Abstract
Objective
To elucidate the clinical impact of humanized CCR4 antibody (mogamulizumab) on adult T-cell leukemia-lymphoma (ATL), we retrospectively analyzed the clinical and pathological features and treatment outcomes of aggressive ATL.
Methods
Twenty-two patients (median age: 65 years) with aggressive ATL [acute- (n=16) or lymphoma-type (n=6)] had their characteristics analyzed. All cases were treated with mogamulizumab at our institution from 2012 to 2018. In addition, we subjected 14 specimens of ATL to histological, immunological, and genetic analyses.
Results
Regarding the patient outcomes, the overall response rates were 68.1% and 31.8% after 4 and 8 courses (or after the final courses), respectively. The median overall survival (OS) was 95.5 days, while the OS rates at 6 and 12 months were 31.5% and 21.1%, respectively. Concerning patient pathological characteristics, 6 of the 14 patients examined (42.9%) had CCR4 mutations. Regarding the clinicopathological findings related to the mogamulizumab response, notably, the cases with somatic CCR4 mutation tended to have a poorer response (16.7%) than those with wild-type CCR4 (62.5%) after 4 cycles of mogamulizumab. Furthermore, the CCR4 global score tended to be higher in the responder cases than in the non-responder cases.
Conclusion
The present findings suggest that the CCR4 expression may be related to the mogamulizumab response, although no other significant predictive markers were identified in this study. Further studies will be needed in order to identify more markers related to the mogamulizumab response.
Keywords: ATL, mogamulizumab, somatic CCR4 mutation, NS mutation and FS mutation, overall response rate, overall survival
Introduction
Adult T-cell leukemia-lymphoma (ATL) is a T-cell neoplasm induced by human T-cell leukemia virus type I (HTLV-I) (1-4). For a quarter of a century, the discovery and establishment of the clinical entity of ATL, its pathogenesis, and remarkable progress in its treatment, including chemotherapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT), have been achieved worldwide thanks to skilled research pioneers and clinicians (1-18). However, despite remarkable advances in the pathogenesis and treatment of ATL, the prognosis remains poor.
A newly developed humanized CCR4 antibody (mogamulizumab) was recently reported to be effective for ATL as a molecular-targeted therapy. A phase 2 trial, post-marketing surveillance of mogamulizumab, and case-series reports in clinical practice have confirmed the safety and adverse effects of the drug for ATL treatment (19-26). The mechanism of action of mogamulizumab on ATL involves antibody-dependent cellular cytotoxicity and natural killer cells (27). In addition, mogamulizumab has also been reported to be effective in immunotherapy via immune check-point inhibition by increasing the numbers of cytotoxic T-cells under conditions of depletion of effector-type regulatory T-cells (T-regs) (28). Gain-of-function mutations in somatic CCR4 in ATL have recently been reported from the view point of treatment outcomes, with the mutation reportedly acting as a predictive marker for a favorable response to mogamulizumab (29-31).
To elucidate the effectiveness of the humanized CCR4 antibody mogamulizumab for ATL and determine the effects of CCR4 mutations under antibody treatment, we retrospectively analyzed the clinical features, pathological features, and treatment outcomes of aggressive ATL in clinical practice.
Materials and Methods
Cases
This retrospective study was conducted in compliance with good clinical practices and the ethical principles of the Declaration of Helsinki. Prior approval was obtained from the ethics review board at Miyazaki Prefectural Hospital.
We retrospectively analyzed 22 patients with aggressive ATL who had been treated at Miyazaki Prefectural Hospital (our institution) with weekly cycles of mogamulizumab for 8 weeks (from January 1, 2012, to August 31, 2018). According to Shimoyama's criteria for the diagnosis of ATL, based on the clinical features and prognostic factors (4), we classified the 22 cases of aggressive ATL as either acute (16) or lymphoma-type ATL (6).
Patients were selected for mogamulizumab therapy based on the following criteria: those with disease that was refractory to the initial treatment, those who required salvage therapy, and those who had difficulty continuing the initial treatment because of severe side effects, such as prolonged thrombocytopenia. All of the analyzed cases had already received several different cytotoxic chemotherapies, such as the vincristine, cyclophosphamide, doxorubicin, and prednisone (VCAP)-doxorubicin, ranimustine, and prednisone (AMP)-vindesine, etoposide, carboplatin, and prednisone (VECP) and cyclophosphamide CHOP regimens.
Consequently, 22 ATL patients were each administered 8 weekly cycles of mogamulizumab therapy at a dose of 1.0 mg/kg as a monotherapy. After 4 courses of mogamulizumab, the treatment response was evaluated. We then added an additional 4 courses of mogamulizumab for the ATL patients attaining complete remission (CR), partial remission (PR), and stable disease (SD). Finally, we evaluated the efficacy of mogamulizumab for the ATL patients based on the best overall response, which was the best response recorded from the initiation of the treatment until disease progression during the mogamulizumab treatment (19). The response to treatment was evaluated according to the Japan Clinical Oncology Group treatment response criteria for ATL.
We retrospectively analyzed the clinical manifestations, treatment, and prognosis of these 22 patients who required treatment with mogamulizumab. Treatment was performed based on the findings of a phase II study that showed a 50% response rate with acceptable toxicity profiles under these conditions (19). Before the administration of mogamulizumab, we confirmed the expression of CCR4 on ATL cells by flow cytometry or an immunohistochemical analysis in all of the ATL patients. Cases 1-14 were described in our previous report (23).
Adverse events (AEs) were evaluated using the National Cancer Institute Common Terminology Criteria for AEs, version 3.0, according to a phase II trial.
Tissue specimens, histology, and immunohistochemistry
Pathological analyses
Fourteen samples, including 1 skin, 2 bone marrow, and 11 lymph nodes lesions (LNS), were available for the pathological analyses. Tissue samples were fixed in 10% formalin, embedded in paraffin, cut into 2-μm-thick sections, and stained with Hematoxylin and Eosin (H & E) (32,33). After the histological assessment, a tissue microarray was created containing the 14 samples in a formalin-fixed paraffin block, with each sample placed in a 2-mm (diameter) area. These tissue microarray specimen slides were prepared according to a previous study. Formalin-fixed paraffin sections were used for immunoperoxidase studies performed using the avidin-biotin-peroxidase complex method. The following monoclonal antibodies were used: anti-CCR4 (Poteligeo test: Kyowa Medex, Tokyo, Japan), anti-Fox P3 (SP97; Abcam, Tokyo, Japan), anti-PD-1 (NAT 105; Abcam), and anti-human leukocyte antigen (HLA) class 1 ABC antibody [EMR 1161 (2); Abcam]. For the immunochemical analysis, CCR4-immunolabelled sections were also evaluated semi-quantitatively using a scoring scale based on the extent and intensity of staining (30). The extent and intensity scores were then multiplied to yield a unique global score, range 0-12 as described previously (30).
Mutation analyses for CCR4
We extracted tumor DNA from the available 14 tissue samples using the GeneRead DNA FFPE Kit (QIAGEN, Tokyo, Japan) following the manufacturer's protocol. According to our previous report, the primer sets used for the polymerase chain reaction (PCR) analysis and sequencing analyses were the same (30). Mutation analyses were performed, and Sanger sequencing was applied to detect mutations in the 14 ATL case samples, as previously reported (30).
Statistical analyses
The Kaplan-Meier method was used to estimate the probabilities of surviving after mogamulizumab treatment [overall survival (OS)]. The OS was defined as the time from the first day of mogamulizumab administration to the day of death or last follow-up. We excluded the patients who underwent allo-HSCT after mogamulizumab therapy from the survival analysis (Supplementary material 1). Fisher's exact test was used to examine the CCR4 mutation and treatment response for mogamulizumab, yielding statistical significance at p <0.05.
Results
Clinical characteristics of ATL patients
The clinical characteristics of the ATL cases are summarized in Table 1. The patients (median age: 65 years old) were classified as having acute (n=16) or lymphoma-type (n=6) ATL. Previous treatment regimens included CHOP, VCAP-AMP-VECP, DeVIC, CHASE, THP-COP, and GDP, and the analyzed patients had received a median of 1 regimen (range: 1-3). Previous disease responses to treatments were partial remission (n=3) and progressive disease (n=19). The overall response rate (ORR), OS, and adverse effects in the present study were consistent with our previous report. The ORRs were 68.1% and 31.8% after 4 and 8 courses (or after the final courses), respectively. The median OS rates at 6 and at 12 months were 31.5% and 21.1%, respectively. All of the patients with acute-type ATL who showed a response to treatment had an early response. Notably, 12 of the 22 ATL patients showed a somewhat prolonged survival (>100 days). Relapse or disease progression in the peripheral blood, central nervous system, lymph nodes, skin, and/or bone occurred within a relatively short period after treatment in some of the analyzed cases. Four cases maintained a CR status with a median survival time of 2.5 years, although 2 of them underwent allo-HSCT after mogamulizumab treatment (Supplementary material 1). The adverse effects were tolerable and included lymphopenia, cytomegalovirus infection, and skin rash.
Table 1.
Patient and disease characteristics in ATL cases.
| Clinical characteristics (n=22) | ||
| Age median (range) | 65 (46-80) | |
| Sex | ||
| Male (%) | 8 (36) | |
| Subtype | ||
| Lymphoma | 6 | |
| Acute | 16 | |
| sATL-PI | ||
| Low | 0 | |
| Intermediate | 13 | |
| High | 9 | |
| Median numbers of prior regimens including allo-SCT (range) | 1 (1-3) | |
| Allo-SCT (%) | 1 (5) | |
| Clincal status prior mogamulizumab | ||
| PR | 3 | |
| PD | 19 | |
| Response after 4 cycles of mogamulizumab | ||
| CR | 8 | |
| PR | 7 | |
| SD | 2 | |
| PD | 5 | |
| Best over all response during mogamulizumab | ||
| CR | 8 | |
| PR | 7 | |
| SD | 2 | |
| PD | 5 | |
| Pathological findings (n=14) | ||
| CD3, positive (%) | 13 (93) | |
| CD4, positive (%) | 11 (79) | |
| CD8, positive (%) | 2 (14) | |
| FOXP3, positive (%) | 5 (36) | |
| CCR4, positive (%) | 12 (86) | |
| CCR4 global score, average (range) | 6.5 (0-12) | |
| HLA class1, positive (%) | 10 (71) | |
| HLA class2, positive (%) | 6 (43) | |
| PDL1, positive in neoplastic cells (%) | 2 (14) | |
| PDL1, positive in microenvironment (%) | 7 (50) | |
| PD-1 positive TIL, counts/HPF average (range) | 3.8 (0-20) |
sATL-PI: simplified Adult T-cell leukemia/lymphoma prognostic index, SCT: stem cell transplantation, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, TIL: tumor-infiltrating lymphocyte
Pathological characteristics of ATL patients
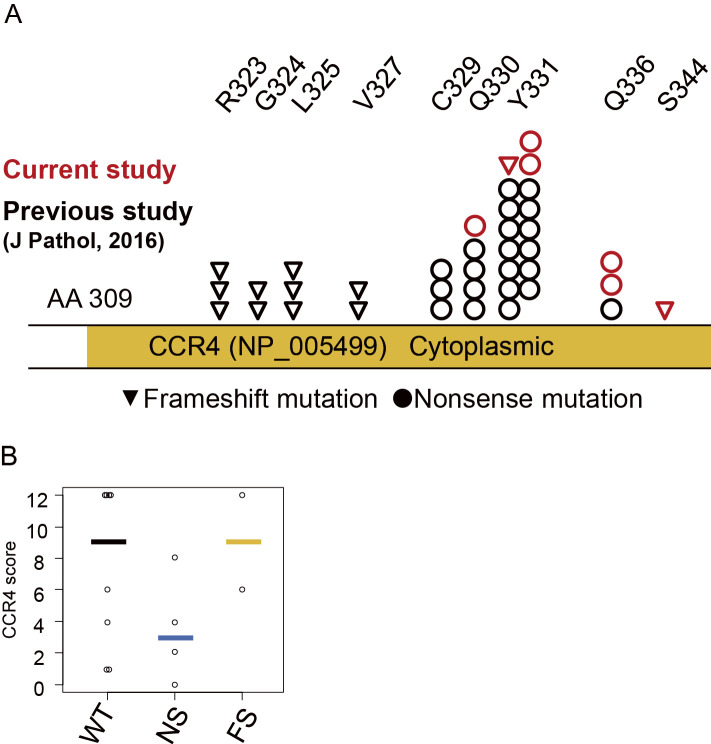
To elucidate the poor outcome of ATL, we investigated 14 specimens (13 LNS and 1 skin) of ATL classified as having acute- (n=9) or lymphoma-type (n=5) ATL using histological, immunological, and genetic analyses. The somatic CCR4 mutation sites of ATL cases are shown in Fig. 1A. Six of the 14 analyzed patients (42.9%) had somatic CCR4 mutations [nonsense (NS) in 4 cases and frame shift (FS) in 2 cases]. The mutated site of CCR4 was point 6 in all cases (Fig. 1A and Supplementary material 2). Case 3 had NS mutations at both Y331 and Q336. As Case 14 had a silent mutation (Y338Y), we regarded the case as not having a CCR4 mutation.
Figure 1.
The CCR4 status and expression in the current study. A) Mutation sites identified in the current study: circles, positions of nonsense mutations; inverted triangle, position of the frameshift mutation; red and black, current and previous cases, respectively (Yoshida et al., 2016). B) The comparison of the CCR4 global scores among ATL cases based on the CCR4 status. Bold bars indicate the mean score in each group.
In the immunohistochemical studies, the expression patterns of several antigens were consistent with those in previous reports (Table 1) (32,33). We previously found that CCR4 mutations, especially NS mutations of CCR4, were related to the expression (30). Indeed, the CCR4 expression in cases with NS tended to be lower than in the cases without CCR4 mutations based on the results of a semi-quantitative CCR4 protein analysis (Fig. 1B).
Clinicopathological findings related to mogamulizumab response
CCR4 mutations have been reported to be predictive markers of the mogamulizumab response (31). However, the OS was not significantly different between the cases with somatic CCR4 mutations and those with wild-type CCR4 in the present study (Fig. 2A). In addition, stratification by the CCR4 mutation status did not reflect the clinical course (Fig. 2B). Notably, cases with somatic CCR4 mutations tended to have a poorer response (16.7%) than those with wild-type CCR4 (62.5%) after 4 cycles of mogamulizumab (Fig. 2C). In our study, the best overall response was consistent with the treatment response after 4 courses of mogamulizumab (Table 1). Consequently, 12 of the 22 analyzed cases had a response to mogamulizumab, but almost all relapsed later.
Figure 2.
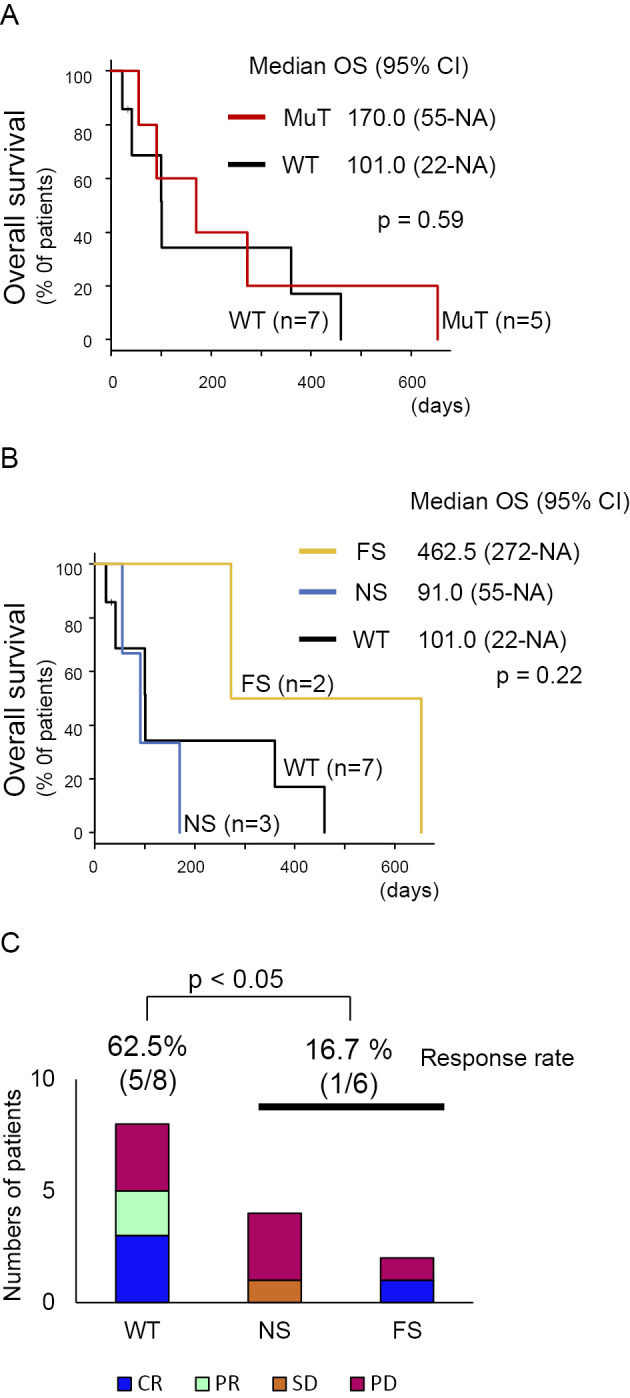
A) Kaplan-Meier plot of the overall survival among acute and lymphoma ATL cases, stratified by the presence of a CCR4 mutation. B) Kaplan-Meier plot of the overall survival among acute and lymphoma ATL cases, stratified by the CCR4 status. C) The examination of the relationship between the clinical response to mogamulizumab and the CCR4 status. The difference was analyzed by Fisher’s exact test.
In order to analyze the predictive markers related to the mogamulizumab response, we retrospectively compared the clinical laboratory findings, histological findings, and somatic CCR4 mutation between the responders (CR + PR) and non-responders [SD + progressive disease (PD)] (Table 2). Although the difference was not significant, the CCR4 global score tended to be higher in the responders than in the non-responders, suggesting that the intensity and proportion of the CCR4 expression may be related to the response. Notably, 1 case (Case 14) that did not express CCR4 (10% of tumor cells) showed a CR after mogamulizumab and remained alive without disease.
Table 2.
Clinicopathological and Genomic Findings Based on the Clinical Responses to Mogamulizumab.
| Reponder group (CR+PR) |
Non-Responder group (SD+PD) |
p value | ||||
|---|---|---|---|---|---|---|
| Clinical characteristics (n=22) | (n=15) | (n=7) | ||||
| Age Median (range) | 67 (46-79) | 63 (53-80) | 0.48 | |||
| Sex | ||||||
| Male (%) | 5 (33) | 3 (43) | ||||
| Subtype | 0.334 | |||||
| Lymphoma | 3 | 3 | ||||
| Acute | 12 | 4 | ||||
| sATL-PI | 0.648 | |||||
| Low | 0 | 0 | ||||
| Intermediate | 8 | 5 | ||||
| High | 7 | 2 | ||||
| Clinical status prior mogamulizumab | 0.523 | |||||
| PR | 3 | 0 | ||||
| PD | 12 | 7 | ||||
| Pathological characteristics (n=14) | (n=8) | (n=6) | ||||
| CD3, positive (%) | 7 (88) | 6 (100) | 1 | |||
| CD4, positive (%) | 6 (75) | 5 (83) | 1 | |||
| CD8, positive (%) | 2 (25) | 0 (0) | 0.473 | |||
| FOXP3, positive (%) | 3 (38) | 2 (33) | 1 | |||
| CCR4, positive (%) | 7 (86) | 5 (83) | 1 | |||
| CCR4 global score, average (range) | 8.4 (1-12) | 4.2 (0-12) | 0.099 | |||
| HLA class1, positive (%) | 6 (75) | 4 (67) | 1 | |||
| HLA class2, positive (%) | 3 (38) | 3 (50) | 1 | |||
| PDL1, positive in neoplastic cells (%) | 1 (13) | 1 (17) | 1 | |||
| PDL1, positive in microenvironment (%) | 4 (50) | 3 (50) | 1 | |||
| PD-1 positive TIL, counts/HPF average (range) | 1.9 (0-10) | 6.4 (0-20) | 0.156 | |||
| CCR 4 mutation | 2 (25) | 4 (67) | 0.277 |
Discussion
In the present study, we analyzed the clinicopathological findings, including the CCR4 mutations, in ATL cases treated with mogamulizumab therapy. The best overall response rate was 68% in the current study, with 2 of the analyzed cases living without any disease for more than 3 years. These findings were consistent with those of a phase II study showing a 50% response rate with acceptable toxicity profiles (19). The CCR4 expression may be related to the mogamulizumab response, but no other significant predictive markers were identified in the current study.
We showed that CCR4 mutations did not act as predictive markers in the current study. Given that the identified CCR4 mutations were consistent with those noted in previous reports, including our own (29-31,34), these mutations are considered gain-of-function mutations. Our previous study showed that the prognosis in ATL cases with an FS mutation of CCR4 was significantly poorer than in other cases without mogamulizumab treatment (30). The current results suggest that this mutation may provide certain benefits under mogamulizumab therapy, as previously reported (31), as the FS mutation seemed to have no adverse effect on the prognosis. However, the prominent predictive impact of CCR4 mutations identified in the previous study (31) was not found in our analysis. It is well recognized that complete remission rates of mogamulizumab therapy vary among target lesions, being high in peripheral blood and low in lymph nodes (19). All of the cases analyzed in the current study had lymph node lesions, and two of the CCR4-mutated cases showed PD at the lymph node lesions after four cycles of mogamulizumab treatment. This finding may indicate that tumors in lymph nodes are not sensitive to mogamulizumab, even in cases with CCR4 mutations. It is therefore speculated that the patient background, especially the tumor involvement sites, may differ between the previous (31) and current studies. We believe that the full impact of CCR4 mutations on the mogamulizumab response is still unclear, and further studies should be performed.
The CCR4 expression may also reflect the response, as responders to mogamulizumab tended to show a higher CCR4 global score than non-responders. Although our previous data showed that cases with NS mutations had higher CCR4 global scores than other cases (30), such differences were not detected in the present study (Fig. 1B). Other studies have also revealed that CCR4 mutations cause impaired internalization of CCR4, leading to a sustained CCR4 expression (29,34). Indeed, one case (case 21) with an NS mutation (Q336) lacked CCR4 expression in the LNS of our immunohistochemistry study, even though CCR4 expression was detected in the peripheral blood by a flowcytometric analysis; no such cases were detected in our previous study (30). Another case with NS mutations (Y331 and Q336) had CCR4 expression, but the expression was quite low. In line with the findings of a previous study (19), those cases showed PD only in the lymph node lesions during mogamulizumab treatment. Umino et al. previously found that clonal evolution of ATL occurred in lymph nodes, and a fraction of these clones was detected in the peripheral blood according to a genomic analysis (35). The current case also suggests a difference in the clones between the lymph nodes and peripheral blood. In order to clarify the utility of CCR4 mutations as a predictive marker concerning the response to mogamulizumab treatment, multiple samples from identical cases and a detailed analysis of CCR4 mutations should be performed in the future.
Of note, we detected one case (case 14) without CCR4 mutation that showed a good response to mogamulizumab, although the case had low levels of CCR4, HLA-I, HLA-II, and tumor infiltrating lymphocytes. Mogamulizumab exerts antibody-dependent cellular cytotoxicity on ATL cells (36), but the current results suggest that other mechanisms may underlie the anti-ATL effect of mogamulizumab.
In conclusion, no apparent predictive markers for mogamulizumab sensitivity were identified, although 68% of the relapsed ATL cases showed a response. Further studies will be needed in order to identify more markers related to the mogamulizumab response. Our current results also suggest the importance of preparing several validation cohorts to determine the definite impacts of genomic alterations on the clinical courses and molecular aspects.
The authors state that they have no Conflict of Interest (COI).
Noriaki Kawano and Noriaki Yoshida contributed equally to this work.
Supplementary Materials
Summary of clinical and pathological findings in analyzed cases.
CCR4 mutations identified in the current study.
References
- 1. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50: 481-492, 1977. [PubMed] [Google Scholar]
- 2. Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA 81: 25342537, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tajima K, Kuroishi T. Estimation of rate of incidence of ATL among ATLV (HTLV-I) carriers in Kyushu, Japan. Jpn J Clin Oncol 15: 423-430, 1985. [PubMed] [Google Scholar]
- 4. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol 79: 428-437, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Yamada Y, Tomonaga M, Fukuda H, et al. . A new G-CSF supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 113: 375-382, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Tsukasaki K, Utsunomiya A, Fukuda H, et al. . VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25: 5458-5464, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Tsukasaki K, Hermine O, Bazarbachi A, et al. . Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol 27: 453-459, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishitsuka K, Tamura K. Treatment of adult T-cell leukemia/lymphoma: past, present, and future. Eur J Haematol 80: 185-196, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Uozumi K. Treatment of adult T-cell leukemia. J Clin Exp Hematop 50: 9-25, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Tsukasaki K, Tobinai K. Clinical trials and treatment of ATL. Leuk Res Treatment 2012: 101754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukushima T, Miyazaki Y, Honda S, et al. . Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19: 829-834, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Okamura J, Utsunomiya A, Tanosaki R, et al. . Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood 105: 4143-4145, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Tanosaki R, Uike N, Utsunomiya A, et al. . Allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning for adult T cell leukemia/lymphoma: impact of antithymocyte globulin on clinical outcome. Biol Blood Marrow Transplant 14: 702-708, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Hishizawa M, Kanda J, Utsunomiya A, et al. . Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood 116: 1369-1376, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Ishida T, Hishizawa M, Kato K, et al. . Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 120: 1734-1741, 2012. [DOI] [PubMed] [Google Scholar]
- 16. Yamada Y, Atogami S, Hasegawa H, et al. . Nationwide survey of adult T-cell leukemia/lymphoma (ATL) in Japan. Rinsho Ketsueki (Jpn J Clin Hematol) 52: 1765-1771, 2011(in Japanese, Abstaract in English). [PubMed] [Google Scholar]
- 17. Kawano N, Yoshida S, Kuriyama T, et al. . Clinical features and treatment outcomes of 81 patients with aggressive type adult T-cell leukemia-lymphoma at a single institution over a 7-year period (2006-2012). Intern Med 54: 1489-1498, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Taniguchi H, Imaizumi Y, Makiyama J, et al. . Outcome of patients with relapsed/refractory adult T-cell leukemia-lymphoma after salvage therapy. Rinsho Ketsueki (Jpn J Clin Hematol) 54: 21592166, 2013(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 19. Ishida T, Joh T, Uike N, et al. . Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30: 837842, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Jo T, Ishida T, Takemoto S, et al. . Randomoized phase II study of mogamulizumab (KW-0761) plus mLSG 15 versus mLSG 15 alone for newly diagnosed aggressive ATL. J Clin Oncol 31: 8506, 2013. [Google Scholar]
- 21. Ishida T, Ito A, Sato F, et al. . Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia/lymphoma. Cancer Sci 104: 647-650, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yonekura K, Kanzaki T, Gunshin K, et al. . Effect of anti-CCR4 monoclonal antibody (mogamulizumab) on adult T-cell leukemia-lymphoma: cutaneous adverse reactions may predict the prognosis. J Dermatol 41: 239-244, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Kawano N, Kuriyama T, Sonoda KH, et al. . Clinical impact of a humanized CCR4 antibody (mogamulizumab) in 14 patients with aggressive adult T-cell leukemia-lymphoma treated at a single institution during a three-year period (2012-2014). Intern Med 55: 1439-1445, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Sekine M, Kubuki Y, Kameda T, et al. . Effects of mogamulizumab in adult T-cell leukemia/lymphoma in clinical practice. Eur J Haematol 98: 501-507, 2017. [DOI] [PubMed] [Google Scholar]
- 25. Ishitsuka K, Yurimoto S, Kawamura K, et al. . Safety and efficacy of mogamulizumab in patients with adult T-cell leukemia-lymphoma in Japan: interim results of postmarketing all-case surveillance. Int J Hematol 106: 522-532, 2017. [DOI] [PubMed] [Google Scholar]
- 26. Ishida T, Utsunomiya A, Jo T, et al. . Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: updated follow-up analysis of phase I and II studies. Cancer Sci 108: 2022-2029, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niwa R, Shoji-Hosaka E, Sakurada M, et al. . Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res 64: 2127-2133, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Sugiyama D, Nishikawa H, Maeda Y, et al. . Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 110: 17945-17950, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakagawa M, Schmitz R, Xiao W, et al. . Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med 211: 2497-2505, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshida N, Miyoshi H, Kato T, et al. . CCR4 frameshift mutation identifies a distinct group of adult T cell leukaemia/lymphoma with poor prognosis. J Pathol 238: 621-626, 2016. [DOI] [PubMed] [Google Scholar]
- 31. Sakamoto Y, Ishida T, Masaki A, et al. . CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood 132: 758-761, 2018. [DOI] [PubMed] [Google Scholar]
- 32. Miyoshi H, Kiyasu J, Kato T, et al. . PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128: 1374-1381, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Asano N, Miyoshi H, Kato T, et al. . Expression pattern of immunosurveillance-related antigen in adult T cell leukaemia/lymphoma. Histopathology 72: 945-954, 2018. [DOI] [PubMed] [Google Scholar]
- 34. Kataoka K, Nagata Y, Kitanaka A, et al. . Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 47: 1304-1315, 2015. [DOI] [PubMed] [Google Scholar]
- 35. Umino A, Nakagawa M, Utsunomiya A, et al. . Clonal evolution of adult T-cell leukemia/lymphoma takes place in the lymph nodes. Blood 117: 5473-5478, 2011. [DOI] [PubMed] [Google Scholar]
- 36. Ueda R. Clinical application of anti-CCR4 monoclonal antibody. Oncology 89 (Suppl 1): 16-21, 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of clinical and pathological findings in analyzed cases.
CCR4 mutations identified in the current study.