Supplemental Digital Content is available in the text
Keywords: fatigue, MET inhibitors, meta-analysis, systematic review
Abstract
Background:
The N-methyl-N′-nitroso-guanidine human osteosarcoma transforming gene (MET) inhibitors show a surprising survival benefit in the treatment of numerous tumors especially in MET-high tumor. Besides their impressive efficacy, fatigue reduced by MET inhibitors is still the safety issue during treatment. Thus, an understanding of this risk in the context of expanding MET-inhibitors use is an important cost and patient safety issue.
Methods:
We searched PubMed, Embase, and the Cochrane Library databases for relevant studies up to October 2017. Eligibility criteria included phase II/III trials of MET inhibitors that reported adequate safety profiles of fatigue. The principal summary measures were incidence and relative risk (RR) of all-grade (grade 1–4) and high-grade (grade 3–4) fatigue, respectively. Random-effects model was applied to consider within-study and between-study variation.
Results:
A total of 5028 patients from 17 clinical trials were identified. The results revealed that the incidences of MET inhibitors-associated all-grade and high-grade fatigue were 41.9% and 9.6%, respectively. The RR of high-grade fatigue was (RR = 1.37; 95% confidence interval, 1.14–1.66; P = .0009), whereas the RR of all-grade fatigue was (RR = 1.02; 95% confidence interval, 0.91–1.15; P = .71).
Conclusion:
Our meta-analysis has demonstrated that MET inhibitors-based treatment is associated with an increased risk of high-grade fatigue compared with control.
1. Introduction
Fatigue is classically reported as a more frequently symptom than any others in patients with underlying malignancy, which causes the most interference with function during survivorship phases.[1] Cancer-related fatigue has been defined as a distressing, persistent, subjective sense of tiredness or exhaustion that is not proportional to recent activity and exertion.[2] These toxicities of mild-to-moderate severity are frequently long-lasting, debilitating and thus, impact the functional status and decrease health-related quality of life.[3,4]
The N-methyl-N′-nitroso-guanidine human osteosarcoma transforming gene (MET) has emerged as a critical intermediate in cell-signaling pathways commonly deregulated in solid tumors. MET tyrosine kinase is the cell-surface receptor for hepatocyte growth factor (also known as scatter factor) that is involved in regulating tumorigenic growth, metastasis, and therapeutic resistance.[5] MET activity is normally detected in defined stages of embryogenesis and organogenesis.[5] Furthermore, MET pathway engages in crosstalk with the epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and Kirsten rat sarcoma viral oncogene signaling pathway, which in part explained MET amplification in various primary tumors, including medulloblastoma, colorectal cancer, hepatocellular carcinoma, gastric cancer, and NSCLC with resistance to EGFR inhibitors.[6–10] Based on this scientific rationale, targeting approaches to the Met signaling axis is mostly comprised of monoclonal antibodies (mAbs) and small-molecule tyrosine kinase inhibitors (TKIs), such as crizotinib, cabozantinib, tivantinib, onartuzumab, and rilotumumab that have been developed as MET inhibitors.[5] Presently, cabozantinib has been approved for treatment of renal cell carcinoma, medullary thyroid cancer, and lung adenocarcinoma, whereas crizotinib is approved for medullary thyroid carcinoma and lung cancer.[11–14]
According to several comprehensive global phase III study and a meta-analysis, patients with MET-high tumor show better survival benefit when a MET inhibitor was added into an EGFR TKI.[15–17] Besides their impressive efficacy, there have been concerns regarding the safety of MET inhibitors. The onset of severe cancer-related fatigue has been the major dose-limiting toxicity of TKIs.[18] Thus, an understanding of this risk in the context of expanding MET-inhibitors use is an important cost and patient safety issue. In this study, we reported a meta-analysis of trials of MET inhibitors in patients with cancer and compared the incidence of fatigue among the cohorts with different tumors types and between the groups treated with various types of MET inhibitors.
2. Methods
2.1. Data sources and study identification
A literature search utilizing PubMed, Embase, and the Cochrane Library databases was conducted from inception to October 2017, using Boolean logic that consisted of the key terms “c-Met,” “MET,”“cabozantinib,” “onartuzumab,” “rilotumumab,” and “tivantinib” being searched. We did not include “crizotinib” because it was approved only for the treatment of anaplastic lymphoma kinase-positive NSCLC. Medical subject headings in Pubmed and EMTREE terms in Embase were used to identify synonyms. Meeting abstracts from the American Society of Clinical Oncology and not published full-text original articles of the European Society of Medical Oncology Conference were not eligible. Updated manufacturer's package inserts, Clinical Trials Registry Platform, and ClinicalTrials.gov was also searched to identify relevant information. Finally, references in reports of all included publications will be tracked to retrieve relevant citations. This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement recommendation.[19] Ethical approval for this study was unnecessary because it was a review of existing literature and did not involve any handling of individual patient data. The primary goal of the analysis was to establish any association between fatigue and exposure to MET inhibitors; therefore, trials with hormonal agents and corticosteroids use were excluded as they can modulate fatigue. Eligible trials that fulfill the following concepts were included: prospective phase II and III trials involving adults with malignancy; random assignment to MET-inhibitors-based treatment or control treatments (placebo or concurrently chemotherapeutic or best supportive care); and available data reporting safety and sample size for treatment-emergent, nondisease-related, all-grade and high-grade fatigue.
2.2. Date extraction and clinical end points
Data extraction was conducted independently by 2 reviewers (HT and YZ) using a predesigned, pilot-tested extraction form. For each study, the following baseline demographic and clinical characteristics were extracted from each study: the first author's name, year of publication, study design, trial phase, underlying malignancy, population size, treatment and dosing regimens, median age, median treatment duration, masking, number of patients available for analysis, and number of relevant adverse events. Count data for fatigue were defined and recorded according to the common toxicity criteria of adverse events version 3.0 or 4.0, which are widely used in grading the relevant adverse events. Discrepancies between the reviewers were settled by consensus or revisiting original trials. Whether the toxicities attributed to treatment related or disease related depended on the decision of investigators in the original articles. Data were extracted from intention-to-treat analyses wherever possible. We also initially aimed to collect the median follow-up duration of all-grade and high-grade fatigue, only to find that this was rarely reported. If there were arms that exposed patients to different doses and schedules of MET inhibitors/control, those arms were pooled together. Study quality was assessed by using the Jadad 7-item scale including randomization, double-blinding, and withdrawals,[20] with disagreements adjudicated by a senior reviewer (YL).
2.3. Statistical analysis
All statistical analyses were carried out by using RevMan (version 5.3; the Nordic Cochrane Centre, Copenhagen), STATA (version 12.0; StataCorp, College Station, TX), and the “R” statistical software version 2.15.2. The principal summary measures were incidence and relative risk (RR) of all-grade (grade 1–4) and high-grade (grade 3–4) fatigue, respectively. We first calculated the incidence of fatigue by using the total number of patients evaluable for the safety profiles and the number of patients experiencing fatigue in selected randomized clinical trials (RCTs). For RR analysis, data were extracted from prospective clinical trials, comparing the incidence of outcomes in patients assigned to MET inhibitors vs control arm in the same trial. Due to substantial clinical heterogeneity between studies such as diagnosis, random assignment, and in exposure to prior treatments, a random-effects model (DerSimonian and Laird) that considered within-study and between-study variation was applied.[21] Cochrane Q test (χ2) was used to qualitatively describe the heterogeneity and I2 was used to quantitate the inconsistency.[22] When substantial heterogeneity was observed, prespecified exploratory subgroup analysis was conducted and stratified to examine the potential risk factors. The subgroups were only chosen for their clinical relevance and importance. A continuity correction was applied in RCTs with zero events. Presence of potential publication bias was assessed using funnel plots with Begg and Egger tests (if more than 10 studies were available).[23,24] All statistical tests were set as 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Literature search results
Following study inclusion (Fig. 1), our search identified 523 potentially relevant articles, describing the use of MET inhibitors from the initial databases (Tables S1–S3). After eligibility assessment, 17 trials were considered to be most relevant for the meta-analysis,[3,16,25–40] (including 13 phase II trials (n = 1823) and 4 phase III trials (n = 3205). Literature review articles, irrelevant topics, case reports, and preclinical studies were excluded. The characteristics of trials and patients are listed in Table 1.
Figure 1.
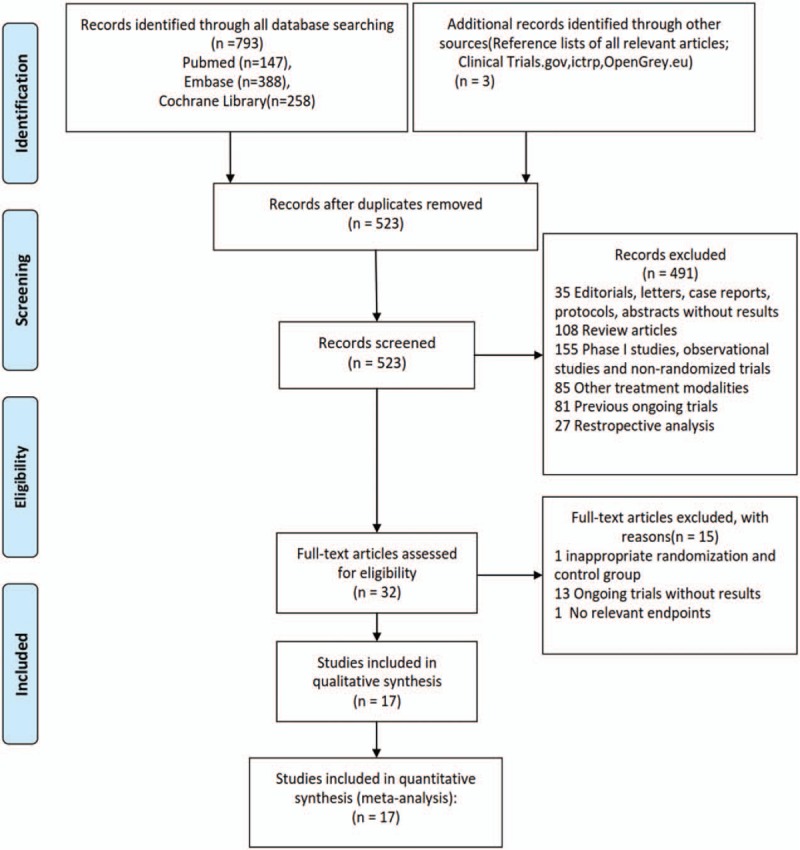
Flow diagram of the process of selecting eligible studies.
Table 1.
Baseline characteristics of the prospective randomized trials included in the meta-analysis.
3.2. Population characteristics
A total of 5028 cancer patients were available for the meta-analysis, of which 1832 patients had NSCLC (36%), 455 (9%) had colorectal cancer, 803 (16%) had renal cancer, 1161 (23%) had prostate cancer, and 777 15%) were treated for other malignancies. All studies were well randomized with adequate follow-up. All selected trials included patients with an Eastern Cooperative Oncology Group performance status of 2 or less and were required to have an adequate renal, hepatic, and hematologic function. For the RR analysis, 2784 patients were treated with MET inhibitors (1169 patients treated with cabozantinib, 660 with onartuzumab, 737 with tivantinib, and 218 with rilotumumab), and the rest with control/placebo treatment. In tivantinib studies, all patients were given 360 mg twice daily. Onartuzumab was administered at a dose of 10 and 15 mg/kg in 2 or 3-week cycles, and rilotumumab was administered 15/10/7.5 mg/kg daily continuously in 3 trials. Cabozantinib was given 60 or 40 mg daily continuously in studies. The baselines characteristics of included studies are presented in Table 1. We graded the quality of each study reported by Jadad et al.[20]
3.3. Quality of studies
Randomized treatment allocation sequences were generated in all trials. The Jadad scores of the included 7 randomized, controlled, and double-blinded trials ranged from 4 to 5,[27,30,32,37–40] whereas there were 2 phase II, randomized, open-labeled studies that were assigned a Jadad score of 3.[26,35] Therefore, quality of these studies was fair and acceptable.
3.4. Overall incidence of all-grade and high-grade fatigue
For the incidence analysis, we considered arms receiving MET inhibitors or MET inhibitor-based combination (due to the limited use of MET inhibitors monotherapy treatment and potential events associated with concomitant therapy). Thus, a total of 2685 patients from 16 trials were available from the included trials. The summary incidences of all-grade fatigue ranged between 10% and 86%, with the highest incidence seen in a phase III trial among patients with colorectal cancer.[34] Using a random-effects model, the calculated overall incidence of all-grade fatigue among patients receiving MET inhibitors was 41.9% (95% confidence interval, CI, 34.6–49.6) (I2 = 92.1%, P < .01), whereas the overall incidences of high-grade fatigue ranged between 4% and 23%, and the highest incidence was observed in a trial of patients with colorectal cancer.[29] The calculated overall incidence of high-grade fatigue among patients receiving MET inhibitors was 9.6% (95% CI, 7.1–12.8) (I2 = 79.5%, P < .01) (Fig. 2). Further exploratory analysis was conducted to evaluate the incidence of fatigue based on underlying malignancy and type of MET inhibitors. Data stratified by tumor type showed that the incidence of all-grade fatigue was higher in RCC patients (74%), whereas high-grade fatigue was higher in gastric or esophagogastric junction adenocarcinoma (14%). When stratified by MET inhibitors, the incidence of all-grade and high-grade fatigue was higher in patients using cabozantinib (68% and 13%, respectively). The incidence of severity adverse events varied significantly with cancer type (P < .001) and type of MET inhibitors (P < .001) (Figs. S1–S3).
Figure 2.
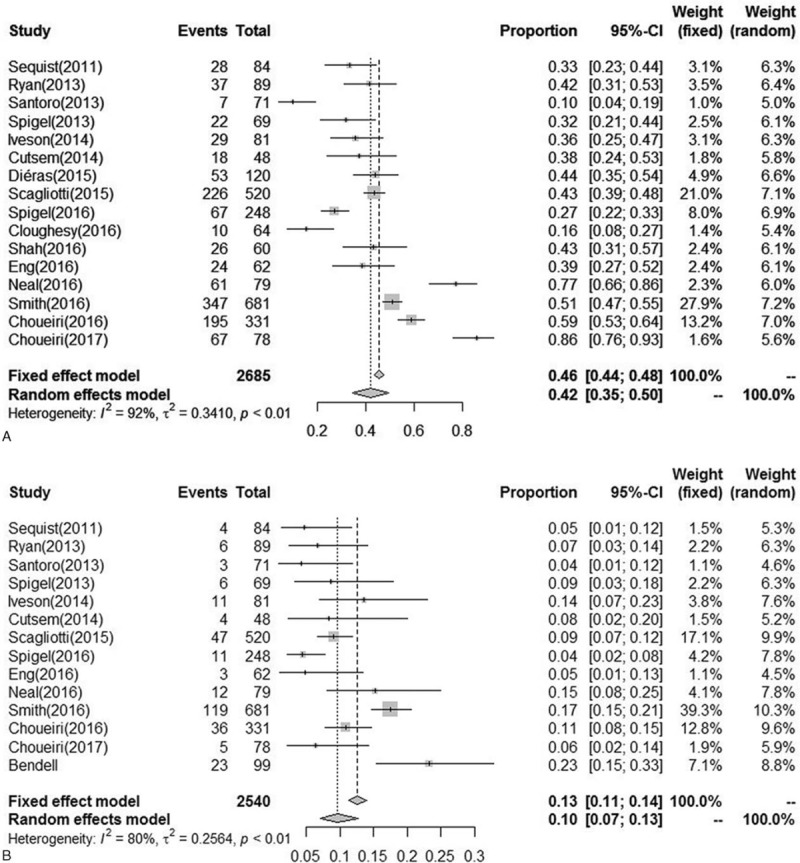
Incidence of all-grade and high-grade fatigue.
3.5. RR of all-grade and high-grade fatigue
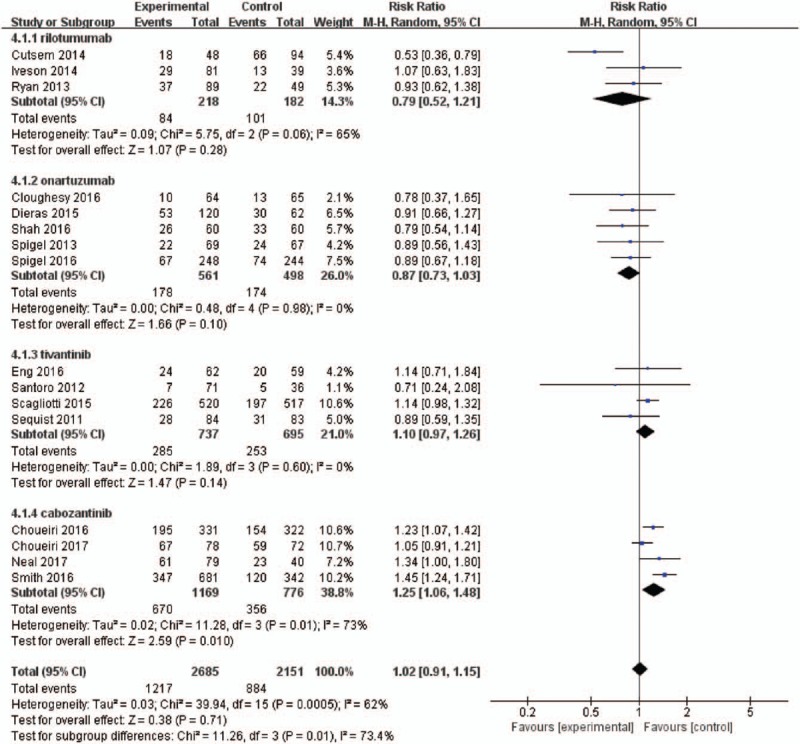
To evaluate the specific contribution of MET inhibitors to the development of fatigue, 16 and 14 randomized trials were available to calculate the RR of all-grade and high-grade fatigue in patients who received MET inhibitors or controls (concurrent chemotherapy or placebo or targeted therapy). The summary of RR of developing all-grade and high-grade fatigue with the administration of MET inhibitors vs controls was 1.02 (95% CI, 0.91–1.15; P = .71) and 1.37 (95% CI, 1.14–1.66; P = .0009), respectively (Figs. 3–6). This estimate was obtained by using random-effects model and fixed-effects model as the heterogeneity tested by I2 statistics in the increased risk of all-grade fatigue (χ2 = 39.94, P = .0005, I2 = 62%) and high-grade fatigue (χ2 = 18.58, P = .14, I2 = 30%). Considering the confounding factors, a sensitivity analysis was performed to examine the stability and reliability of pooled RRs. The result indicated that the pooled RRs were not materially altered by changing the pooling models and by omitting any single study from the sequence (Fig. S4). Prespecified subgroup analysis was then conducted to explore the potential cause of heterogeneity.
Figure 3.
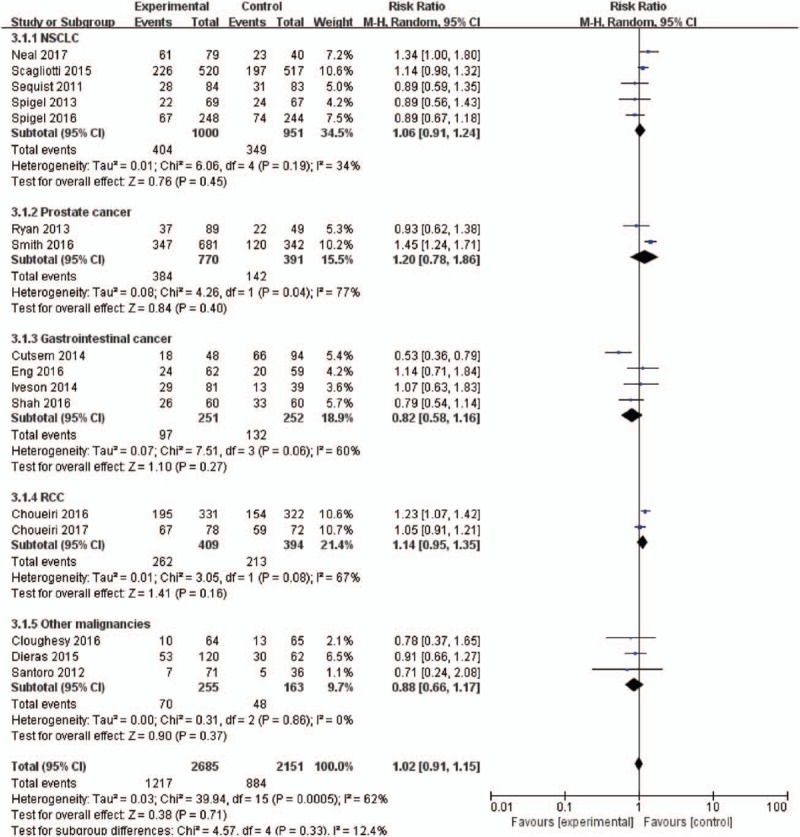
Forest plots for subgroup for the risk of all-grade fatigue stratified by tumor type.
Figure 6.
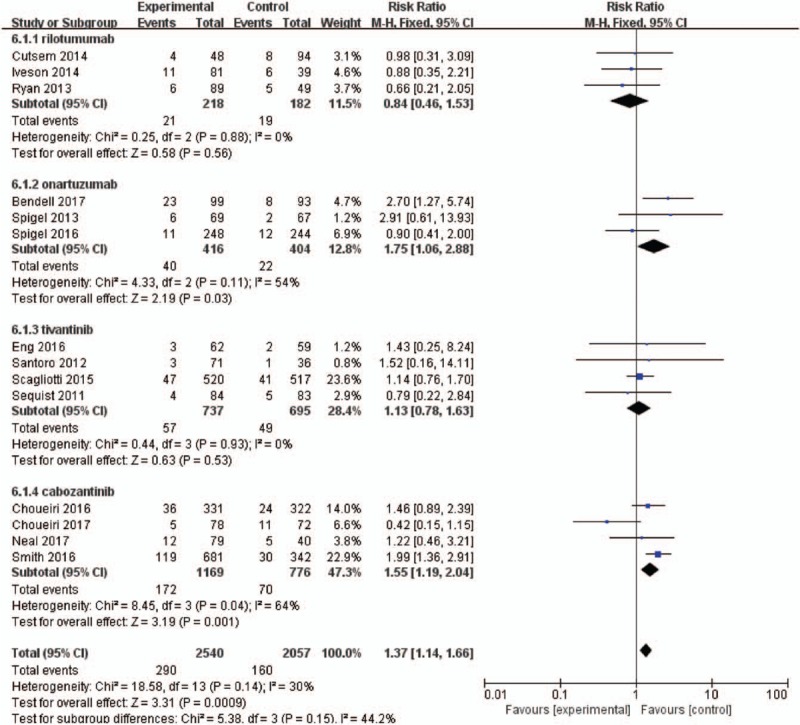
Forest plots for subgroup for the risk of high-grade fatigue according to the type of MET inhibitors.
3.6. Subgroup analyses
To examine whether the observed RRs of developing all-grade and high-grade fatigue were the result of confounding bias, prespecified subgroup analyses were performed according to the underlying malignancy, type of MET inhibitors, and controlled therapy. As suggested in the Cochrane Handbook, we tried to limit the number of subgroup analyses.
3.7. Influence of tumor type
Different tumor biological behavior and associated treatment might affect the risk of fatigue. We thus evaluated RRs of all-grade and high-grade fatigue with MET inhibitors according to tumor types (Figs. 3 and 4). For all-grade fatigue, there was no significantly increased risk in the included trials, 1.02 (95% CI, 0.91–1.15; P = .71). Interestingly, the effect size was significantly greater in the RR analysis of high-grade fatigue considering only the trials in prostate cancer than other cancers, with RRs of 1.81 (95% CI, 1.27–2.58; P = .001) and 1.22 (95% CI, 0.97–1.52; P = .08).
Figure 4.
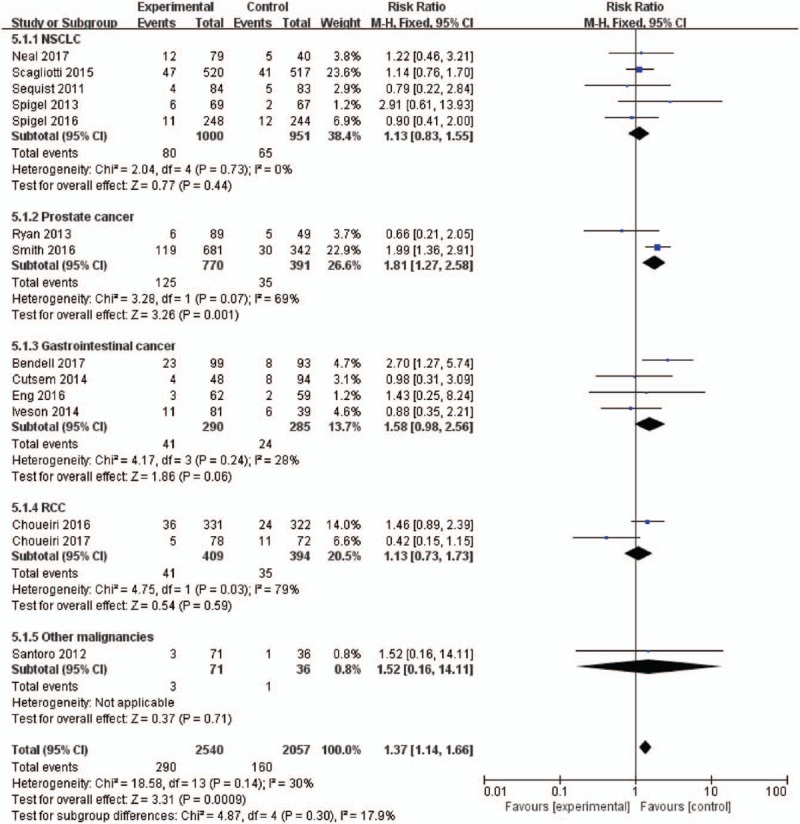
Forest plots for subgroup for the risk of high-grade fatigue stratified by tumor type.
3.8. Influence of MET inhibitors
The RRs of all-grade and high-grade fatigue events might be related to MET inhibitors (Figs. 5 and 6). Our results demonstrated that the use of cabozantinib significantly increased the risk of all-grade fatigue (RR = 1.25; 95% CI, 1.06–1.48; P = .01). Additionally, there was significant increased risk of high-grade fatigue among patients who received onartuzumab (RR = 1.75; 95% CI, 1.06–2.88; P = .03) and cabozantinib (RR = 1.55; 95% CI, 1.19–2.04; P = .001). The use of other MET inhibitors did not increase the risk of all-grade fatigue (RR = 0.91; 95% CI, 0.79–1.04; P = 0.17) and high-grade fatigue (RR = 1.04; 95% CI, 0.76–1.43; P = .41).
Figure 5.
Forest plots for subgroup for the risk of all-grade fatigue according to the type of MET inhibitors.
3.9. Influence of controlled therapy
As an exploratory analysis, patients were stratified according to the type of treatment (MET inhibitors monotherapy vs MET-based combination; Fig. S3). We found no statistically significant difference between the 2 groups regarding all-grade fatigue (P = .05). With regard to RRs of high-grade events, we found a significant difference between MET inhibitor monotherapy vs control (RR = 1.58; 95% CI, 1.20–2.09; P = .001), whereas no differences between RR of MET inhibitor combination vs control were found (P = .14).
3.10. Publication bias
For the meta-analysis of all-grade fatigue, visual examination of Begg funnel plot indicated a degree of asymmetry, confirmed by Egger test (P = .025). No evidence of publication bias was observed in any category of the toxicities of high-grade fatigue. The P value of Egger test for the association between MET inhibitors and high-grade fatigue was 0.24, thus suggesting a minor influence of bias in the identification of high-grade fatigue. Funnel plots are presented in Figure S5.
4. Discussion
MET transmembrane glycoprotein has been demonstrated to be overexpressed in a wide variety of tumor, and it correlates with more advanced disease, poor survival, and the presence of metastases.[41] Meanwhile, the signaling pathway is also associated with chemotherapy, radiotherapy, and targeted therapy including EGFR and VEGF inhibitors.[42,43] Therefore, inhibition of the MET signaling pathway could be an attractive therapeutic target for cancer therapy. MET inhibitor can enhance the cytotoxic effects of chemotherapy or chemoradiotherapy in MET expressing cell carcinoma.[43] Several drugs that target MET signaling pathway, including antibodies and small molecule inhibitors, have reached clinical evaluation and showed promise in animal models.[44] Results from the clinical studies indicated that those patients who exhibit c-Met gene amplification and mutations respond more successfully to targeted therapy and achieve maximum therapeutic outcome.[45] Furthermore, the most promising clinical data comes from combination therapies in which MET agent is believed to participate in mechanisms of drug resistance.[46]
Fatigue represents a fundamental and essential issue in oncology practice since it correlates with poor life quality in patients with underlying malignancies. Cancer treatment-related fatigue is a multifactorial process, and the precise underlying pathophysiology remains unclear.[18] Fatigue can arise as a result of cancer induced or as an adverse event of drugs. Fatigue occurs through putative mechanisms that include cytokine dysregulation, dysregulation of the hypothalamic–pituitary–adrenal axis, alterations in the autonomic nervous system, anemia, neurotransmitter dysregulation, as well as patient-related factors such as psychosocial states and demographic and medical factors.[47] Furthermore, fatigue might be a consequence of TKI-induced anemia or endocrine disorders, such as adrenal dysfunctions, thyroid alterations, mineral, gonadal, and other metabolic alterations.[48] Additionally, TKI inhibitors can also lead to hypothyroidism, cardiotoxicity, skeletal muscle atrophy, and pneumonitis, which indirectly cause fatigue. In our meta-analysis, fatigue was reported in a substantial proportion of patients in the control arms, which provides a benchmark for future research attempting to mitigate fatigue. According to the National Comprehensive Cancer Network guidelines, initial evaluation of cancer patients suffering from fatigue should include focused disease status as well as fatigue assessment. Moreover, endocrinologic evaluation, hematological function, and hepatic and renal function are always warranted.[49] The guidelines published by the American Society of Clinical Oncology recommended that fatigue assessment should be conducted for all cancer survivors from the point of diagnosis onwards.[50]
Fatigue in relation to the use of MET inhibitors was reported in the overwhelming majority of completed clinical trials. These trials have highlighted how fatigue and people reported adverse events emerged as fatal when weighing the efficacy and safety of these inhibitors. However, both subjective outcome scales of fatigue assessment and long-term effect of cancer treatment may limit the accurate quantification of the events for fatigue in cancer patients. Additionally, concurrent therapies might exacerbate fatigue. For this reason, it is crucial for both clinicians and patients to properly understand the risk of MET inhibitors-related fatigue to promptly allow early management and take the appropriate measures to face these events.
Previously, the prevalence of fatigue in cancer survivors has been examined in reviews, with the use of immune checkpoint inhibitors, mammalian target of rapamycin (mTOR), and VEGF receptor.[51,52] The results demonstrated that the use of mTOR and VEGF receptor inhibitors is associated with a significantly increased risk of all-grade fatigue, but not for high-grade fatigue. However, to date, only a narrative review examined the safety results of MET inhibitors, which was not estimated with a meta-analysis.[5] In this article, we tried to assess the risk of fatigue, as evaluated by treating investigators using the common toxicity criteria of adverse events version 2.0 or 3.0, treated by several developed MET TKI and mAbs. This meta-analysis confirms that cancer patients treated with a MET inhibitor-containing regimen are at higher risk of high-grade fatigue relative to control treatment. It is noteworthy that incidence of all-grade and high-grade fatigue varied among patients with different types of tumors. Similarly, we found higher incidence values in the cabozantinib and tivantinib subgroup compared to other groups, by 22% (P < .001). This data analysis also showed that MET inhibitors were linked to a higher risk of high-grade fatigue compared with the control groups, whereas all-grade fatigue showed no significant differences. Nevertheless, the above results should be interpreted with cautious while some potential heterogeneity associated with this analysis. To overcome the potential bias, sensitive analysis and subgroup analyses have been performed.
In line with a meta-analysis of clinical studies, our sensitivity analysis excluded patients receiving crizotinib, which was approved as an anaplastic lymphoma kinase inhibitor.[53] In the subgroup analysis, numerical higher RRs of both all-grade and high-grade fatigue were found in trials of prostate cancer. This may be because these patients are more prone to develop drug-related renal toxic effects and endocrine-induced fatigue. Stratified by type of MET inhibitors, the different risk of fatigue among TKI and mAbs in the current analysis highlights that cabozantinib and tivantinib have a deep impact on their toxicity of fatigue. The different risk of hepatotoxicity among MET inhibitors in the current analysis highlights the importance of not dealing with MET inhibitor as a single entity, but rather classifying them according to their mechanism of action and the combinations used, which would have a profound impact on their efficacy and toxicity. Furthermore, subanalyses did not identify any differential impacts in all-grade fatigue based on the line of therapy, age, and median duration of treatment (data not shown). Unlike a small increase in a critical toxicity such as death, these findings are not likely to affect the therapy given by an individual physician. However, high-grade fatigue may always contribute to dose reductions, treatment interruptions, or treatment discontinuation. As no prior review has applied meta-analytic methods to assess the MET-inhibitors-associated fatigue, these results of potential interactions may have an indirect impact on the level of management of these toxicities and consequently the optimal continuation of cancer treatment. Psychosocial and pharmacologic interventions could be offered, and the reported outcomes may help to inform treatment choice in certain tumors.
4.1. Our meta-analysis weakness
This study has several potential limitations. First, there was apparent heterogeneity between studies. This was likely to be derived from different underlying malignancies, concomitant therapies, control regimens, phase of trials, and schedule doses. Moreover, comparing fatigue among trials is inherently difficult as the diverse criteria were used to define measurement instrument, clinical time points, and heterogeneity in tumor type and disease stage.[54] The influence was minimized by using prespecified subgroup analyses to explore the possible reasons for the heterogeneity. Second, safety profile is usually not the primary outcome measure in clinical trials; all-grade and high-grade fatigue reported to be suboptimal and variable. Third, this is a meta-analysis at study level, and confounding variables at the patient level could not be incorporated into the analysis. Fourth, it is uncertain if these findings can be generalized to drugs-related side effects because fatigue is the well-known cancer-related symptom. It is also noteworthy that the stringent eligibility criteria of RCTs may exclude patients with comorbidities. Practicing oncologists need to be aware of this risk and provide continuous monitoring to patients.
5. Conclusion
Our meta-analysis indicates that the RR of high-grade fatigue is higher in patients receiving MET inhibitors than the control groups. Early detection and effective management of high-grade fatigue that can occur in cancer patients with MET inhibitors are crucial for the safer use of these drugs. The findings of this meta-analysis should be considered when assessing the benefits and risks of MET inhibitors in clinical practice.
Author contributions
Data curation: Hongxuan Tong, Yutian Zhu, Yihua Liu.
Investigation: Yutian Zhu.
Resources: Hongxuan Tong.
Software: Yutian Zhu.
Visualization: Yihua Liu.
Writing – original draft: Hongxuan Tong, Yihua Liu.
Writing – review & editing: Hongxuan Tong, Yihua Liu.
Hongxuan Tong orcid: 0000-0003-4788-1768.
Supplementary Material
Footnotes
Abbreviations: EGFR = epidermal growth factor receptor, ESMO = European Society of Medical Oncology Conference, mAbs = monoclonal antibodies, MET = the N-methyl-N′-nitroso-guanidine human osteosarcoma transforming gene, TKI = tyrosine kinase inhibitor, VEGF = vascular endothelial growth factor.
The involvement of MET in solid tumor has been demonstrated in preclinical studies and clinical trials suggest that MET inhibitors are likely to be critical to future combination-based therapies. Ideally, biomarkers of MET detected and the publication of more randomized trials report these toxicities will provide the basis for personalized treatment regimens.
HT and YZ equally contributed to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Berger AM, Mitchell SA, Jacobsen PB, et al. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA Cancer J Clin 2015;65:190–211. [DOI] [PubMed] [Google Scholar]
- [2].Mock V, Atkinson A, Barsevick A, et al. NCCN Practice Guidelines for cancer-related fatigue. Oncology 2000;14(11A):151–61. [PubMed] [Google Scholar]
- [3].Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 2000;5:353–60. [DOI] [PubMed] [Google Scholar]
- [4].Larkin JM, Pyle LM, Gore ME. Fatigue in renal cell carcinoma: the hidden burden of current targeted therapies. Oncologist 2010;15:1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013;39:793–801. [DOI] [PubMed] [Google Scholar]
- [6].Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504–16. [DOI] [PubMed] [Google Scholar]
- [7].Di Renzo MF, Olivero M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995;1:147–54. [PubMed] [Google Scholar]
- [8].Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89–103. [DOI] [PubMed] [Google Scholar]
- [9].Sattler M, Reddy MM, Hasina R, et al. The role of the c-Met pathway in lung cancer and the potential for targeted therapy. Ther Adv Med Oncol 2011;3:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang R, Ferrell LD, Faouzi S, et al. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol 2001;153:1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nishio M, Kim DW, Wu YL, et al. Crizotinib versus chemotherapy in Asian patients with advanced ALK-positive non-small cell lung cancer. Cancer Res Treat 2018;50:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choueiri TK, Pal SK, McDermott DF, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol 2014;25:1603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim JH, Kim HS, Kim BJ. MET inhibitors in advanced non-small-cell lung cancer: a meta-analysis and review. Oncotarget 2017;8:75500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoshioka H, Azuma K, Yamamoto N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol 2015;26:2066–72. [DOI] [PubMed] [Google Scholar]
- [18].Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. Oncologist 2007;12Suppl 1:22–34. [DOI] [PubMed] [Google Scholar]
- [19].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [20].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [21].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [24].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spigel DR, Edelman MJ, O’Byrne K, et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol 2017;35:412–20. [DOI] [PubMed] [Google Scholar]
- [26].Neal JW, Dahlberg SE, Wakelee HA, et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial. Lancet Oncol 2016;17:1661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Scagliotti G, von Pawel J, Novello S, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol 2015;33:2667–74. [DOI] [PubMed] [Google Scholar]
- [28].Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 2011;29:3307–15. [DOI] [PubMed] [Google Scholar]
- [29].Bendell JC, Hochster H, Hart LL, et al. A phase II randomized trial (GO27827) of first-line FOLFOX plus bevacizumab with or without the MET inhibitor onartuzumab in patients with metastatic colorectal cancer. Oncologist 2017;22:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eng C, Bessudo A, Hart LL, et al. A randomized, placebo-controlled, phase 1/2 study of tivantinib (ARQ 197) in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with wild-type KRAS who have received first-line systemic therapy. Int J Cancer 2016;139:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Van Cutsem E, Eng C, Nowara E, et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res 2014;20:4240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol 2016;34:3005–13. [DOI] [PubMed] [Google Scholar]
- [33].Ryan CJ, Rosenthal M, Ng S, et al. Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin Cancer Res 2013;19:215–24. [DOI] [PubMed] [Google Scholar]
- [34].Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917–27. [DOI] [PubMed] [Google Scholar]
- [36].Shah MA, Cho JY, Tan IB, et al. A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist 2016;21:1085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014;15:1007–18. [DOI] [PubMed] [Google Scholar]
- [38].Cloughesy T, Finocchiaro G, Belda-Iniesta C, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O(6)-methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol 2017;35:343–51. [DOI] [PubMed] [Google Scholar]
- [39].Dieras V, Campone M, Yardley DA, et al. Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer. Ann Oncol 2015;26:1904–10. [DOI] [PubMed] [Google Scholar]
- [40].Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol 2013;14:55–63. [DOI] [PubMed] [Google Scholar]
- [41].Liu X, Newton RC, Scherle PA. Development of c-MET pathway inhibitors. Expert Opin Investig Drugs 2011;20:1225–41. [DOI] [PubMed] [Google Scholar]
- [42].Garajova I, Giovannetti E, Biasco G, et al. c-Met as a target for personalized therapy. Transl Oncogenomics 2015;7Suppl 1:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ko B, He T, Gadgeel S, et al. MET/HGF pathway activation as a paradigm of resistance to targeted therapies. Ann Transl Med 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maroun CR, Rowlands T. The Met receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther 2014;142:316–38. [DOI] [PubMed] [Google Scholar]
- [45].Parikh PK, Ghate MD. Recent advances in the discovery of small molecule c-Met kinase inhibitors. Eur J Med Chem 2018;143:1103–38. [DOI] [PubMed] [Google Scholar]
- [46].Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16:553–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013;30Suppl:S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Santoni M, Conti A, Massari F, et al. Treatment-related fatigue with sorafenib, sunitinib and pazopanib in patients with advanced solid tumors: an up-to-date review and meta-analysis of clinical trials. Int J Cancer 2015;136:1–0. [DOI] [PubMed] [Google Scholar]
- [49].Abdel-Rahman O, Fouad M. Risk of fatigue in patients with solid tumors treated with everolimus, temsirolimus or ridaforolimus: a comparative meta-analysis. Expert Rev Anticancer Ther 2015;15:477–86. [DOI] [PubMed] [Google Scholar]
- [50].Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 2014;32:1840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ghatalia P, Je Y, Nguyen PL, et al. Fatigue with vascular endothelial growth factor receptor tyrosine kinase inhibitors and mammalian target of rapamycin inhibitors in patients with renal cell carcinoma (RCC) and other malignancies: a meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 2015;95:251–63. [DOI] [PubMed] [Google Scholar]
- [52].Abdel-Rahman O, Helbling D, Schmidt J, et al. Treatment-associated fatigue in cancer patients treated with immune checkpoint inhibitors; a systematic review and meta-analysis. Clin Oncol 2016;28:e127–38. [DOI] [PubMed] [Google Scholar]
- [53].Ye S, Li J, Hao K, et al. The efficacy and risk profile of c-Met inhibitors in non-small cell lung cancer: a meta-analysis. Sci Rep 2016;6:35770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vardy JL, Dhillon HM, Pond GR, et al. Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol 2016;27:1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


