Abstract
Introduction:
Intrapancreatic accessory spleen (IPAS) has been rarely noted radiologically because the spatial resolution of conventional images was low. The infrequent presence of the accessory spleen in the pancreatic tissue could lead to inappropriate diagnosis, thereby necessitating a therapeutic approach. The present study reported such cases and summarized the available imaging findings to reduce unnecessary invasive surgeries.
Patient concerns:
The patient's complaint was “a pancreatic mass was found for half a month.”
Diagnosis:
IPAS was eventually diagnosed by pathology.
Interventions:
Laparoscopic spleen-preserving pancreatic resection.
Outcomes:
Postoperative course was uneventful and the patient was discharged from our hospital after 10 days.
Conclusions:
When an asymptomatic pancreatic mass is detected, the diagnosis of IPAS should not be excluded, especially if the lesion has the same imaging features as the spleen. As a definite diagnosis of IPAS is difficult by a single examination, multiple techniques might be essential.
Keywords: image, intrapancreatic accessory spleen, laparoscopic
1. Introduction
Accessory spleen (AS) is a congenital anomaly in which splenic tissue is found outside the spleen. This anomaly typically occurs in the fifth week of intrauterine fetal development.[1] Accessory spleens occur in about 10% of normal population and can also be found ubiquitously in the abdominal cavity. In a majority of the cases, it was localized in the splenic hilum, gastrosplenic ligament, and greater momentum. The less frequent localizations include gastric wall, small intestine, mesenterium, pancreas, ovary, and testicle.[2] The intrapancreatic accessory spleens (IPASs) are uncommon with a prevalence rate of 11% to 17% for all ASs, which were localized in the pancreatic tail according to the autopsy studies.[3] The AS was reported in approximately 10% to 20% of the individuals,[1] with a prevalence of IPAS between 1.1% and 3.4%. It has been estimated that around 75 million people were affected by IPAS worlwide.[3] As it is a benign entity, associated with seldom symptoms, IPAS does not require any treatment, unless it is accompanied by idiopathic thrombocytopenic purpura or symptoms owing to compression, torsion, and spontaneous rupture of hemorrhage.[4] The previously reported cases with IPASs were inaccurately diagnosed with primary pancreatic tumors, such as pancreatic neuroendocrine tumor (PNET), solid pseudopapillary tumor, pancreatic ductal adenocarcinoma, or hypervascular metastases after unnecessary sugery.[5] Therefore, an accurate preoperative diagnosis is essential to avoid unnecessary surgeries (Figures 1–3).
Figure 1.
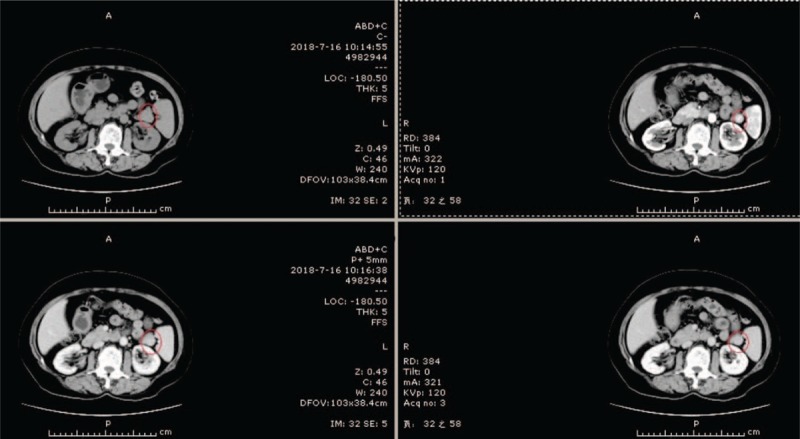
Enhanced CT imaging of pancreatic tumor.
Figure 3.
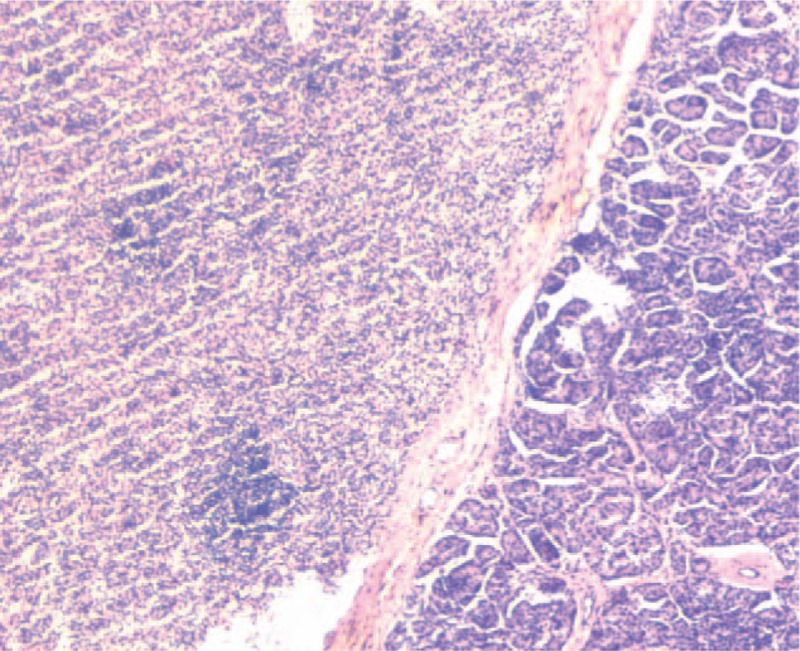
Histopathological section (hematoxylin and eosin stain, ×40) shows the interface between pancreatic and splenic tissues.
Figure 2.
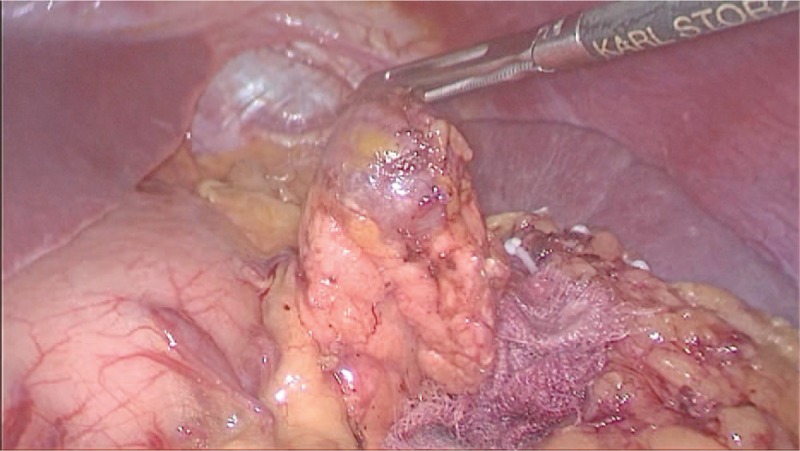
During laparoscopic surgery, the tumors were localized at the tail of the pancreas with clear margins and similar color to the spleen.
2. Case report
A 62-year-old female was admitted to our Hospital with a mass lesion on the pancreatic tail that was detected by computed tomography (CT) during a health check-up owing to liver cirrhosis. Any history of trauma or pancreatitis was not recorded. Laparoscopic cholecystectomy and appendectomy were performed 5 years ago. Also, cases of hepatitis B for 30 years and liver cirrhosis for 5 years were recorded. The initial laboratory data did not show any abnormalities, including tumor markers and hormone assays, such as carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), or carbohydrate antigen 19-9 (CA19-9). Because the patient had recently undergone endoscopic gastric polypectomy and was not able to undergo abdominal magnetic resonance imaging (MRI), the abdominal multiphase enhanced CT was performed. It revealed a well-defined cystic neoplasm of 14 × 15 mm2 localized in the tail of the pancreas, associated with a mild enhancement in the arterial phase (AP), the portal phase, and the delayed phase. Simultaneously, no pancreatic mass was detected by abdominal ultrasonography during cholecystectomy 5 years ago, and also, no pancreatic mass was found by abdominal ultrasonography when checked every 6 months after cholecystectomy. Therefore, the lesion was interpreted as a nonfunctioning PNET for making the decision for surgical procedure. The patient underwent a laparoscopic spleen-preserving pancreatic resection. Macroscopically, the lesion was oval, encapsulated, node-like, dark reddish-brown, 17 × 15 × 11 mm3 in size, and resembled that of the splenic tissue. Histopathological analysis revealed the true nature of the lesion, and IPAS was diagnosed. The postoperative course was uneventful, and the patient was discharged from our hospital after 10 days. Three months after surgery, the patient was disease-free.
3. Ethics approval
The study was approved by the Ethics Committee, and the ethical approval number was 2019013. Written informed consent was obtained from the patient for utilizing the details and images for research purposes.
4. Discussion
The ectopic spleen can be divided into 2 types: AS results from congenital fusion failure of splenic anlages[1] and splenosis that is the benign acquired condition of heterotopic autotransplantation of splenic tissue in another anatomical compartment of the body after splenic rupture.[6] AS is typically localized around the embryonic origin or along the migration path,[7] whereas splenosis can be found anywhere in the abdomen, pelvis, and chest.[6] In autopsy studies,[2,8] approximately 10% of the patients had >1 AS, whereas all the IPASs in the present study were solitary cases.
This patient had the following characteristics. First, early repeated color Doppler ultrasound did not show any lesions, which were related to small lesions and the deep location of the pancreas and intestinal gas. Second, the patient presented cirrhosis. IPAS is more common in liver cirrhosis. Third, the enhancement mode of CT in this case was atypical, but consistent with the enhancement mode of the spleen. Four, laparoscopic spleen-preserving pancreatic resection was carried out. The tumors were dark red during operation, and local excision was safe and reliable.
Hitherto, a total of 86 articles and 122 cases of IPAS were published in the English language.[9–14] In 2018, Li et al [9] reported that 87% of the cases were diagnosed with nonfunctioning PNET. The splenic parenchyma consisted of red and white pulp. The red pulp contains several splenic blood sinuses, and white pulp consists of lymphoid follicles and reticular epithelial cells between the splenic blood sinuses. The different proportions of red and white pulps in IPAS are the anatomical basis of modified imaging.[15] Herein, we summarized the published images to date for an improved diagnosis of IPAS:
-
1.
CT scanning: It is mainly similar to that of the spleen, but higher than that of the pancreas. The first characteristic is that AS tissue tends to exhibit similar attenuation on both non-contrast and post-contrast scans.[15] The second feature is a heterogeneous enhancement pattern during AP.[16] Notably, a heterogeneous enhancement pattern might not be detected on AP,[16] especially when the IPAS is limited. The addition of CTA to routine CT might be helpful by demonstrating the inhomogeneous enhancement in small lesions[11] because of the precise time control and excellent spatial resolution. The enhancement was not obvious in this case, but the time–density curve was consistent with that of splenic enhancement. In this case, the imaging examination showed that the tumors had no calcification, whereas PNET showed calcification, which was an aspect of differential diagnosis.
-
2.
MRI: Signal intensities (SI) of IPAS on MRI are identical to that of the spleen on multiple pulse sequences with dynamic gadolinium enhancement of the normal spleen. The SI of the intrapancreatic spleen was low in T1-weighted image (T1WI) and high in T2WI and diffusion-weighted imaging (DWI). Strikingly, high b-value (b = 600 s/mm2) images based on DWI technique provided greater soft tissue contrast than T2WI with respect to SI.[17] While conducting DWI using a high b-value, some endocrine neoplasms presented high SI and low apparent diffusion coefficient values,[18] whereas IPAS homogeneously exhibited high signals in DWI. The superparamagnetic iron oxide (SPIO)-enhanced MRI confirmed the diagnosis of IPAS by the loss of SI similar to the spleen and negative contrast enhancement, thereby presenting the advantage of better spatial resolution than scintigraphy.[15]
-
3.
Nuclear medicine: Technetium-99m-red blood cell scintigraphy is a highly specific method for detecting splenic tissue as up to 90% of the injected cases were trapped based on the splenic tissue. The drawback of this technique is a poor anatomic resolution as compared to the CT scanning and MRI.[15] No uptake was observed in neuroendocrine tumors or metastases using either of the two techniques.[19] However, similar to scintigraphy, single photon emission computed tomography (SPECT) offers an inferior spatial resolution than the cross-sectional imaging modalities, and a small IPAS typically has minimal functional splenic tissue that might achieve false-negative results. Therefore, scintigraphy and SPECT are frequently used in conjunction with other cross-sectional imaging modalities to diagnose and precisely localize an AS.[9]
-
4.
Positron emission tomography/diagnostic computed tomography (PET/CT): The 68Ga-DOTA-TOC is a highly appropriate method for the diagnosis of PNET owing to the critical expression of receptors of somatostatin on lymphocytes. Therefore, a physiological accumulation of 68Ga-DOTA-TOC is commonly observed in splenic tissue.[20] 18F-prostate-specific membrane antigen (PSMA) PET/CT scanning can detect the IPAS as it possesses several advantageous physical features. This includes a longer half-life and larger allowable mean injected activity. It also enables imaging at the later time points, making a higher clearance rate and less nonspecific binding, thereby causing high signal to noise ratios. In addition, 18F has lower mean positron emission energy, resulting in higher resolution and an accurate quantification.[12]
-
5.
Endoscopic ultrasonography (EUS): In the largest study of diagnosis of IPAS performed using EUS-guided fine-needle aspiration biopsy (EUS-FNA), the technique was successful in 90% of the cases, and no complications were reported.[21] The cytological features of the splenic tissue were characterized by small lymphocytes, associated with a mixed inflammatory infiltration with the appearance of the white pulp and in the presence of thin-walled blood vessels, which represented the splenic sinuses. Another predominant feature is endothelial cell is CD8- and CD31-positive in cell block sections.[22] This staining was highly specific, as systemic endothelial cells and hemangioma are negative.[23] Moreover, negative staining associates with chromogranin and low-molecular-weight cytokeratin against PNET.[24] Occasionally, a biopsy can inadvertently pick up the fragments of gastric mucosa or benign pancreatic islet cells, decreasing the specificity of the results.[23,25] In addition, FNA harbors the risk of severe complications, such as intra-abdominal bleeding and pancreatic fistula, and although it might not provide a representative tissue sample in every patient,[26] it is a second-line modality as compared to other nontraumatic techniques. Qualitative and quantitative EUS elastography might be useful to support the diagnosis of IPAS. Reportedly, the benign pancreatic masses have increased elasticity similar to the malignant lesions, thereby displaying an elastographic pattern.[27,28] In addition to the qualitative and quantitative EUS elastography, imaging follow-up may be appropriate when EUS-FNA cannot be performed.
Nevertheless, the present study has some limitations. First, PNET has the possibility of calcification[29]; however, the available reports did not show any calcification in IPAS. Second, IPAS was found to be gradually increased after splenectomy.[13,14] Therefore, whether lesions can be excised together as AS is yet to be investigated. Third, when IPAS was highly suspected intraoperatively, laparotomy, biopsy, or surgical resection needs to be decided.
Therefore, when an asymptomatic pancreatic mass is detected, IPAS should be diagnosed, especially if the lesion has the same imaging features as the spleen. The knowledge about specific multimodality imaging characteristics using EUS, CT scanning, MRI, PET/CT, and nuclear medicine examinations can prevent unnecessary biopsy and/or surgery.
Author contributions
Conceptualization: Jinming Chen.
Data curation: Zhonghua Liu.
Formal analysis: Qiang Li.
Project administration: Jinming Chen, Zhonghua Liu.
Resources: Qiang Li.
Supervision: Le Li, Ying Shi.
Visualization: Ying Shi.
Writing – original draft: Le Li, Xiaohua Liu.
Writing – review & editing: Le Li.
Footnotes
Abbreviations: AP = arterial phase, AS = accessory spleen, CA125 = carbohydrate antigen 125, CA19-9 = carbohydrate antigen 19-9, CEA = carcinoembryonic antigen, CT = computed tomography, CTA = computed tomography angiography, DWI = diffusion-weighted imaging, EUS = endoscopic ultrasonography, EUS-FNA = EUS-guided fine needle aspiration biopsy, IPAS = intrapancreatic accessory spleen, MRI = magnetic resonance imaging, PET/CT = positron emission tomography/diagnostic computed tomography, PNET = pancreatic neuroendocrine tumor, PSMA = prostate-specific membrane antigen, SI = signal intensities, SPECT = single photon emission computed tomography, SPIO = superparamagnetic iron oxide, T1WI = T1-weighted image.
LL and XL are co-first authors.
The authors report no conflicts of interest.
References
- [1].Dodds WJ, Taylor AJ, Erickson SJ, et al. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol 1990;155:805–10. [DOI] [PubMed] [Google Scholar]
- [2].Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol 1959;32:165–8. [DOI] [PubMed] [Google Scholar]
- [3].Kavic SM, Park A. Intrapancreatic accessory spleen: deficiency in diagnosis or therapeutic success? J Gastrointest Surg 2009;13:396[author reply 397]. [DOI] [PubMed] [Google Scholar]
- [4].Obuchi T, Takagane A, Sato K, et al. Single-incision laparoscopic excision of symptomatic accessory spleen in the pelvis: an initial report. J Minim Access Surg 2017;13:321–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sica GT, Reed MF. Case 27: intrapancreatic accessory spleen. Radiology 2000;217:134–7. [DOI] [PubMed] [Google Scholar]
- [6].White JD, West AN, Priebat DA. Splenosis mimicking an intraabdominal malignancy. Am J Med 1989;87:687–90. [DOI] [PubMed] [Google Scholar]
- [7].Lehtinen SJ, Schammel CM, Devane M, et al. Intrapancreatic accessory spleen presenting as a pancreatic mass. J Gastrointest Oncol 2013;4:E23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Unver Dogan N, Uysal II, Demirci S, et al. Accessory spleens at autopsy. Clin Anat 2011;24:757–62. [DOI] [PubMed] [Google Scholar]
- [9].Li BQ, Xu XQ, Guo JC. Intrapancreatic accessory spleen: a diagnostic dilemma. HPB(Oxford) 2018;20:1004–11. [DOI] [PubMed] [Google Scholar]
- [10].Issa M, Bradshaw L, Loveluck M, et al. Laparscopic distal pancreatectomy for intrapancreatic accessory spleen: a case report. ANZ J Surg 2017;12. [DOI] [PubMed] [Google Scholar]
- [11].Miyayama S, Matsui O, Yamamoto T, et al. Intrapancreatic accessory spleen: evaluation by CT arteriography. Abdom Imaging 2003;28:862–5. [DOI] [PubMed] [Google Scholar]
- [12].Singh D, Horneman R, Nagra NK. More than the prostate: intrapancreatic accessory spleen and papillary thyroid cancer detected with 18F-PSMA PET/CT. Hell J Nucl Med 2018;21:145–7. [DOI] [PubMed] [Google Scholar]
- [13].Tatekawa Y. An intrapancreatic accessory spleen presenting as a rapidly growing pancreatic mass after splenectomy in a patient with hereditary spherocytosis: a case report and literature review. J surg Case Rep 2018;2018:rjy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Uchida D, Tsutsumi K, Kato H, et al. An intrapancreatic accessory spleen that was difficult to diagnose due to temporal changes after splenectomy. Intern Med 2018;57:681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim SH, Lee JM, Han JK, et al. Intrapancreatic accessory spleen: findings on MR imaging, CT, US and scintigraphy, and the pathologic analysis. Korean J Radiol 2008;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kawamoto S, Johnson PT, Hall H, et al. Intrapancreatic accessory spleen: CT appearance and differential diagnosis. Abdom Imaging 2012;37:812–27. [DOI] [PubMed] [Google Scholar]
- [17].Ding Q, Ren Z, Wang J, et al. Intrapancreatic accessory spleen: evaluation with CT and MRI. Exp Ther Med 2018;16:3623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kurata Y, Kido A, Moribata Y, et al. Diagnostic performance of MR imaging findings and quantitative values in the differentiation of seromucinous borderline tumour from endometriosis-related malignant ovarian tumour. Eur Radiol 2017;27:1695–703. [DOI] [PubMed] [Google Scholar]
- [19].Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol 2010;83:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Santos MPD, Rezende AP, Santos PVD Filho. Intrapancreatic accessory spleen. Einstein (Sao Paulo) 2017;15:366–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ardengh C, Lopes V, Kemp R, et al. Pancreatic splenosis mimicking neuroendocrine tumors: microhistological diagnosis by endoscopic ultrasound guided fine needle aspiration. Arq Gastroenterol 2013;50:10–4. [DOI] [PubMed] [Google Scholar]
- [22].Kraus MD. Splenic histology and histopathology: an update. Semin Diagn Pathol 2003;20:84–93. [DOI] [PubMed] [Google Scholar]
- [23].Saunders TA, Miller TR, Khanafshar E. Intrapancreatic accessory spleen: utilization of fine needle aspiration for diagnosis of a potential mimic of a pancreatic neoplasm. J Gastrointest Oncol 2016;7Suppl 1:S62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Okun SD. Lewin DN: Non-neoplastic pancreatic lesions that may mimic malignancy. Semin Diagn Pathol 2016;33:31–42. [DOI] [PubMed] [Google Scholar]
- [25].Bastidas AB, Holloman D, Lankarani A, et al. Endoscopic ultrasound-guided needle-bases probe confocal laser endomicroscopy (nCLE) of inrapancreatic ectopic spleen. ACG Case Rep J 2016;3:196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Matthaei H, Schmelzle M, Braunstein S, et al. Pancreatic incidentalomas: a growing clinical challenge exemplified by an intrapancreatic accessory spleen. Wien Klin Wochenschr 2011;123:186–8. [DOI] [PubMed] [Google Scholar]
- [27].Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc 2009;70:1101–8. [DOI] [PubMed] [Google Scholar]
- [28].Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology 2010;139:1172–80. [DOI] [PubMed] [Google Scholar]
- [29].Horton KM, Hruban RH, Yeo C, et al. Multi-detector row CT of pancreatic islet cell tumors. RadioGraphics 2006;26:453–64. [DOI] [PubMed] [Google Scholar]


