Abstract
The number of Takayasu arteritis (TAK) cases being diagnosed at an advanced age has increased, including some who develop ischemic lesions without inflammation of the involved arteries long after the onset of TAK. However, few histopathological analyses of such patients without immunosuppressive therapy have been reported. We herein report a 92-year-old woman with atypical aortic coarctation complicating TAK who underwent bypass graft surgery and survived for 23 years without immunosuppressive therapy. Microscopic findings at the autopsy revealed clear differences between the affected and unaffected arteries. This case suggests that inflammation severe enough to destroy the structure of the aorta may not inherently be sufficient to promote systemic atherosclerosis.
Keywords: Takayasu arteritis, atypical aortic coarctation, inactive disease
Introduction
Takayasu arteritis (TAK) is a chronic inflammatory disease that involves mainly the aorta and its major branches. Many TAK cases show a fever, fatigue, weight loss, arthralgia and myalgia. In laboratory findings anemia and inflammation are often noted. However, because of these nonspecific symptoms and laboratory findings, its diagnosis is sometimes delayed (1). Some patients are diagnosed with burn-out of inflammation in the scarring stage of TAK, when ischemic symptoms of the involved organs develop without active inflammation many years after the onset of TAK.
Atypical aortic coarctation (AAC) is a representative manifestation of TAK (2). It may develop anywhere along the length of the aorta (3). Severe hypertension of the upper extremities is characteristic of AAC complicated with TAK. If not treated, the prognosis is poor (4).
While immunosuppressive therapy is chosen as the initial treatment for TAK generally, surgical treatment is employed in some cases with ischemia caused by stenosis of the affected arteries. Immunosuppressive therapy includes glucocorticoid, immunosuppressants such as methotrexate and calcineurin inhibitors (5), and biologics such as interleukin 6 blockers (6). In contrast, surgical treatment is applied to TAK cases with severe stenosis, which includes AAC, vascular claudication, hypertension caused by severe stenosis of renal arteries, cerebral ischemia caused by severe stenosis of cerebrovascular arteries, and myocardial ischemia with detected cardiovascular stenosis (7,8).
While the number of TAK cases diagnosed at an advanced age has increased (9), they include some who develop ischemic lesions due to vascular stenosis without inflammation of the involved arteries long after the onset of TAK. Such cases are surgically treated without immunosuppressive therapy and are expected to have a favorable prognosis. However, very few histopathological analyses of the affected and unaffected arteries long after successful surgical therapy have been conducted.
We herein report a patient with TAK who survived for 23 years after bypass graft surgery without immunosuppressive therapy in whom a detailed histopathological analysis was performed of both the affected and unaffected arteries at the autopsy.
Case Report
A 92-year-old woman was admitted to our hospital because of congestive heart failure. Thirty years previously, she had also been admitted to another hospital because of congestive heart failure. Although a high systolic blood pressure (sBP) (270 mmHg) was noted, it improved (160 mmHg) with anti-hypertensive medication. Twenty-four years previously, she had been found to have systolic hypertension (220 mmHg) again. Since she had been experiencing orthopnea, she was admitted to an affiliated hospital.
A physical investigation revealed an extreme blood pressure difference between the upper and lower limbs (sBP; right upper limb 202 mmHg, left upper limb 184 mmHg, right lower limb 112 mmHg, left lower limb 118 mmHg). There was no heart murmur, but a bruit around the abdominal aorta was heard. She was afebrile. A blood examination showed no inflammation; C reactive protein (CRP) 0.1 mg/dL and erythrocyte sedimentation rate 8.0 mm/h. Human leukocyte antigen (HLA)-typing was not done. Enhanced computed tomography (CT) revealed calcification and coarctation in the entire circumference throughout the thoracoabdominal aorta without enhanced aortic wall thickening. Angiography revealed irregular narrowing of the descending aorta involving the celiac artery to 5 cm below the renal artery branch, along with stenosis of the ostium of the celiac and superior mesentery artery. No stenosis of other aortic branches, including the renal arteries, was detected. Conspicuous coarctation at the Th11/Th12 level with a significant blood pressure change across the damaged aorta (180 mmHg vs. 120 mmHg) was also revealed. Renal vascular hypertension was excluded because renal venous sampling revealed no laterality in the plasma renin activity level, although renal scintigraphy revealed laterality in the renal blood flow (right 34 mL/min, left 61 mL/min). A diagnosis of hypertension due to AAC complicating TAK was made according to the Japanese criteria (2).
No immunosuppressant, including glucocorticoid, was prescribed because of the inactive nature of the disease. Since she was refractory to four kinds of antihypertensive drugs, thoracoabdominal aortic bypass grafting and celiac and superior mesenteric artery reconstruction were performed (Fig. 1). A histological analysis of the unaffected thoracic and abdominal aorta that had been resected when making the anastomosis showed no inflammation.
Figure 1.
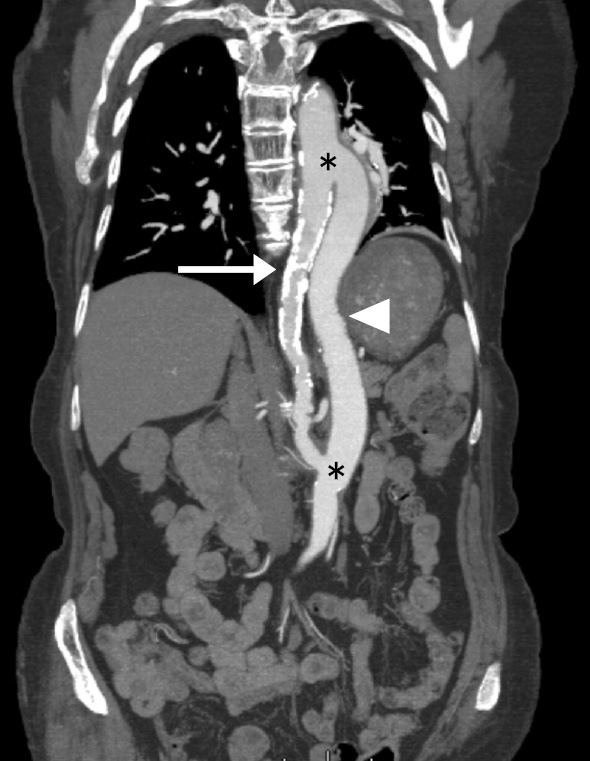
Enhanced CT image after thoracoabdominal aortic bypass grafting. Calcification and coarctation were seen in the entire circumference throughout the affected thoracoabdominal aorta (arrow). The graft showed good patency (arrowhead). Anastomoses of the bypass graft and aorta are indicated with asterisks.
After the operation, the blood pressure difference between the upper and lower limbs decreased to the normal range, and her blood pressure was controlled well with antihypertensive medication (sBP 130 mmHg). No postoperative complications, such as anastomotic aneurysm, anastomotic stenosis or graft deterioration, occurred. TAK did not recur for 23 years despite no immunosuppressants being administered from the time of her diagnosis of TAK. Starting around three years before her death, she began to experience repeated episodes of congestive heart failure accompanied by sick sinus syndrome. She died of chronic heart failure at 92 years of age, and an autopsy was performed. She had never been treated with glucocorticoids or any other immunosuppressants during her lifetime.
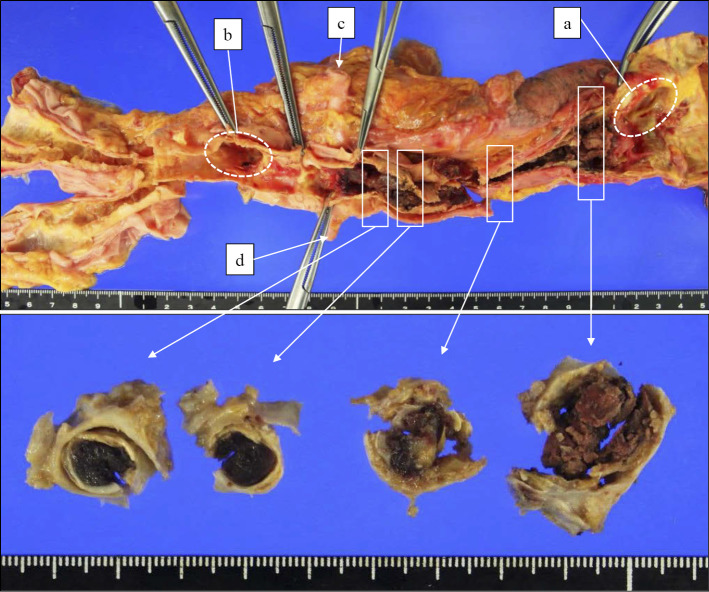
The graft showed good patency. The affected descending aorta had diffuse calcification, sclerosis and stenosis. The minimum diameter of the aorta was about 1 cm. Thrombotic occlusion over 14 cm in the descending aorta was found from just below the anastomosis of the thoracic aorta bypass graft to just above the renal artery branch (Fig. 2). Stenosis was also found in the right renal artery but not in other aortic branches. The right kidney revealed mild atrophy (right 70 g vs. left 85 g). No dilated arteries including aneurysms were detected. Microscopically, the affected aorta demonstrated fibrous thickening and calcification in the intima, rupture and disappearance of the medial elastic fibers, and fibrous thickening of the adventitia and feeding vessels (Fig. 3). The infiltration of lymphocytes and plasma cells in the adventitia along the aortic wall was slight. No granulomatous inflammation was detected. The same findings were found in the right renal artery and common iliac arteries. These findings were consistent with the scarring stage of TAK. In the ascending aorta, aortic arch and other aortic branches, including the celiac, superior mesenteric, common carotid, brachiocephalic, subclavian and left renal artery, the medial elastic fibers remained almost normal except for slight atherosclerosis, suggesting no involvement of TAK (Fig. 4). Slight infiltration of lymphocytes in the adventitia was noted in the boundary regions between the affected and unaffected arteries. Based on these pathological findings, a diagnosis of type III TAK involving the thoracoabdominal aorta, right renal artery and common iliac arteries was made (Fig. 5) (10). In marked contrast, the findings of the non-diseased arteries were those of arteriosclerosis associated with aging rather than with vasculitis.
Figure 2.
Macroscopic findings of the affected aorta. (a) Anastomosis of the bypass graft and thoracic aorta. (b) Anastomosis of the bypass graft and abdominal aorta. (c) Left renal artery. (d) Right renal artery. The affected descending aorta showed stenosis with a minimum diameter of about 1 cm (lower photos). Thrombosis over 14 cm in the descending aorta was found from just below the anastomosis of the thoracic aorta bypass graft to just above the renal artery branch. The renal blood flow seemed to be maintained by backflow from the bypass graft.
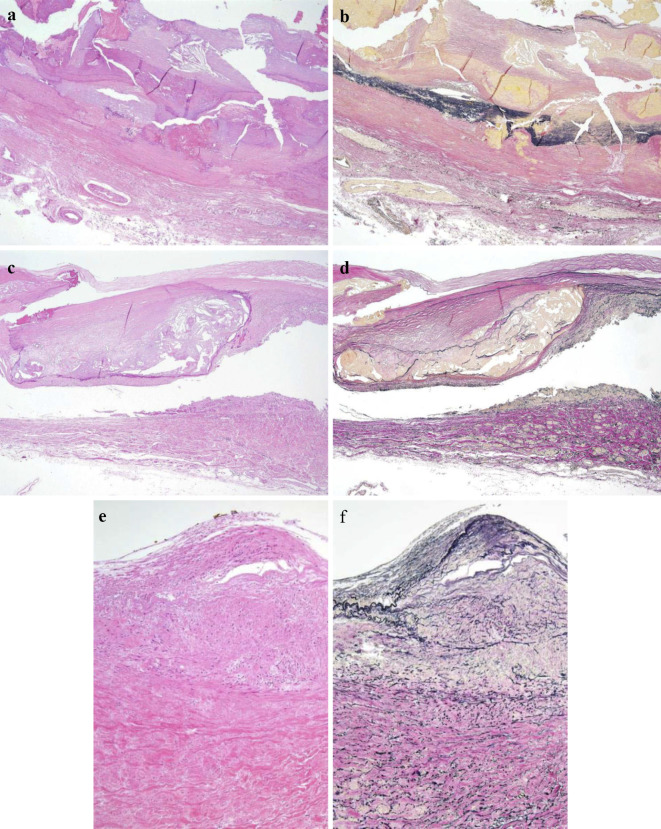
Figure 3.
Histopathological findings of the affected aorta. (a, b) Thoracoabdominal aorta, (c, d) common iliac artery, (e, f) right renal artery. Fibrous thickening and calcification were demonstrated in the intima, with rupture and disappearance of the medial elastic fibers. Fibrous thickening of the adventitia and feeding vessels in the affected aorta was found. Inflammatory cell infiltration in the adventitia was very slight. (a, c, d) Hematoxylin and Eosin staining, (b, d, f) Elastica van Gieson (EVG) stain. (a-d) ×20, (e-f) ×100
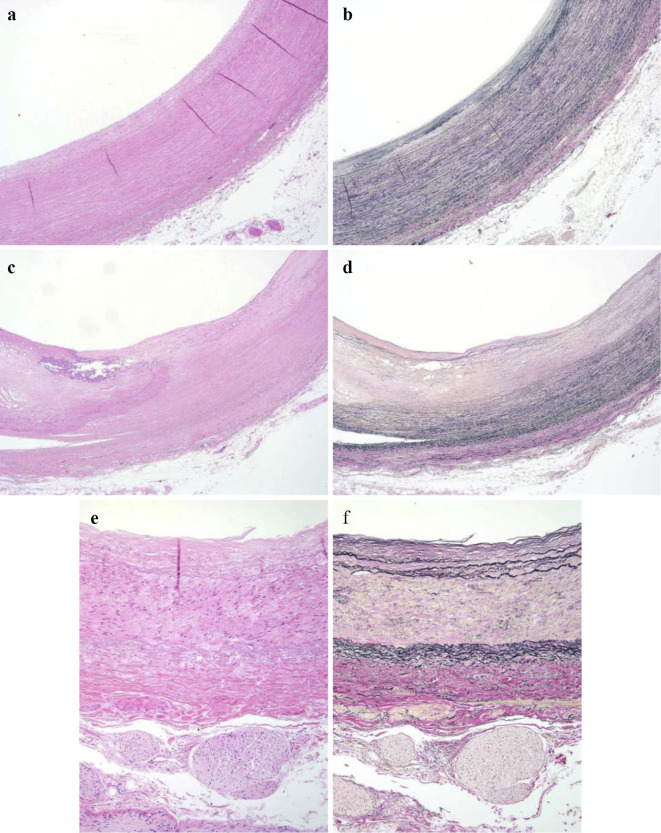
Figure 4.
Histopathological findings of unaffected arteries. (a, b) Brachiocephalic artery, (c, d) left subclavian artery, (e, f) left renal artery. The medial elastic fibers were preserved with only slight atherosclerosis. (a, c, e) Hematoxylin and Eosin staining. (b, d, f) EVG stain. (a-d) ×20, (e-f) ×100
Figure 5.
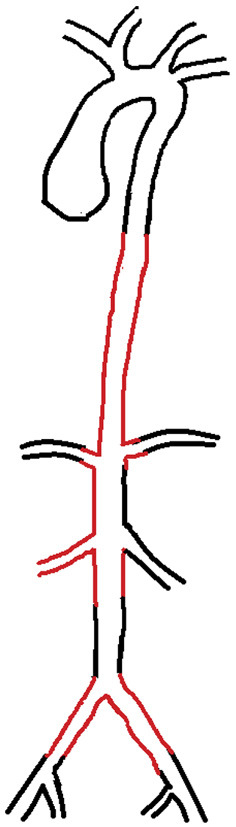
Angiogram of this TAK case. Involvement of the thoracoabdominal aorta, right renal artery and common iliac arteries (red line) indicated type III TAK [Adapted from Hata A et al. Int J Cardiol 1996; 54: (Suppl) S155-S163 (10)].
Discussion
We experienced a patient with AAC complicated by TAK who had had inactive disease since the diagnosis and enjoyed a prolonged survival after surgery without immunosuppression therapy at any time. She survived for 23 years after surgical treatment with only antihypertensive medication despite a history of severe hypertension in the upper half of the body (sBP 220 mmHg). Histopathological findings demonstrated characteristic lesions of TAK in the thoracoabdominal aorta, right renal artery and common iliac arteries, which were pathologically different from those of mere arteriosclerosis associated with aging. In contrast, the non-diseased arteries showed only slight atherosclerosis, although they had been exposed to the same degree of hypertension for many years before the surgery. Although the patient was long suspected of having been exposed to high blood pressure and inflammation sufficiently severe to destroy the arterial structures despite the non-use of glucocorticoid or immunosuppressants, the remote effect of persistent localized inflammation was unexpectedly limited and appeared to exert only minimal influence on the progression of atherosclerosis. Although limited to a single case, this finding raises the possibility that long-term persistent and severe inflammation alone is not necessarily sufficient to promote the development of arterial damage, such as atherosclerosis, at least in some cases.
The present case enjoyed a prolonged survival after surgery with only antihypertensive medication. AAC is common in TAK (2), which is often treated by surgery with a satisfactory prognosis (3). Her long-term survival was attributed to various favorable prognostic factors, including the absence of inflammation at surgery (11), good control of blood pressure after surgery (3), lack of post-surgical complications (7) and the lack of side effects, such as infection related to glucocorticoid or other immunosuppressants.
Although the number of TAK cases diagnosed at an advanced age has increased recently, their prognosis is not well understood. In a report on 1,372 TAK cases diagnosed in Japan from 2001 to 2011 (9), the average age at the disease onset was 35±3 years, and 43% were more than 40 years old at the diagnosis. In another report, 7% of TAK cases were stated to have been diagnosed at over 50 years of age (12). As the present case had developed congestive heart failure 30 years previously, presumably due to arteritis, the onset of TAK likely occurred at an age considerably younger than 62 years old. Although an elderly onset suggests the possibility of giant cell arteritis (GCA), the present case had not complained of headache at any time during her life, so we did not investigate the temporal arteries at the autopsy. Furthermore, no case showing spontaneous burn out with such ischemic lesions without inflammation of the involved arteries long after the onset of GCA has been described. In addition, we were unable to evaluate the disease activity of TAK in its early stage but consider it likely that the present patient had prolonged inflammation sufficiently severe to destroy the structure of the aorta before the diagnosis.
The frequency of TAK cases whose arteritis burned out without immunosuppressive therapy is unclear. However, TAK cases diagnosed at an advanced age include many with inactive disease and no inflammation without immunosuppression therapy. In the report of 11 TAK cases who were diagnosed at >40 years of age (57±6 years) (13), 73% had no inflammation, and none of them had been treated with immunosuppressive therapy. Aortography in elderly cases showed an irregular luminal surface, kinking and calcification (13). As TAK flare is infrequent in cases with inactive disease at the diagnosis, they usually do not receive any immunosuppressive therapy (14-18). Although the 5-year survival rate was 80% in elderly TAK cases in 1984 (13), the details of their long-term prognosis remain to be elucidated.
The present case showed the scarring stage of TAK based on a histopathological analysis in which the diseased vessels could be clearly discriminated from non-diseased ones. While it is often difficult to discriminate the scarring stage of TAK from arteriosclerosis, the former demonstrates rupture and fibrosis of the medial elastic fibers, fibrous thickening of adventitia and characteristic cell infiltration (19). Kerr et al. reported histopathological evidence of vasculitis in 44% of clinically inactive cases (14). The present case showed these histopathological findings of the scarring stage of TAK with typical distribution involving the thoracoabdominal aorta, right renal artery and common iliac arteries. The carotid and coronary arteries showed slight atherosclerosis rather than rupture of the medial elastic fibers, suggesting arteriosclerosis related to aging. From the nature and distribution of these histopathological findings, the diagnosis of the scarring stage of TAK was made. In contrast, the slight arteriosclerosis noted in the non-diseased arteries despite exposure to severe hypertension and suspected severe inflammation for many years was also a salient feature of this case.
We encountered a case of AAC complicating TAK that manifested inactive disease at the diagnosis and survived for a long period with only surgical treatment for AAC. The histopathological findings demonstrated characteristic lesions and distribution of the scarring stage of TAK in the diseased vessels while also showing unexpectedly slight arteriosclerosis in the non-diseased ones. This case suggests that a long-term survival after surgery for AAC with good control of blood pressure without immunosuppressive therapy may be possible in some appropriately treated elderly TAK cases with inactive disease at the diagnosis and that inflammation sufficiently severe to destroy the structure of the aorta may not be inherently sufficient to promote systemic atherosclerosis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank John Gelblum for his critical reading of the manuscript.
References
- 1. Arend WP, Michel BA, Bloch DA, et al. . The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33: 1129-1134, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Katsumura T. A report on the diagnostic criteria and therapeutic strategy of aortitis syndrome. In: 1991 Annual Report of the Intractable Arteritis Research Committee of Japan Organized by the Ministry of Health and Welfare, Ministry of Health and Welfare. Tokyo, Japan, 1991: 9-12(in Japanese). [Google Scholar]
- 3. Taketani T, Miyata T, Morota T, Takamoto S. Surgical treatment of atypical aortic coarctation complicating Takayasu's arteritis-experience with 33 cases over 44 years. J Vasc Surg 41: 597-601, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Cohen JR, Birnbaum E. Coarctation of the abdominal aorta. J Vasc Surg 8: 160-164, 1988. [PubMed] [Google Scholar]
- 5. Ogino H, Matsuda H, Kitamura S, et al. . Overview of late outcome of medical and surgical treatment for Takayasu arteritis. Circulation 118: 2738-2747, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Nakaoka Y, Isobe M, Nishimoto N, et al. . Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 77: 348-354, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mason JC. Takayasu arteritis: surgical interventions. Curr Opin Rheumatol 27: 45-52, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Saadoun D, Lambert M, Cacoub P, et al. . Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation 125: 813-819, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 132: 1701-1709, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol 54 (Suppl): S155-S163, 1996. [DOI] [PubMed] [Google Scholar]
- 11. Fields CE, Bower TC, Cherry KJ Jr, et al. . Takayasu's arteritis: operative results and influence of disease activity. J Vasc Surg 43: 64-71, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Ohigashi H, Haraguchi G, Isobe M, et al. . Improved prognosis of Takayasu arteritis over the past decade-comprehensive analysis of 106 patients. Circ J 76: 1004-1011, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Morooka S, Saito Y, Nonaka Y, Gyotoku Y, Sugimoto T. Clinical features and course of aortitis syndrome in Japanese women older than 40 years. Am J Cardiol 53: 859-861, 1984. [DOI] [PubMed] [Google Scholar]
- 14. Kerr GS, Hallahan CW, Hoffman GS, et al. . Takayasu arteritis. Ann Intern Med 120: 919-929, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Vanoli M, Daina E, Bertolini G, et al. . Takayasu's arteritis: a study of 104 Italian patients. Arthritis Rheum 53: 100-107, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Lee GY, Jang SY, Kim DK, et al. . Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol 159: 14-20, 2012. [DOI] [PubMed] [Google Scholar]
- 17. Park MC, Lee SW, Park YB, Chung NS, Lee SK. Clinical characteristics and outcomes of Takayasu's arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol 34: 284-292, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Hong S, Bae SH, Yoo B, et al. . Outcome of takayasu arteritis with inactive disease at diagnosis: the extent of vascular involvement as a predictor of activation. J Rheumatol 42: 489-494, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Numano F, Kishi Y, Tanaka A, Ohkawara M, Kakuta T, Kobayashi Y. Inflammation and atherosclerosis. Atherosclerotic lesions in Takayasu arteritis. Ann N Y Acad Sci 902: 65-76, 2000. [DOI] [PubMed] [Google Scholar]