positive correlation (r = 0.86, P < .001) but strongly negative correlation with transferrin saturation (P < .001).
In this study, we presented 2 strategies for excluding the risk of ferritin induction due to inflammatory condition. First, many factors that induce inflammatory conditions have been set as exclusion criteria on health status questionnaires. Second, for allogeneic use, each donation undergoes blood laboratory screening tests to safeguard patient health. These include Hepatitis B surface antigen, HBV DNA, Anti-HCV, HCV RNA, Anti-HIV, HIV RNA, anti-HTLV-I/II, syphilis and alanine aminotransferase. All previous laboratory screening tests must be normal for a donor to donate again. If one of the results is abnormal, the donor is deferred or rejected. As infection can cause inflammation, there are more inflammatory factors that are criteria for exclusion among regular donors than among first-time donors. The results of this study suggested that ferritin represents iron status rather than inflammation marker among regular male blood donors in Taiwan. BMI may be one of the factors influencing ferritin level. In addition, lipid storage and lipogenesis gene expression levels are associated with iron metabolism.[20] Therefore, higher BMI was associated with higher ferritin content in this study. Obesity (high BMI), one of the metabolism syndromes, was reportedly associated with nonalcoholic fatty liver disease (NAFLD), which is a major risk factor of hepatocellular carcinoma (HCC).[21] In the present study, high ferritin levels were noted in high BMI male blood donors. After blood donation, which is a kind of phlebotomy, ferritin levels decreased in these people. Iron has been widely implicated in the pathogenesis of NAFLD and represents a potential target for treatment.[22] Thus, we speculate that iron decreases after blood donation in high BMI people, which may decrease the risk of NAFLD, thereby decreasing HCC risk.
High-frequency blood donors are associated with decreased iron stores, decreased oxidative stress, and improved vascular function when compared with low-frequency donors.[23] Over time, iron overload results in excess iron accumulation in organs, which may cause liver failure, diabetes, and heart failure.[24] The results of this study showed reduced mean ferritin in regular male blood donors and an association between abnormal SBP/DBP and increased ferritin level in first-time male blood donors. A number of previous studies have revealed that cardiovascular risk factors are correlated with body iron stores. Our findings support the beneficial effects of regular blood donations as abnormal SBP/DBP is associated with increased ferritin level only in first-time male blood donors.
Our study has several limitations. First, Hb screening for blood donors was based only on copper sulfate to determine if potential donors meet the criterion to donate. We do not have quantitative fingerstick Hb or hematocrit data. The reason is that the Ethical Review Board of the Taiwan Blood Services Foundation approved the taking of only 6 mL of blood in SST tube to protect the health of blood donors. From each blood donation, 32 mL blood sample is required for laboratory screening tests and archive sample. To avoid too much blood loss, only 6 mL blood of blood was collected in SST tube, which was not enough for Hb quantitative test. Second, smoking causes significant reduction in vitamin C in the body and influences the absorption of iron. In this study, we did not ask participants whether they smoked or not, as this item was not included in the health status questionnaire.
In conclusion, the regular male blood donors demonstrated decreased ferritin and transferrin saturation levels. Based on the criteria for blood donor eligibility in Taiwan, the mean values of ferritin and transferrin saturation are still within the safe range. There were differences in ferritin level between BMI ≥24 kg/m2 and BMI <24 kg/m2 subgroups both for first-time and regular male donors, but no difference was found in transferrin saturation values. Abnormal SBP/DBP was associated with increased ferritin level only in first-time male blood donors.
Acknowledgment
We would like to thank all of the participants who donated blood for this study. We would also like to thank Dr Chi-Ling Lin who provided medical information and Mr Kuo-Chou Chen who collected the data.
Author contributions
Conceptualization: Jiunn-Liang Ko.
Methodology: Hsuan-Hui Wang, Li-Na Liao, Ci-Wen Chang, Yu-Chang Chang, Kang-Hsi Wu.
Writing – original draft: Hsuan-Hui Wang.
Writing – review and editing: Kang-Hsi Wu, Jiunn-Liang Ko.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, DBP = diastolic blood pressure, DNA = deoxyribonucleic acid, Hb = hemoglobin, HCC = hepatocellular carcinoma, ID = iron deficiency, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, TIBC = total iron binding capacity.
J-LK and K-HW contributed equally to this study.
This research was supported by the Taiwan Blood Services Foundation (PM-102-TC-119, PM-104-TC-148) and the China Medical University Hospital (DMR-108-197).
The authors have no conflicts of interest to disclose.
References
- [1]. [Accessed October 7, 2018]. Organization, W.H., Global Status Report on Blood Safety and Availability 2016, 2017. ISBN 978-92-4-156543-1. File: “WHO. 2016-9789241565431-eng. pdf”. Available at: http://apps.who.int/iris/bitstream/10665/254987/1/9789241565431-eng.pdf. [Google Scholar]
- [2].Lattimore S, Wickenden C, Brailsford SR. Blood donors in England and North Wales: demography and patterns of donation. Transfusion 2015;55:91–9. [DOI] [PubMed] [Google Scholar]
- [3].Wiersum-Osselton JC, Marijt-van der Kreek T, Brand A, et al. Risk factors for complications in donors at first and repeat whole blood donation: a cohort study with assessment of the impact on donor return. Blood Transfus 2014;12Suppl 1:s28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shuchman M. Frequent blood donors risk iron deficiency. CMAJ 2014;186:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vuk T, Magnussen K, De Kort W, et al. International forum: an investigation of iron status in blood donors. Blood Transfus 2017;15:20–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao L, Zhang X, Shen Y, et al. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev 2015;16:1081–93. [DOI] [PubMed] [Google Scholar]
- [7].Nead KG, Halterman JS, Kaczorowski JM, et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 2004;114:104–8. [DOI] [PubMed] [Google Scholar]
- [8].del Giudice EM, Santoro N, Amato A, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab 2009;94:5102–7. [DOI] [PubMed] [Google Scholar]
- [9].Sanchez A, Rojas P, Basfi-Fer K, et al. Micronutrient deficiencies in morbidly obese women prior to bariatric surgery. Obes Surg 2016;26:361–8. [DOI] [PubMed] [Google Scholar]
- [10].Cheng HL, Bryant C, Cook R, et al. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev 2012;13:150–61. [DOI] [PubMed] [Google Scholar]
- [11].Ozata M, Mergen M, Oktenli C, et al. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem 2002;35:627–31. [DOI] [PubMed] [Google Scholar]
- [12].Li B, Lin W, Lin N, et al. Study of the correlation between serum ferritin levels and the aggregation of metabolic disorders in non-diabetic elderly patients. Exp Ther Med 2014;7:1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Risko P, Pláteník J, Buchal R, et al. Long-term donors versus non-donor men: iron metabolism and the atherosclerotic process. Atherosclerosis 2018;272:14–20. [DOI] [PubMed] [Google Scholar]
- [14].Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II donor iron status evaluation (RISE) study. Transfusion 2012;52:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goldman M, Uzicanin S, Osmond L, et al. A large national study of ferritin testing in Canadian blood donors. Transfusion 2017;57:564–70. [DOI] [PubMed] [Google Scholar]
- [16].O’Meara A, Infanti L, Stebler C, et al. The value of routine ferritin measurement in blood donors. Transfusion 2011;51:2183–8. [DOI] [PubMed] [Google Scholar]
- [17].Rushton DH, Dover R, Sainsbury AW, et al. Why should women have lower reference limits for haemoglobin and ferritin concentrations than men? BMJ 2001;322:1355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van den Hurk K, Zalpuri S, Prinsze FJ, et al. Associations of health status with subsequent blood donor behaviour – an alternative perspective on the Healthy Donor Effect from Donor InSight. PLoS One 2017;12:e0186662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khan A, Khan WM, Ayub M, et al. Ferritin is a marker of inflammation rather than iron deficiency in overweight and obese people. J Obes 2016;2016:1937320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Segrestin B, Moreno-Navarrete JM, Seyssel K, et al. Adipose tissue expansion by overfeeding healthy men alters iron gene expression. J Clin Endocrinol Metab 2019;104:688–96. [DOI] [PubMed] [Google Scholar]
- [21].Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol 2014;20:9217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Britton LJ, Subramaniam VN, Crawford DH. Iron and non-alcoholic fatty liver disease. World J Gastroenterol 2016;22:8112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng H, Patel M, Cable R, et al. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol 2005;25:1577–83. [DOI] [PubMed] [Google Scholar]
- [24].Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


