Abstract
This study means to investigate a combination of noninvasive methods in diagnosis of minimal or mild endometriosis expecting to narrow down the range of laparoscopic exploration for female infertility.
It is a retrospective case control study of totally 447 patients suspected unexplained infertility before surgery were eligible from May 2012 to February 2017. Of these, 299 patients were laparoscopy-proved minimal or mild endometriosis group, the remaining 148 patients served as control group (normal pelvis). Preoperative age, duration of infertility, type of infertility, body mass index, baseline follicle-stimulating hormone, anti-Müllerian hormone, serum CA125, clinical symptoms, findings on vagino-recto-abdominal examinations and pregnancy prognosis had been recorded. Every variable and their combinations were evaluated.
Any single factor had limited diagnostic value. The cut-off value for CA125 was 19.25 IU/L. Parallel testing had a higher sensitivity at 81.3%. Serial tests of vagino-recto-abdominal examination combined with dysmenorrhea or positive CA125 got reasonable sensitivity (51.4% and 49%), remarkable high specificities (95.7% and100%) and Positive Predictive Value (96.4% and 100%). Multivariate logistic regression identified the following factors in decreasing order of importance: (1) vagino-recto-abdominal examinations, (2) CA125, (3) dysmenorrhea, their ORs being 16.148, 3.796, and 2.809, respectively. The spontaneous pregnancy rate (50.8%) in minimal or mild endometriosis was higher than control (35.6%, P = .043).
A combination of noninvasive diagnostic methods had certain preoperative diagnostic value of minimal or mild endometriosis, which might benefit some patients from avoiding laparoscopic surgery.
Keywords: diagnosis, endometriosis, infertility, laparoscopy, noninvasive methods
1. Introduction
Endometriosis, a common gynecological disorder in which the endometrial-like glands and stroma grows outside the uterus,[1] most commonly in the pelvis,[2] is associated with pelvic pain, subfertility, dysmenorrhea and dyspareunia.[3,4] It has been reported that the monthly fecundity rate is lower in women with minor endometriosis than in those with unexplained infertility.[5] On other hand, the infertile women have been proved wide range (14–67%) of endometriosis by laparoscopy.[6,7]
The optimal means of accurately diagnosing endometriosis in a timely manner is controversial. Diagnosis of endometriosis is usually delayed by an average of 8 to 11 years, which has significant consequences in terms of disease progression.[8] Endometriosis can be classified into different phenotypes including ovarian endometriosis, peritoneal endometriosis, deep endometriosis and other types. It has been established that ultrasound and pelvic examination can quite accurately discriminate ovarian endometriosis from other ovarian cysts.[9,10] They are also highly sensitive and specific for diagnosis of deep endometriosis.[11] However, these noninvasive methods have limited capacity to diagnose superficial peritoneal disease and endometriosis-associated adhesions, mostly classified as minimal or mild endometriosis, namely the revised American Fertility Society (rAFS) classification system stages I and II; these patients are often classified as having unexplained infertility prior to surgery.
The current gold standard procedure for diagnosing endometriosis is laparoscopy.[12,13] Laparoscopic surgery could give consideration to both diagnosis and treatment. The surgical destruction of minimal to mild endometriosis and associated adhesions was indeed demonstrated to enhance fecundity comparing with diagnostic laparoscopy alone.[14] It was reported that the fertility rate was significantly improved after the removal of minimal and mild endometriosis (4.7 per 100 person-months in the group with laparoscopic treatment group and 2.4 per 100 person-months in the diagnostic laparoscopy group).[15] A study in 2014 concluded that women who had undergone complete removal of endometriotic lesions (n = 399) subsequently had a higher implantation rate (30.9% versus 23.9%, P = .02), pregnancy rate (40.1% vs 29.4%, P = .004), and live-birth rate per ovum retrieval cycle (27.7% vs 20.6%, P = .04) than women who underwent diagnostic laparoscopy only (n = 262).[16] While, the disadvantages of IVF cannot be ignored, including the great expense (especially if more than one cycle is required), the inconvenience for frequent injections and monitoring for several weeks and the risks of multiple pregnancy and ovarian hyper-stimulating syndrome.[17] But non-selective laparoscopy is also waste of medical resource as one-half to two-third of negative finding, let alone the complication of operation.[18]
In the present study, we aimed to
-
(1)
evaluate the diagnostic value of a combination of noninvasive methods including evaluation of clinical symptoms, vagino-recto-abdominal examination and serum CA125 (carbohydrate antigen 125) concentration for minimal and mild endometriosis so that can narrow down the population who need laparoscopic exploration and
-
(2)
verify the pregnancy prognosis after therapeutic laparoscopy.
2. Materials and methods
This is a retrospective case control study of patients who attended the Reproductive Center of the Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University between May 2012 and February 2017. This study was approved by the Reproductive Medical Ethics Committee of Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
2.1. Patients
During the study period, 447 women were enrolled in this study. The flow chart was shown in Figure 1. They were selected from women who had undergone laparoscopy for evaluation of suspected unexplained infertility before surgery in our Laparoscopy Unit.
Figure 1.
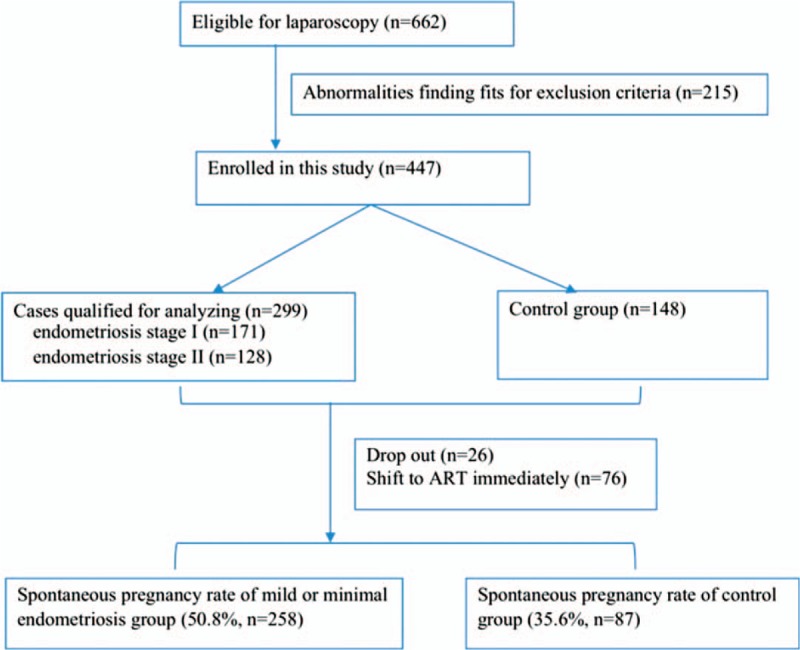
Flow chart.
Women who had undergone laparoscopy for evaluation of infertility were divided into 2 groups according to their laparoscopic findings:
-
(1)
mild or minimal endometriosis group, endometriosis stage I or II according to the rAFS classification system;[14] and
-
(2)
control group, normal pelvis. The revised inclusion and exclusion criteria are as follow:
Inclusion criteria were: suspected unexplained infertility prior to surgery, no pregnancy after more than 1 year without contraception, reproductive age between 20 and 40 years, regular menstrual cycles and partner with normal semen test.
Exclusion criteria were: finding tubal abnormality and inflammation related pelvic adhesion, acute abdomen, malignant tumor, uterine leiomyoma, adenomyosis, uterine malformation, rAFS endometriosis stage III or IV according to the America Fertility Society classification and other benign ovarian tumors during laparoscopy examination, and previous surgical treatment or medications for endometriosis.
2.2. Data collection and analysis
Data collected included age, duration of infertility, type of infertility, body mass index (BMI), baseline follicle-stimulating hormone (FSH) (day 3–5 of menstrual cycle), anti-Müllerian hormone (AMH), dysmenorrhea, other related symptoms (anal bulge and other intestinal symptoms, and/or dyspareunia, and/or chronic pelvic pain), positive signs on vagino-recto-abdominal examinations (tender posterior fornix nodules/pains, and/or tender uterosacral ligament nodules/pains) and pregnancy outcomes after surgery.
Vagino-recto-abdominal examinations were all performed by the same experienced chief Gynecologist within three days prior to surgery to ensure the examination was accurate. Serum CA125 concentrations were measured during the mid-follicular phase within 30 days before surgery. FSH was measured within days 3 to 5 of the menstrual cycle before surgery.
2.3. Operative procedure
The whole abdomen was explored, including the pelvic peritoneum, reproductive organs, colonic space, sub septum, and hepatic and splenic surfaces. Minimal or mild endometriosis was diagnosed when typical red, blue, and/or brown endometriotic lesions were identified on the pelvic peritoneum and/or organ surfaces. Eradication by excision or ablation of all endometriotic lesions, relaxing of adhesions and restoration of normal anatomy were performed wherever possible, after which the laparoscopic diagnosis of endometriosis was confirmed by histopathological examination of the operative specimens. The pelvis was irrigated with 1000 mL normal saline before closure.
2.4. Pregnancy prognosis
Patients were followed up until March 2018. Only spontaneous pregnancies were analyzed. Cases were censored when the patient failed to follow up or underwent any assisted reproductive technology (ART).
2.5. Statistical analysis
Data were analyzed using SPSS 21.0 software. ROC curves were used to calculate the cut-off point for serum CA125 in diagnosis of infertile women with minimal or mild endometriosis. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each method was calculated. Variables were compared between groups by the χ2 test. Logistic regression analysis was used to analyze combinations of various methods. The P value of <.05 was considered significant.
3. Results
Of the 447 women undergoing laparoscopy for investigation of suspected unexplained infertility, 299 patients had confirmed minimal or mild endometriosis finally, including endometriosis stage I (n = 171) or II (n = 128) according to the revised American Fertility Society classification system and the averages of the scoring were 2.62 ± 1.24 and 10.00 ± 3.13, respectively. The other 148 was as control group (normal pelvis). No major complications of laparoscopy were recorded.
3.1. Preoperative patient characteristics
No differences were identified between the groups in mean age, duration of infertility, BMI, baseline FSH and AMH (all P > .05). Whereas, there showed significant differences in type of infertility, vagino-recto-abdominal examination, dysmenorrhea, other related symptoms and serum CA125 concentration (all P < .05, in Table 1).
Table 1.
Selected factors according to study group.

3.2. ROC curve for serum CA125 and minimal or mild endometriosis
Table 1 showed that the average CA125 of minimal or mild endometriosis group with infertility was 25.19 ± 14.94 IU/L, and the control group was 14.12 ± 7.93 IU/L, which were both lower than that of the traditional cut-off value of CA125 (35 IU/L). Therefore, it could be better to find out a more appropriate cut-off value.
The cut-off value for serum CA125 determined by ROC curve for diagnosing infertility with minimal or mild endometriosis was 19.25 which were calculated according to the maximum Youden index, and the area under the curve was 0.781 (Fig. 2).
Figure 2.
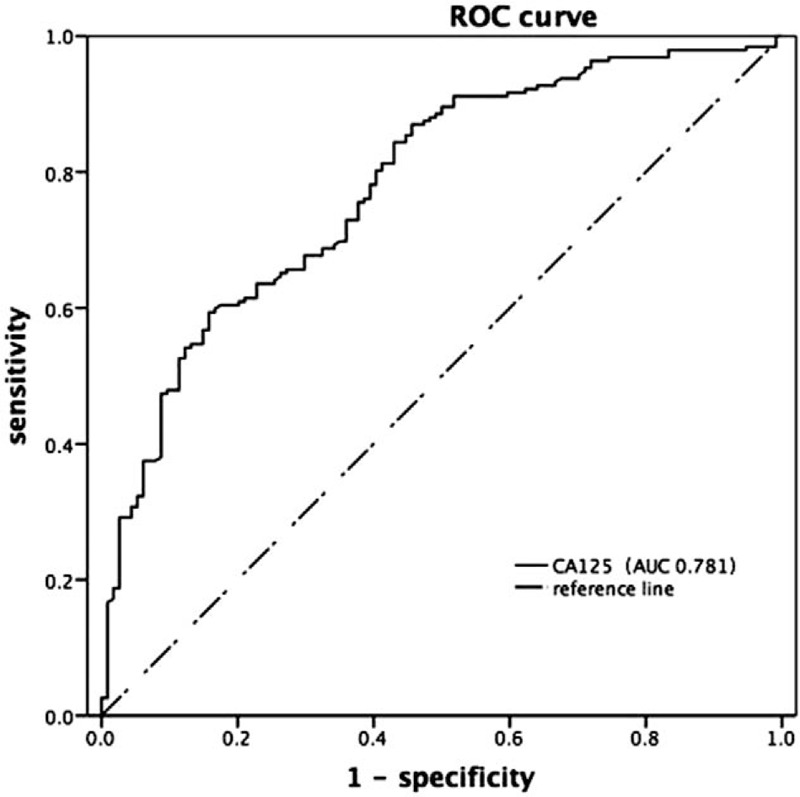
ROC curve of serum CA125 in diagnosis of infertility with minimal or mild endometriosis.
3.3. Diagnostic value of any single method
Any single method had limited diagnostic value, as summarized in Table 2. The sensitivities of dysmenorrhea, other related symptoms, vagino-recto-abdominal examination, and CA125> 19.25 IU/L for diagnosis of infertility with minimal or mild endometriosis were 59.9%, 13.0%, 71.2%, and 59.4%, respectively, all of which are insufficient, especially other related symptoms. However, their specificities were relatively better as follows: 74.3%, 95.9%, 89.2%, and 83.3%, respectively.
Table 2.
Individual methods for diagnosing minimal or mild endometriosis before surgery.

3.4. Diagnostic value of combinations of noninvasive methods
When parallel testing is used for diagnosis, it is positive if one of several indexes is diagnosed as positive. When serial testing is used for diagnosis, it is positive if all factors are positive. The sensitivity and specificity of combinations of diagnostic methods were assessed with the aim of identifying a combination that might be superior to any single variable.
-
(1)
Parallel testing had a high sensitivity of 81.3%, but an insufficient specificity of 58.8%.
-
(2)
Serial tests between two methods all presented high specificities (95.7–100%) and PPV (94.1–100%) values, but insufficient sensitivities of 9.7%-51%. The two combinations with a slightly higher sensitivity were vagino-recto-abdominal examination + dysmenorrhea and vagino-recto-abdominal examination + CA125, as shown in Table 3.
Table 3.
Combined use of 2 methods for diagnosing minimal or mild endometriosis before surgery.

3.5. Logistic regression analysis of various diagnostic methods
To further evaluate the ability of various methods to diagnose infertility with minimal or mild endometriosis, logistic regression analysis was carried out by using the groups as the dependent variable and the following factors, which were statistically significant according to univariate analysis, as independent variables: vagino-recto-abdominal examination, other related symptoms, type of infertility, dysmenorrhea, and serum CA125 concentration. Multivariate logistic regression identified the following variables in decreasing order of importance:
-
(1)
vagino-recto-abdominal examination,
-
(2)
CA125,
-
(3)
dysmenorrhea, their ORs being 16.148, 3.796, and 2.809, respectively.
Other related symptoms and the type of infertility did not qualify for inclusion in the model. The result of logistic regression analysis is shown in Table 4.
Table 4.
Multivariate logistic regression analysis of methods used to diagnose infertility with minimal or mild endometriosis.

3.6. Outcome of spontaneous pregnancies
Women unable to contact (n = 26) or directly asked for ART postoperatively were excluded (n = 76). Thus, 345 women were assessed for spontaneous pregnancy outcomes, including 258 in endometriosis group and 87 unexplained infertilities in control group.
The spontaneous pregnancy rate was 50.8% (131/258) in the endometriosis group, which appeared higher than in the unexplained infertility group (35.6%, 31/87) and the difference was statistically significant (P = .043).
4. Discussion
Most women with minimal or mild endometriosis have no specific symptoms, hence it is easy for it to escape diagnosis or be misdiagnosed.[7] The current gold standard procedure for diagnosing endometriosis is laparoscopy,[12,13] which also offers the option of simultaneous treatment of lesions and/or adhesions.[19] The additional advantages of laparoscopic surgery by the public: treatment can be completed during the procedure, lower monetary and time cost, fewer adverse effects and complications, and mostly a single fetus.[17] Unfortunately, being an invasive procedure, laparoscopy is associated with rare but significant potential risks.[20] While it is not feasible for all infertile women to undergo laparoscopic diagnosis. A lack of proven noninvasive diagnostic methods has hindered the ability of clinicians to choose appropriate treatment, including surgery.
To the best of our knowledge, our study is the first to identify a combination of positive pelvic signs, serum biomarkers, and clinical symptoms for diagnosing minimal or mild endometriosis in infertile women. The most important goal of this study was to avoid missing the correct diagnosis in any infertile women with endometriosis or associated adhesions or other related pathology who might benefit from surgery.
4.1. Single noninvasive diagnostic methods
Few studies on diagnosis of minimal or mild endometriosis have been reported. In our series, any single noninvasive method had limited sensitivity (13.0–71.2%) for such diagnoses, but good specificity (74.3–95.9%). Vagino-recto-abdominal examination was the most valuable of these methods, having 71.2% sensitivity and 89.2% specificity, reminding clinicians of the importance of careful pelvic examination, especially vagino-recto-abdominal examination, in assessment of a woman with suspected endometriosis. Vagino-recto-abdominal examination is low-risk and assists in locating disease, especially that in regions within easy reach of examining fingers such as the uterosacral ligaments and posterior compartment.[21]
Serum biomarkers can be useful in women with a history of chronic pelvic pain and/or subfertility without evidence of endometriosis on ultrasound.[8] CAl25 is the most widely-used serum-screening marker for endometriosis; however, its diagnostic value has always been controversial. One study has shown that using the usual cut-off for CA125 (35 IU/L), the sensitivity for the diagnosis of endometriosis were poor (27%).[22] Other study has shown that serum CAl25 concentration is a sensitive diagnostic indicator of stage III and IV endometriosis, but not of stage I or II or deep invasive endometriosis.[23] Several studies have found high CA125 concentrations during menstruation and the premenstrual late secretory phase,[24] especially in women with endometriosis.[16] However, fluctuations in CA125 concentration during the menstrual cycle may affect the accuracy of this test. In our study, we measured CA125 concentration during the mid-follicular phase to optimize the reliability of our assay and calculated a cut-off point for serum CA125 of 19.25 for diagnosing minimal or mild endometriosis by constructing a ROC curve. Using this cut-off point, the specificity (83.3%) and PPV (85.7%) were good, whereas the sensitivity (59.4%) and NPV (54.9%) were poor. Other markers for diagnosing endometriosis comprise CA72, CA153, TAG72, and CA199, all of which reportedly have low sensitivity.[25]
4.2. Combinations of diagnostic methods
As stated above, any single method has limited diagnostic accuracy. Several authors have investigating combinations of diagnostic methods. The combination of high serum CA125 with detection of pelvic nodules reportedly has a sensitivity of 87% for diagnosing endometriosis, but does not assist its classification.[26] Another study showed that noninvasive tools are useful in identifying women with ovarian, but not non-ovarian endometriosis.[8] As to minimal or mild endometriosis, a few studies have reported diagnosis by noninvasive methods, mostly serum biomarkers. One study showed a combination of CA125 (19.9 IU/L) and prolactin concentrations (14.8 ng/mL) allowed the diagnosis of minimal or mild endometriosis with sensitivity and specificity of 77% and 88%.[22]
It would be fantastic to have a diagnostic method with high sensitivity and specificity; however, this is usually impossible. In our study, parallel testing increased the diagnostic sensitivity to 81.3%, thus reducing the rate of misdiagnoses, whereas serial tests of two indicators significantly improved specificity (from 95.7% to 100%) and PPV (from 94.1% to 100%), thus greatly reducing the misdiagnosing rate, especially the vagino-recto-abdominal examination plus dysmenorrhea or CA125. So we believe these 2 noninvasive serial tests will get a passable sensitivity and a wonderful low misdiagnosis rate for infertility with minimal or mind endometriosis besides laparoscopic exploration.
In our study, the ratio of primary infertility is higher in endometriosis group, which may due to the retrospective study with bias in sample selection. But, the infertility type was not an influential factor of diagnosis in the multivariate logistic regression analysis, which was carried out to quantify that vagino-recto-abdominal examination had the highest diagnostic value, followed by CA125, clinical symptoms, which remind us to improve the gynecological examination skills, especially the vagino-recto-abdominal examination for the well-beings of patients.
Above all, we can say that a combination of noninvasive diagnostic methods had certain preoperative diagnostic value of minimal or mild endometriosis, which might benefit some patients from avoiding laparoscopic surgery.
4.3. Outcomes of spontaneous pregnancies
Our study verified a substantial spontaneous pregnancy rate (50.8%) in women with minimal or mild endometriosis, which was higher than control group (35.6%, P = .043). It might suggest that suspected minimal or mild endometriosis seem to be choosing laparoscopy firstly regarding of the good spontaneous pregnancy rate and the advantages of laparoscopy. While the unexplained infertile women with normal pelvis might search for a more effective method to get pregnancy, because of low spontaneous pregnancy rate.
4.4. Limitations of present study
There are some limitations in our paper regretfully. First, it is possible that recall bias existed as a retrospective study, so that the data relating to diagnosis of minimal or mild endometriosis and the spontaneous pregnancy rate should be considered preliminary, expecting to be confirmed by further prospective studies. Second, our result is based on a single center in a clear geographical area. It would be of interest to compare the data with other centers to determine if the same pattern could be observed elsewhere. Third, the accuracy of vagino-recto-abdominal examinations was depended on the Gynecologist's technical level, and there would be a learning curve to generalize this pre-surgical diagnosis method.
Acknowledgments
We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
Conceptualization: Baijia Li, Songying Zhang, Xiaona Lin.
Data curation: Huaying Yu, Baijia Li.
Formal analysis: Huaying Yu, Baijia Li.
Investigation: Huaying Yu.
Supervision: TinChiu Li, Songying Zhang, Xiaona Lin.
Writing – original draft: Huaying Yu.
Writing – review & editing: Huaying Yu, TinChiu Li, Songying Zhang, Xiaona Lin.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, AMH = anti-Müllerian hormone, ART = assisted reproductive technology, BMI = body mass index, CA125 = carbohydrate antigen 125, FSH = follicle-stimulating hormone, NPV = negative predictive value, OR = odds ratio, PPV = positive predictive value, rAFS = the revised American Fertility Society.
This study was funded by the Key Research and Development Program of Zhejiang Province (2017C03022) and the National Natural Science Foundation of China (81671435, 81471505).
The authors have no conflicts of interest to disclose.
References
- [1].Signorile PG, Baldi F, Bussani R, et al. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res 2009;28:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sharkwy IAEE. Combination of non-invasive and semi-invasive tests for diagnosis of minimal to mild endometriosis. Arch Gynecol Obstetr 2013;288:793–7. [DOI] [PubMed] [Google Scholar]
- [3].Milingos S, Mavrommatis C, Elsheikh A, et al. Fecundity of infertile women with minimal or mild endometriosis. a clinical study. Arch Gynecol Obstet 2002;267:37–40. [DOI] [PubMed] [Google Scholar]
- [4].Sinaii N, Plumb K, Cotton L, et al. Differences in characteristics among 1000 women with endometriosis based on extent of disease. Fertil Steril 2008;89:538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Opøien HK, Fedorcsak P, Byholm T, et al. Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod Biomed Online 2011;23:389–95. [DOI] [PubMed] [Google Scholar]
- [6].Forman RG, Robinson JN, Mehta Z, et al. Patient history as a simple predictor of pelvic pathology in subfertile women. Hum Reprod 1993;8:53. [DOI] [PubMed] [Google Scholar]
- [7].Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a pro- spective study. Hum Reprod 1996;11:387–91. [DOI] [PubMed] [Google Scholar]
- [8].Gajbhiye R, Bendigeri T, Ghuge A, et al. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis: a multicenter study. Reprod Sci. 24 (3). doi: 10.1177/1933719116657190. [DOI] [PubMed] [Google Scholar]
- [9].Eskenazi B, Warner M, Bonsignore L, et al. Validation study of nonsurgical diagnosis of endometriosis. Fertil Steril 2001;76:929–35. [DOI] [PubMed] [Google Scholar]
- [10].H Holsbeke CV, Calster BV, Guerriero S, et al. Imaging in gynaecology: how good are we in identifying endometriomas? Facts Views Vis Obgyn 2009;1:7. [PMC free article] [PubMed] [Google Scholar]
- [11].Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril 2007;108:886–94. [DOI] [PubMed] [Google Scholar]
- [12].Buchweitz O, Wülfing P, Malik E. Interobserver variability in the diagnosis of minimal and mild endometriosis ☆. Eur J Obstet Gynecol Reprod Biol 2005;122:213–7. [DOI] [PubMed] [Google Scholar]
- [13].Angelos, Daniilidis, George, et al. Comments on the ESHRE recommendations for the treatment of minimal endometriosis in infertile women. Reprod Biomed Online. 2017; 36, 1. doi: 10.1016/j.rbmo.2017.10.103. [DOI] [PubMed] [Google Scholar]
- [14].Daniilidis A, Pados G. Comments on the ESHRE recommendations for the treatment of minimal endometriosis in infertile women. Reprod Biomed Online 2017;S1472648317305734. [DOI] [PubMed] [Google Scholar]
- [15].Duffy J, Arambage K, Fjs C, et al. Laparoscopic surgery for endometriosis. Clin Obstetr Gynecol 2009;52:351. [DOI] [PubMed] [Google Scholar]
- [16].Kafali H, Artuc H, Demir N. Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report. Eur J Obstet Gynecol Reprod Biol 2004;116:85–8. [DOI] [PubMed] [Google Scholar]
- [17].Practice Committee of the American Society for Reproductive Medicine. Role of tubal surgery in the era of assisted reproductive technology: acommittee opinion. Fertil Steril 2015;103:e37. [DOI] [PubMed] [Google Scholar]
- [18].Listed N. Revised American society for reproductive medicine classification of endometriosis. Fertil Steril 1997;67:817–21. [DOI] [PubMed] [Google Scholar]
- [19].Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis and treatment. Drugs 2001;61:1735. [DOI] [PubMed] [Google Scholar]
- [20].Pahlajani G, Falcone T. Laparoscopic surgery for endometriosis-associated infertility: a pathophysiologic approach. Gynecol Surg 2010;7:319–28. [Google Scholar]
- [21].Hudelist G, Oberwinkler KH, Singer CF, et al. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum Reprod 2009;24:1018–24. [DOI] [PubMed] [Google Scholar]
- [22].Bilibio JP, Souza CAB, Rodini GP, et al. Serum prolactin and CA-125 Levels as biomarkers of peritoneal endometriosis. Gynecol Obstetr Investigat 2014;78:45–52. [DOI] [PubMed] [Google Scholar]
- [23].Jäger W, Diedrich K, Wildt L. Elevated levels of ca-125 in serum of patients suffering from ovarian hyperstimulation syndrome. Fertil Steril 1987;48:675–8. [DOI] [PubMed] [Google Scholar]
- [24].Zeimet AG, Muller-Holzner E, Marth C, et al. Tumor marker CA-125 in tissues of the female reproductive tract and in serum during the normal menstrual cycle. Fertil Steril 1993;59:1028–35. [DOI] [PubMed] [Google Scholar]
- [25].Muscatello R, Cucinelli F, Fulghesu A, et al. Multiple serum marker assay in the diagnosis of endometriosis. Gynecol Endocrinol 1992;6:265–9. [DOI] [PubMed] [Google Scholar]
- [26].Koninckx PR, Meuleman C, Oosterlynck D, et al. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil Steril 1996;65:280–7. [PubMed] [Google Scholar]


