Abstract
Viral hepatitis is caused by different etiological agents with distinct epidemiological, clinical, and laboratory characteristics accounting for significant worldwide morbidity and mortality. Since 1996, the Brazilian Department of Sexually Transmitted Infections (STIs), Acquired Immune Deficiency Syndrome (AIDS) and Viral Hepatitis (DIAHV) in collaboration with the Ministry of Defense has been conducting periodic serosurveys of conscripts enlisted for the Brazilian army to assess STI prevalence and obtain data on knowledge and risk factors pertaining to STIs. This article aims to present the hepatitis B (hepatitis B surface antigen - HBsAg) and C (anti-HCV) seroprevalence estimates and risk factors as per the 8th edition of the Conscript Survey performed in 2016.
This cross-sectional study was conducted among conscripts across Brazil aged 17 to 22 years from August to December 2016. It included a self-reported questionnaire and blood testing for syphilis, human immunodeficiency virus (HIV), and hepatitis B and C.
In total 38,247 conscripts were enrolled; after applying exclusion criteria, 37,282 conscripts were included. The estimated HBsAg and anti-HCV prevalence rates were 0.22% and 0.28%, respectively. Higher HBsAg and anti-HCV prevalence rates were observed in the North Region (0.49%) and in the Central-west Region (0.65%), respectively. Regarding hepatitis B vaccination, 23.5% (n = 8412) of the individuals reported being unvaccinated and 47.4% (n = 16,970) did not know if they had been vaccinated. Among the anti-HCV positive conscripts, 53% (n = 51, 0.56%, P = .049) reported that they had never had sexual intercourse. Regarding self-reported STI status, most of the positive anti-HCV (n = 100, 0.29%, P < .01) and positive HBsAg (n = 76, 0.22%, P = .205) conscripts reported not having a STI. From those who tested positive for HBsAg, 89% (n = 42, 0.28%, P = .005) reported not making consistent use of condoms with steady partners.
Our data suggest a low prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections among Brazilian young men, and relatively low rates of self-reported HBV immunization. History of STIs, higher number of partners, inconsistent use of condoms, and lack of awareness of routes of transmission were significantly associated with HBV and HCV infections. To achieve the World Health Organization's goal of viral hepatitis elimination, access to hepatitis information, testing, and surveillance need to be improved.
Keywords: army, Brazil, conscripts, hepatitis, prevalence, sexual behavior, young men
1. Introduction
Viral hepatitis is an infectious disease caused by different etiological agents that present distinct epidemiological, clinical, and laboratory characteristics accounting for significant worldwide morbidity and mortality. Globally, in 2015, an estimated 257 million people were living with chronic hepatitis B virus (HBV) infection, and 71 million people with chronic hepatitis C virus (HCV) infection.[1] From 1999 to 2017, 218,257 HBV and 160,105 HCV confirmed infection cases have been reported in Brazil.[2] Worldwide, mortality due to hepatitis B and C was comparable to that due to tuberculosis and higher than that due to HIV.[1] In Brazil, deaths related to HCV infection are the leading cause of deaths among viral hepatitis patients; the number of deaths due to this etiology has been increasing over the years in all Brazilian regions. From 2010 to 2016, 50,179 deaths related to HCV and 14,172 deaths related to HBV have been reported.[2]
The major modes of viral hepatitis transmission include either mucous membrane contact or percutaneous exposure to infected blood or other body fluid (ie unsterile medical injections, needle stick injuries, blood transfusions, tattooing, body piercing, dental care, injecting drug use, and sexual intercourse).[3–5] In more recent years, as increased screening of blood products and the use of sterile equipment for medical injection has reduced transmission via these routes, injection drug use has become proportionately more important as a vector for viral hepatitis transmission.[3,4]
HCV and HBV infections can lead to chronic hepatitis, cirrhosis, and the development of hepatocellular carcinoma if patients are not diagnosed and do not receive timely and effective clinical management. HBV infection requires lifelong treatment whereas HCV can be cured using highly effective direct-acting antivirals.[1] In both cases, an efficient response to the hepatitis epidemics, especially the hepatitis C epidemic, requires a well-structured public health policy. For this purpose, up to date epidemiological information is essential.
Since 1996, the Brazilian Department of Sexually Transmitted Infections (STI), Acquired Immune Deficiency Syndrome (AIDS) and Viral Hepatitis (DIAHV) in collaboration with the Ministry of Defense has been conducting periodic serosurveys of conscripts enlisted for the Brazilian army to assess STI prevalence and to obtain data on knowledge and risk factors pertaining to STIs. Assessment of knowledge about viral hepatitis was included in the 6th edition of the serosurvey performed in 2002 [6,7] while HCV and HBV serological testing was included only in the 2016 edition. Data from these surveys are used to monitor STI trends in young men and are applied as a proxy to estimate the prevalence of STIs among adults in the overall population.
This article aims to present the hepatitis B (hepatitis B surface antigen - HBsAg) and C (anti-HCV) seroprevalence estimates and risk factors of the 8th edition of the Conscript Survey performed in 2016.
2. Methods
2.1. Study design, setting, and participants
This cross-sectional study was conducted among young men across Brazil aged 17 to 22 years who were in compulsory enlistment for military service from August to December 2016.
In total, 39,996 conscripts were selected to participate in the survey by following a sampling plan based on stratification in 2 selection stages previously described.[8] Exclusion criteria for this study were as follows: illiterate conscripts; conscripts outside the age range of 17 to 22 years; lack of information regarding age, origin (municipality), or educational levels; and refusal to provide informed consent.
2.2. Data collection and laboratory assays
Participants completed a self-reported anonymous questionnaire and provided blood samples for Human Immunodeficiency Virus (HIV), hepatitis B and C, and syphilis infection testing. The questionnaire contained 74 questions and included questions about sociodemographic characteristics, sexual behavior practices, problems related to STIs, and the use of licit/illicit drugs.
Participants were classified as men who have sex with men (MSM) if they reported having sex “only with men” or “with men and women”. All questionnaires were processed at the Laboratório de Pesquisa em HIV/AIDS (Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil) using OpenText TeleForm 11.1 software (Waterloo, ON, Canada).
Specimens obtained from recruited conscripts were tested for hepatitis B and C using the Elecsys HBsAg II (Roche Diagnostics, Mannheim, Germany) and the Elecsys Anti-HCV II (Roche Diagnostics, Mannheim, Germany) assays, respectively. A signal-to-cut-off ratio (S/CO) equal to or greater than 1.00 is suggested to be positive for anti-HCV and HBsAg by the manufacturer. All hepatitis tests were performed according to the manufacturer's instructions by trained technicians and were conducted at a central laboratory (Vespasiano, MG, Brazil).
2.3. Statistical analysis
All analyses were performed using SPSS Statistics, version 22.0 software (IBM Corp, Armonk, NY). The analyses incorporated data weighting, clustering (as selection commissions with different sizes were included), and stratification. Since the dataset was obtained using a complex sampling procedure that combined stratification and clustering, the design of the survey was incorporated into the statistical analysis of the data. Additionally, a calibration procedure was applied for the samples according to the census distribution by population size of the city of residence (less than 80,000 inhabitants, 80,000–199,999 inhabitants, and equal to or greater than 200,000 inhabitants), and educational levels.
Qualitative variables were presented as absolute and relative frequencies, and quantitative variables were presented as means and standard deviations (SD). P values < .05 were considered statistically significant. The hepatitis B and C prevalence rates were expressed with a binomial confidence interval (CI) of 95%.
2.4. Ethics
This study was approved by the Brazilian National Commission of Ethics in Research (CONEP), register number 278.616 on May 21, 2013. This study also obtained approval from the local Institutional Review Board of the coordinating center (Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil; register number 1.074.338) on May 22, 2015; updated on February 24, 2016 (register number 1.422.093). All participants signed a written consent form.
3. Results
3.1. General characteristics
From an estimated sample size of 39,996 conscripts, 38,247 individuals aged 17 to 22 years were enrolled in the study; 965 (2.5%) were excluded due to lack of information regarding age, origin (municipality), or educational levels. Thus, 37,282 (93.2%) conscripts across Brazil were included in the study. The general characteristics of the study population have previously been described in detail.[8] Briefly, the mean age of the participants was 18 years (SD: 0.8), 98.2% (n = 36,436) were single, and 93.6% (n = 34,894) lived with their parents or relatives. Regarding educational levels, 93.5% (n = 34,860) of conscripts completed elementary education, 50.7% (n = 18,908) completed high school, and 67.0% (n = 24,852) were still in school.
3.2. Hepatitis B and C prevalence
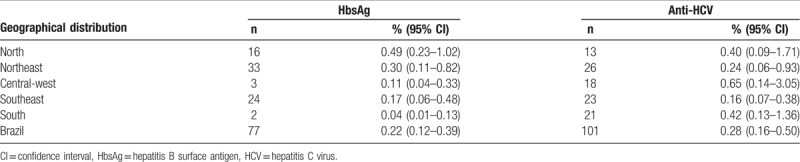
The estimated HBsAg and anti-HCV seroprevalence rates across the country by region are shown in Table 1. The estimated HBsAg rate was 0.22% (n = 77), ranging from 0.04% in the South to 0.49% in the North Region. The estimated anti-HCV seroprevalence rate was 0.28% (n = 101). The Central-west Region presented the highest anti-HCV seroprevalence rate (0.65%, n = 18), followed by the South (0.42%, n = 21) and North Regions (0.40%, n = 13). From 101 anti-HCV positive individuals, 96 presented a low-positive result (S/CO < 10.00) and 5 presented a high-positive result (S/CO≥10.00). No patients were found to be simultaneously HBsAg and anti-HCV positive.
Table 1.
Estimated seroprevalence of hepatitis B surface antigen and anti-hepatitis C virus antibodies among conscripts by region in Brazil, 2016.
3.3. Risk factors
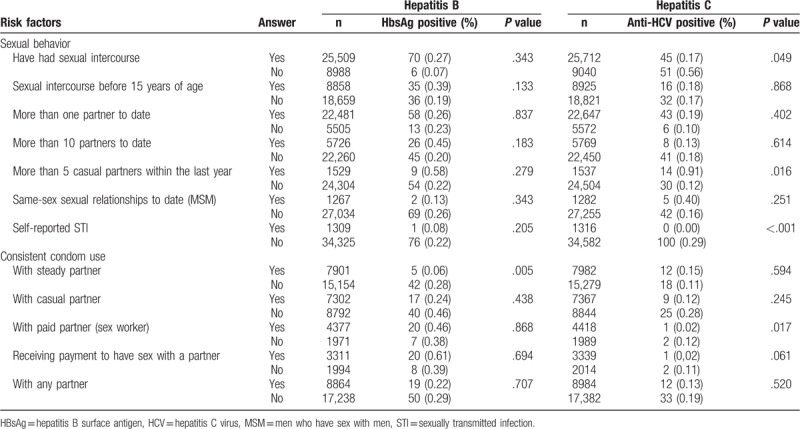
Table 2 summarizes the risk factors associated with a positive HBsAg and an anti-HCV positive status among conscripts, when compared to a negative HBsAg and anti-HCV status. Among the anti-HCV positive conscripts, 53% (n = 51, 0.56%, P = .049) reported they had never had sexual intercourse, vs 12% (n = 6, 0.07%, P = .343) in the positive HBsAg group. Regarding self-reported STI status, the vast majority of positive anti-HCV (n = 100, 0.29%, P < .01) and positive HBsAg (n = 76, 0.22%, P = .205) conscripts reported not having a STI. From those conscripts who tested positive for HBsAg, 89% (n = 42, 0.28%, P = .005) reported not making consistent use of condoms with steady partners. Although not statistically significant, this behavior was conserved with any partner, except with paid partners (sex workers) or when receiving payment to have sex, where condom use was 26% (n = 7, 0.38%, P = .868) and 29% (n = 8, 0.39%, P = .694), respectively. Among anti-HCV positive conscripts, 40% (n = 12, 0.15%, P = .594) reported the use of condoms with steady partners, 36% (n = 9, 0.12%, P = .245) with casual partners, and only 26.7% (n = 12, 0.13%, P = .520) with any partner.
Table 2.
Risk factors associated with a positive hepatitis B surface antigen and anti-hepatitis C virus antibody test result among Brazilian conscripts, 2016.
Regarding hepatitis B vaccination, 23.5% (n = 8,412) of the individuals reported being unvaccinated and 47.4% (n = 16,970) did not know if they had been vaccinated. From the 10,388 (29%) conscripts who reported having taken the vaccine, only 788 (7.9%) reported having taken the 3 recommended doses, while 1987 (19.8%) took 2 doses, 1935 (19.3%) received 1 dose, and the remaining conscripts did not know how many doses they had taken (n = 5,327; 53.1%).
Of note, 63.3% (n = 21,717) of the young male conscripts reported not knowing of any health services where tests for HBV and HCV could be performed free of charge, even though such free services are offered as part of the public health system.
3.4. Knowledge about hepatitis transmission
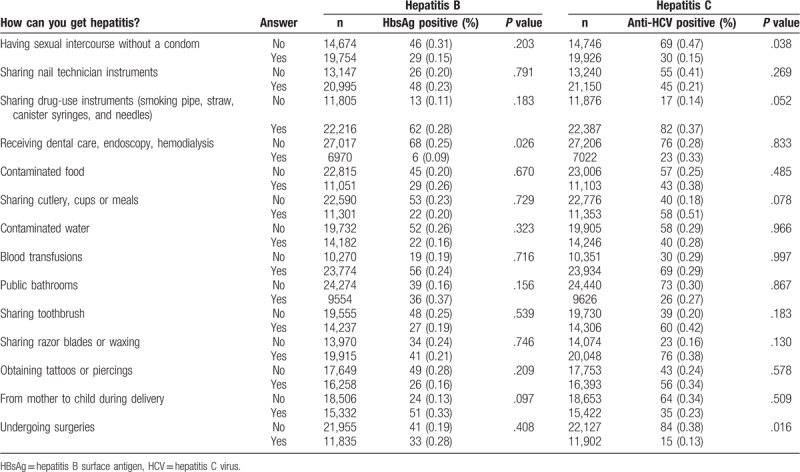
Table 3 presents the results of the questions which verified the knowledge about hepatitis transmission according to the serological status for hepatitis B and C. In the anti-HCV positive group, it is noteworthy that most of conscripts answered that hepatitis cannot be transmitted by sexual relations (n = 69, 0.47%, P = .038), nor by undergoing surgeries (n = 84, 0.38%, P = .016). Similarly, in the HBsAg-positive group, most of the conscripts answered that hepatitis cannot be transmitted when receiving dental care, endoscopy, or hemodialysis (n = 68, 0.25%, P = .026).
Table 3.
Association between knowledge about hepatitis transmission and a positive hepatitis B surface antigen and anti-hepatitis C virus antibody test result among Brazilian conscripts, 2016.
4. Discussion
Since 2003, all cases of viral hepatitis detected in Brazil are subject to compulsory notification on the national notifiable diseases information system (SINAN). Data from the passive notification-based system are not sufficiently reliable to estimate infection rates in the general population since a large number of viral infections are asymptomatic, the etiology of notified symptomatic cases cannot always be confirmed, and most surveys are conducted in restricted geographical areas.[9] In 2016, the WHO released the Global Health Sector Strategy on Viral Hepatitis, 2016–2021 (WHO, 2016). The goal of the strategy is to eliminate viral hepatitis as a major public health challenge by 2030.[10] Brazil has signed on to implement the goals outlined in this document, and has since established its own action plans towards meeting these goals. One of the action plans involves estimating cases of hepatitis at the national level, based on epidemiological data.[2] The current study is the first to report HBsAg and anti-HCV seroprevalence rates among Brazilian army male conscripts at the national level.
The WHO classifies hepatitis endemicity according to the prevalence of the HBsAg, and the anti-HCV, a serological marker of viremia and a serological marker of past or present infection, respectively.[3,4] Currently, Brazil has intermediate to low hepatitis endemicity depending on the region, state, and city.[11]
We found a HBsAg seroprevalence of 0.22% among the Brazilian conscripts in this study. Data from a systematic review performed by Schweitzer et al showed a hepatitis B seroprevalence rate of 0.65% for both sexes in all age groups in Brazil. Higher HBsAg seroprevalences of 0.49% (95% CI 0.23–1.02) and 0.30% (95% CI 0.11–0.82) were observed in the North and Northeast regions, respectively.[12]
In our study, the estimated anti-HCV seroprevalence rate among the Brazilian conscripts was 0.28% (n = 101). This prevalence is lower than the overall HCV antibody prevalence of 0.7% obtained in a previous survey among Brazilian conscripts (Villar, 2015) or the estimated prevalence of 1.38% for both sexes in the Brazilian population, reported from a cross-sectional study.[13,14] Our data show that the Central-west Region presented the highest anti-HCV seroprevalence rate (0.65%, n = 18), followed by the South (0.42%, n = 21) and the North (0.40%, n = 13).
The risk factors associated with a positive anti-HCV status were having had sexual intercourse, self-reported history of having a STI and having more than 5 casual partners within the last year, while the risk factor associated with a positive HBsAg status was not making consistent use of condoms with steady partners. Although most of the risk factors assessed in our study are well-described predictors[3,4] for viral hepatitis, we did not observe such association among Brazilian conscripts.
The association between the number of sexual partners and positivity of serologic markers of hepatitis B and other STIs has often been reported in Brazilian studies conducted at blood donation centers, in specific groups, or in the general population.[15] Data available at the 2018 Brazilian Epidemiological Bulletin of Viral Hepatitis indicated that during 2017, sexual transmission was the probable source of infection in 21.2% and 9.2% of the of HBV and HCV infections, respectively. Thus, in Brazil, sexual transmission overtook drug use (8.1%), and blood transfusions (6.8%) as the source of transmission of these infections in 2017.[2]
Sexual transmission of viral hepatitis B plays a major role in the spread of the hepatitis B epidemic, whereas sexual transmission of viral hepatitis C, although possible, is much less common. Special attention should be given to certain populations such as men who have not been vaccinated against hepatitis B virus and heterosexual persons with multiple sexual partners. Safer sex practices, including minimizing the number of sexual partners and consistently and correctly using male and female condoms, offer powerful protection against viral hepatitis B and C and a wide range of other STIs.[16]
Hepatitis B is preventable with the currently available safe and effective vaccines. The main objective of hepatitis B immunization strategies is to prevent chronic infection and its consequences, including liver cirrhosis and hepatocellular carcinoma. According to the WHO, the complete vaccine series induces protective antibody levels in more than 95% of infants, children and young adults, for acute and chronic infections. Protection lasts at least 20 years and is probably lifelong. Nevertheless, it is known that a proportion of vaccinated children (5–10%) have a poor response to vaccination, and will remain susceptible as adults to acquisition of HBV infection.[3] Approximately 2/3 (29.0%, n = 10,388) of the conscripts self-reported that they have received the hepatitis B vaccine. In Brazil, hepatitis B vaccination was incorporated into the national immunization program in 1996 for all children younger than 1 year of age, but it was only widely available in 1998 due to a vaccine shortage; the vaccine is also recommended for citizens up to 49 years of age.[17] Thus, it would be expected that a higher proportion of participants had reported receiving hepatitis B vaccination; however, due to the young age at which patients receive vaccination, many of the participants did not remember whether they had taken it. Recent studies reported that the vaccine coverage in Brazil in adolescents and young adults is approximately 55% to 60%, based on serological evidence of hepatitis B immunization (anti-HBs titers above 10 UI/L and a negative HBsAg and/or anti-hepatitis B core antigen (anti-HBc) status).[18] Other studies reported that hepatitis B vaccination coverage among military personnel in the Southeast and South regions of Brazil is between 57% and 70%.[13,19] Regarding hepatitis C, as there is no vaccine currently available, primary prevention is aimed at reducing or eliminating transmission due to unsafe injection drug use, unprotected sex with multiple partners, exposure to blood in healthcare settings, and other risky behaviors (for example, tattooing, and body piercing).[4,20]
Additionally, while the majority of primary healthcare units as well as testing and counseling centers offer HBV and HCV testing services, there is nevertheless a low demand for these services among young men; this may be because these young men are unaware of the availability of these services, revealing that there is an urgent need to provide the population with information about the availability of no-cost HBV and HCV testing services.
Both HBV and HCV infections are hepatotropic infections and share common routes of transmission.[21] Being unaware of these routes of transmission imply a high risk of acquiring these infections and also unknowingly transmitting them to others. Although the major HBV transmission route in Brazil is unprotected sexual intercourse,[2] less than half (n = 14,674) of the conscripts were aware of this information. Concerning HCV transmission, sharing drug-use instruments[2] accounts for most of the infections acquired in the country; however, approximately one-third (n = 11,876) of the study population was unaware of this information. We also observed lack of knowledge regarding well-known routes of transmission[3,4] (i.e., blood transfusions, sharing nail technician instruments, sharing razor blades or waxing and obtaining tattoos or piercings). Similarly, other studies have already demonstrated a low level of knowledge about viral hepatitis, suggesting the need for strategies to increase access to information, such as awareness campaigns targeted towards this population.[22,23]
Early diagnosis of hepatitis infections is critical for effective treatment and care. The DIAHV has recommended that to define a seropositive HCV infection status, a positive anti-HCV screening test result should be confirmed by a positive HCV RNA assay.[24] HCV RNA testing was not performed on the specimens in the current study. Nevertheless, recent studies have proposed the use of signal-to-cut-off (S/CO) ratios of screening-test-positive results as an alternative to a supplemental test in some circumstances, minimizing the number of specimens that require supplemental testing and providing a result that has a high probability of reflecting the person's true antibody status.[25,26] Recent studies have shown that a Elecsys Anti-HCV II S/CO ratio within 12.27 to 19.0 is predictive of a true positive result in ≥95% of the cases.[27,28]
This study was subject to some limitations:
-
1.
the screening methodology for anti-HCV employed in this study shows high sensitivity, which may have resulted in false-positive results, and consequently an overestimation of HCV seroprevalence levels;
-
2.
data regarding Hepatitis B vaccination were self-reported by the conscripts;
-
3.
illiterate conscripts were dismissed from military service and were not enrolled in the study;
-
4.
self-reported questionnaires were used, which may have led to losses due to inadequate responses regarding relevant information.
Nevertheless, these limitations were minimized by the use of standardized data collection instruments and laboratory measurements, the large size of the sample, and by the study design applied. Noteworthy, the DIAHV designed this study to assess the frequency and distribution of the STIs allowing the generalizability of the results at national level.
In conclusion, our data suggest a low prevalence of HBV and HCV infections (0.22% and 0.28%, respectively) and relatively low rates of self-reported HBV immunization among Brazilian young men. History of STIs, high number of sex partners, inconsistent use of condoms, and lack of awareness regarding the routes of transmission were significantly associated with HBV and HCV infections.
In order to achieve the goal of eliminating viral hepatitis, efforts to improve access to HCV and HBV information, testing, and surveillance are indicated. Thus, as more infected patients are diagnosed and the understanding of the distribution of these viral infections in the general population increases, groups at increased risk may be identified more easily and may be targeted for implementing prevention and control programs.
Further research among the illiterate male population is advised. Also, the inclusion of other serological markers for HBV that allow for stratifying patients based on phases of infection, and the use of HCV RNA testing to prevent overestimation in the HCV seroprevalence rates are recommended.
Acknowledgments
The authors acknowledge the Brazilian Ministry of Defense and all study participants. They also thank Dr Célia Landmann Szwarcwald and her team at FioCruz, Rio de Janeiro, RJ, Brazil for statistical assistance.
Author contributions
Conceptualization: Gerson Fernando Mendes Pereira, Adele Schwartz Benzaken.
Data curation: Sérgio Kakuta Kato.
Formal analysis: Sérgio Kakuta Kato.
Funding acquisition: Leonardo Rapone da Motta, Rosa Dea Sperhacke, Gerson Fernando Mendes Pereira, Adele Schwartz Benzaken.
Investigation: Leonardo Rapone da Motta, Aline De Gregori Adami, Sérgio Kakuta Kato.
Methodology: Gerson Fernando Mendes Pereira.
Project administration: Leonardo Rapone da Motta, Rosa Dea Sperhacke.
Resources: Leonardo Rapone da Motta, Rosa Dea Sperhacke.
Supervision: Leonardo Rapone da Motta, Aline De Gregori Adami, Rosa Dea Sperhacke, Gerson Fernando Mendes Pereira.
Validation: Leonardo Rapone da Motta, Sérgio Kakuta Kato, Machline Paim Paganella, Gerson Fernando Mendes Pereira.
Visualization: Leonardo Rapone da Motta, Aline De Gregori Adami, Rosa Dea Sperhacke, Sérgio Kakuta Kato, Machline Paim Paganella.
Writing – original draft: Leonardo Rapone da Motta, Aline De Gregori Adami, Sérgio Kakuta Kato, Machline Paim Paganella.
Writing – review & editing: Leonardo Rapone da Motta, Aline De Gregori Adami, Rosa Dea Sperhacke, Machline Paim Paganella, Gerson Fernando Mendes Pereira, Adele Schwartz Benzaken.
Leonardo Rapone da Motta orcid: 0000-0003-3673-2687.
Footnotes
Abbreviations: AIDS = Acquired Immune Deficiency Syndrome, anti-HBc = anti-hepatitis B core antigen, CI = confidence interval, CONEP = Brazilian National Commission of Ethics in Research, DIAHV = Department of Surveillance, Prevention and Control of STI, HIV/AIDS and Viral Hepatitis, Secretariat of Health Surveillance, Ministry of Health of Brazil, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, MSM= men who have sex with men, OR = odds ratio, S/CO = signal-to-cut-off ratio, SD = standard deviation, SINAN = national notifiable diseases information system, STI = sexually transmitted infection, WHO = World Health Organization.
All participants signed a written consent form.
The authors certify that this manuscript is a unique submission that has not been published in part or in full in any form and is not being considered for publication by any other source in any medium.
This study was approved by Brazilian National Commission of Ethics in Research (CONEP), register number 278.616 on May 21, 2013. This study also obtained approval from the local Institutional Review Board of the coordinating center (Universidade de Caxias do Sul, Caxias do Sul, RS, Brazil), register number 1.074.338 on May 22, 2015 and updated on February 24, 2016 (register number 1.422.093).
Financial support for this study was provided by the Brazilian Ministry of Health, through its Secretariat for Health Surveillance and its Department of Prevention, Surveillance, and Control of Sexually Transmitted Infections, HIV/AIDS, and Viral Hepatitis.
The authors have no conflicts of interest to disclose.
References
- [1].World Health Organization. Global Hepatitis Report, 2017. Geneva: World Health Organization; 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ [Acessed December 3, 2018]. [Google Scholar]
- [2].Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Prevenção e Controle das IST do HIV/Aids e das Hepatites Virais. Boletim Epidemiológico Hepatites Virais 2018. Vol 49; 2018. Available at: http://www.aids.gov.br/ptbr/pub/2018/boletim-epidemiologico-de-hepatites-virais-2018 [Acessed December 4, 2018]. [Google Scholar]
- [3].World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. Available at: https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [Acessed May 14, 2019]. [PubMed] [Google Scholar]
- [4].World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis C Infection. Geneva: World Health Organization; 2018. Available at: https://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/ [Acessed May 14, 2019]. [PubMed] [Google Scholar]
- [5].Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine 1999;17:1730–3. [DOI] [PubMed] [Google Scholar]
- [6].Szwarcwald CL, de Carvalho MF, Barbosa Júnior A, et al. Temporal trends of HIV-related risk behavior among Brazilian military conscripts, 1997–2002. Clinics (Sao Paulo) 2005;60:367–74. [DOI] [PubMed] [Google Scholar]
- [7].Szwarcwald CL, Andrade CLTde, Pascom ARP, et al. HIV-related risky practices among Brazilian young men, 2007. Cad Saude Publica 2011;27 Suppl. 1:S19–26. [DOI] [PubMed] [Google Scholar]
- [8].Sperhacke RD, da Motta LR, Kato SK, et al. HIV prevalence and sexual behavior among young male conscripts in the Brazilian army, 2016. Medicine (Baltimore) 2018;971S Suppl. 1:S25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ximenes RA, de A, Pereira LMB, et al. Methodology of a nationwide cross-sectional survey of prevalence and epidemiological patterns of hepatitis A, B and C infection in Brazil. Cad Saude Publica 2010;26:1693–704. [DOI] [PubMed] [Google Scholar]
- [10].World Health Organization. Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021. Geneva: World Health Organization; 2016. Available at: https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/ [Acessed December 12, 2018]. [Google Scholar]
- [11].Pereira VRZB, Wolf JM, Luz CA, et al. Risk factors for hepatitis B transmission in south Brazil. Mem Inst Oswaldo Cruz 2017;112:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [13].Villar LM, do Ó KMR, Scalioni LP, et al. Prevalence of hepatitis B and C virus infections among military personnel. Braz J Infect Dis 2015;19:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pereira LMMB, Martelli CMT, Moreira RC, et al. Prevalence and risk factors of Hepatitis C virus infection in Brazil, 2005–2009: a cross-sectional study. BMC Infect Dis 2013;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ximenes RAA, Figueiredo GM, Cardoso MRA, et al. Population-based multicentric survey of hepatitis B infection and risk factors in the North, South, and Southeast Regions of Brazil, 10–20 years after the beginning of vaccination. Am J Trop Med Hyg 2015;93:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Glob Hepat Program Dep HIV/AIDS. 2016; (June):56. Available at: https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ [Acessed January 4, 2019]. [Google Scholar]
- [17].Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Programa Nacional de Imunizações (PNI): 40 Anos. 1 ed. Brasília: Ministério da Saúde; 2013. Available at: http://bvsms.saude.gov.br/bvs/publicacoes/programa_nacional_imunizacoes_pni40.pdf [Acessed January 7, 2019]. [Google Scholar]
- [18].Souto FJD. Distribution of hepatitis B infection in Brazil: the epidemiological situation at the beginning of the 21 st century. Rev Soc Bras Med Trop 2016;49:11–23. [DOI] [PubMed] [Google Scholar]
- [19].Passos AM, Treitinger A, Spada C. Hepatitis B immunity and vaccination coverage among young adult males in the Air Force in South Brazil. Vaccine 2011;29:9284–8. [DOI] [PubMed] [Google Scholar]
- [20].Manns MP, Buti M, Gane E, et al. Hepatitis C virus infection. Nat Rev Dis Prim 2017;3:17006. [DOI] [PubMed] [Google Scholar]
- [21].World Health Organization. Prevention & Control of Viral Hepatitis Infection: Framework for Global Action. Geneva; 2012. Available at: https://www.who.int/hiv/pub/hepatitis/Framework/en/ [Acessed January 8, 2019]. [Google Scholar]
- [22].Cruz HM, Barbosa JR, Baima Colares JK, et al. Cross-sectional study to determine viral hepatitis knowledge in different urban populations in Brazil. World J Hepatol 2018;10:867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Oliveira SB, Sabidó M, Pascom ARP, et al. State of viral hepatitis knowledge and testing uptake in Brazil: findings from the National Survey of Knowledge, Attitudes and Practices (PCAP-2013). Hepatol Med Policy 2016;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST Aids e Hepatites Virais. Manual Técnico Para o Diagnóstico Das Hepatites Virais. Brasília, DF: Ministério da Saúde; 2018. Available at: http://www.aids.gov.br/pt-br/pub/2015/manualtecnicopara-o-diagnostico-das-hepatites-virais [Accessed January 17, 2019]. [Google Scholar]
- [25].Lai KKY, Jin M, Yuan S, et al. Improved reflexive testing algorithm for hepatitis C infection using signal-to-cutoff ratios of a hepatitis C virus antibody assay. Clin Chem 2011;57:1050–6. [DOI] [PubMed] [Google Scholar]
- [26].Granados-García V, Contreras AM, García-Peña C, et al. Cost-effectiveness analysis of different testing strategies that use antibody levels to detect Chronic Hepatitis C in blood donors. PLoS One 2016;11:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang R, Guan W, Wang Q, et al. Performance evaluation and comparison of the newly developed Elecsys anti-HCV II assay with other widely used assays. Clin Chim Acta 2013;426:95–101. [DOI] [PubMed] [Google Scholar]
- [28].Pan J, Li X, He G, et al. Reflex threshold of signal-to-cut-off ratios of the Elecsys anti-HCV II assay for hepatitis C virus infection. J Infect Dev Ctries 2016;10:1031–4. [DOI] [PubMed] [Google Scholar]


