Abstract
No specific endoscopic features for eosinophilic gastroenteritis (EGE) have been reported previously. This study therefore evaluated the endoscopic findings of six patients with EGE. The diagnosis was confirmed based on gastrointestinal symptoms, pathological findings on biopsy, and the absence of other diseases. The site of the lesion was identified based on eosinophilic infiltration with ≥20 cells per high-power field during a pathological specimen analysis. Flattening of the small intestinal villi was observed in four patients; we speculate that this may be a specific feature in the diagnosis of EGE.
Keywords: endoscopy, eosinophilic gastroenteritis, eosinophils, esophagus, gastroenteritis, intestines
Introduction
Eosinophils differentiate and proliferate from stem cells in the bone marrow, circulate in the peripheral blood, and finally accumulate in various target organs to exert their functions. They are also associated with diseases induced by aggravation of the Th2 immune responses, such as parasitic infections and allergic diseases (1).
In eosinophilic gastrointestinal diseases, the infiltration of eosinophils occurs in several parts of the gastrointestinal tract, resulting in abnormalities in its morphology and function. At present, eosinophilic esophagitis (EoE) is diagnosed only by the presence of eosinophilic infiltration (EI) in the esophagus, while eosinophilic gastroenteritis (EGE) is diagnosed only by the presence of EI in the gastrointestinal tract. In the United States, the prevalence of EGE is 22 to 28 out of 100,000 people (2). Kinoshita et al. (3) reported 26 cases of EoE and 144 cases of EGE over 5 years in Japan. Accordingly, the frequency of EGE was five times higher than that of EoE. Under both conditions, the age of susceptibility is 40 years, and the ratio of men to women was 13:11 (3). Klein et al. (4) classified EGE into three types: mucosal dominant, muscular dominant, and serosal dominant, depending on the depth of EI into the mucosa of the gastrointestinal tract. The primary site of lesions in the mucosal dominant type is the mucosa, and symptoms include abdominal pain, diarrhea, vomiting, bleeding, and easy fatigability. In the muscular dominant type, the primary site of lesions is the muscular layer, which causes thickening of the wall and narrowing of the gastrointestinal tract, resulting in obstructive symptoms, such as abdominal pain and vomiting. The main site of lesions in the serosal dominant type is the serosa, and symptoms include abdominal pain, sense of distension, diarrhea, vomiting, and, in some cases, ascites. Endoscopic findings are diverse but non-specific and include erythema, erosion, ulceration, edema, nodules, polyps, and stenosis (5).
We herein report the clinical symptoms and endoscopic features of EGE that may be specific features in the diagnosis of EGE.
Case Report
Between April 1, 2012, and March 31, 2017, we examined the clinical features and endoscopic findings of six patients diagnosed with EGE in our department. Saitama Medical University Hospital is a central hospital in Saitama Prefecture, and the number of gastrointestinal endoscopy examinations performed over the 5-year period was approximately 35,000. The ratio of EGE was 6/35,000 patients, or 17/100,000, which was similar to the ratio in previous report (2). The diagnosis for EGE was based on gastrointestinal symptoms, the infiltration of eosinophils in a pathological specimen, and the absence of other diseases (6). There are no specific symptoms of EGE; however, we strongly suspect EGE in cases of abdominal symptoms, co-existence of allergic diseases, and an increased serum eosinophil count.
The differential diagnoses of EGE include ulcerative colitis, Crohn's disease, intestinal malignant lymphoma, collagenous colitis, scirrhous gastric cancer, Menetrier's disease, celiac disease, and protein-losing gastroenteropathy. Intestinal malignant lymphoma cells were not found on biopsy specimens, and computed tomography (CT) showed no findings of lymphoma. Collagenous colitis was excluded based on a pathological evaluation, with no collagenous fibrous bands exceeding 10 μm noted below the mucosal epithelium. There were also no findings of scirrhous stomach cancer endoscopically or pathologically. Menetria's disease shows similar symptoms as EGE. The feature of Menetria's disease is massive gastric folds, however, no such findings were noted endoscopically. Patients with celiac disease have an autoimmune response to gluten contained in wheat, so celiac disease was excluded based on the lack of a history of wheat allergy. Protein-losing gastroenteropathy was also excluded based on all six patients showing a normal serum total protein level.
The pathological lesion was defined as the site at which ≥20 cells per high-power field showed EI in the biopsy specimens. A biopsy was performed at random different sites for each case. Informed consent was obtained from all patients included in this study, and the study protocol was approved by the Institutional Review Board of our university.
Results
The study patients were between 20 and 80 years of age and included 1 man and 5 women (Table 1). The onset was defined as acute if noted within the past 1 week, subacute if noted between 1 and 3 weeks, and chronic if noted for 3 weeks or longer, and cases that had previous episodes were defined as recurrent. Two of the six patients were subacute, two were chronic, and two were recurrent. All patients had some type of allergic disease, including bronchial asthma (n=3), urticaria (n=2), chronic eosinophilic pneumonia (n=1), eosinophilic rhinitis (n=1), and eosinophilic otitis media (n=1). In addition, drug allergies were confirmed in five cases. Gastrointestinal symptoms, such as abdominal pain (n=4) and diarrhea (n=3), were also observed. In one case, mild ascites was documented. The serum eosinophil levels were raised in all cases and markedly increased in three cases (Table 2). The immunoglobulin E levels in the blood were elevated in four cases.
Table 1.
Patient Demographic Data.
| Case | Age | Sex | Symptoms | Drug allergy | Complication | Helicobacter pylori | Site of eosinophilic infiltration on biopsy | Ascites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | F | Epigastric pain | Aspirin | Acute pancreatitis Hives Bronchial asthma Eosinophilic sinusitis | (+) | Esophagus Stomach Duodenum Ileum Colon | (-) | ||||||||
| 2 | 44 | F | Dyspnea Diarrhea Abdominal pain | Over-the-counter medicine | Bronchial Asthma Chronic eosinophilic pneumonia Hives Eosinophilic otitis | Unknown | Ileum | (-) | ||||||||
| 3 | 80 | F | Diarrhea Fever Malaise | Panipenem Betamipron | None | (-) | Ileum Colon | (-) | ||||||||
| 4 | 63 | F | Abdominal pain Diarrhea | Aspirin | Bronchial asthma Type 2 diabetes | (+) | Esophagus Stomach Duodenum Ileum | (-) | ||||||||
| 5 | 20 | M | Epigastric pain | Acetaminophen Carbamazepine | Fabry disease | Unknown | Colon Abdominal Dropsy | (-) | ||||||||
| 6 | 51 | F | Exertional dyspnea | None | Ovarian cystoma Bronchial asthma | Unknown | Ileum Ascending colon Rectum | (+) |
Table 2.
Blood Laboratory Results.
| Patient No. | WBC | Eo (/μ/) | TP (g/dL) | IgE (U/mL) | CRP (mg/dL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7,020 | 1,797 | 6.2 | 850 | 0.10 | |||||
| 2 | 4,290 | 1,458 | 6.5 | 195 | 0.10 | |||||
| 3 | 69,590 | 63,326 | 6.6 | 508 | 4.48 | |||||
| 4 | 20,670 | 10,955 | 6.5 | 239 | 1.13 | |||||
| 5 | 10,560 | 1,689 | 7.1 | 379 | 1.38 | |||||
| 6 | 45,220 | 34,367 | 7.2 | 784 | 2.2 |
Endoscopic features of EoE, such as sulcus circularis insulae, longitudinal groove, and white spots, were not observed in the esophagus in any cases as evaluated by esophagogastroduodenoscopy (EGD). However, EI was observed in the pathological tissues of two patients. In the stomach, erosion was observed in two cases and erythema in one. In one patient with erosion, EI was observed on the histopathological specimens; however, EI was also observed in another patient with no endoscopic abnormalities. In one patient, erythema and erosion were observed in the duodenum, and EI was observed in the pathological tissue. In addition, shortening and flattening of the villi were observed in one patient (Fig. 1) along with EI. EI was also observed in the histopathological specimens of one patient with no abnormal findings on EGD. Mucosal edema without EI in the duodenum was observed in one patient. Colonoscopy demonstrated erythema in two cases, erosion in two cases, and mucosal edema in two cases. EI was observed in the pathological tissue biopsies in two of three patients with these endoscopic abnormalities as well as in two of three patients with no endoscopic abnormalities. In summary, endoscopic findings did not necessarily correlate with the EI observed on the biopsy.
Figure 1.
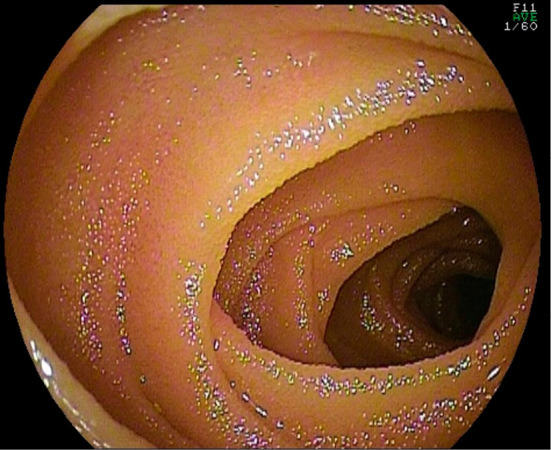
Esophagogastroduodenoscopy demonstrating shortening and flattening of villi in the overall biopsy specimen of the duodenum in one case. Eosinophil infiltration was also observed in this patient.
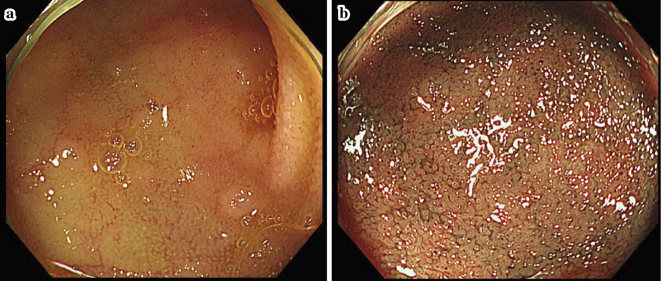
On colonoscopy, mucosal edema of the terminal ileum was observed in two patients, and erythema of the terminal ileum was observed in one patient. In four of six patients, flattening of the villi was observed (Fig. 2). Among the six patients, three showed EI in the tissue, including in one patient who did not show flattening of the villi. In addition, in one patient, while erythema and erosion were not observed in either the duodenum or jejunum, flattening of the villi was observed (Fig. 3) on oral double-balloon endoscopy, and a tissue biopsy demonstrated EI.
Figure 2.
(a, b) Colonoscopy showing mucosal edema and erythema in the terminal ileum. In four of six patients, flattening of the villi was observed in the overall biopsy specimens.
Figure 3.
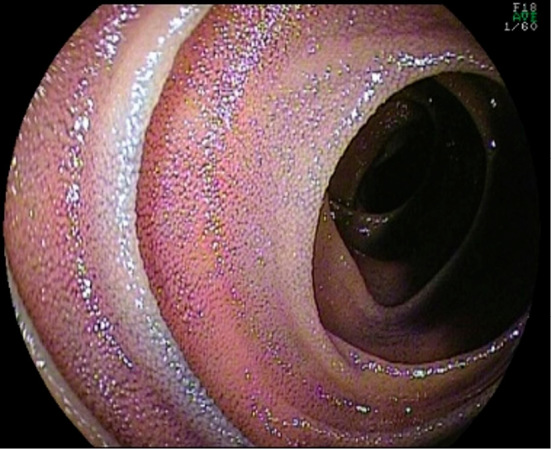
Oral double-balloon endoscopy showing flattening of the villi in the overall biopsy specimen of the jejunum.
Discussion
There were more women than men (five and one, respectively) in the present study; however, Kinoshita et al. reported no significant difference in the ratio of men and women in EGE (3). The sex bias in our study may have been due to our small sample size. Approximately half of all patients with EGE experience complications, including allergic disease; indeed, three of six patients in our study exhibited allergic complications. Only one patient demonstrated mild ascites. Five cases were classified as the mucosal dominant type and one case as the serosal dominant type because of ascites.
The endoscopic findings of EGE are varied, non-specific, and include erythema, erosion, ulceration, edema, nodules, polyps, and stenosis. Among the six patients in our study, three demonstrated erythema or erosion in the stomach and duodenum, while only one patient exhibited EI on a tissue biopsy, despite showing no endoscopic abnormalities. In one case, despite no endoscopic abnormalities, EI was observed in the esophagus, stomach, and duodenum on a histopathological examination, suggesting that confirmation of EI via a biopsy is useful, even in the absence of endoscopic abnormalities. In addition, we observed flattening of the villi in the duodenum and EI in the pathological tissue in one case. Therefore, when flattening of the villi in the duodenum is observed, it may be important to assess the degree of EI by a biopsy. Similar findings were observed in the jejunum in this case, with EI was confirmed in the pathological specimens. Careful observation of the small intestine using balloon endoscopy was therefore considered useful for the diagnosis. Similar endoscopic findings have been reported previously (7); as such, flattening of the villi in the duodenum may be a useful finding in the diagnosis of EGE.
EI was confirmed by a pathological examination in two of three patients with erythema or erosion, as observed via colonoscopy. Therefore, if such findings are observed, biopsies should be performed to confirm the presence of EI for a diagnosis. Conversely, even in the absence of endoscopic abnormalities, EI was observed in two patients; therefore, it may be important to confirm EI via a biopsy in patients with no abnormal findings endoscopically.
In addition, in four of six patients, flattening of the small intestinal villi was observed in the terminal ileum; three of these demonstrated EI on a biopsy. Therefore, the observation of the terminal ileum by colonoscopy may provide specific findings of EGE. Conversely, even when no abnormalities were observed during colonoscopy, EI was observed in the tissue in one patient; therefore, it may be useful to examine tissue biopsy results for a diagnosis. Previous studies have reported flattening or dropping out of the small intestinal villi or scattered erosion of the small intestine based on observations of the small intestine using a capsule or double-balloon endoscope (8); therefore, these findings may also be characteristic of EGE.
Other diseases or conditions in which flattening of the small intestinal villi is observed include congenital microvillous atrophy (9), prolonged central intravenous nutrition, celiac disease (10), and tropical sprue (11). Atrophy of the small intestinal villi during long-term central intravenous nutrition is believed to be due to apoptosis of villous epithelial cells, as reported in an animal study (12). Atrophy of the small intestinal villi in celiac disease is caused by inflammation of the small intestine that is triggered by immune reactions and destruction of the villus structure (13). Thus, the mechanism underlying small intestinal villi atrophy varies among diseases. Several reports have noted that, in epithelial Sel1L-deficient mice, the villi of the small intestine are flattened, and eosinophils infiltrate into the lamina propria of the mucosa, suggesting a relationship between epithelial Sel1L and villous atrophy in animals (14). Although the precise mechanism behind this observation is not clear, chronic inflammation caused by EI in the mucosa may cause flattening of the intestinal villi in EGE. However, this hypothesis needs to be validated in future studies.
The standard treatment for EGE is oral steroids [prednisolone (PSL) 0.5 to 1.0 mg/kg/day]; the dosage is gradually decreased every 1 to 2 weeks, based on the treatment regimen of inflammatory bowel disease. While this standard treatment has been reported to be effective in approximately 90% of cases, many cases of recurrence have been reported after tapering and discontinuation of steroids (15). In all six patients, the administration of oral steroids was effective; however, steroid tapering resulted in aggravation of the disease in one patient in our study. Therefore, the continuous administration of a small dose of PSL (4 mg/day) for a prolonged period was required to stabilize the disease.
Based on our findings, we propose that flattening of the intestinal villi may be caused by EI in the mucosa in EGE, and this change may be a feature of this disease. However, further studies will be required to validate this, as the present study had several limitations, including the small sample size and lack of an objective marker to indicate the degree of flattening.
Conclusion
EGE is not associated with any specific features in the upper gastrointestinal tract and colon; however, the detection of modest abnormalities, such as flattening of the small intestinal villi, may be important for obtaining an accurate diagnosis of EGE. Even in cases where no abnormalities are observed on endoscopy, confirmation of EI via a biopsy is important for establishing a diagnosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 105: 651-663, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr 52: 300-306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol 48: 333-339, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Klein NC, Hargrove RL, Sleisenger MH, et al. Eosinophilic gastroenteritis. Medicine 49: 299-319, 1970. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita Y, Furuta K, Ishimaura N, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol 48: 333-339, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Rached A, Hajj E. Eosinophilic gastroenteritis: approach to diagnosis and management. World J Gastrointest Pharmacol Ther 7: 513-523, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur S, Rosen JM, Kriegermeier AA, et al. Utility of gastric and duodenal biopsies during follow-up endoscopy in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 65: 399-403, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pungpapong S, Stark ME, Camgemi JR, et al. Protein losing enteropathy from eosinophilic enteritis diagnosed by wireless capsule endoscopy and double-balloon enteroscopy. Gastrointest Endosc 65: 917-918, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Kucinskiene R, Janciauskas D, Puzas A, et al. Microvillous inclusion disease. Medicina 40: 864-867, 2004. [PubMed] [Google Scholar]
- 10.Kaur A, Shimoni O, Wallach M, et al. Celiac disease: from etiological factors to evolving diagnostic approaches. J Gastroenterol 52: 1001-1012, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Lo A, Guelrud M, Essenfeld H. Classification of villous atrophy with enhanced magnification endoscopy in patients with celiac disease and tropical sprue. Gastrointest Endosc 66: 377-382, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Shaw D, Gohil K, Basson MD, et al. Intestinal mucosal atrophy and adaptation. World J Gastroenterol 44: 6357-6375, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozumi H, Hokari R, Miura S. Role of gastrointestinal endoscopy in malabsorption syndrome. Gastroenterol Endosc 56: 1511-1519, 2014. [Google Scholar]
- 14.Sun S, Loulie R, Sara B, et al. Epithelial Sel1L is required for the maintenance of intestinal homeostasis. Mol Biol Cell 27: 483-490, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambrun GP, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 9: 950-956, 2011. [DOI] [PubMed] [Google Scholar]