Abstract
The aim of this study was to examine predictors of discharge of hospitalized stroke patients to either an acute inpatient rehabilitation facility (IRF) or subacute skilled nursing facility (SNF).
A retrospective cohort study was done in a large multicampus urban academic medical center of individuals hospitalized for stroke between January 1, 2015 and December 31, 2015 and who were discharged to either an IRF (n = 84) or SNF (n = 59). A set of characteristics and scales were collected on each patient and assessed using univariate and multivariate regression analyses.
Although univariate analyses revealed multiple measures were associated with discharge destination, the most predictive multivariate logistic regression model for discharge to SNF incorporated age (odds ratio [OR] = 1.09, 95% confidence interval [CI], 1.05–1.13), premorbid physical disability (OR 7.52, 95% CI 1.66–34.14), and inability to ambulate before discharge (OR 5.84, 95% CI 2.01–16.92) with an overall c-statistic of 0.85.
Increasing age, premorbid physical disability, and inability to ambulate increase the overall likelihood of discharge to a SNF. These findings need to be replicated in larger samples to determine whether they are generalizable.
Keywords: cerebrovascular accident, discharge, prediction, rehabilitation, stroke, stroke survivor
1. Introduction
Approximately 795,000 people sustain a new or recurrent stroke each year and stroke is the fifth leading cause of death in the United States.[1,2] Stroke is also one of the leading causes of long-term disability, leaving more than half of stroke survivors aged ≥65 years with impaired mobility.[1,2] In 2014, the average hospital length of stay before discharge for a patient with a primary diagnosis of a stroke was 4.7 days,[1] reflecting a trend towards shorter hospital stays and earlier discharge to post-acute care. The average annual cost from 2013 to 2014 of direct healthcare for stroke patients was estimated to be $23.6 billion, which included outpatient visits, inpatient stays, emergency department visits, medications, and home healthcare.[1] Informal caregivers such as family or friends provide much of the post-discharge care to stroke survivors.[3] This incurs not only a financial, but physical and psychological burden for these informal caregivers leading to risk of depression, cardiovascular diseases, increased mortality, and a decreased quality of life.[3–5]
Although independent mobility before discharge is a predictor of discharge home,[6–8] there are no detailed predictive models assessing multiple patient characteristics to determine post-acute stroke rehabilitation care. Rehabilitation facilities include inpatient rehabilitation facilities (IRFs) and skilled nursing facilities (SNFs). Based on Medicare regulations that govern the care of most post-stroke care, IRFs require patient to participate in at least 3 hours of rehabilitation therapy per day with frequent physician encounters and medical necessity for a hospital level of care.[9] SNFs are short-term facilities or units within nursing homes that provide variable amounts of rehabilitation therapy and typically include infrequent physician services.[9,10] Accurate prediction of post-acute discharge destination earlier in a patient's stay could assist with the determination of rehabilitation goals and facilitate the discharge planning process resulting in a more efficient and targeted manner.[11]
Previous studies have assessed various factors that influence discharge location, focusing on home versus post-acute discharge destinations. These factors have included age, sex, race, type of stroke, stroke severity, length of stay, stroke interventions, medical comorbidities, National Institutes of Health Stroke Scale (NIHSS), Motor Assessment Scale, Functional Independence Measure (FIM), Activity Measure for Post Acute Care (AMPAC), neurocognitive dysfunction as well as environmental and socioeconomic factors.[8,11–19] Other studies have examined the factors that distinguish patients who receive post-acute care after stroke at IRF versus SNF and found that age, pre-morbid function, activity of daily living (ADL) impairments, sociodemographics and geographic location contribute.[9,20–23] Most of these studies have used administrative data and have lacked detailed clinical information that might influence discharge destination selection. We therefore undertook this study to examine the factors that influence selection of post-acute level of care for stroke survivors.
2. Methods
This was an institutional review board–approved retrospective analysis of patients at Columbia University Medical Center system. Data were retrospectively collected from patients with the primary diagnosis of a stroke. They were included if they were admitted to 1 of 2 campuses (community or academic) of a large multicampus urban medical center during 2015 and if they were 18 years or older. Of this cohort, patients were excluded based on several criteria: admission from a rehabilitation facility or group home, discharge to hospice or long-term care facilities, discharge after 2015, transfer from another hospital or index stroke (first stroke in the year of 2015) occurred at another hospital, not given the final diagnosis of a new stroke, developed a stroke during a hospital admission for another medical condition, active malignancy or life-threatening condition other than a stroke, transient ischemic accident or subdural hemorrhage, the clinical examination and radiology findings not consistent with one another, or expired during hospitalization.
Members of the study team reviewed the medical records of each patient admitted from January 1, 2015 to December 31, 2015 with a primary diagnosis of a stroke. A set of standardized data abstraction forms were created in Research Electronic Data Capture (REDCap) and used to collect information about age, sex, time between stroke onset and admission, length of hospital stay, discharge site, stroke type and location, thrombolytic therapy, modified Charlson Comorbidity Index (mCCI) scores, insurance type, rehabilitation service consultation, family support, inability to tolerate 3 hours of therapy, premorbid physical disability, premorbid dementia, inability to ambulate before hospital discharge, and barriers affecting discharge. Additionally, the last recorded values for NIHSS scores, AMPAC scores of basic mobility (BM) and daily activity (DA), and Modified Rankin Scale (mRS) scores were assessed. Of note, Charlson Comorbidity typically includes stroke as one point in the score, but was modified to not be included in our study given that every patient in the study carried this diagnosis. Also, physical medicine & rehabilitation service consultation was initially analyzed, but was eventually excluded because of a clear institutional bias to obtain these consultations mainly for patients being assessed for admission to the hospital's IRF.
Initial univariate analyses were performed to test for significance and association between individual patient characteristics and discharge level of care. Categorical variables were tested using the χ2 test or Fisher exact test, as appropriate. Continuous variables were analyzed using 2-tailed t tests. To assess for compliance of the t test assumptions, Levene test for equality of variances was used. Of note, NIHSS were converted from continuous to categorical variables. NIHSS was also analyzed as both a continuous and dichotomous variable based on scores of ≥16, or ≤15, representing the commonly used threshold between moderate and severe strokes. A P value <.05 in the univariate analysis was used to identify significant variables for regression analysis. A binary logistic regression model was used to assess for variable association with discharge destination and relative significance in the overall model in comparison to covariables. The final model reveals associations that were only considered significant at P values <.05. All analyses were performed using SPSS Version 24 (IBM Corp. Released 2016, IBM SPSS Statistics for Mac, Version 24.0, Armonk, NY).
3. Results
3.1. Cohort description
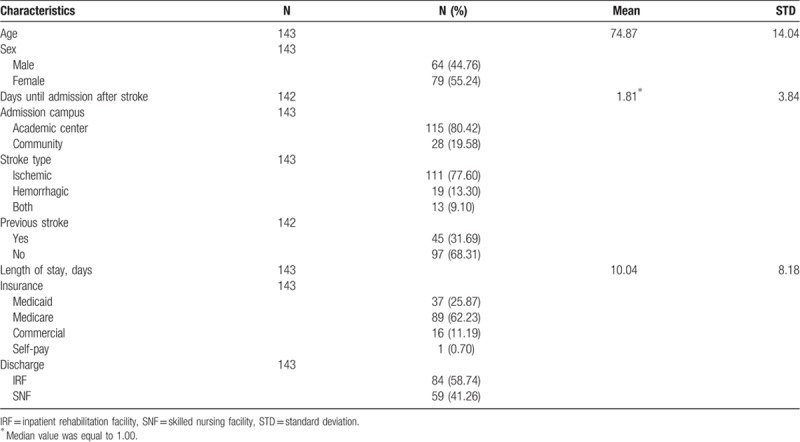
The complete study included 2892 patients. After exclusion, a total of 143 patients were analyzed in the study based on data from the subjects’ acute hospitalization course (Fig. 1). Of these subjects, 84 (58.7%) were discharge to IRF and 59 (41.3%) were discharged to SNF. Demographic characteristics are summarized in Table 1.
Figure 1.
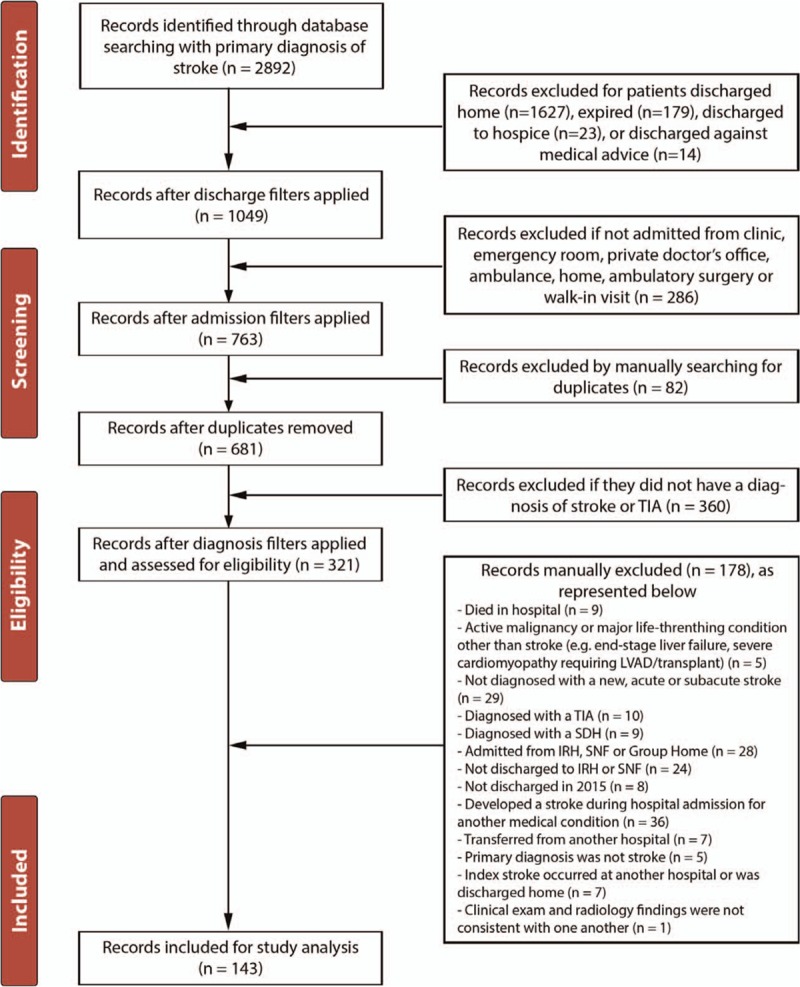
Flow diagram for the selection of eligible patients for the study. IRH = inpatient rehabilitation facitilty, LVAD = left ventricular assist device, SDH = subdural hematoma, SNF = skilled nursing facility, TIA = transient ischemia accident.
Table 1.
Demographics.
3.2. Univariate analysis
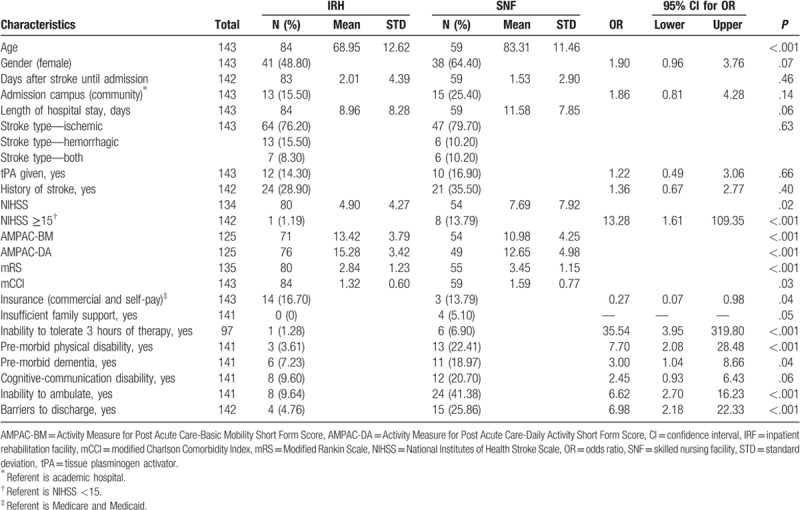
A univariate analysis was performed on all continuous and categorical variables. Significance was found for age, hospital length of stay, NIHSS, NIHSS score >15, AMPAC-BM, AMPAC-DA, mRS, mCCI, inability to tolerate 3 hours of rehabilitation therapy per day, premorbid physical disability, inability to ambulate, and barriers affecting discharge, as shown in Table 2.
Table 2.
Univariate analysis.
3.3. Multivariate analysis
Multiple binary regression models were performed on significant variables from the univariate analyses. Given the sample size limitation, a maximum of 5 variables were used per model. Each model included age, premorbid functional disability and inability to ambulate based on previous research determining that these are key factors as well as clinical experience demonstrating that these are foundational variables used in the clinical decision-making process. The last variable was analyzed with a variation of each of the functional scales (mRS, AM-PAC, NIHSS, mCCI). The most predictive model was selected based on all variables being statistically significant (P < .05) and a high c-statistic. Additionally, collinearity of the variables was evaluated before the finalization of the model (Table 3). Table 4 illustrates the adjusted odds ratios (ORs) and 95% confidence interval (CI) results of the regression models.
Table 3.
Colinearity analysis of predictive model (model 1).
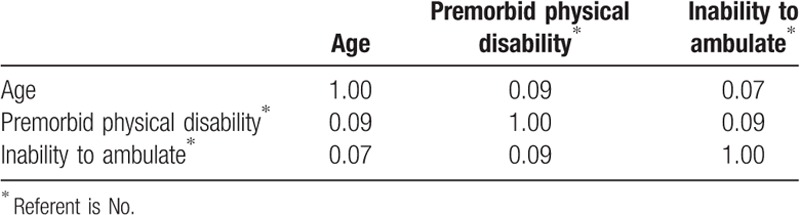
Table 4.
Predictors of discharge to subacute rehabilitation (multivariate analysis).
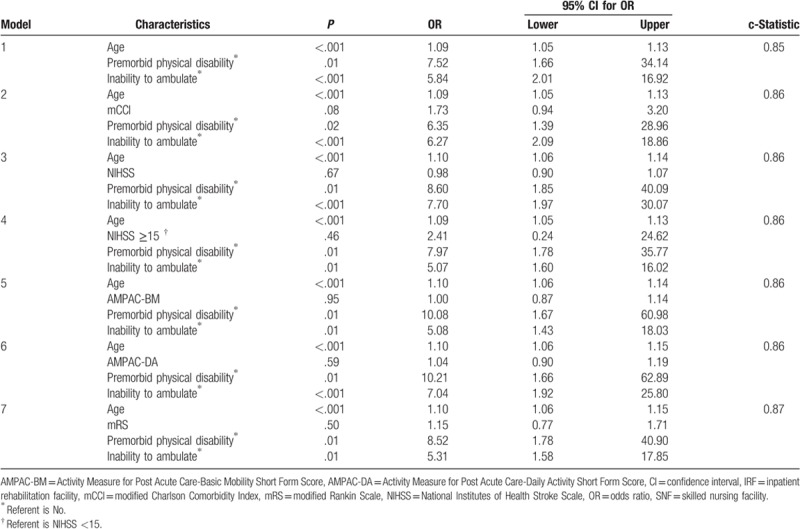
The most predictive model demonstrated that for every-year increase in a subject's age they were 1.09 times more likely to be discharged to SNF (OR 1.09, 95% CI 1.05–1.13) and if the subject had a premorbid physical disability before their stroke, they were 7.52 times more likely to be discharged to SNF (OR 7.52, 95% CI 1.66–34.14). Finally, patients unable to ambulate before discharge were 5.84 times more likely to be discharged to SNF (OR 5.84, 95% CI 2.01–16.92). The model was found to have an overall c-statistic of 0.85 (Table 4 - Model 1).
4. Discussion
Previous studies have examined factors such as NIHSS, pre-morbid disability, and age predicting discharge destination. However, most of these studies do not distinguish between IRF and SNF for discharge to a post-acute rehabilitation facility. The results of this study reveal that age, premorbid physical disability, and inability to ambulate predict discharge to SNF with an overall c-statistic of 0.85. This model allows a simplified method of assessment that may help predict the ultimate post-acute rehabilitation discharge destination.
We found that age is a predictor of discharge to SNF. Our predictive model reveals that the odds of patients within this older group being discharged to SNF is 1.09 times more likely for every year increase in the patient's age. The elderly often require a higher level of care for longer periods of time and may be less able to care for themselves upon discharge. The Northern Manhattan Stroke Study found that the probability of discharge to a nursing facility for patients older than 65 years of age was 2.4-fold.[24] Other studies have similarly found that there is an association between increased age and reduced likelihood of discharge to IRF compared with SNF.[7,20]
Patients with a premorbid physical disability and inability to ambulate before discharge were found to be 7.52 and 5.84 times, respectively, more likely to be discharged to SNF. These findings are unsurprising because of the bias for IRFs in accepting patients who are likely to return to the community. Premorbid physical disability included patients that had alterations in their ADLs and instrumental ADLs (iADLs) requiring some level of assistance ranging from assistive devices to family members and home health aids directly providing care. Of note, AMPAC-BM is also a measure of a patient's mobility given various tasks such as walking 1 mile, making sharp turns and running short distances.[25] However, inability to ambulate is easier to measure and more ubiquitously available. The results reveal that these patients likely require a longer rehabilitation course to achieve a level of functioning necessary to be safely discharged home and are at a higher risk of requiring long-term care owing to the cumulative effects of previous disability compounded by the index stroke.
Finally, the mCCI is a compilation of serious medical conditions that not only predict 1-year mortality, but also serve as a surrogate for medical complexity of a patient.[26,27] Other studies have used various scales such as mRS, AM-PAC, FIM, and NIHSS to identify characteristics of patients discharged to post-acute rehabilitation facilities.[13–15,28–30] Multiple multivariate regression models were run utilizing mCCI, mRS, AM-PAC, and NIHSS, which revealed no statistically significant contribution to discharge prediction. This is likely a result of increasing age being correlated with a patient's likehood of having increasing medical complixity.
5. Limitations
Our study has several potential limitations. First, our study included subjects within a single institution representing a particular geographic region, which would limit our study's generalizability to all stroke survivors discharged to post-acute rehabilitation facilities. Additionally, given the retrospective nature of the study, it is limited by data contained within the electronic medical records, which could not be corroborated by assessing the actual patients.
Finally, an important limitation is that there is no generally accepted “criterion standard” to determine which patients should receive IRF versus SNF care. Our study assessed existing patterns of care, but we cannot determine whether these patterns represent the optimal level of care selection for these patients. Some guidance is provided by the American Heart Association Stroke Rehabilitation Guidelines,[20] but our sample predated those guidelines. Thus, there are no published studies examining how consistently these stroke guidelines are being followed for this purpose.
6. Conclusion
This research study demonstrates that increasing age, premorbid physical disability, and inability to ambulate increase the overall likelihood of discharge to a SNF. Of all the scales compared, NIHSS, AMPAC-BM and DA, mRS, and mCCI were found to be significant in the univariate analyses. However, these scales were found to be not statistically significant when it came to prediction of discharge destination.
Our analyses reflect current practice at one institution, rather than optimal practice. Our observations reflect actual practice rather than optimal care, and should not be used as a guide to determine which level of post-acute care stroke survivors should receive. Our findings can nonetheless inform future research on developing optimal care paths for stroke survivors by highlighting existing practice patterns and subjecting these to scrutiny and possible modification. Ultimately, outcome studies that incorporate the patient outcomes of post-acute care, both medically (eg, hospital readmission and recurrent stroke) and functionally (return to community, activities of daily living, and mobility status), are needed to determine the “right” level of rehabilitation for various subpopulations of stroke survivors.
Author contributions
Conceptualization: Neal Rakesh, Daniel Boiarsky, Shaliesha Hinds, Joel Stein.
Data curation: Neal Rakesh, Daniel Boiarsky, Ammar Athar, Joel Stein.
Formal analysis: Neal Rakesh, Daniel Boiarsky, Ammar Athar, Joel Stein.
Investigation: Neal Rakesh, Ammar Athar, Shaliesha Hinds, Joel Stein.
Methodology: Neal Rakesh, Shaliesha Hinds, Joel Stein.
Project administration: Joel Stein.
Software: Neal Rakesh, Daniel Boiarsky, Joel Stein.
Supervision: Joel Stein.
Validation: Neal Rakesh, Daniel Boiarsky, Shaliesha Hinds, Joel Stein.
Visualization: Neal Rakesh, Daniel Boiarsky, Ammar Athar, Shaliesha Hinds, Joel Stein.
Writing – original draft: Neal Rakesh, Joel Stein.
Writing – review & editing: Neal Rakesh, Joel Stein.
Footnotes
Abbreviations: ADL = activities of daily living, AMPAC = activity measure for post acute care, AMPAC-BM = activity measure for post acute care-basic mobility, AMPAC-DA = activity measure for post acute care-daily activity, CI = confidence interval, FIM = functional independence measure, IADL = instrumental activities of daily living, IRF = acute inpatient rehabilitation facility, mCCI = Modified Charlson Comorbidity Index, NIHSS = National Institutes of Health Stroke Scale, OR = odds ratio, REDCap = Research Electronic Data Capture, SNF = skilled nursing facility.
Prior Work: Portions of these data have been presented in abstract form at the Association of Academic Physiatry and the American Academy of Physical Medicine and Rehabilitation.
Dr. Joel Stein has received research support from Neurolutions, Inc., and Revance, Inc., both unrelated to this study. The other authors report no conflicts of interest.
References
- [1].Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics—2018 Update: a report from the American Heart Association; 2018. [Google Scholar]
- [2].Stroke. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/stroke/facts.htm. Published September 6, 2017. [Google Scholar]
- [3].Dewey H, Thrift A, Mihalopoulos C, et al. Informal care for stroke survivors: results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2002;33:1028–33. [DOI] [PubMed] [Google Scholar]
- [4].Jonsson A-C, Lindgren I, Hallstrom B, et al. Determinants of quality of life in stroke survivors and their informal caregivers. Stroke 2005;36:803–8. [DOI] [PubMed] [Google Scholar]
- [5].Tooth L, Mckenna K, Barnett A, et al. Caregiver burden, time spent caring and health status in the first 12 months following stroke. Brain Inj 2005;19:963–74. [DOI] [PubMed] [Google Scholar]
- [6].Prvu Bettger J, McCoy L, Smith EE, et al. Contemporary trends and predictors of postacute service use and routine discharge home after stroke. J Am Heart Assoc 2015;4:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stein J, Bettger JP, Sicklick A, et al. Use of a standardized assessment to predict rehabilitation care after acute stroke. Arch Phys Med Rehabil 2015;96:210–7. [DOI] [PubMed] [Google Scholar]
- [8].Roberts PS, Mix J, Rupp K, et al. Using functional status in the acute hospital to predict discharge destination for stroke patients. Am J Phys Med Rehabil 2016;95:416–24. [DOI] [PubMed] [Google Scholar]
- [9].MedPAC. Medicare Payment Policy. Rep to Congr 2016;333–59. [Google Scholar]
- [10].Centers for Medicare and Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Centers Medicare Medicaid Serv 2015;8–9. https://www.medicare.gov/Pubs/pdf/10153.pdf [Google Scholar]
- [11].Brauer SG, Bew PG, Kuys SS, et al. Prediction of discharge destination after stroke using the motor assessment scale on admission: a prospective, multisite study. Arch Phys Med Rehabil 2008;89:1061–5. [DOI] [PubMed] [Google Scholar]
- [12].Van Der Cruyssen K, Vereeck L, Saeys W, et al. Prognostic factors for discharge destination after acute stroke: a comprehensive literature review. Disabil Rehabil 2015;37:1214–27. [DOI] [PubMed] [Google Scholar]
- [13].Elwood D, Rashbaum I, Bonder J, et al. Length of stay in rehabilitation is associated with admission neurologic deficit and discharge destination. PM R 2009;1:147–51. [DOI] [PubMed] [Google Scholar]
- [14].Schlegel DJ, Tanne D, Demchuk AM, et al. Multicenter rt PASSG. Prediction of hospital disposition after thrombolysis for acute ischemic stroke using the National Institutes of Health Stroke Scale. Arch Neurol 2004;61:1061–4. [DOI] [PubMed] [Google Scholar]
- [15].Thorpe ER, Garrett KB, Smith AM, et al. Outcome measure scores predict discharge destination in patients with acute and subacute stroke: a systematic review and series of meta-analyses. J Neurol Phys Ther 2018;42:2–11. [DOI] [PubMed] [Google Scholar]
- [16].Burton JK, Ferguson EEC, Barugh AJ, et al. Predicting discharge to institutional long-term care after stroke: a systematic review and metaanalysis. J Am Geriatr Soc 2017;161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jackson JP, Whisner S, Wang EW. A predictor model for discharge destination in inpatient rehabilitation patients. Am J Phys Med Rehabil 2013;92:343–50. [DOI] [PubMed] [Google Scholar]
- [18].Ouellette DS, Timple C, Kaplan SE, et al. Predicting discharge destination with admission outcome scores in stroke patients. NeuroRehabilitation 2015;37:173–9. [DOI] [PubMed] [Google Scholar]
- [19].Deutsch A, Granger CV, Heinemann AW, et al. Poststroke rehabilitation: Outcomes and reimbursement of inpatient rehabilitation facilities and subacute rehabilitation programs. Stroke 2006;37:1477–82. [DOI] [PubMed] [Google Scholar]
- [20].Kramer A, Holthaus D, Goodrish G, Epstein A. A study of stroke post-acute care costs and outcomes. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. Accessed May 22, 2019 at https://aspe.hhs.gov/basic-report/study-stroke-post-acute-care-costs-and-outcomes-final-report; 2006. [Google Scholar]
- [21].Winstein CJ, Stein J, Arena R, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. [DOI] [PubMed] [Google Scholar]
- [22].Kane RL, Lin W-C, Blewett LA. Geographic variation in the use of post-acute care. Health Serv Res 2002;37:667–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alcusky M, Ulbricht CM, Lapane KL. Postacute care setting, facility characteristics, and poststroke outcomes: a systematic review. Arch Phys Med Rehabil 2017;99:1124–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rundek T, Mast H, Hartmann A, et al. Predictors of resource use after acute hospitalization: The Northern Manhattan Stroke Study. Neurology 2000;55:1180–7. [DOI] [PubMed] [Google Scholar]
- [25].Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Medical Care 2004;1:I49–61. [DOI] [PubMed] [Google Scholar]
- [26].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [27].Charlson M, Charlson RE, Briggs W, et al. Can disease management target patients most likely to generate high costs? The impact of comorbidity. J Gen Intern Med 2007;22:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Belagaje SR, Sun CHJ, Nogueira RG, et al. Discharge disposition to skilled nursing facility after endovascular reperfusion therapy predicts a poor prognosis. J Neurointerv Surg 2015;7:99–103. [DOI] [PubMed] [Google Scholar]
- [29].Chan L. Does post-acute care site matter? Assessing functional recovery after a stroke. Arch Phys Med 2014;94:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Keith RA, Wilson DB, Gutierrez P. Acute and subacute rehabilitation for stroke: a comparison. Arch Phys Med Rehabil 1995;76:495–500. [DOI] [PubMed] [Google Scholar]


