Abstract
Whether statin use has any impact on survival of esophageal cancer patients remains controversial. Therefore, we conducted a meta-analysis focusing on current topic for the first time.
We systematically searched the following databases for relevant studies comparing survival between statin users and non-users among esophageal cancer patients up to March 16, 2019: Pubmed, Embase, and Web of Science. We extracted data of hazard ratio (HR) with 95%confidence interval (CI) of all-cause and cancer-specific mortality for analysis. We used the STATA 12.0 software to perform this meta-analysis.
We finally included a total of 4 cohort studies involving a total of 20,435 esophageal cancer patients (5319 statin users and 15116 non-users). Our meta-analysis found that statin use after diagnosis of esophageal cancer was significantly correlated to decreased all-cause (random effects: HR = 0.81, 95%CI: 0.75–0.89, P < .001; I2 = 68.1%) and cancer-specific mortality (fixed effects: HR = 0.84, 95%CI: 0.78–0.89, P < .001; I2 = 46.6%) in esophageal cancer patients. When stratified by pathological subtypes, the protective effect of statin use after diagnosis of esophageal cancer was observed in both esophageal adenocarcinoma patients and esophageal squamous cell carcinoma patients. Moreover, statin use before diagnosis of esophageal cancer was also confirmed to have favorable survival benefit for esophageal cancer patients.
Statin use was significantly correlated to lower mortality risk of esophageal cancer patients regardless of the time when statins were taken and pathological subtypes of esophageal cancer. Statins may serve as promising adjunctive anticancer agents for treating esophageal cancer.
Keywords: esophageal cancer, HMG-CoA reductase inhibitors, meta-analysis, mortality, statins
1. Introduction
Esophageal cancer still remains to be a heavy disease burden worldwide, with 455,800 new esophageal cancer cases and 400,200 deaths occurring in 2012.[1] In pathology, esophageal cancer mainly consisted of esophageal adenocarcinoma (EA) and esophageal squamous cell carcinoma (SCC).[2] Despite of the advancement of therapeutic strategies, the prognosis of esophageal still remains dismal with a 5-year survival rate of about 15% to 34%.[3] Therefore, investigation on novel potential medications that have anticancer effects for esophageal cancer seems to be greatly important, which might help improve the survival of esophageal cancer patients.
Statins, as the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, have been widely utilized for the primary and secondary prevention of atherosclerotic cardiovascular disease, with more than 200 million people taking them worldwide.[4] However, recent evidence has showed that statins might have the anticancer effects such as antiproliferation and proapoptosis[5] and as a results, more and more studies have focused on the impact of statins on both risk and prognosis of various cancers. Previously, statins has been shown to lower the risk of esophageal cancer among people taking them, especially the risk of esophageal adenocarcinoma in patients with Barrett esophagus.[6] However, as for the impact of statins on survival of esophageal cancer patients, previous studies have drawn conflicting conclusions due to various biases.[7–10] Some studies found that statin use was significantly correlated with decreased all-cause and cancer-specific mortality in esophageal cancer patients with both EA and esophageal SCC[9] while others found that statin use had little evidence of cancer-specific mortality reduction in esophageal cancer patients.[8] Moreover, some even found that statin use could only reduce the all-cause and cancer-specific mortality in patients with EA but not in patients with esophageal SCC.[7] Therefore, the impact of statin use on prognosis of esophageal cancer remains unclear. Hence, in this study, we tried to conduct a systematic review and meta-analysis focusing on the impact of statin use on long-term survival of esophageal cancer patients by pooling all these up-to-date evidence together. To our knowledge, this is the first meta-analysis focusing on the correlation between statin use and survival of esophageal cancer patients.
2. Methods and materials
2.1. Literature search
We performed a comprehensive literature search in the following online databases systematically: Pubmed, Embase, and Web of Science and The time period was up to March 16, 2019. We used the following search terms: ((((((statin) OR statins) OR hydroxymethylglutaryl coenzyme reductase inhibitors) OR HMG CoA reductase inhibitors)) AND ((((esophageal) OR oesophageal) OR esophagus) OR oesophagus)) AND ((((cancer) OR carcinoma) OR tumor) OR neoplasm). We also manually searched the reference lists of relevant studies for potential study retrieval.
2.2. Study inclusion and exclusion
We would include either randomized controlled trials (RCTs) or observational studies that met the fowling inclusion criteria:
-
1.
exploring the effects of statins on survival of esophageal cancer patients by comparing statin users and non-users among esophageal cancer patients;
-
2.
providing sufficient data of hazard ratio (HR) with 95% confidence interval (CI) for all-cause mortality or cancer-specific mortality;
-
3.
if studies were conducted from the same population, only the most recent one was included.
We would exclude studies that met the following exclusion criteria:
-
1.
including patients with other types of cancer apart from esophageal cancer;
-
2.
no sufficient data for meta-analysis;
-
3.
not published in English language;
-
4.
reviews, case reports, conference abstracts, experimental studies.
2.3. Data extraction and the assessment of risk of bias
In order to minimize potential analyzing bias, we developed a standardized data collection form, which was utilized for data extraction from 2 of our authors independently. The data collected from these included studies consisted the following baseline characteristics: first author name, year of publication, country of origin, age, statin use status, total sample size, treatment strategies, follow-up information, sample size in statin user group and non-user group, and study design. Our main outcomes for analysis included all-cause mortality and cancer-specific mortality, which were expressed as HR with 95%CI comparing survival between statin users and non-users. For quality assessment and risk of bias analysis of each study, we would apply the Jadad scale[11] to evaluate the quality of RCTs, which consisted of randomization (0–2 points), blinding of the studies (0–2 points), and withdrawals (0–1 point). For observational studies, we would use the Newcastle–Ottawa Scale (NOS)[12] for risk of bias analysis. In this scale, it consisted of 3 factors: patient selection, comparability of the study groups,[12] and assessment of outcome and a score of 0 to 9 (allocated as stars) would be assigned to each observational study based on the scale. And a high-quality study was defined as one with a quality score of no less than 7. For identification, we used the name of first author and publication year for representing each study throughout the meta-analysis.
2.4. Data synthesis and statistical analysis
We performed our meta-analysis by applying the STATA 12.0 package (StataCorp, College Station, TX, USA) in accordance with the PRISMA guidelines.[13] We extracted HRs with 95%CI directly from the text of original study for comparison of all-cause mortality and cancer-specific mortality between statin users and non-users among esophageal cancer patients. Here we explored the impact of statin use on survival of esophageal cancer patients by not only focusing on these taking statins after the diagnosis of esophageal cancer but also these before the diagnosis. Moreover, we also conducted subgroup analysis based on pathological subtypes of esophageal cancer. We used the χ2-based Q statistics and I2 test for assessing the between-study heterogeneity and defined a high heterogeneity as P < .1 or I2 > 50%. If we encountered high heterogeneity during analysis, we would use the random effects models. If no high heterogeneity was observed, the fixed effects models would be applied for analysis. To test the stability of our results, we also conducted a sensitivity analyses by removing each study sequentially. Finally, we applied a funnel plot as tested by Begg and Egger tests[14] to evaluate potential publication bias. Statistical significance was as a two-sided P value of less than .05.
3. Results
3.1. Search results
After careful searching in above online databases, we initially retrieved a total of 429 papers. Our process for study evaluation was presented in Figure 1. After excluding these duplicate papers, we had a total of 269 papers for evaluation. After evaluation of the titles and abstracts of these paper, we excluded these unrelated studies, conference abstracts, and studies not published in English. As a result, we had a total of 7 papers for further evaluation of full text. One systematic review focusing on all types of cancers was excluded[15] and another 2 studies exploring the impact of statin use on either short-term outcomes[16] or cost-utility effects[17] in surgically treated esophageal cancer patients were also excluded. Finally, we included a total of 4 cohort studies[7–10] involving a total of 20,435 esophageal cancer patients (5319 statin users and 15116 non-users) for meta-analysis.
Figure 1.
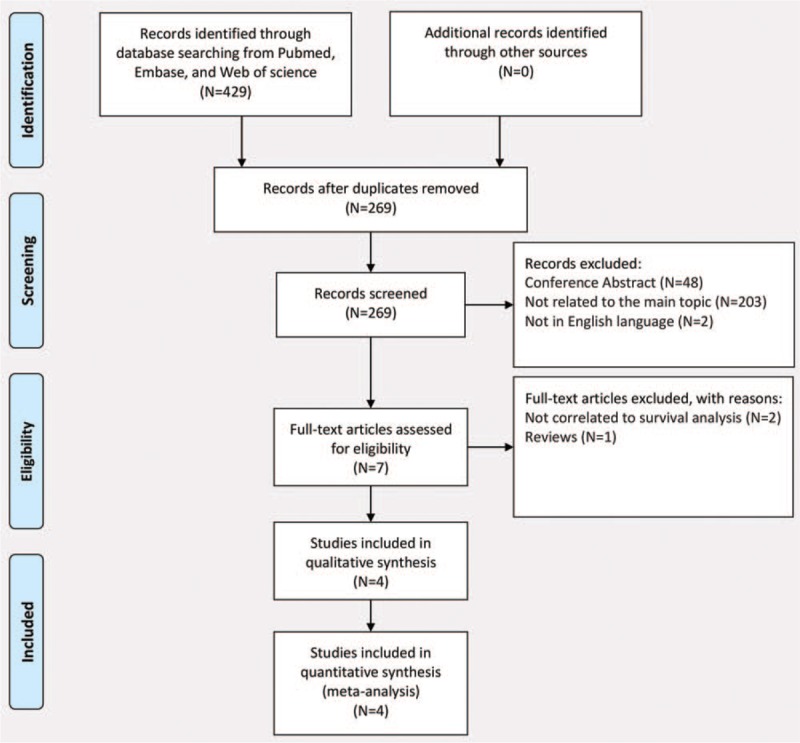
PRISMA flow diagram demonstrating the progress of study evaluation throughout the review.
3.2. Characteristics of the included studies
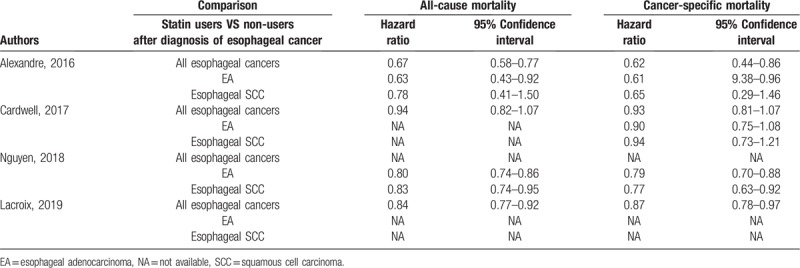
We presented the baseline characteristics of each included study in Table 1. All studies included patients with esophageal cancer treated with surgery, chemotherapy, or radiotherapy. The follow-up was actually relatively long even though the absolute time of follow-up time was not given out in some studies.[7–10] All studies had a relatively large sample size and appropriate adjustment for comparing survival between statin users and non-users. The main data for meta-analysis extracted from each included study were presented in Table 2. Two studies reported HRs of mortality for all esophageal cancers as well as EA and esophageal SCC individually, while 1 study only reported for all esophageal cancers without subgroup data for EA and esophageal SCC individually[10] and another 1 only reported HRs for EA and esophageal SCC individually.[9]
Table 1.
Characteristics of the included studies in this meta-analysis.
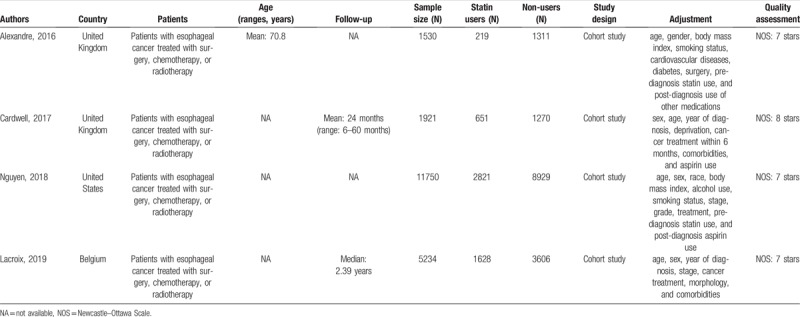
Table 2.
Main outcomes extracted from the studies included in our meta-analysis.
3.3. Quality assessment and risk of bias analysis
Because there was no RCTs available for analysis, we could only include above cohort studies for meta-analysis. Therefore, we used the NOS to evaluate the quality and risk of bias of these studies. The results of quality assessment were listed in Table 1. All these included studies yielded a quality score of no less than 7, which suggested a low risk of bias in our meta-analysis.
3.4. The impact of statin use after diagnosis on mortality of esophageal cancer patients
All studies reported HRs of mortality for comparing survival between statin users and non-users among esophageal cancer patients either stratified by pathological subtypes or not. Our meta-analysis found that statin use after diagnosis of esophageal cancer was significantly correlated to decreased all-cause mortality (random effects: HR = 0.81, 95%CI: 0.75–0.89, P < .001; I2 = 68.1%) in esophageal cancer patients (Fig. 2A). Moreover, statin use after diagnosis of esophageal cancer was also found to be significantly correlated to decreased cancer-specific mortality (fixed effects: HR = 0.84, 95%CI: 0.78–0.89, P < .001; I2 = 46.6%) in esophageal cancer patients (Fig. 2B). However, significant heterogeneity was observed during the analysis.
Figure 2.
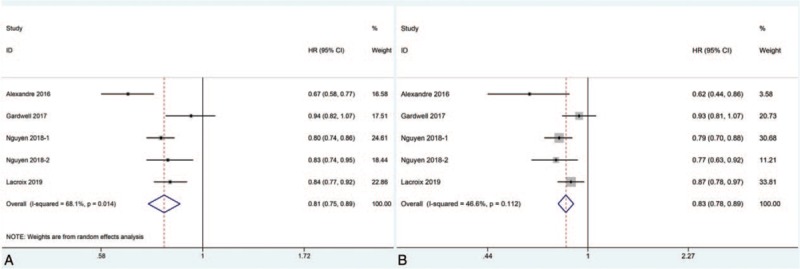
Forest plots of (A): all-cause mortality and (B): cancer-specific mortality demonstrating the impact of statin use after diagnosis of esophageal cancer on all esophageal cancer patients. NOTE: Those studies with the same author and year of publication were extracted from the same article analyzing subgroup based on different pathological subtypes; therefore, they shared the same identification code.
3.5. Subgroup analysis based on pathological subtypes of esophageal cancer
Because of significant heterogeneity observed above, considering the innate difference of EA and esophageal SCC, we conducted the subgroup analysis based on different pathological subtypes of esophageal cancer. However, our meta-analysis still found that statin use after diagnosis of esophageal cancer was significantly correlated to decreased all-cause mortality in both patients with EA (fixed effects: HR = 0.79, 95%CI: 0.74–0.85, P < .001; I2 = 31.5%) and these with esophageal SCC (fixed effects: HR = 0.83, 95%CI: 0.73–0.94, P = .003; I2 = 0%) (Fig. 3A). And statin use after diagnosis of esophageal was also found to be significantly correlated to decreased cancer-specific mortality in both patients with EA (fixed effects: HR = 0.81, 95%CI: 0.74–0.89, P < .001; I2 = 31.1%) and these with esophageal SCC (fixed effects: HR = 0.82, 95%CI: 0.71–0.95, P = .009; I2 = 0%) (Fig. 3A). Moreover, no significant heterogeneity was observed during subgroup analysis.
Figure 3.
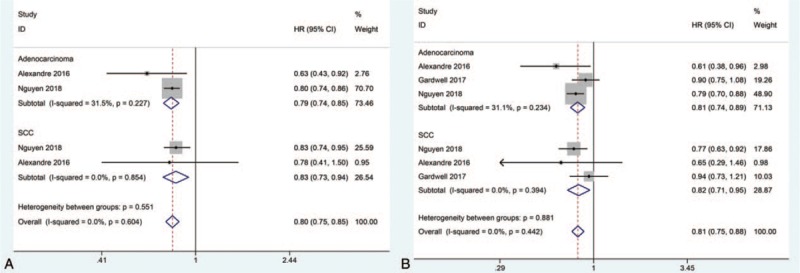
Forest plots of (A): all-cause mortality,and (B): cancer-specific mortality demonstrating the impact of statin use after diagnosis of esophageal cancer on esophageal cancer patients stratified by pathological subtypes. SCC = squamous cell carcinoma.
3.6. The impact of statin use before diagnosis on mortality of esophageal cancer patients
Three studies reported the impact of statin use before diagnosis of esophageal cancer on mortality of esophageal cancer patients. Our meta-analysis found that statin use before diagnosis of esophageal cancer was still significantly correlated to decreased all-cause mortality (fixed effects: HR = 0.86, 95%CI: 0.81–0.90, P < .001; I2 = 0%) in esophageal cancer patients (Fig. 4A). Moreover, statin use before diagnosis of esophageal cancer was also found to be significantly correlated to decreased cancer-specific mortality (fixed effects: HR = 0.85, 95%CI: 0.79–0.91, P < .001; I2 = 0%) in esophageal cancer patients (Fig. 4B). Moreover, no significant heterogeneity was observed during analysis.
Figure 4.
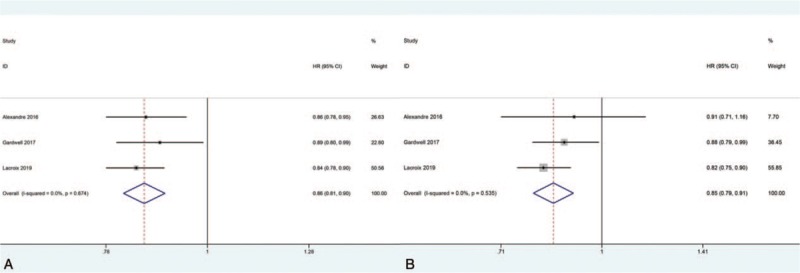
Forest plots of (A): all-cause mortality and (B): cancer-specific mortality demonstrating the impact of statin use before diagnosis of esophageal cancer on all esophageal cancer patients.
3.7. Sensitivity analysis and publication bias
For sensitivity analysis, we removed each study sequentially to test the stability of our results based on the analysis of all-cause and cancer-specific mortality. Sensitivity analysis showed that omitting each study sequentially did not change our primary results regarding the effects of statin use on mortality of esophageal cancer patients (Fig. 5). Moreover, we drew a funnel pot to evaluated publication bias based on all-cause mortality, which showed a symmetrical appearance, and no statistical significance was observed (Begg test: P = 1.00; Egger test: P = .91), indicating a very low publication bias (Fig. 6).
Figure 5.
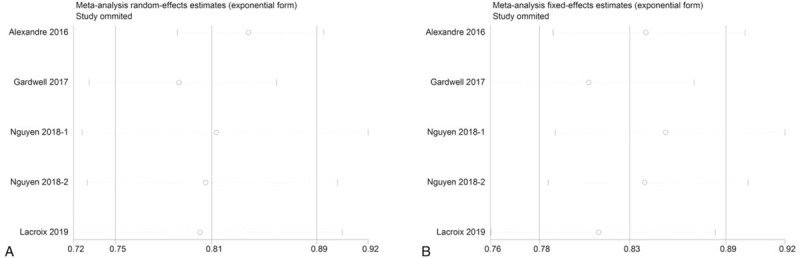
Sensitivity analysis for (A): all-cause mortality and (B): cancer-specific mortality demonstrating the impact of statin use after diagnosis of esophageal cancer on all esophageal cancer patients. NOTE: Those studies with the same author and year of publication were extracted from the same article analyzing subgroup based on different pathological subtypes; therefore, they shared the same identification code.
Figure 6.
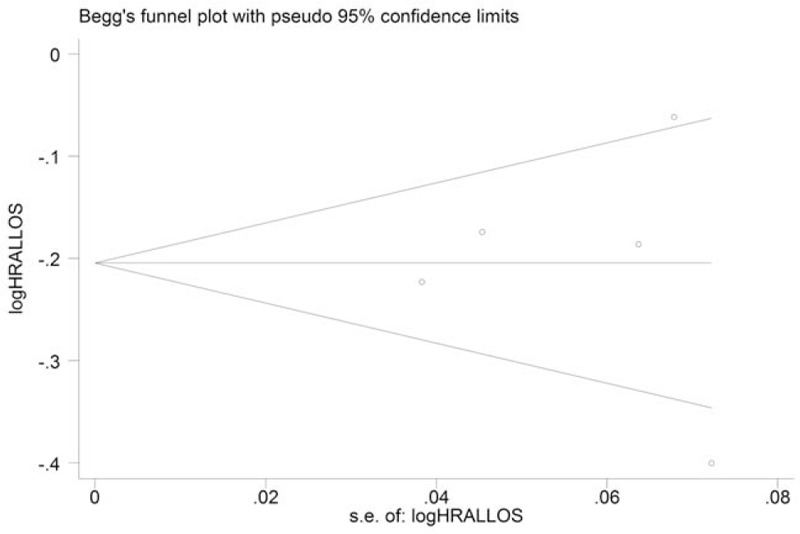
Funnel plot of the included studies based on the analysis of all-cause mortality. Begg test: P = 1.00; Egger test: P = .91.
4. Discussions
Statins, as the HMG-CoA reductase inhibitors, which could inhibit the endogenous mevalonate pathway and thus inhibit the downstream cholesterol biosynthesis and numerous isoprenoid metabolites,[18] are commonly used as cholesterol-lowering agents in cardiovascular diseases.[19] However, previous evidence has shown that apart from their cholesterol-lowering effects, statins also exhibited pleiotropic effects such as antiproliferative, pro-apoptotic, and anti-invasive effects in cancer cells.[19,20] Therefore, more and more studies have been carried out to explore the impact of statin use on survival of cancer patients.[15] Overwhelming evidence has shown that statin use was significantly correlated to decreased mortality of patients with various cancers, such as prostate cancer,[21] breast cancer,[22] and lung cancer.[23] However, only a few studies[7–10] have focused on the impact of statin use on survival of esophageal cancer patients with conflicting conclusions because of different sample size as well as statistical methods and different adjustment for confounding factors. Therefore, the impact of statin use on survival of esophageal cancer patients remains unclear. Hence, in this study, we conducted the first comprehensive systematic review and meta-analysis to figure out the actual effects of statin use on esophageal cancer patients.
In this meta-analysis, we included only a total of 4 cohort studies involving a total of 20,435 esophageal cancer patients (5319 statin users and 15,116 non-users). Our meta-analysis found that statin use after diagnosis of esophageal cancer was significantly correlated to decreased all-cause (HR = 0.81, 95%CI: 0.75–0.89, P < .001) and cancer-specific (HR = 0.84, 95%CI: 0.78–0.89, P < .001) mortality in esophageal cancer patients regardless of pathological subtypes (either EA or esophageal SCC). Moreover, statin use before diagnosis of esophageal cancer was also significantly correlated to decreased all-cause (HR = 0.86, 95%CI: 0.81–0.90) and cancer-specific (HR = 0.85, 95%CI: 0.79–0.91, P < .001) mortality in esophageal cancer patients. Therefore, our studies proved that statin use could significantly decrease the mortality risk of esophageal cancer patients regardless of the time when statins were taken or pathological subtypes of esophageal cancer.
Preclinical studies have shown that statins could exert their anticancer effects via both HMG-CoA reductase-dependent and HMG-CoA reductase-independent signal pathways.[6] In the HMG-CoA reductase-dependent pathways, statins inhibit the HMG-CoA reductase and subsequently block the conversion of HMG-CoA into mevalonte, thus decreasing the downstream products such as Ras/Rho families of the movalonate pathway which are crucial in regulating cell signal cascades mediating cell proliferation and growth.[6,24,25] In the HMG-CoA reductase-independent signal pathways, statins could block the lymphocyte function associated-1 and intercellular adhesion molecule-1 interaction and thus inhibit cancer cell migration and invasion. Moreover, statins could also inhibit proteasome, which could limit the breakdown of cyclin-dependent kinase inhibitors p21 and p27.[6,25] Moreover, statins could also exert anti-inflammatory and immunomodulatory effects via either HMG-CoA reductase-dependent pathways or HMG-CoA reductase-independent signal pathways.[6,18,24,25]
In esophageal SCC cells, HMG-CoA reductase was found to play an important role in tumorigenecity of esophageal cancer as knockdown of the expression of HMG-CoA reductase could inhibit the growth and migration of esophageal SCC cells,[26] which supported the application of statins as anticancer agents for esophageal cancer. Moreover, the mevalonate pathway was confirmed to be up-regulated in esophageal SCC cells and the application of statins could decrease extracellular signal-regulated kinase activation and proliferating cell nuclear antigen as well as cyclin D1 expression and thus induce cell apoptosis in esophageal SCC cells.[27] Statins could also decrease cell viability and proliferation as well as expression of key metastatic markers and induce apoptosis in EA cells.[28,29] In addition, statins could increase radiosensitivity and reverse epithelial-mesenchymal transition in esophageal cancer cells and thus exhibit potential effects of overcoming radioresistance of esophageal cancer.[30] As a result, it is reasonable to find that statin use was significantly correlated lower risk of esophageal cancer.[6] Moreover, in esophageal cancer patients undergoing esophagectomy, statin use could significantly decrease inflammation biomarkers and pulmonary epithelial and systemic endothelial injury, which may lead to improved perioperative outcomes.[16] Adjuvant statin therapy was also found to yield lower costs and better quality-adjusted life years in EA patients and it was a promising cost-saving treatment.[17] Therefore, taken our results together, we believe that statin use may improve the prognosis of esophageal cancer patients. As a result, statins may serve as promising adjunctive anticancer agents in treating esophageal cancer patients, particular these with cardiovascular diseases. Therefore, our meta-analysis highlighted the need of well-conducted RCTs to verify the efficacy of statins in treating esophageal cancer.
However, several limitations existed in our meta-analysis. First, we could only include 4 observational studies for analysis, which might decrease the validity of our results. Second, potential heterogeneity was observed during analysis. Even though we conducted subgroup analyses, the sample size in each subgroup analysis remained relatively small. Therefore, well-conducted RCTs exploring the anticancer effects of statins in esophageal cancer patients are required.
5. Conclusions
We conducted the first meta-analysis to investigate the impact of statin use on survival of esophageal cancer patients. We found that statin use was significantly correlated to decreased all-cause and cancer-specific mortality of esophageal cancer patients. Statins may serve as adjunctive anticancer agents in treating esophageal cancer. Further well-designed RCTs are badly needed to confirm and update our conclusions.
Author contributions
Conceptualization: Han-Yu Deng, Xi Zheng, Panpan Zha, Jie Zhou, Xiao-Ming Qiu.
Data curation: Ru-Lan Wang.
Formal analysis: Han-Yu Deng, Xi Zheng.
Methodology: Xi Zheng, Panpan Zha, Jie Zhou, Ru-Lan Wang, Rui Jiang.
Supervision: Xiulin Lan, Xiao-Ming Qiu.
Writing – original draft: Han-Yu Deng, Jie Zhou, Rui Jiang, Xiao-Ming Qiu.
Writing – review & editing: Xiulin Lan, Xiao-Ming Qiu.
Footnotes
Abbreviations: CI = confidence interval, EA = esophageal adenocarcinoma, HMG-CoA = 3-hydroxy-3-methylglutaryl-coenzyme A, HR = hazard ratio, NOS = the Newcastle–Ottawa Scale, RCT = randomized controlled trial, SCC = esophageal squamous cell carcinoma.
H-YD and XL contributed equally to the study and were co-first authors.
The authors declare no competing interests.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Deng HY, Wang ZQ, Wang YC, et al. Oesophageal adenocarcinoma has a higher risk of lymph node metastasis than squamous cell carcinoma: a propensity score-matched study. Eur J Cardiothorac Surg 2017;52:958–62. [DOI] [PubMed] [Google Scholar]
- [3].Deng HY, Wang WP, Wang YC, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg 2017;51:421–31. [DOI] [PubMed] [Google Scholar]
- [4].Desai CS, Martin SS, Blumenthal RS. Non-cardiovascular effects associated with statins. BMJ (Clinical research ed) 2014;349:g3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003;9:10–9. [PubMed] [Google Scholar]
- [6].Singh S, Singh AG, Singh PP, et al. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alexandre L, Clark AB, Bhutta HY, et al. Association between statin use after diagnosis of esophageal cancer and survival: a population-based cohort study. Gastroenterology 2016;150:854–65. .e851; quiz e816-857. [DOI] [PubMed] [Google Scholar]
- [8].Cardwell CR, Spence AD, Hughes CM, et al. Statin use after esophageal cancer diagnosis and survival: a population based cohort study. J Gastrointest Cancer 2017;48:124–30. [DOI] [PubMed] [Google Scholar]
- [9].Nguyen T, Khan A, Liu Y, et al. The association between statin use after diagnosis and mortality risk in patients with esophageal cancer: a retrospective cohort study of United States Veterans. Aliment Pharmacol Ther 2018;113:1310. [DOI] [PubMed] [Google Scholar]
- [10].Lacroix O, Couttenier A, Vaes E, et al. Statin use after diagnosis is associated with an increased survival in esophageal cancer patients: a Belgian population-based study. Cancer Causes Control 2019. [DOI] [PubMed] [Google Scholar]
- [11].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [12].Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203–10. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mei Z, Liang M, Li L, et al. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer 2017;140:1068–81. [DOI] [PubMed] [Google Scholar]
- [16].Shyamsundar M, McAuley DF, Shields MO, et al. Effect of simvastatin on physiological and biological outcomes in patients undergoing esophagectomy: a randomized placebo-controlled trial. Ann Surg 2014;259:26–31. [DOI] [PubMed] [Google Scholar]
- [17].Fong Soe Khioe R, Skedgel C, Hart A, et al. Adjuvant statin therapy for esophageal adenocarcinoma: a cost-utility analysis. Pharmacoeconomics 2018;36:349–58. [DOI] [PubMed] [Google Scholar]
- [18].Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 2012;64:102–46. [DOI] [PubMed] [Google Scholar]
- [19].Altwairgi AK. Statins are potential anticancerous agents (Review). Oncol Rep 2015;33:1019–39. [DOI] [PubMed] [Google Scholar]
- [20].Licarete E, Sesarman A, Banciu M. Exploitation of pleiotropic actions of statins by using tumour-targeted delivery systems. J Microencapsul 2015;32:619–31. [DOI] [PubMed] [Google Scholar]
- [21].Larsen SB, Dehlendorff C, Skriver C, et al. Postdiagnosis statin use and mortality in danish patients with prostate cancer. J Clin Oncol 2017;35:3290–7. [DOI] [PubMed] [Google Scholar]
- [22].Borgquist S, Broberg P, Tojjar J, et al. Statin use and breast cancer survival - a Swedish nationwide study. BMC Cancer 2019;19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cardwell CR, Mc Menamin U, Hughes CM, et al. Statin use and survival from lung cancer: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2015;24:833–41. [DOI] [PubMed] [Google Scholar]
- [24].Alexandre L, Clark AB, Cheong E, et al. Systematic review: potential preventive effects of statins against oesophageal adenocarcinoma. Aliment Pharmacol Ther 2012;36:301–11. [DOI] [PubMed] [Google Scholar]
- [25].Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005;5:930–42. [DOI] [PubMed] [Google Scholar]
- [26].Zhong C, Fan L, Yao F, et al. HMGCR is necessary for the tumorigenecity of esophageal squamous cell carcinoma and is regulated by Myc. Tumour Biol 2014;35:4123–9. [DOI] [PubMed] [Google Scholar]
- [27].Shi J, Zhu J, Zhao H, et al. Mevalonate pathway is a therapeutic target in esophageal squamous cell carcinoma. Tumour Biol 2013;34:429–35. [DOI] [PubMed] [Google Scholar]
- [28].Sadaria MR, Reppert AE, Yu JA, et al. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg 2011;142:1152–60. [DOI] [PubMed] [Google Scholar]
- [29].Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett's esophageal adenocarcinoma cells. Am J Gastroenterol 2008;103:825–37. [DOI] [PubMed] [Google Scholar]
- [30].Jin Y, Xu K, Chen Q, et al. Simvastatin inhibits the development of radioresistant esophageal cancer cells by increasing the radiosensitivity and reversing EMT process via the PTEN-PI3K/AKT pathway. Exp Cell Res 2018;362:362–9. [DOI] [PubMed] [Google Scholar]


