Abstract
Background:
The relationship between metformin use and the risk of prostate cancer is still inconclusive. Therefore, we performed a systematic review and meta-analysis of all eligible cohort studies to evaluate a potential association of metformin use with prostate cancer risk.
Methods:
A comprehensive literature search was performed in PubMed and Web of Science databases through July 2018. A DerSimonian and Laird random-effects model was applied to calculate the pooled relative risk (RR) and its 95% confidence interval (CI).
Results:
Eighteen cohort or nested case-control studies were included in this study with a total of 52,328 cases. In a random-effect pooled analysis, metformin use was not significantly associated with the risk of prostate cancer (RR 0.97, 95% CI 0.80–1.16, P = .711). Statistically significant heterogeneity was identified among included studies (P < .001, I2 = 98.1%). Sensitivity analysis indicated that no single study dominated the pooled RR.
Conclusion:
The present large meta-analysis of cohort studies did not find an association between metformin use and prostate cancer risk.
Keywords: cohort, meta-analysis, metformin, prostate cancer
1. Introduction
Prostate cancer is the second most common male cancer in the world, with a total of 1,111,700 new cases and 307,500 deaths estimated in 2012.[1] The most well-established risk factors for prostate cancer are age, race/ethnicity, and family history of prostate cancer.[2] Physical activities, coffee consumption, statin use, intake of certain vegetables also have been linked with the prevention of prostate cancer.[3–7] Recently, it was proposed that metformin had additional beneficial anticarcinogenic effects in human cancers,[8,9] including prostate cancer.[10]
Metformin, a biguanide, is the most widely prescribed antidiabetic drug worldwide due to its clinical effectiveness and tolerability.[11] Emerging evidence indicated that metformin might reduce insulin-stimulated cancer growth.[12] Previous large observational studies on commonly diagnosed cancers found inverse associations between metformin use and colon cancer,[8] liver cancer,[13] and lung cancer.[14] However, findings from previous epidemiologic studies on the association of metformin use with prostate cancer risk are inconsistent. Preston et al,[15] Haring et al,[16] and Ruiter et al[17] reported a significant inverse relationship between metformin therapy and the risk of prostate cancer, while many other epidemiological studies failed to find this association.
A few previous meta-analyses[10,18,19] were performed on this topic and indicated a protective effect of metformin use on the risk of prostate cancer. Since then, several new large cohort studies[16,20–22] were published in recent years but the results were still inconsistent. Considering the conflicting findings in the literature, we carried out the present systematic review and meta-analysis of all available cohort studies to summarize evidence on the association of metformin use with the risk of prostate cancer.
2. Materials and methods
2.1. Literature review
We searched for all relevant studies that examined the effects of metformin on the risk of prostate cancer in July 2018 based on PubMed and Web of Science databases with the following search algorithm: (“metformin” or “biguanide” or “dimethylbiguanidine”) and (“prostatic neoplasms” or “prostatic cancer” or “prostate neoplasms” or “prostate cancer”). In addition, the cited references of the screened articles and reviews were examined to identify any additional relevant studies. No language or publication date limitation was applied. This systematic review and meta-analysis were designed, performed, and reported in accordance with the standards of quality for reporting meta-analyses.[23] This study was based on previously published studies and did not have original data. Therefore, no ethical approval and patient consent are required.
2.2. Study selection criteria
An eligible study must meet all the following criteria:
-
(1)
the exposure of interest was metformin use;
-
(2)
the outcome of interest was prostate cancer risk;
-
(3)
the study design was cohort or nested case-control; and
-
(4)
the risk estimate with its 95% confidence interval (CI) was provided.
-
(5)
If multiple studies used the same population data, the study with the largest sample size was included.
2.3. Study quality assessment
Newcastle–Ottawa scale (NOS, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was adopted to evaluate the study quality by 2 independent reviewers (ZF and XZ). NOS is a 9-star system including the following 3 broad perspectives: selection, comparability, and outcome. The full score is 9 stars and a study with ≥7 awarded stars is regarded as high quality.
2.4. Data extraction
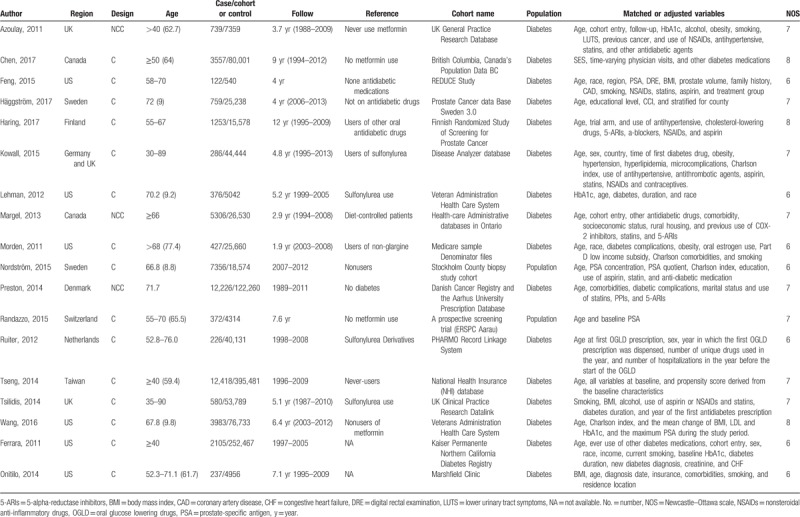
Two reviewers (ZF and XZ) independently collected and recorded the following information: first author's surname, the region where the study was performed, publication year, study design (cohort or nested case–control), age of study population, number of cases and cohorts/controls, fully adjusted risk estimates with their corresponding 95% CIs, and matched or adjusted confounding variables in the study design or statistical analysis. Any discrepancies were resolved by consensus.
2.5. Statistical methods
A combined relative risk (RR) with its 95% CI was used to determine the strength of the relationship between metformin use and prostate cancer risk. A DerSimonian and Laird random-effects model,[24] which takes into account both within- and between-study variability, was introduced to calculate the pooled risk estimates. Subgroup analyses were performed by geographical region, study design, study quality, and number of cases. Statistical heterogeneity across studies was evaluated by Cochran Q test with the level of significance set at 0.1.[25] The I2 score was used to measure the degree of heterogeneity (I2 < 25%: no heterogeneity; I2 = 25–50%: moderate heterogeneity; I2 > 50%: large or extreme heterogeneity).[25] Meta-regression analysis was performed to explore the potential source of heterogeneity. Sensitivity analysis was performed by removing each study in turn and recalculating the pooled RR of remainder studies. Potential publication bias was evaluated by funnel plots, Begg test (rank correlation method),[26] and Egger test (linear regression method).[27] All statistical analyses were performed with STATA 11.0 (StataCorp, College Station, TX), using 2-sided P values (set at .05).
3. Results
3.1. Literature search and study main characteristics
Figure 1 shows a flow diagram of the literature review. A total of 18 eligible studies[15–17,20–22,28–39] were ultimately included in our meta-analysis aimed to comprehensively evaluate the association of metformin use with prostate cancer risk. These studies were published between 2011 and 2017, with a total of 52,328 cases. Of the studies included, 15 were cohort studies and 3 were nested case–control studies. These studies were performed in the following geographical regions: Europe (n = 9), North America (n = 8), and Asia (n = 1). Sixteen of the 18 studies analyzed the risk of prostate cancer in patients with diabetes. The study quality was evaluated by the NOS. Scores ranged from 6 to 8, with a mean of 6.78. Table 1 summaries the characteristics of studies analyzed in this meta-analysis.
Figure 1.
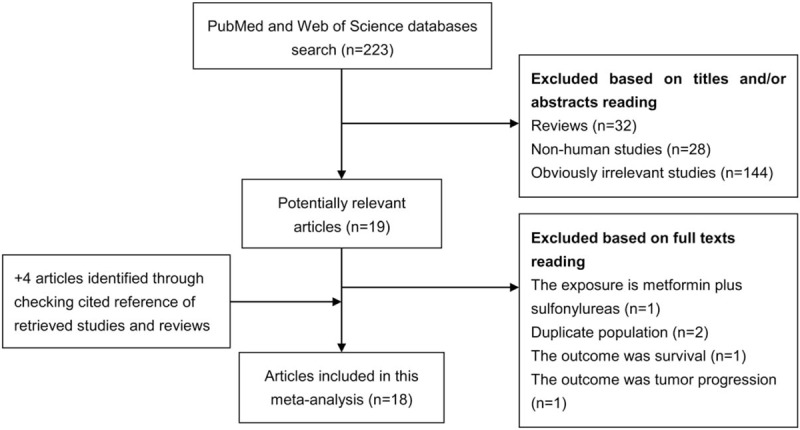
Literature search and study selection.
Table 1.
Characteristics of the studies included in this meta-analysis.
3.2. Overall analysis and heterogeneity assessment
Multivariable-adjusted RRs with their corresponding 95% CIs for each individual study and for the combination of all included studies are presented in Figure 2. In a random-effects meta-analysis of these studies, metformin use was not significantly associated with the incidence of prostate cancer (RR 0.97, 95% CI 0.80–1.16, P = .711). Statistically significant heterogeneity was identified among included studies (P < .001 for heterogeneity, I2 = 98.1%). We then assessed heterogeneity across studies using meta-regression analysis. As a result, none of the following factors was identified as a possible source of heterogeneity in the pooled analysis: geographical region, study design, study quality, and sample size (all P for interaction >.05).
Figure 2.
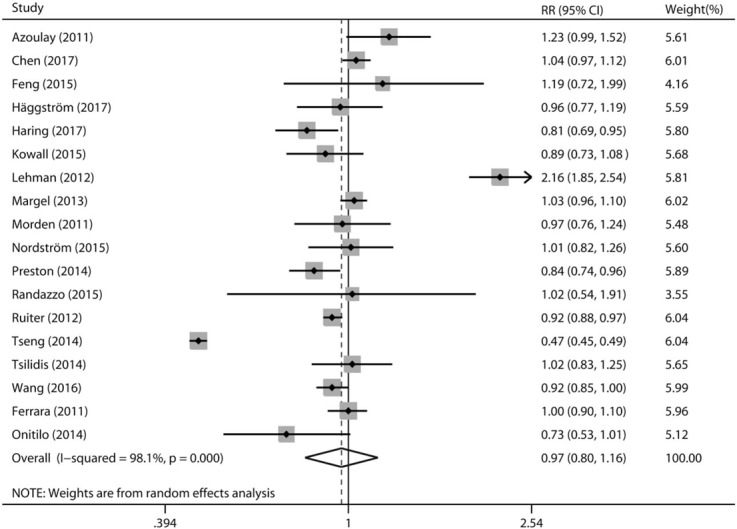
A forest plot showing the relative risk of prostate cancer associated with metformin use. The size of each square is proportional to the study's weight (inverse of variance). Weights are from random effects analysis.
3.3. Stratified analysis
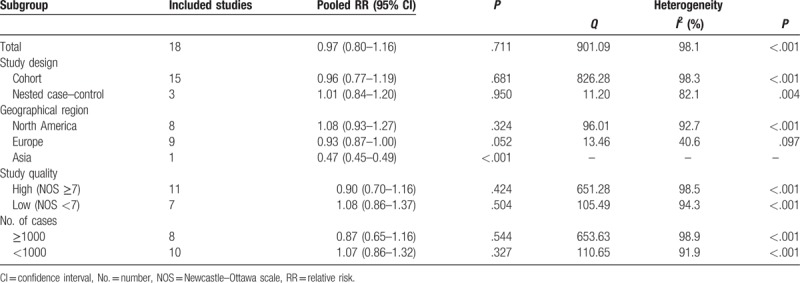
We first analyzed the risk of prostate cancer in terms of geographical region, there was no protective effect of metformin use against prostate cancer in the following geographical populations: North America (RR 1.08, 95% CI 0.93–1.27, P = .324) and Europe (RR 0.93, 95% CI 0.87–1.00, P = .052). When further subgroup by study design, the analysis of cohort studies yielded a RR of 0.96 (95% CI 0.77–1.19, P = .681) and the analysis of nested case-control studies yielded a RR of 1.01 (95% CI 0.84–1.20, P = .950). In the stratified analysis by study quality, no significant association was observed either in studies with low quality (RR 1.08, 95% CI 0.86–1.37, P = .504) or in studies with high-quality (RR 0.90, 95% CI 0.70–1.16, P = .424). Finally, in the stratified analyses by sample size, metformin use was not related with prostate cancer risk in small studies (RR 1.07, 95% CI 0.86–1.32, P = .327) as well as in large studies (RR 0.87, 95% CI 0.65–1.16, P = .544). The effects of metformin use on prostate cancer risk in subgroup meta-analyses have been shown in Table 2.
Table 2.
Subgroup analyses of the association between metformin use and prostate cancer risk.
3.4. Sensitivity analysis and publication bias
The impact of each individual study on the combined RR was determined by repeating the meta-analysis after omitting each study in turn. As shown in Figure 3, no single study dominated the pooled RR with the 18 study-specific RRs ranging from a low of 0.92 (95% CI 0.77–1.10) to a high of 1.01 (95% CI 0.92–1.11) via removing the study by Lehman et al[36] and the study by Tseng et al,[33] respectively. Finally, significant publication bias was observed in Egger test (P = .010), but not in Begg test (P = .081). In addition, a certain degree of asymmetry was observed upon visual inspection of the funnel plot (Fig. 4).
Figure 3.
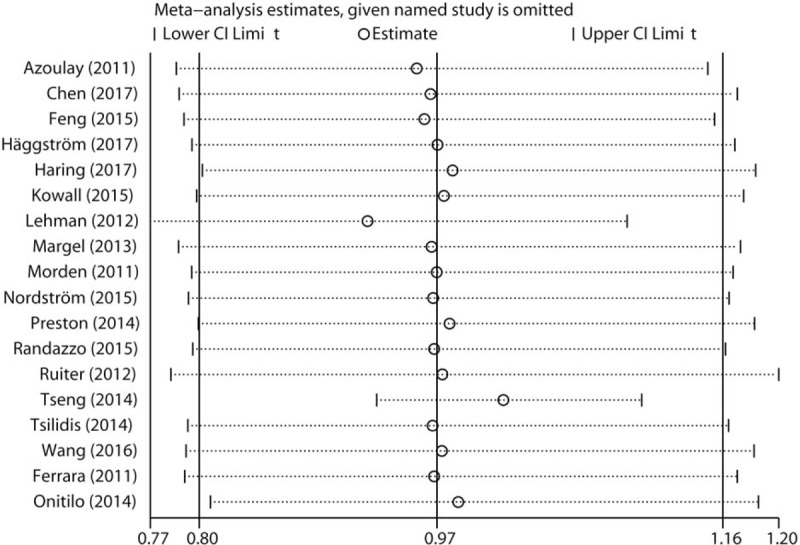
Sensitivity analysis was performed whereby each study was omitted in turn and the pooled relative risks were recalculated to determine the influence of each study.
Figure 4.
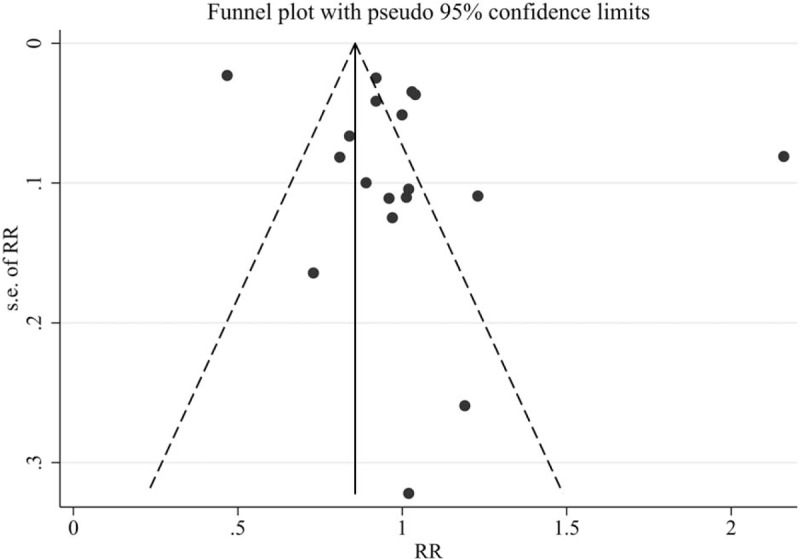
Publication bias was evaluated by the funnel plot.
4. Discussion
The present systematic review and meta-analysis of relevant cohort studies aimed to evaluate the association of metformin use with the incidence of prostate cancer. A total of 18 eligible cohort studies with 52,328 cases were ultimately included in this study. The results of pooled analysis and subgroup analysis indicated that there was no association between metformin use and the risk of prostate cancer.
Various animal and cell line studies have indicated that metformin is able to inhibit tumor development and progression in prostate cancer. Tong et al[40] reported that metformin suppressed castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. The study by Dirat et al[41] suggested that metformin had antimigratory effects in prostate cancer cells by inhibition of the GTPase Rac1. Akinyeke et al[42] found that metformin targeted c-MYC oncogene to prevent prostate cancer. Recently, Hayashi et al[43] reported that metformin was able to inhibit prostate cancer growth induced by a high-fat diet in Pten-deficient model mice and Liu et al[44] found that metformin inhibited prostate cancer progression by regulating tumor-associated inflammatory infiltration. Additional studies also indicated that metformin could improve the radiosensitivity in prostate cancer cells.[45,46] Zaidi et al[47] reviewed the literature regarding the anticancer potential of metformin on prostate cancer and proposed that metformin may have a future role in the treatment protocol of prostate cancer. However, although the antitumor role of metformin in prostate cancer was supported by few cohort studies, the results of most epidemiological studies, as well as the present meta-analysis, were null.
Previously, 3 meta-analyses have evaluated the role of metformin use in the prevention of prostate cancer. Yu et al[19] reported in 2014 that metformin use was significantly associated with a decreased cancer risk with 14 datasets and 963,991 male subjects. Deng et al study[18] also supported that metformin therapy was associated with a significantly decreased incidence of prostate cancer with 13 studies involving 334,532 participants. Wu et al[10] suggested in 2015 that metformin therapy was associated with a significant reduced risk of prostate cancer among cohort studies rather than among case–control studies.
During the peer review process of this manuscript, Ghiasi et al[48] also summarized the relationship between prostate cancer and metformin consumption but only included 11 observational studies. Compared with these previous studies, our meta-analysis only included cohort and nested case–control studies and the results indicated that metformin use was not related with the incidence of prostate cancer. Metformin therapy has been associated with a reduced risk of endometrial cancer,[49] liver cancer,[13] lung cancer,[14] pancreatic cancer,[9] and colorectal cancer,[8] but not related with breast cancer.[50] Our meta-analysis indicated that there was no association of metformin use with prostate cancer risk, which further enriched the knowledge of relationship between metformin therapy and cancer development.
Our study had several important strengths. First, this meta-analysis only included cohort/nested case–control studies, which had the advantage of being less subject to potential selection and recall bias than case-control studies. Second, our meta-analysis included a total of 18 published studies with 52,328 prostate cancer cases, which might improve statistical power. Third, various stratified analyses, sensitivity analysis, and publication bias analysis were performed to comprehensively evaluate the robustness of the combined effect size estimate. Finally, the results of our study were not consistent with the previous meta-analysis on same topics, which had important implications for future studies.
4.1. Limitations
Several limitations should be acknowledged for this meta-analysis. First, obvious heterogeneity was observed across studies (P < .001 for heterogeneity, I2 = 98.1%), which was attributed to the variation in study design, study population, the methods of exposure and outcome assessment, and so on. Second, Egger test indicated the existence of some publication bias, which was inevitable as studies with small sample size and null results were less likely to be published. Third, the quality of a systematic review and meta-analysis depended on each individual study, which usually was not able to adjust for all confounding factors and thus might bias the risk estimate.
4.2. Future directions
Numerous biological studies have indicated the anticancer potential of metformin on prostate cancer. However, many epidemiological studies, as well as our meta-analysis, did not support that metformin had a protective effect against prostate cancer. Therefore, further large well-designed cohort studies are still warranted to examine the potential role of metformin on progression and mortality of prostate cancer.
5. Conclusion
In summary, this large meta-analysis of cohort studies did not find an association between metformin use and risk of prostate cancer.
Author contributions
Conceptualization: Zhaohan Feng, Xiaofeng Zhou, Jianfeng Wang, Xin Xu.
Data curation: Zhaohan Feng, Xiaofeng Zhou, Naibo Liu, Jianfeng Wang, Xin Xu.
Formal analysis: Zhaohan Feng, Xiaofeng Zhou, Xing Chen.
Investigation: Zhaohan Feng, Xiaofeng Zhou, Jianfeng Wang, Xing Chen, Xin Xu.
Methodology: Zhaohan Feng, Xiaofeng Zhou, Jianfeng Wang, Xing Chen, Xin Xu.
Project administration: Zhaohan Feng, Xiaofeng Zhou, Xing Chen.
Resources: Zhaohan Feng, Xiaofeng Zhou, Naibo Liu, Jianfeng Wang.
Software: Zhaohan Feng, Naibo Liu.
Supervision: Zhaohan Feng, Xiaofeng Zhou, Naibo Liu.
Validation: Zhaohan Feng, Naibo Liu.
Visualization: Zhaohan Feng, Xiaofeng Zhou, Naibo Liu.
Writing – original draft: Zhaohan Feng, Xiaofeng Zhou, Naibo Liu, Xin Xu.
Writing – review and editing: Zhaohan Feng.
Footnotes
Abbreviations: CI = confidence interval, NOS = Newcastle–Ottawa scale, RR = relative risk.
The authors declare that there are no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet 2016;387:70–82. [DOI] [PubMed] [Google Scholar]
- [3].Xu X, Cheng Y, Li S, et al. Dietary carrot consumption and the risk of prostate cancer. Eur J Nutr 2014;53:1615–23. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Hu F, Li D, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol 2011;60:1029–44. [DOI] [PubMed] [Google Scholar]
- [5].Liu B, Mao Q, Cao M, et al. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol 2012;19:134–41. [DOI] [PubMed] [Google Scholar]
- [6].Liu H, Hu GH, Wang XC, et al. Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutr Cancer 2015;67:392–400. [DOI] [PubMed] [Google Scholar]
- [7].Bansal D, Undela K, D’Cruz S, et al. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 2012;7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu F, Yan L, Wang Z, et al. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Oncotarget 2017;8:16017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Z, Lai ST, Xie L, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:19–26. [DOI] [PubMed] [Google Scholar]
- [10].Wu GF, Zhang XL, Luo ZG, et al. Metformin therapy and prostate cancer risk: a meta-analysis of observational studies. Int J Clin Exp Med 2015;8:13089–98. [PMC free article] [PubMed] [Google Scholar]
- [11].Alexander GC, Sehgal NL, Moloney RM, et al. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zakikhani M, Dowling RJ, Sonenberg N, et al. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369–75. [DOI] [PubMed] [Google Scholar]
- [13].Ma S, Zheng Y, Xiao Y, et al. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 2017;96:e6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhu N, Zhang Y, Gong YI, et al. Metformin and lung cancer risk of patients with type 2 diabetes mellitus: a meta-analysis. Biomed Rep 2015;3:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Preston MA, Riis AH, Ehrenstein V, et al. Metformin use and prostate cancer risk. Eur Urol 2014;66:1012–20. [DOI] [PubMed] [Google Scholar]
- [16].Haring A, Murtola TJ, Talala K, et al. Antidiabetic drug use and prostate cancer risk in the Finnish Randomized Study of Screening for Prostate Cancer. Scand J Urol 2017;51:5–12. [DOI] [PubMed] [Google Scholar]
- [17].Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 2012;35:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Deng D, Yang Y, Tang X, et al. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: evidence from a meta-analysis. Diabetes Metab Res Rev 2015;31:595–602. [DOI] [PubMed] [Google Scholar]
- [19].Yu H, Yin L, Jiang X, et al. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One 2014;9:e116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haggstrom C, Van Hemelrijck M, Zethelius B, et al. Prospective study of Type 2 diabetes mellitus, anti-diabetic drugs and risk of prostate cancer. Int J Cancer 2017;140:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen CB, Eurich DT, Majumdar SR, et al. Metformin and the risk of prostate cancer across racial/ethnic groups: a population-based cohort study. Prostate Cancer Prostatic Dis 2017;20:122–6. [DOI] [PubMed] [Google Scholar]
- [22].Wang CP, Lehman DM, Lam YF, et al. Metformin for reducing racial/ethnic difference in prostate cancer incidence for men with type II diabetes. Cancer Prev Res (Phila) 2016;9:779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [24].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [25].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [26].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [27].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Randazzo M, Beatrice J, Huber A, et al. Influence of metformin use on PSA values, free-to-total PSA, prostate cancer incidence and grade and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol 2015;33:1189–96. [DOI] [PubMed] [Google Scholar]
- [29].Nordstrom T, Clements M, Karlsson R, et al. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer 2015;51:725–33. [DOI] [PubMed] [Google Scholar]
- [30].Kowall B, Stang A, Rathmann W, et al. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf 2015;24:865–74. [DOI] [PubMed] [Google Scholar]
- [31].Feng T, Sun X, Howard LE, et al. Metformin use and risk of prostate cancer: results from the REDUCE study. Cancer Prev Res (Phila) 2015;8:1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsilidis KK, Capothanassi D, Allen NE, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care 2014;37:2522–32. [DOI] [PubMed] [Google Scholar]
- [33].Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer 2014;50:2831–7. [DOI] [PubMed] [Google Scholar]
- [34].Onitilo AA, Stankowski RV, Berg RL, et al. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur J Cancer Prev 2014;23:134–40. [DOI] [PubMed] [Google Scholar]
- [35].Margel D, Urbach D, Lipscombe LL, et al. Association between metformin use and risk of prostate cancer and its grade. J Natl Cancer Inst 2013;105:1123–31. [DOI] [PubMed] [Google Scholar]
- [36].Lehman DM, Lorenzo C, Hernandez J, et al. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care 2012;35:1002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Morden NE, Liu SK, Smith J, et al. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older medicare patients. Diabetes Care 2011;34:1965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ferrara A, Lewis JD, Quesenberry CP, Jr, et al. Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 2011;34:923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Azoulay L, Dell’Aniello S, Gagnon B, et al. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev 2011;20:337–44. [DOI] [PubMed] [Google Scholar]
- [40].Tong D, Liu Q, Liu G, et al. Metformin inhibits castration-induced EMT in prostate cancer by repressing COX2/PGE2/STAT3 axis. Cancer Lett 2017;389:23–32. [DOI] [PubMed] [Google Scholar]
- [41].Dirat B, Ader I, Golzio M, et al. Inhibition of the GTPase Rac1 mediates the antimigratory effects of metformin in prostate cancer cells. Mol Cancer Ther 2015;14:586–96. [DOI] [PubMed] [Google Scholar]
- [42].Akinyeke T, Matsumura S, Wang X, et al. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis 2013;34:2823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hayashi T, Fujita K, Matsushita M, et al. Metformin inhibits prostate cancer growth induced by a high-fat diet in Pten-deficient model mice. Int J Urol 2019;26:307–9. [DOI] [PubMed] [Google Scholar]
- [44].Liu Q, Tong D, Liu G, et al. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res 2018;24:5622–34. [DOI] [PubMed] [Google Scholar]
- [45].Gonnissen A, Isebaert S, McKee CM, et al. The effect of metformin and GANT61 combinations on the radiosensitivity of prostate cancer cells. Int J Mol Sci 2017;18:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang T, Zhang L, Fan J, et al. Metformin sensitizes prostate cancer cells to radiation through EGFR/p-DNA-PKCS in vitro and in vivo. Radiat Res 2014;181:641–9. [DOI] [PubMed] [Google Scholar]
- [47].Zaidi S, Gandhi J, Joshi G, et al. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis 2019;doi: 10.1038/s41391-018-0085-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [48].Ghiasi B, Sarokhani D, Najafi F, et al. The relationship between prostate cancer and metformin consumption: a systematic review and meta-analysis study. Curr Pharm Des 2019;doi: 10.2174/1381612825666190215123759. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [49].Meireles CG, Pereira SA, Valadares LP, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol 2017;147:167–80. [DOI] [PubMed] [Google Scholar]
- [50].Yang T, Yang Y, Liu S. Association between metformin therapy and breast cancer incidence and mortality: evidence from a meta-analysis. J Breast Cancer 2015;18:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


