Highlights
-
•
NOX is a key player of modulating both acute and chronic neurological disorders.
-
•
Prevention of the assembling of cytosolic and membrane subunits of the NOX is very important to inhibit NOX activity.
-
•
Both specific and non-specific NOX inhibitors are found to be effective in modulating chronic and neurological disorders.
Keywords: Ischemic stroke, NADPH oxidase, Superoxide, NOX inhibition
Abstract
Oxidative stress is a key player in both chronic and acute brain disease due to the higher metabolic demand of the brain. Among the producers of free radicals, NADPH-oxidase (NOX) is a major contributor to oxidative stress in neurological disorders. In the brain, the superoxide produced by NOX is mainly found in leukocytes. However, recent studies have reported that it can be found in several other cell types. NOX has been reported to regulate neuronal signaling, memory processing, and central cardiovascular homeostasis. However, overproduction of NOX can contribute to neurotoxicity, CNS degeneration, and cardiovascular disorders. Regarding the above functions, NOX has been shown to play a crucial role in chronic CNS diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS), and in acute CNS disorders such as stroke, spinal cord injury, traumatic brain injury (TBI), and related cerebrovascular diseases. NOX is a multi-subunit complex consisting of two membrane-associated and four cytosolic subunits. Thus, in recent years, inhibition of NOX activity has drawn a great deal of attention from researchers in the field of treating chronic and acute CNS disorders and preventing secondary complications. Mounting evidence has shown that NOX inhibition is neuroprotective and that inhibiting NOX in circulating immune cells can improve neurological disease conditions. This review summarizes recent studies on the therapeutic effects and pharmacological strategies regarding NOX inhibitors in chronic and acute brain diseases and focuses on the hurdles that should be overcome before their clinical implementation.
Introduction
Mammalian brain has a high oxidative metabolic rate with a low level of antioxidant and neuroprotective enzyme activity. The presence of copious readily oxidizable membrane polyunsaturated fatty acids (PUFAs) and endogenous production of reactive oxygen species (ROS) makes the central nervous system (CNS) vulnerable to oxidative stress damage (Schonfeld and Reiser, 2013). These PFAs and ROS are mediators of several chronic and acute neurodegenerative diseases. Chronic CNS disorders which are related to oxidative stress include dopaminergic motor neuron disease in the brain substantia nigra leading to Parkinson’s disease (PD); dementia-related disorders that include issues such as language problem, disorientation, depression, lack of motivation, behavioral issues in Alzheimer’s disease (AD); hereditary mutation of the huntingtin (Htt) gene that causes changes in mood and mental abilities in Huntington’s disease (HD); chronic CNS inflammatory disease, multiple sclerosis (MS); and inherited non-cell-autonomous disease called amyotrophic lateral sclerosis (ALS), and also in acute CNS disorders such as stroke, spinal cord injury, and traumatic brain injury (TBI).
Loss of blood supply in the brain due to hemorrhage or blood vessel occlusion is known as stroke. Subsequent neuronal cell death in the vascular territory of the stroke is caused by the energy depletion due to occlusion of the residual arteries. The severity of cellular death causes long-term disability, leading to ischemic stroke being ranked as the third most frequent case of death following heart disease and cancer at the 1st and 2nd, respectively (Kriz and Lalancette-Hebert, 2009; Lakhan et al., 2009). However, the pathophysiology of ischemic stroke is less understood and demands more attention to reduce infarct progression over time. In the acute phase, along with decreased cerebral blood flow, the disrupted cellular ionic homeostasis causes increased intracellular calcium concentrations and stimulation of glutamate release leading to excitotoxicity with inflammation and edema in the ischemic brain (Anrather et al., 2006; Barone and Feuerstein, 1999; Dirnagl et al., 1999). Again, ROS produced by the oxidative stress triggered by ischemic condition can destroy membranes, mitochondria, and DNA, which is also a leading cause of cellular death.
Superoxide generation by the immune cells and their consequences have been suggested to play a key role in the progression of neurodegenerative disorders. The soluble superoxide can combine with other ROS to induce different neurological disease conditions and form toxic particles in the diseased environment. In this review, among several other ROS, we will discuss the superoxide produced by the activation of NADPH oxidase (NOX) and its inhibitors in both acute and chronic CNS disorders (Chen et al., 2009). NOX has been reported to produce ROS more than any other known ROS-producing enzyme including lipoxygenase, cyclooxygenases (COX), xanthine oxidase, cytochrome P450, and substrate-coupled nitric oxide synthetase (Chan, 2004). Thus, many researchers studying CNS disorders and cell death have paid great attention to the research of the production of ROS by NOX and its mechanism in cell death in the recent years. The new discovery of the diverse NOX homologs have helped researchers in the study of the NOX enzyme and allowed them to investigate more targeted therapeutic approaches. However, to date, there are few therapeutic approaches to treat the NOX-induced ROS-related CNS disorders. This review will discuss NOX and its inhibitors in relation to CNS diseases and the therapeutic potential in detail (Table 1).
Table 1.
List of NOX inhibitors and their role on inhibition.
| Pharmacological compounds | Mode of inhibition | Disease Experimented | References |
|---|---|---|---|
| Diphenyleneiodonium (DPI) | Flavoprotein inhibition | Stroke. Spinal Cord Injury (SCI) | Nagel et al., 2012, Zehendner et al., 2013 |
| Alzheimer’s Disease (AD) Parkinson’s Disease (PD) | He et al., 2013; Khayrullina et al., 2015; Qin et al., 2002 | ||
| Wang et al., 2015 | |||
| Blocks NOX | Stroke. SCI | Heumuller et al., 2008 | |
| Apocynin | Blocks p47phox to migration. | AD, PD | Kleinschnitz et al., 2010 |
| Act as antioxidant and anti-inflammatory. | ALS | Tang et al., 2011; Lull et al., 2011 | |
| Ghosh et al., 2012, | |||
| Philippens et al., 2013 | |||
| Trumbull et al., 2012 | |||
| Honokiol and Plumbagin | Increases cytosolic p47phox | Stroke. SCI | Chen et al., 2016 |
| AD, PD | Liu et al., 2015 | ||
| Decreases membrane p22phox | Son et al., 2010 | ||
| Blocks the interaction of p47phox to NOX2 | Stroke. SCI, Traumatic brain injury (TBI) | Kumar et al., 2016a,b | |
| Nox2ds-tat | AD, PD | Khayrullina et al., 2015; Cooney et al., 2014, | |
| He et al., 2011; Askarova et al., 2011; Pal et al., 2016 | |||
| Serine protease inhibitors AEBSF | Effect directly on the plasma membrane NOX components | AD | Citron et al., 1996 |
| Asthma | Gillibert et al., 2005 | ||
| Interrupts cytosolic p47phox and p67phox binding | |||
| Phenylarsine oxide (PAO) | Interacts with the vicinal cysteine residues and inhibits NOX2 building | Paw edema | Doussiere et al., 1998, Roussin et al., 1997, |
| AD | Sardar Sinha et al., 2018 | ||
| Gliotoxin (GT) | Blocks p47phox phosphorylation | Dagenais and Keller, 2009 | |
| Blocks cytoskeleton fusion | Tsunawaki et al., 2004 | ||
| Inhibits of the p67phox, p47phox, and p40phox translocation | |||
| Ebselen | Block the p47phox and p67phox translocation to the plasma membrane | Smith et al., 2012 | |
| Herin et al., 2001; Koizumi et al., 2011; Jia et al., 2018 | |||
| VAS2870 | Inhibit phorbol-12-myristate 13-acetate-dependent NOX stimulation | Bupivacaine-induced spinal neurotoxicity | Ten frey et al., 2006 |
| (interfere downstream assembly process but not the p47phox) | Oshima et al., 2015 | ||
| HMGCoA reductase inhibitors | Inhibit NOX indirectly | Stroke | Becker et al., 2008 |
| AD, PD | Ghosh et al., 2009 | ||
| Padala et al., 2012 | |||
| Longenberger and Shah, 2011 |
ROS and NOX in the CNS
ROS play a critical role in normal cellular function in both the developing and adult brain. Oxygen, a provider of chemical energy, is also responsible for the formation of ROS through cellular metabolic activity. ROS can be produced from the both endogenous and exogenous sources. Endogenously, can be produced in cellular organelles such as the mitochondria (mostly in complex I and III), endoplasmic reticulum, and peroxisome. Enzymes that regulate the non-mitochondrial ROS production include NADPH oxidase, lipoxygenase, COX, xanthine oxidase, cytochrome P450, and nitric oxide synthetase (Angelova and Abramov, 2018). In the normal physiological condition, ROS is a by product of the metabolic system and cellular antioxidant system can defy ROS’s deteriorative effect and prevent the cellular death. In conditions of oxidative stress, ROS overproduction becomes injurious, contributing to various acute diseases such as ischemic stroke and myocardial infarction and chronic diseases related to the cardiovascular system such as hypertension, atherosclerosis, and neurodegeneration (e.g., AD, PD).
Among the sources of the cellular ROS production the mitochondrial system and the NOX system are the major contributor. Mitochondria use about 1–2% of the daily total cellular oxygen consumption during cellular respiration to produce superoxide anion radicals in the normal physiological condition in most tissues (Cadenas and Davies, 2000). Mitochondrial ROS has two sources which are, the mitochondrial respiratory chain and the mitochondrial outer membrane flavoprotien named as monoamine oxidase (MAO). The mitochondrial respiratory chain has 5 complexes in the inner mitochondrial membrane (IMM), complex I–V (Kim et al., 2015; Quinlan et al., 2013). These membrane complexes show thermodynamic properties which reduce the O2 to O2•−, these disproportionating O2•− are the main source of the H2O2. Usually, Complex I is stimulated by the succinate, a substrate of the Complex II, produce O2•− in the mitochondrial matrix, and Complex III in both side of the IMM (Orrenius, 2007). MAO is mostly produces the highly permeable H2O2, and due to its highly permeable property it can contribute to the both cytosol and mitochondrial matrix. Due to the absence of the histone wrapping (as in the nuclear DNA) and closer proximity the ROS production site, mitochondrial DNA (mtDNA) is a sensitive target for the oxygen radical attack (Cadenas and Davies, 2000).
NOX, one of the most important enzymes that utilizes molecular oxygen as a substrate, regulates ROS production in CNS disease when it is activated. To date, seven NOX paralogues have been reported by different research groups comprising NOX 1–5, and dual oxidase 1/2 (Duox1/2) (Eun et al., 2017). In the CNS, among the different cell types neurons, microglia, and intracranial vessels express few of the NOX paralogues, most prominently NOX2, NOX3, and NOX4 (Chan, 2004). Among the NOX family, NOX1 and NOX2 are particularly highly expressed in microglia and NOX3 is least studied and found expressed in the inner ear but not in blood vessels (Ago et al., 2005; Infanger et al., 2006). NOX4 is less expressed in neurons and astrocytes but is highly expressed in the kidneys and vascular smooth muscle cells. NOX5 is mostly expressed in the spleen and fetal tissues and has been found to be the least functional isoform of NOX in gene deletion experiments in rodent (Bedard and Krause, 2007). Duox1/2 were first found in the thyroid but then later in the immune system, gut, and lung. However, they are also found in the human neuroblastoma cell line (MO3-13) and rat brain.
In the mammalian nervous system, NOX paralogues are widely distributed throughout the cortex, hippocampus, and cerebellum. Recent studies have revealed that the NOX enzyme complex comprises four independent cytosolic subunits, p40phox, p47phox, p67phox, and Rac, and the membrane-bound p91phox and p22phox subunits (Panday et al., 2015). The activation of the enzyme depends on the proper interaction of the enzyme complexes where the cytoplasmic p47phox subunit acts as the major contributor by activating, binding, and docking the other cytoplasmic subunits to p22phox (Groemping et al., 2003; Groemping and Rittinger, 2005). The phosphorylation of the p47phox subunit is very important prior to the activation of the NOX enzyme and diverse upstream serine kinases, such as p21 and p38-activated kinases (PAK), PKB/AKT, mitogen-activated kinase (MAPK), cAMP-dependent kinases, and protein kinase C (PKC) isoforms (α, β, δ, ζ), are known to activate p47phox (Groemping and Rittinger, 2005). Among these enzymes, the PKC isoforms are most responsible for the phosphorylation of p47phox. Upon phosphorylation and binding of p47phox to p91phox, electron transfer from FAD to heme occurs through redox coupling to generate superoxide (Tang et al., 2012). However, the p67phox activator subunit is needed for electron transfer from NADPH to FAD. The hydrogen peroxide form within phagosomes is also reactive in nature. In the vascular system, the endothelial, adventitial, and vascular smooth muscle cells (VSMC) are the main source of ROS, primarily produced by NOX by catalyzing formation of the superoxide anion (O2•−) in the reaction: 2O2 + NADPH → 2 O2•−+ NADP + H+ where one electron reduces the oxygen (Lassegue and Clempus, 2003). The mechanism of cellular death by superoxide remains poorly understood, but may act via modulation of the functional properties of critical metabolic processes allowing nonselective generation of oxidants such as the hydroxyl radical. However, the dramatic attenuation of virulence in SOD gene deletion studies in bacteria showed that superoxide may directly kill the bacteria. The inflammatory transcription factor, nuclear factor kappa B (NFκB) can regulate the expression of NOX and can also induce gp91phox expression when stimulated by lipopolysaccharide (LPS) (Anrather et al., 2006). In the non-phagocytic cells AGE-RAGE [advanced glycation end-product (AGE), receptor of AGE] signaling is related with the controlled production of ROS via NOX activation (Koulis et al., 2015; Yamagishi et al., 2012). RAGE signaling also suggested to be involved in the ROS production via mitochondrial respiratory chain in AGE treated endothelial cells (Basta et al., 2005).
Studies suggested that mitochondrial ROS and NOX-ROS are related to each other and one may increase the production of another and vice versa (Dikalov, 2011). Both mitochondrial and NOX-ROS system are physically related with the endoplasmic system and in the normal physiological condition they are very site specific, and exerts their normal physiological function and on signaling (Santos et al., 2009). The interaction between the NOX and mitochondrial ROS system constitutes a feed-forward cycle by NOX-derived O2•− in which NOX-ROS promote the production of Mitochondrial ROS followed by additional NOX activation and O2•− production (Boulden et al., 2006). Emerging evidence suggested that the ROS produced by the mitochondria were considered to be unwanted by product of oxidative metabolism in contrast to the amount of the ROS produced by the NOX system (Dan Dunn et al., 2015). Maintenance of the mitochondrial ROS found to be beneficiary in the cognitive impairment (Glade, 2010) and also preservation of the high locomotor and exploratory activity, and less anxiety in animals (Head, 2009; Stefanova et al., 2010). On the other hand, higher NOX activity, and ROS production were evident in the AD and PD patients where the neuron and microglial cells might responsible for the over ROS production and neurological damages (Basta et al., 2005; Park et al., 2005). Antioxidant treatment targeted the mitochondrial and NOX-derived ROS production found to be beneficiary in the oxidative damage induced cognitive and locomotive impairment (Head, 2009; Wilkinson and Landreth, 2006). However, further studies targeting mitochondrial and NOX-derived ROS production in CNS disorder are warranted.
Function of NOX in acute and chronic CNS disorders
The presence of NOX isoforms in brain cells and its vasculature make it a potential therapeutic target in both acute and chronic brain diseases. NOX enzymes are not only responsible for disease conditions, but are also involved in essential events in stem cell biology, CNS development, and mature neurons, warranting related therapeutic research in CNS disorders. The superoxide produced by NOX in NMDA excitotoxicity is a popular concept when considering CNS disease progression. Recently, many studies have reported the involvement of ROS produced by NOX in acute and chronic disorders.
Ischemic stroke is one of the most critical CNS disorders and is the 3rd leading cause for human death in the world. Stroke is typically divided into two types, hemorrhagic and ischemic. Again the ischemic stroke can be divided into thrombotic or embolic; ROS production and ROS-mediated cellular damage is observed in all types of ischemia. Sudden loss of blood flow in the affected region causes damage to the mitochondria that cannot be reversed even after reperfusion (Aronowski et al., 1997; Kuroda and Siesjo, 1997). The rapidly induced hyperglycemic state in the reperfusion period causes NADPH overproduction through a hexose monophosphate shunt, which causes NOX activation and increases the production of ROS. The role of the ROS following reperfusion is depended on the concentration of the ROS produced. When the ROS production is less it contribute to the normal cellular physiological fictions (Katsuyama et al., 2012). On the other hand, the over production of ROS produces oxidative stress which causes lipid peroxidation, compromised membrane integrity, and organelle damage, which eventually accelerates cell damage (Nita and Grzybowski, 2016). Moreover, following ischemia, the cerebral parenchyma is overwhelmed with endogenous microglial activation and infiltration of peripheral leukocytes. The activated and infiltrating immune cells can produce superoxide with the help of a list of enzymes, of which NOX plays the major role. However, mechanism of NOX phosphorylation in ischemia is not well established yet. However, the phosphorylation of the p47phox subunit, including several protein kinase C isoforms, p38, p2, and phagocytic NOX2 are thought to be associated with this phenomenon (Lambeth, 2004). On the other hand, it is well known that in the inflammatory conditions the ROS is produced by the infiltrating immune cells via increased expression of pro-inflammatory cytokines, such as TNFα (Qin et al., 2004), Interleukin-1β (Mander et al., 2006), Interleukin-4 (Park et al., 2008a) and Interleukin-13 (Park et al., 2009) or PGE2 (Wang et al., 2004). In the injured CNS microglia, the expression of NOX2 is increased followed by their hypersensitivity nature to the proinflammatory cytokines which eventually resulted in the higher ROS generation (Surace and Block, 2012). Pawate et al., suggested that inhibition of the NOX2 in microglial cell line can suppress the expression of the pro-inflammatory cytokines such as TNFα, Interleukin-1β, and also attenuate the MAP kinase, and NFκB phosphorylation which is associated with the reduced ROS production (Pawate et al., 2004). As in ischemic stroke, oxidative stress and ROS are the major players with respect to cellular damage in traumatic brain injury (TBI). In ischemic stroke and TBI models, gp91phox (NOX2) expression was found to be increased whereas inflammation, oxidative stress, cellular damage, and secondary damage seem to be attenuated by NOX2 deletion (Tang et al., 2011; Wang et al., 2013). As TBI is a more direct injury than any other mechanism, superoxide injury is more dependent on NOX than any other signaling pathway. In another mechanism of oxidative injury in acute brain disease, NMDA receptor activation has been suggested to be responsible for ROS generation (Wang et al., 2013). In a previous study, mice with NOX2 deficiency in circulating blood cells but intact brain NOX2 and vice versa were subjected to strokes showing that mice with intact brain NOX2 but lacking circulating NOX2 had better functional outcomes than mice with intact circulating NOX2, but deficient brain NOX2 (Park et al., 2007, 2005; Park et al., 2008b).
Chronic inflammation and oxidative stress are related to the progression of most chronic CNS diseases in which the NOX enzyme family plays a pivotal role. NOX enzymes modulate the disease progression of chronic diseases like Alzheimer’s disease (AD) Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). In the astrocytes and microglia of the AD brain, NOX expression has been suggested to be increased by β-amyloid plaques (Bruce-Keller et al., 2010; Clayton et al., 2017; Ma et al., 2017). However, NOX was not found to have any direct relationship with the development of β-amyloid plaques or neurofibrillary tangles, which are important biomarkers of AD. The activation of NOX enzymes in AD is suggested to be by the phosphorylation of the p47phox subunit and subsequent translocation to the membrane by β-amyloid plaques (Shimohama et al., 2000). Recently, it has been suggested that NOX derived ROS (mostly H2O2) in AD are the central regulator of the cholesterol oxidation product, 24-hydrocholesterol (Gamba et al., 2011). 24-hydrocholesterol is considered to potentiate the Aβ mediated pro-apoptotic and pro-necrogenic effects in AD. Microglial NOX expression and ROS production have been observed in PD patients and animal models. Yet, neuroinflammation and oxidative stress were considered to be the root cause of dopaminergic neuron death and PD progression. Although studies regarding PD pathogenesis suggested that oxidative stress related to mitochondrial dysfunction (mitochondrial ROS production) are the leading cause of this disease and the role od NOX derived oxidative stress is still poorly understood. Hernan MS. et al., found that in 6-hydroxydopamine induced PD, higher membrane translocation of the NOX subunit p67phox which evident the involvement of NOX-derived ROS in PD pathogenesis (Hernandes and Britto, 2012). Rotenone induced higher activation of the NOX2 found to increase the accumulation of the alpha-synuclein aggregation elevating the alpha-synuclein toxicity, a well known hallmark of the PD (Pal et al., 2016). Excessive activation of the NOX2 by rotenone, increases the production of NOX2-induced ROS that impairs the autophagic flux by reducing the lysosomal activity. Autophagic flux is a crucial process for the clearance of the alpha-synuclein aggregates. In an another PD study, Zawada et al. reported that angiotensin II receptor (Ang II/AT1) levels were increased according to the disease condition in postmortem examination of pre-PD and PD patients’ substantia nigra (Zawada et al., 2015). They also suggested that elevated AT1 levels may activate NOX4, which may be the reason for substantia nigra neuronal death in PD, suggesting the role of NOX in PD development and progression. In the spinal cord microglia of animal ALS (a motor neuron disease) models, the expression of NOX2 mediates protein oxidation and modulates the neuronal insulin-like growth factor 1 (IGF-1)/Akt survival pathway to ameliorate neuronal damage (D’Ambrosi et al., 2018; Wu et al., 2006). SOD1, a regulator of NOX, was also found at increased levels in familial ALS. Marden et al. showed that mice with G93A SOD1 mutation crossed with NOX1- and NOX2-null mice can increase the life span of NOX2-null mice over that of NOX1-null mice (Marden et al., 2007). Thus, the researchers suggested that NOX is one of the components of ALS pathology. In the prion (misfolded, aggregated prion protein) infected disease such as Creutzfeldt-Jakob disease (a transmissible spongiform encephalopathies), microglial NOX2 related excessive ROS production is the key player of pathogenesis of this disease. The authors found that in the ex-vivo cortical slices ablation of the microglial cells reduced the NOX2-derived ROS, and slower the disease pathogenesis and increse in the neuronal life span (Sorce et al., 2014). In the early stages of the Huntington's disease (HD), accessive production of the NOX-derived (specially NOX2) ROS suggested to be a key player (Valencia et al., 2013). In their study, the authors suggested that the level of ROS was increased with the higher NOX activity in HD140Q/140Q (a model of HD mice) primary neurons and also the rise in the mitochondrial superoxide was depended with the rise in NOX activity. In chronic CNS diseases, NMDA excitotoxicity can activate NOX, which produces superoxide, and is also seen in acute CNS disease. The NOX-mediated ROS production via NMDA receptor overactivation may also be a major mechanism for chronic CNS disease development and progression. Thus, NOX inhibition and its subsequent effects have great potential as a target of therapeutic research in acute and chronic CNS diseases.
Pharmacological compounds to inhibit NOX enzymes
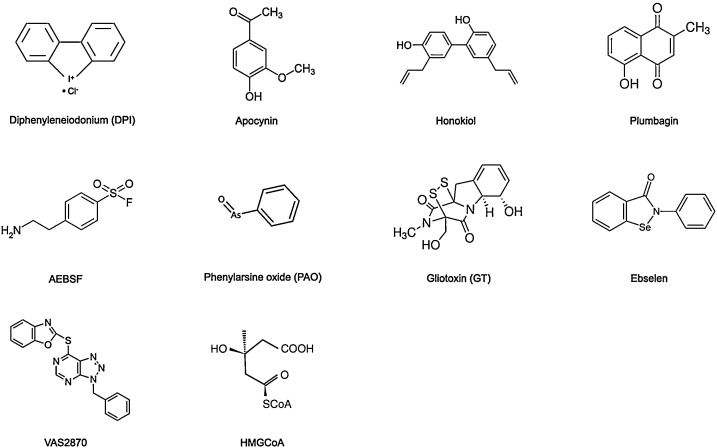
Due to the potential role of NOX in the development and progression of CNS disorders, NOX inhibition is a possible target for the treatment of various CNS disease. In recent years, a number of chemical compounds with NOX-inhibitory properties have been studied in the treatment of acute and chronic CNS disease (Fig. 1). The lower specificity, selectivity, and relative toxicities of these compounds impede their use in humans. However, there are a number of specific NOX inhibitors found to be safe, and safer alternatives are under development to be used in humans.
Fig. 1.
Chemical structure of NOX inhibitors.
Diphenyleneiodonium (DPI)
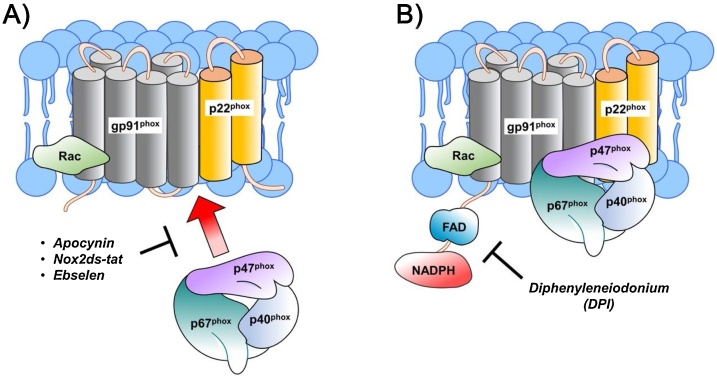
DPI, a well-known NOX inhibitor, inhibits superoxide production through flavoprotein inhibition (Fig. 2B). As a flavoprotein inhibitor, DPI not only inhibits NOX but also other flavin-dependent enzymes such as nitric oxide synthase (NOS), xanthine oxidase, and NADH coenzyme Q reductase. In ischemic stroke, it is also suggested to protect the brain from stroke by directly suppressing cerebral immunological responses, maintaining blood-brain barrier (BBB) integrity. The extracellular matrix proteins MMP-2 and MMP-9 are known to disrupt the BBB. DPI administration in an animal stroke model led to a reduction in the inflammatory responses and ROS production, along with MMP-2 and MMP-9 expression, while also protecting tight-junction integrity (Nagel et al., 2007, 2012; Zehendner et al., 2013). In spinal cord injury, DPI also reduced lesion size, post-injury inflammation, and the rate of oligodendrocyte and oligodendrocyte precursor death (Byrnes et al., 2011; He et al., 2013; Khayrullina et al., 2015)
Fig. 2.
Mode of inhibition of NOX. Apocynin, Nox2ds-tat and Eblesen block p47Phox translocation to the membrane. DPI inhibit the NOX by flavoprotein inhibition.
Enhanced beta-amyloid toxicity caused by microglia in cortical and mesencephalic neurons (mixed culture) has been reduced by treatment with DPI (Qin et al., 2002). Beta-amyloid stimulated microglia release of ATP, which induces ROS production and eventually neuronal death that is primarily dependent on NOX2. DPI inhibits neuronal death by inhibiting NOX2 ROS generation and mitochondrial depolarization in beta-amyloid stimulated astrocytes and neurons (Abramov et al., 2004). Bianca et al. also reported that DPI is capable of inhibiting H2O2 production induced by beta-amyloid in immune cells like the microglia, monocytes, and neutrophils (Bianca et al., 1999). In Parkinson's disease, pretreatment with an ultra-low dose of DPI has been found to reduce progression of neurodegeneration via attenuating microglia-induced chronic neuroinflammation by inhibiting NOX (Wang et al., 2015). Along with inhibition of the microglia, pretreatment with DPI is also capable of inhibiting proinflammatory factors and α-synuclein aggregation. However, due to the inhibitory effect of DPI on many other metabolic pathway component flavoenzymes in vivo, clinical implementation of DPI has been limited (Kahles et al., 2007).
Apocynin
Apocynin (4-hydroxy-3-methoxy-acetophenone), also known as acetovanillone, a naturally occurring organic compound of the root of Canadian hemp (Apocynum cannabinum) and Picrorhiza kurroa, is the most prominent NOX inhibitor widely used in the treatment of inflammatory diseases (Sun et al., 2008). The antioxidant and anti-inflammatory methoxy-substituted catechol, apocynin, can block NOX in both phagocytic and non-phagocytic cells (Zhang et al., 2005), but does not interfere with the phagocytosis or intracellular killing performed by neutrophils (Stolk et al., 1994). Apocynin does not block NOX directly, rather its inhibitory function involves myeloperoxidase (MPO), which was made evident by MPO deletion experiments. Together with hydrogen peroxide, MPO can facilitate the dimerization of the apocynin that oxidizes thiols in NOX. Thus, apocynin dimer blocks p47phox from migrating to the membrane, hampering the formation of the enzyme complex, eventually inhibiting the release of superoxide (Fig. 1A). However, in MPO deficient cells apocynin can act as a non-specific oxidative scavenger to ameliorate oxidative stress without NOX inhibition (Heumuller et al., 2008). Besides MPO, apocynin dimerization can also be catalyzed by other peroxidases such as horseradish peroxidase with successive NOX inhibition. On the other hand, supplementation with glutathione or cysteine as a source of thiols blocks the inhibitory function of apocynin. Thus, it has been suggested that to inhibit NOX activity, formation of the apocynin dimer is necessary (Van den Worm et al., 2001).
Several research groups, including ours, have reported that apocynin treatment in ischemic stroke can reduce brain hemorrhage, lesion size, and restore neurological functions. The systemic delivery of apocynin has also been shown to penetrate the BBB and exert its function without any impairment (Tang et al., 2008). In our previous study, we reported that pre-treatment (prior to reperfusion) or post-treatment (1.5 h after ischemic stroke) with apocynin (2.5 mg/kg) successfully suppressed superoxide generation in the brain while reducing infarct size and restoring neurological function (Tang XN,Cairns B,Cairns N and Yenari MA, 2008). On the other hand, a higher dose of apocynin (3.75 and 5 mg/kg), tends to increase the damage after brain hemorrhage. Other research groups found that apocynin shows somewhat beneficiary effects at a higher dose of 40–50 mg/kg (Tang et al., 2007), and some along us found no effect apocynin using a dose of about 4 mg/kg (Kleinschnitz et al., 2010). Thus, this non-specific dose range limits the therapeutic usage of apocynin. However, apocynin was found to be tolerable in single oral doses as high as 1000 mg/kg in normal mice (Pandey et al., 2009). Interestingly, in our NOX2 deletion study, we found that the beneficial effect of apocynin was absent in NOX2-deficient mice subjected to stroke, which may suggest that the function of apocynin is specific to NOX2 (Tang et al., 2011).
The NOX inhibitor, apocynin, has been reported to have neuroprotective effects in different chronic CNS diseases. In in vitro beta-amyloid-treated primary neurons, apocynin increased cellular survivability by reducing expression of proinflammatory cytokines such as TNF-alpha and IL-1beta (Jekabsone et al., 2006; Li et al., 2004). Apocynin was also reported to attenuate the cytotoxic effect in familial AD. However, apocynin alone is not capable of reducing cytotoxicity, but rather functions in the presence of another NOX inhibitor, oxypurinol (Abe et al., 2004). Dumont et al. found that a higher dose of apocynin (300 mg/kg/day) in Tg19959 mice (hAPP695 with the London and Swedish mutations) did not affect AD plaque formation or microglial proliferation but reduced the protein carbonyl levels and increased Rac1 expression. However, a lower dose of apocynin (10 mg/kg/day) in hAPP (751)SL mice (neuron-specific expression of hAPP751 with the London and Swedish mutations) reduced the microglial number and plaque size (Lull et al., 2011). The above results suggest that apocynin at a lower dose could be a therapeutic alternative for AD (Simonyi et al., 2012). In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, researchers found that apocynin is capable of blocking microglial and astroglial activation, along with expression of p91phox in the substantia nigra (Ghosh et al., 2012). The oral dose of apocynin (100 mg/kg/day) improved the hand-eye coordination and also increased the substantial neuronal survivability to 8.5% indicating the blocking of the PD progression in MPTP mouse PD model (Philippens et al., 2013). Like in AD and PD, apocynin has been found to be effective in the ALS animal models. In the SOD1G93A transgenic mice model of ALS, apocynin blocked the oxidative stress and increased the life span compared to the wild type mice (Harraz et al., 2008). Marchetto et al. showed that apocynin can reduce motor neuron loss caused by mutant SOD1 astrocytes in an in vitro co-culture ALS model (Marchetto et al., 2008). Prophylactic treatment of apocynin in SOD1G93A transgenic mouse model of ALS exhibited an increase in the number of neurons in the spinal cord and life span prolonged by up to 50% (Harraz et al., 2008). However, another research group suggested that apocynin or diapocynin has a somewhat limited benefit to SOD1G93A mice (Trumbull et al., 2012). There is not much evidence of the therapeutic use of apocynin in humans except in a handful of studies in asthmatics patients who received nebulized apocynin (Stefanska et al., 2010). Further studies are needed to clarify the functional specificity of apocynin on NOX isomers and to determine a functional dose for therapeutic use.
Honokiol and plumbagin
Honokiol ((3′,5-di-(2-propenyl)-1,1′-biphenyl-2,2′-diol) and plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), the plant quinoid, are natural bioactive NOX inhibitors derived from Magnolia spp. and the root of the Plumbago zeylanica L (also known as chitrak), respectively. In both in vivo and in vitro studies honokiol was found to be neuroprotective against lipid peroxidation and neutrophil infiltration, which is also accompanied by reduced ROS production (Liou et al., 2003a,b; Liou et al., 2003a,b). In cancer cell lines, honokiol increased cytosolic p47phox accumulation and decreased membrane p22phox, resulting in the blocking of NOX1 activation (Prasad et al., 2016). In stroke, honokiol also inhibits TNF-alpha-induced neutrophil infiltration of the injury core by inhibiting neutrophil adhesion to the cerebral endothelial cells (Chen et al., 2016). In spinal cord injury, honokiol attenuated proinflammatory cytokine expression, microglial activation, and neutrophil infiltration, eventually improving the functional outcome (Liu et al., 2015). Plumbagin is the only NOX inhibitor to date that interacts directly with a specific NOX homolog (Ding et al., 2005). Plumbagin inhibited the NOX activity in HEK293 and LN229 cells, two lines that only express NOX4 in a time- and dose-dependent manner. Superoxide production in NOX4 transfected cells is also blocked by plumbagin through its direct interaction with NOX4 (Ding et al., 2005). The mechanism of NOX4 inhibition is not well understood yet. However, Son et al. suggested that in an in vitro stroke model, plumbagin may activate the nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway, which provides protection against oxidative and metabolic insults to neurons (Son et al., 2010). They also suggested that plumbagin provides protection to neurons in focal ischemic injury. ROS production by NOX and NOX homolog expression in different cancers warrants NOX inhibitors function being referred to as anti-cancer. Due to its superoxide production blocking property, plumbagin has been suggested to have significant anti-cancer activity (Cheng et al., 2001; Hazra et al., 2002; Parimala and Sachdanandam, 1993).
In AD mice, honokiol reduced ROS production, mitochondrial membrane potential, and increased hippocampal neuronal survivability, eventually reducing the beta amyloid-induced learning and memory impairment (Matsui et al., 2009; Talarek et al., 2017; Wang et al., 2017). The molecular mechanism by which honokiol counteracts the deleterious effect of beta amyloid in AD is still unknown. However, researchers have suggested that there are several combined neuroprotective effects including reduction of beta-amyloid-induced ROS production (Hoi et al., 2010; Talarek et al., 2017), attenuation of Ca2+ influx (Lo et al., 1994; Wang et al., 2013), and inhibition of caspase-3 (Hoi et al., 2010). In the 6−OHDA-lesioned PD mouse model, honokiol modulated NOS signaling and showed both protective and therapeutic effects by protecting motor function and dopaminergic cellular viability (Chen et al., 2018). Treatment with plumbagin in the streptozotocin (STZ)-induced mouse AD model shows improvement of cognitive function in an Nrf2/ARE-dependent manner by suppressing astrogliosis and β-secretase enzyme function (Rodriguez et al., 1998). Plumbagin inhibits NOX4, the dominant NOX homolog in vascular smooth muscle cells, explaining its anti-atherosclerotic effect.
Nox2ds-tat
To date, chimeric peptide NOX2ds-tat (NOX2 docking sequence-tat), formerly known as gp91ds-tat, is the most selective and effective NOX inhibitor and inhibits NOX in both in vitro and in vivo experiments (Rey et al., 2001). It is a chimeric 18-amino acid-containing peptide that was constructed from the sequence of gp91phox; a nine-amino acid NOX2ds-tat portion of NOX is designed to block the interaction of p47phox with NOX2 and eventually block the formation of ROS (Fig. 1A). NOX2ds-tat is linked with the HIV internalization peptide, HIVtat, for intercellular delivery (Rey et al., 2001). NOX1 shares the homology domain with NOX2 and presence of the organizer subunit p47phox in NOX2, and also binding of the B-loop of NOX2 with the c-terminal dehydrogenase (DH) domain of NOX4 suggested that NOX2ds-tat might also block the ROS production by NOX1 and NOX4 (Banfi et al., 2003; Jackson et al., 2010; Rey et al., 2001). Kumar et al. suggested that NOX2ds-tat reduces oxidative neuronal damage and modifies the M1-/M2-like balance toward neuroprotection in TBI mainly regulated by NOX2 (Kumar A et al., 2016; Kumar A et al., 2016). In the rat spinal cord injury model, NOX2 inhibition by NOX2ds-tat blocked free radical formation and pro-inflammatory cytokine expression and also helped the functional recovery (Cooney et al., 2014; Khayrullina et al., 2015). Although NOX2ds-tat does not have any known test performed in stroke, it is known to inhibit oxidative stress that affects the cerebrovascular system (Park et al., 2007).
In the primary neuron and astrocyte culture, beta amyloid-induced NMDA receptor excitotoxicity increased Ca2+ influx, ROS production, and cPLA2 and ERK1/2 and other downstream signaling has been reported to be blocked by the NOX2ds-tat (Askarova et al., 2011; He et al., 2011). In transgenic Tg2576 AD mice, NOX2ds-tat improved neurovascular dysfunction and mitigates the behavioral deficits by reducing vascular and neuronal oxidative stress (Park et al., 2008b). Pal et al. showed that NOX2 da-tat can increase cellular protection by inhibiting rotenone-dependent apoptotic cell death and might be used for treating PD patients (Pal et al., 2016).
Serine protease inhibitors AEBSF
The serine protease enzyme, AEBSF (4-(2-aminoethyl)-benzenesulfonyl fluoride), also known as Pefabloc SC, is an irreversible serine protease inhibitor. AEBSF blocks the formation of the free oxygen radical by NOX in macrophages and in cell-free systems. In the process of NOX inhibition, AEBSF does not either interfere with electron transport or scavenge oxygen radicals but tends to effect NOX components present in the plasma membrane directly and by interrupting the binding of cytosolic components, p47phox and p67phox (Diatchuk et al., 1997). AEBSF is water soluble and stable, and due to its binding preference can modify several proteins through highly preferential covalent attachment on tyrosine, and on lysine, histidine, and the amino-terminus to a lesser extent. There is another irreversible serine protease inhibitor referred to as tosylphenylalanychloromethane, which shares similar NOX inhibitory properties as AEBSF (Gillibert et al., 2005). AEBSF is reported to reduce allergic airway inflammation in allergic asthma, although there are no studies that tested serine protease inhibitors in acute CNS models. Citron et al. claimed that AEBSF is specific for amyloid beta proteins starting with aspartate 1 and capable of directly inhibiting methionine–aspartate (Met-Asp)-cleaving enzyme b-secretase (Citron et al., 1996). However, to date, there are no reports of the role of serine protease inhibitors in chronic CNS disease related to NOX.
Phenylarsine oxide (PAO)
The NOX isoform specificity of PAO has not been determined yet but it is known as a potent NOX2 inhibitor (Le Cabec and Maridonneau-Parini, 1995). PAO interacts with the vicinal cysteine residues and only the NOX2 isoform is suggested to have two neighboring cysteine residues (positions 368 and 370), which suggests PAO may have specificity for NOX2 (Doussiere et al., 1998). PAO inhibits NOX by inhibiting the building of the enzyme complex, suggesting that once NOX is formed, PAO can not inhibit its function. On the other hand, the inhibitory property of the PAO can be reversed by 2,3-dimercaptopropanol and not mercaptoethanol (Kutsumi et al., 1995). PAO has been found to reduce ROS production by rat phagocytes, paw edema induced by carrageenan, and neutrophil infiltration after lipopolysaccharide inhalation (Roussin et al., 1997). However, the relationship of NOX2 with these events has not been established yet.
PAO is suggested to be increase sAPPα shedding and decrease the take-up by exosomes containing toxic amyloid-beta oligomers (Pedrini et al., 2005; Sardar Sinha et al., 2018). The specific function of soluble amyloid precursor protein α (sAPPα) is unknown, but the reduction of toxic amyloid-beta oligomers may decrease neuron-neuron transfer of AD. There are no studies examining the role of PAO in chronic CNS diseases, which requires exploration to increase the therapeutic possibilities for those diseases.
Gliotoxin (GT)
The secondary metabolite, gliotoxin, is the sulfur-containing virulence factor of Aspergillus fumigatus. The mode of action of GT is by blocking p47phox phosphorylation, its fusion with the cytoskeleton, and membrane translocation of p67phox, p47phox, and p40phox, and also reacting with thiol groups, which limits its specificity (Tsunawaki et al., 2004). Thus, GT also blocks NOX by blocking the assembly of NOX not through modulation of activated NOX. It also seems to react with thiol groups, thus limiting its specificity. The mycotoxin GT is suggested to kill microglia, astrocytes, and neurons via apoptosis, and can also block ROS production and phagocytosis (Dagenais and Keller, 2009). The above function of GT and its relationship with NOX has not been tested. To date, no studies appear to examine the therapeutic value of these compounds in acute and chronic CNS disease models.
Ebselen
Ebselen (2-phenyl-1,2-benzisoselenazol-3(2 H)-one) is a synthetically produced organoselenium drug that is also known to inhibit NOX and is in clinical trials. The glutathione peroxidase-mimicking ebselen not only inhibits NOX but also a number of other enzymes such as lipoxygenases, nitric oxide synthases, protein kinase C, and Hz/Kz-ATPase; therefore, it is known as a non-specific NOX inhibitor (Cotgreave et al., 1989). It can also react with peroxynitrite. Ebselen has been suggested to block the translocation of p47phox and p67phox to the plasma membrane in neutrophils (Smith et al., 2012) (Fig. 1A). It is considered to have anti-oxidant, anti-inflammatory, and cytoprotective activity via preventing cellular damage by ROS. It is also reported to be cytoprotective in NMDA receptor-mediated excitotoxicity (Herin et al., 2001; Koizumi et al., 2011). For a couple of decades, ebselen has been used in ischemic stroke clinical trials. It was found to repress lipid peroxidation and expression of iNOS in the cerebral cortex of a rat stroke model (Sui et al., 2005; Yamaguchi et al., 1998). It is more effective in the earlier stages of stroke (Yamaguchi et al., 1998). To date, some centers in Japan use ebselen for the treatment of stroke and currently a multicenter phase 3 ebselen trial is ongoing (www.strokecenter.org). In spinal cord injury, ebselen is suggested to be neuroprotective via improving mitochondrial function and inhibiting mitochondrial apoptosis, which eventually reduces secondary injury (Jia et al., 2018; Kalayci et al., 2005). In a rat TBI model, ebselen improved neurological injury by reducing NO, which also involved the TLR4-mediated p38 MAPK signaling pathway (Wei et al., 2014).
In AD neurons and mouse brain, ebselen reduced the expression of APP and β-secretase, which reduced the accumulation of amyloid-beta and misallocated phosphorylated tau and tau phosphorylation at the Thr231, Ser396, and Ser404 positions (Xie et al., 2017). Treatment with ebselen in the STZ-induced mice AD model markedly reduced the number of apoptotic neurons by reducing oxidative stress (Unsal et al., 2016). In LRRK2-linked PD, ebselen was found to have neuroprotective effects via reduction of superoxide and improving peroxidase function in dopaminergic neurons of drosophila (Angeles et al., 2014).
VAS2870
VAS2870 (3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine) is one of a few recent NOX inhibitors developed by the pharmaceutical industry (ten Freyhaus et al., 2006). It was identified as a specific inhibitor of NOX2 by means of high-throughput screening. However, Kleinschnitz et al. suggested that VAS2870 is also capable of inhibiting NOX4 (Kleinschnitz et al., 2010). Later, it appeared to be am inhibitor of all NOX isoforms, and not a general flavoprotein inhibitor nor ROS scavenger (Warner et al., 2004). VAS2879 appears to be a pan-NOX inhibitor. It has been found to inhibit phorbol-12-myristate 13-acetate-dependent NOX stimulation, which indicates that VAS2870 interacts downstream of the assembly process but not with p47phox (ten Freyhaus et al., 2006; Wingler et al., 2019). In preclinical stroke experiments, VAS2870 has been shown to be neuroprotective against inhibition of NOX4 (Kleinschnitz et al., 2010). A recent study suggested that VAS2870 alkylates cysteine thiol residues, as it was shown to have potential off-target effects. In bupivacaine-induced spinal neurotoxicity, VAS2870 was found to reverse all changes and attenuate all neuronal toxicity by inhibiting p47phox membrane translocation (Li et al., 2017). In uric acid-, indoxyl sulfate-, and methylguanidine-stimulated bulbospinal RVLM neurons, the oxidative stress-induced neurotoxicity has been suggested to be suppressed by the VAS2870 by inhibiting NOX2 and NOX4 (Oshima et al., 2015).
HMGCoA reductase inhibitors
HMGCoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase is a rate-limiting enzyme that is involved in the synthesis of cholesterol in the liver. There are several HMGCoA reductase inhibitors, such as pravastatin, fluvastatin, atorvastatin, simvastatin, and rosuvastatin, that are found as the mainstay of therapy for high LDL cholesterol, coronary disease, and stroke. Along with the HMGCoA reductase inhibitor (statins), angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor-1 blockers (ARBs) can also indirectly inhibit NOX activity (Wagner et al., 2000). All of the above clinically tested drugs have been shown to protect the brain from experimental stroke through different mechanisms. However, statins have been shown to exert their neuroprotective function in experimental stroke via their ability to upregulate endothelial nitric oxide synthase (Li et al., 2014). All of these compounds, besides their lipid lowering capabilities, have been shown to act as anti-inflammatory agents, affect endothelial function, and modulate the coagulation cascade (Bedi and Flaker, 2002). However, whether this is directly due to NOX inhibition seems less likely, with these compounds acting as related immune targets.
Several studies have suggested the use of HMGCoA reductase inhibitors in the treatment of AD. The principle behind statins reducing the prevalence of AD is by lowering the build-up of brain cholesterol, eventually reducing amyloid beta aggregation (Longenberger and Shah, 2011). However, Sparks et al. and a few other studies have suggested that the use of statins would be less advised as statins may reduce the production of essential cholesterols in the AD patients, which may exacerbate AD degeneration and also adversely affect cognition in AD patients (Padala et al., 2012). The statins may modulate PD in several ways such as by inhibiting proinflammatory stimulation (Ghosh et al., 2009), upregulating eNOS (Feron et al., 2001), reducing NOX-mediated production of ROS through the inhibition of geranylgeranylation of Rac (Gao et al., 2003; Wu et al., 2003), and attenuating α-synuclein aggregation (Bar-On et al., 2008; Roy and Pahan, 2011). On the other hand, studies have suggested that the long-term use of statins does not alter the relative risk of developing PD (Becker et al., 2008).
Conclusion
NOX appears to be an important player in CNS disorders, particularly as it is related to the generation of superoxide, which is the major modulator of acute and chronic CNS diseases. Several preclinical studies have reported that the inhibition of NOX improved neurological outcomes and reduced severity of brain injury in both acute and chronic cases. However, most of the available inhibitors may be non-specific with a relatively narrow therapeutic window that warrants greater investigation of NOX inhibitors and studies to develop safe and selective drugs for the treatment of clinical CNS diseases. It is also very important to study the complications and adverse effects of the available NOX inhibitors and the difference in benefits among them as well. Furthermore, with the knowledge that the facts of the role of various NOX isoforms is rapidly increasing, such facts could also drive the development of appropriate therapies as well.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding
This study was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2016M3C7A1905098), and grants from the National Institutes of Health (RO1 NS106441) Department of Defense and the Veteran's Merit Award (I01 BX000589) to MAY.
Author contributions
LJE provided concept, design and overall supervision of this study. SB, JYK1 contributed in the writing and drawing. MAY and LJE participated in the discussion and revision. All authors approved and agreed to be accountable for all aspects of the work.
Acknowledgments
This study was supported by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT(NRF-2016M3C7A1905098)
Contributor Information
Sumit Barua, Email: drsbarua@gmail.com.
Jong Youl Kim, Email: jongyoul74@gmail.com.
Midori A. Yenari, Email: yenari@alum.mit.edu.
Jong Eun Lee, Email: jelee@yuhs.ac.
References
- Abe Y., Hashimoto Y., Tomita Y., Terashita K., Aiso S., Tajima H., Niikura T., Matsuoka M. Cytotoxic mechanisms by M239V presenilin 2, a little-analyzed Alzheimer’s disease-causative mutant. J. Neurosci. Res. 2004;77:583–595. doi: 10.1002/jnr.20163. [DOI] [PubMed] [Google Scholar]
- Abramov A.Y., Canevari L., Duchen M.R. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago T., Kitazono T., Kuroda J., Kumai Y., Kamouchi M., Ooboshi H., Wakisaka M., Kawahara T. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- Angeles D.C., Ho P., Chua L.L., Wang C., Yap Y.W., Ng C., Zhou Z., Lim K.L. Thiol peroxidases ameliorate LRRK2 mutant-induced mitochondrial and dopaminergic neuronal degeneration in Drosophila. Hum. Mol. Genet. 2014;23:3157–3165. doi: 10.1093/hmg/ddu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592:692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- Anrather J., Racchumi G., Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Aronowski J., Strong R., Grotta J.C. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Askarova S., Yang X., Sheng W., Sun G.Y., Lee J.C. Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience. 2011;199:375–385. doi: 10.1016/j.neuroscience.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B., Clark R.A., Steger K., Krause K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- Bar-On P., Crews L., Koob A.O., Mizuno H., Adame A., Spencer B., Masliah E. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J. Neurochem. 2008;105:1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F.C., Feuerstein G.Z. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J. Cereb. Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Basta G., Lazzerini G., Del Turco S., Ratto G.M., Schmidt A.M., De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler. Thromb. Vasc. Biol. 2005;25:1401–1407. doi: 10.1161/01.ATV.0000167522.48370.5e. [DOI] [PubMed] [Google Scholar]
- Becker C., Jick S.S., Meier C.R. Use of statins and the risk of Parkinson’s disease: a retrospective case-control study in the UK. Drug Saf. 2008;31:399–407. doi: 10.2165/00002018-200831050-00004. [DOI] [PubMed] [Google Scholar]
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bedi A., Flaker G.C. How do HMG-CoA reductase inhibitors prevent stroke? Am. J. Cardiovasc. Drugs. 2002;2:7–14. doi: 10.2165/00129784-200202010-00002. [DOI] [PubMed] [Google Scholar]
- Bianca V.D., Dusi S., Bianchini E., Dal Pra I., Rossi F. Beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J. Biol. Chem. 1999;274:15493–15499. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- Boulden B.M., Widder J.D., Allen J.C., Smith D.A., Al-Baldawi R.N., Harrison D.G., Dikalov S.I., Jo H. Early determinants of H2O2-induced endothelial dysfunction. Free Radic. Biol. Med. 2006;41:810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller A.J., Gupta S., Parrino T.E., Knight A.G., Ebenezer P.J., Weidner A.M., LeVine H., 3rd, Keller J.N. NOX activity is increased in mild cognitive impairment. Antioxid. Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes K.R., Washington P.M., Knoblach S.M., Hoffman E., Faden A.I. Delayed inflammatory mRNA and protein expression after spinal cord injury. J. Neuroinflamm. 2011;8:130. doi: 10.1186/1742-2094-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Chan P.H. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Chen H., Song Y.S., Chan P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Chang P.C., Chen C., Chan M.H. Protective and therapeutic activity of honokiol in reversing motor deficits and neuronal degeneration in the mouse model of Parkinson’s disease. Pharmacol. Rep. 2018;70:668–676. doi: 10.1016/j.pharep.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Chen P.J., Wang Y.L., Kuo L.M., Lin C.F., Chen C.Y., Tsai Y.F., Shen J.J., Hwang T.L. Honokiol suppresses TNF-alpha-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IkappaBalpha. Sci. Rep. 2016;6:26554. doi: 10.1038/srep26554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Cao Z., Xu X., van Meir E.G., Lambeth J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Citron M., Diehl T.S., Capell A., Haass C., Teplow D.B., Selkoe D.J. Inhibition of amyloid beta-protein production in neural cells by the serine protease inhibitor AEBSF. Neuron. 1996;17:171–179. doi: 10.1016/s0896-6273(00)80290-1. [DOI] [PubMed] [Google Scholar]
- Clayton K.A., Van Enoo A.A., Ikezu T. Alzheimer’s disease: the role of microglia in brain homeostasis and proteopathy. Front. Neurosci. 2017;11:680. doi: 10.3389/fnins.2017.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney S.J., Zhao Y., Byrnes K.R. Characterization of the expression and inflammatory activity of NADPH oxidase after spinal cord injury. Free Radic. Res. 2014;48:929–939. doi: 10.3109/10715762.2014.927578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotgreave I.A., Duddy S.K., Kass G.E., Thompson D., Moldeus P. Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem. Pharmacol. 1989;38:649–656. doi: 10.1016/0006-2952(89)90211-6. [DOI] [PubMed] [Google Scholar]
- D’Ambrosi N., Cozzolino M., Carri M.T. Neuroinflammation in amyotrophic lateral sclerosis: role of redox (dys)Regulation. Antioxid. Redox Signal. 2018;29:15–36. doi: 10.1089/ars.2017.7271. [DOI] [PubMed] [Google Scholar]
- Dagenais T.R., Keller N.P. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchuk V., Lotan O., Koshkin V., Wikstroem P., Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J. Biol. Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Chen Z.J., Liu S., Che D., Vetter M., Chang C.H. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J. Pharm. Pharmacol. 2005;57:111–116. doi: 10.1211/0022357055119. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Doussiere J., Poinas A., Blais C., Vignais P.V. Phenylarsine oxide as an inhibitor of the activation of the neutrophil NADPH oxidase--identification of the beta subunit of the flavocytochrome b component of the NADPH oxidase as a target site for phenylarsine oxide by photoaffinity labeling and photoinactivation. Eur. J. Biochem. 1998;251:649–658. doi: 10.1046/j.1432-1327.1998.2510649.x. [DOI] [PubMed] [Google Scholar]
- Eun H.S., Cho S.Y., Joo J.S., Kang S.H., Moon H.S., Lee E.S., Kim S.H., Lee B.S. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci. Rep. 2017;7:11060. doi: 10.1038/s41598-017-11280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O., Dessy C., Desager J.P., Balligand J.L. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- Gamba P., Leonarduzzi G., Tamagno E., Guglielmotto M., Testa G., Sottero B., Gargiulo S., Biasi F. Interaction between 24-hydroxycholesterol, oxidative stress, and amyloid-beta in amplifying neuronal damage in Alzheimer’s disease: three partners in crime. Aging Cell. 2011;10:403–417. doi: 10.1111/j.1474-9726.2011.00681.x. [DOI] [PubMed] [Google Scholar]
- Gao H.M., Liu B., Zhang W., Hong J.S. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Kanthasamy A., Joseph J., Anantharam V., Srivastava P., Dranka B.P., Kalyanaraman B., Kanthasamy A.G. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J. Neuroinflamm. 2012;9:241. doi: 10.1186/1742-2094-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Roy A., Matras J., Brahmachari S., Gendelman H.E., Pahan K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009;29:13543–13556. doi: 10.1523/JNEUROSCI.4144-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillibert M., Dehry Z., Terrier M., El Benna J., Lederer F. Another biological effect of tosylphenylalanylchloromethane (TPCK): it prevents p47phox phosphorylation and translocation upon neutrophil stimulation. Biochem. J. 2005;386:549–556. doi: 10.1042/BJ20041475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade M.J. Oxidative stress and cognitive longevity. Nutrition. 2010;26:595–603. doi: 10.1016/j.nut.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Lapouge K., Smerdon S.J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz M.M., Marden J.J., Zhou W., Zhang Y., Williams A., Sharov V.S., Nelson K., Luo M. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra B., Sarkar R., Bhattacharyya S., Ghosh P.K., Chel G., Dinda B. Synthesis of plumbagin derivatives and their inhibitory activities against Ehrlich ascites carcinoma in vivo and Leishmania donovani Promastigotes in vitro. Phytother. Res. 2002;16:133–137. doi: 10.1002/ptr.867. [DOI] [PubMed] [Google Scholar]
- He Y., Cui J., Lee J.C., Ding S., Chalimoniuk M., Simonyi A., Sun A.Y., Gu Z. Prolonged exposure of cortical neurons to oligomeric amyloid-beta impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (-)-epigallocatechin-3-gallate. ASN Neuro. 2011;3 doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.F., Chen H.J., Qian L.H., He L.F., Buzby J.S. Diphenyleneiodonium protects preoligodendrocytes against endotoxin-activated microglial NADPH oxidase-generated peroxynitrite in a neonatal rat model of periventricular leukomalacia. Brain Res. 2013;1492:108–121. doi: 10.1016/j.brainres.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem. Res. 2009;34:670–678. doi: 10.1007/s11064-008-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin G.A., Du S., Aizenman E. The neuroprotective agent ebselen modifies NMDA receptor function via the redox modulatory site. J. Neurochem. 2001;78:1307–1314. doi: 10.1046/j.1471-4159.2001.00517.x. [DOI] [PubMed] [Google Scholar]
- Hernandes M.S., Britto L.R. NADPH oxidase and neurodegeneration. Curr. Neuropharmacol. 2012;10:321–327. doi: 10.2174/157015912804143540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schroder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hoi C.P., Ho Y.P., Baum L., Chow A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010;24:1538–1542. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- Infanger D.W., Sharma R.V., Davisson R.L. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid. Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Jackson H.M., Kawahara T., Nisimoto Y., Smith S.M., Lambeth J.D. Nox4 B-loop creates an interface between the transmembrane and dehydrogenase domains. J. Biol. Chem. 2010;285:10281–10290. doi: 10.1074/jbc.M109.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekabsone A., Mander P.K., Tickler A., Sharpe M., Brown G.C. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J. Neuroinflamm. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z.Q., Li S.Q., Qiao W.Q., Xu W.Z., Xing J.W., Liu J.T., Song H., Gao Z.Y. Ebselen protects mitochondrial function and oxidative stress while inhibiting the mitochondrial apoptosis pathway after acute spinal cord injury. Neurosci. Lett. 2018;678:110–117. doi: 10.1016/j.neulet.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Kahles T., Luedike P., Endres M., Galla H.J., Steinmetz H., Busse R., Neumann-Haefelin T., Brandes R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kalayci M., Coskun O., Cagavi F., Kanter M., Armutcu F., Gul S., Acikgoz B. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem. Res. 2005;30:403–410. doi: 10.1007/s11064-005-2615-2. [DOI] [PubMed] [Google Scholar]
- Katsuyama M., Matsuno K., Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J. Clin. Biochem. Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayrullina G., Bermudez S., Byrnes K.R. Inhibition of NOX2 reduces locomotor impairment, inflammation, and oxidative stress after spinal cord injury. J. Neuroinflamm. 2015;12:172. doi: 10.1186/s12974-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., Barit D., Schwarz T. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010:8. doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H., Fujisawa H., Suehiro E., Shirao S., Suzuki M. Neuroprotective effects of ebselen following forebrain ischemia: involvement of glutamate and nitric oxide. Neurol. Med. Chir. (Tokyo) 2011;51:337–343. doi: 10.2176/nmc.51.337. [DOI] [PubMed] [Google Scholar]
- Koulis C., Watson A.M., Gray S.P., Jandeleit-Dahm K.A. Linking RAGE and Nox in diabetic micro- and macrovascular complications. Diabetes Metab. 2015;41:272–281. doi: 10.1016/j.diabet.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Kriz J., Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- Kumar A., Alvarez-Croda D.M., Stoica B.A., Faden A.I., Loane D.J. Microglial/Macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Barrett J.P., Alvarez-Croda D.M., Stoica B.A., Faden A.I., Loane D.J. NOX2 drives M1-like microglial/macrophage activation and neurodegeneration following experimental traumatic brain injury. Brain Behav. Immun. 2016;58:291–309. doi: 10.1016/j.bbi.2016.07.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S., Siesjo B.K. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin. Neurosci. 1997;4:199–212. [PubMed] [Google Scholar]
- Kutsumi H., Kawai K., Johnston R.B., Jr, Rokutan K. Evidence for participation of vicinal dithiols in the activation sequence of the respiratory burst of human neutrophils. Blood. 1995;85:2559–2569. [PubMed] [Google Scholar]
- Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lassegue B., Clempus R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Le Cabec V., Maridonneau-Parini I. Complete and reversible inhibition of NADPH oxidase in human neutrophils by phenylarsine oxide at a step distal to membrane translocation of the enzyme subunits. J. Biol. Chem. 1995;270:2067–2073. doi: 10.1074/jbc.270.5.2067. [DOI] [PubMed] [Google Scholar]
- Li M., Pisalyaput K., Galvan M., Tenner A.J. Macrophage colony stimulatory factor and interferon-gamma trigger distinct mechanisms for augmentation of beta-amyloid-induced microglia-mediated neurotoxicity. J. Neurochem. 2004;91:623–633. doi: 10.1111/j.1471-4159.2004.02765.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhuang Q.K., Yang J.N., Zhang Y.Y. Statins excert neuroprotection on cerebral ischemia independent of their lipid-lowering action: the potential molecular mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2014;18:1113–1126. [PubMed] [Google Scholar]
- Li Y.J., Zhao W., Yu XJ Li F.X., Liu Z.T., Li L., Xu S.Y. Activation of p47phox as a mechanism of bupivacaine-induced burst production of reactive oxygen species and neural toxicity. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/8539026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou K.T., Shen Y.C., Chen C.F., Tsao C.M., Tsai S.K. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur. J. Pharmacol. 2003;475:19–27. doi: 10.1016/s0014-2999(03)02121-6. [DOI] [PubMed] [Google Scholar]
- Liou K.T., Shen Y.C., Chen C.F., Tsao C.M., Tsai S.K. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhang C., Liu Z., Zhang J., Xiang Z., Sun T. Honokiol downregulates Kruppel-like factor 4 expression, attenuates inflammation, and reduces histopathology after spinal cord injury in rats. Spine (Phila Pa 1976) 2015;40:363–368. doi: 10.1097/BRS.0000000000000758. [DOI] [PubMed] [Google Scholar]
- Lo Y.C., Teng C.M., Chen C.F., Chen C.C., Hong C.Y. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem. Pharmacol. 1994;47:549–553. doi: 10.1016/0006-2952(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Longenberger J., Shah Z.A. Simvastatin and other HMG-CoA reductase inhibitors on brain cholesterol levels in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8:434–442. doi: 10.2174/156720511795745393. [DOI] [PubMed] [Google Scholar]
- Lull M.E., Levesque S., Surace M.J., Block M.L. Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL) mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12:7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander P.K., Jekabsone A., Brown G.C. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J. Immunol. 2006;176:1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- Marchetto M.C., Muotri A.R., Mu Y., Smith A.M., Cezar G.G., Gage F.H. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Marden J.J., Harraz M.M., Williams A.J., Nelson K., Luo M., Paulson H., Engelhardt J.F. Redox modifier genes in amyotrophic lateral sclerosis in mice. J. Clin. Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui N., Takahashi K., Takeichi M., Kuroshita T., Noguchi K., Yamazaki K., Tagashira H., Tsutsui K. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. 2009;1305:108–117. doi: 10.1016/j.brainres.2009.09.107. [DOI] [PubMed] [Google Scholar]
- Nagel S., Genius J., Heiland S., Horstmann S., Gardner H., Wagner S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–217. doi: 10.1016/j.brainres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Nagel S., Hadley G., Pfleger K., Grond-Ginsbach C., Buchan A.M., Wagner S., Papadakis M. Suppression of the inflammatory response by diphenyleneiodonium after transient focal cerebral ischemia. J. Neurochem. 2012;123(Suppl 2):98–107. doi: 10.1111/j.1471-4159.2012.07948.x. [DOI] [PubMed] [Google Scholar]
- Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007;39:443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- Oshima N., Onimaru H., Matsubara H., Uchida T., Watanabe A., Takechi H., Nishida Y., Kumagai H. Uric acid, indoxyl sulfate, and methylguanidine activate bulbospinal neurons in the RVLM via their specific transporters and by producing oxidative stress. Neuroscience. 2015;304:133–145. doi: 10.1016/j.neuroscience.2015.07.055. [DOI] [PubMed] [Google Scholar]
- Padala K.P., Padala P.R., McNeilly D.P., Geske J.A., Sullivan D.H., Potter J.F. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer’s dementia: a prospective withdrawal and rechallenge pilot study. Am. J. Geriatr. Pharmacother. 2012;10:296–302. doi: 10.1016/j.amjopharm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Pal R., Bajaj L., Sharma J., Palmieri M., Di Ronza A., Lotfi P., Chaudhury A., Neilson J. NADPH oxidase promotes Parkinsonian phenotypes by impairing autophagic flux in an mTORC1-independent fashion in a cellular model of Parkinson’s disease. Sci. Rep. 2016;6:22866. doi: 10.1038/srep22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Kour K., Bani S., Suri K.A., Satti N.K., Sharma P., Qazi G.N. Amelioration of adjuvant induced arthritis by apocynin. Phytother. Res. 2009;23:1462–1468. doi: 10.1002/ptr.2803. [DOI] [PubMed] [Google Scholar]
- Parimala R., Sachdanandam P. Effect of Plumbagin on some glucose metabolising enzymes studied in rats in experimental hepatoma. Mol. Cell. Biochem. 1993;125:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- Park K.W., Baik H.H., Jin B.K. Interleukin-4-induced oxidative stress via microglial NADPH oxidase contributes to the death of hippocampal neurons in vivo. Curr. Aging Sci. 2008;1:192–201. doi: 10.2174/1874609810801030192. [DOI] [PubMed] [Google Scholar]
- Park K.W., Baik H.H., Jin B.K. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J. Immunol. 2009;183:4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- Park L., Anrather J., Girouard H., Zhou P., Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Park L., Anrather J., Zhou P., Frys K., Pitstick R., Younkin S., Carlson G.A., Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J. Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L., Zhou P., Pitstick R., Capone C., Anrather J., Norris E.H., Younkin L., Younkin S. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawate S., Shen Q., Fan F., Bhat N.R. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Pedrini S., Carter T.L., Prendergast G., Petanceska S., Ehrlich M.E., Gandy S. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005;2:e18. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippens I.H., Wubben J.A., Finsen B., t Hart B.A. Oral treatment with the NADPH oxidase antagonist apocynin mitigates clinical and pathological features of parkinsonism in the MPTP marmoset model. J. Neuroimmune Pharmacol. 2013;8:715–726. doi: 10.1007/s11481-013-9450-z. [DOI] [PubMed] [Google Scholar]
- Prasad R., Kappes J.C., Katiyar S.K. Inhibition of NADPH oxidase 1 activity and blocking the binding of cytosolic and membrane-bound proteins by honokiol inhibit migratory potential of melanoma cells. Oncotarget. 2016;7:7899–7912. doi: 10.18632/oncotarget.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Liu Y., Cooper C., Liu B., Wilson B., Hong J.S. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J. Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- Qin L., Liu Y., Wang T., Wei S.J., Block M.L., Wilson B., Liu B., Hong J.S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Orr A.L., Brand M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Rodriguez L.L., Owens J.H., Peters C.J., Nichol S.T. Genetic reassortment among viruses causing hantavirus pulmonary syndrome. Virology. 1998;242:99–106. doi: 10.1006/viro.1997.8990. [DOI] [PubMed] [Google Scholar]
- Roussin A., Le Cabec V., Lonchampt M., De Nadai J., Canet E., Maridonneau-Parini I. Neutrophil-associated inflammatory responses in rats are inhibited by phenylarsine oxide. Eur. J. Pharmacol. 1997;322:91–96. doi: 10.1016/s0014-2999(96)00988-0. [DOI] [PubMed] [Google Scholar]
- Roy A., Pahan K. Prospects of statins in Parkinson disease. Neuroscientist. 2011;17:244–255. doi: 10.1177/1073858410385006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.X., Tanaka L.Y., Wosniak J., Laurindo F.R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Sardar Sinha M., Ansell-Schultz A., Civitelli L., Hildesjo C., Larsson M., Lannfelt L., Ingelsson M., Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P., Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J. Cereb. Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S., Tanino H., Kawakami N., Okamura N., Kodama H., Yamaguchi T., Hayakawa T., Nunomura A. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2000;273:5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- Simonyi A., Serfozo P., Lehmidi T.M., Cui J., Gu Z., Lubahn D.B., Sun A.Y., Sun G.Y. The neuroprotective effects of apocynin. Front. Biosci. Elite Ed. (Elite Ed) 2012;4:2183–2193. doi: 10.2741/535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Min J., Ganesh T., Diebold B., Kawahara T., Zhu Y., McCoy J., Sun A. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem. Biol. 2012;19:752–763. doi: 10.1016/j.chembiol.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son T.G., Camandola S., Arumugam T.V., Cutler R.G., Telljohann R.S., Mughal M.R., Moore T.A., Luo W. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J. Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S., Nuvolone M., Keller A., Falsig J., Varol A., Schwarz P., Bieri M., Budka H. The role of the NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N.A., Fursova A., Kolosova N.G. Behavioral effects induced by mitochondria-targeted antioxidant SkQ1 in Wistar and senescence-accelerated OXYS rats. J. Alzheimers Dis. 2010;21:479–491. doi: 10.3233/JAD-2010-091675. [DOI] [PubMed] [Google Scholar]
- Stefanska J., Sokolowska M., Sarniak A., Wlodarczyk A., Doniec Z., Nowak D., Pawliczak R. Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm. Pharmacol. Ther. 2010;23:48–54. doi: 10.1016/j.pupt.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Stolk J., Rossie W., Dijkman J.H. Apocynin improves the efficacy of secretory leukocyte protease inhibitor in experimental emphysema. Am. J. Respir. Crit. Care Med. 1994;150:1628–1631. doi: 10.1164/ajrccm.150.6.7952625. [DOI] [PubMed] [Google Scholar]
- Sui H., Wang W., Wang P.H., Liu L.S. Protective effect of antioxidant ebselen (PZ51) on the cerebral cortex of stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2005;28:249–254. doi: 10.1291/hypres.28.249. [DOI] [PubMed] [Google Scholar]
- Sun A.Y., Wang Q., Simonyi A., Sun G.Y. Botanical phenolics and brain health. Neuromol. Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace M.J., Block M.L. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell. Mol. Life Sci. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarek S., Listos J., Barreca D., Tellone E., Sureda A., Nabavi S.F., Braidy N., Nabavi S.M. Neuroprotective effects of honokiol: from chemistry to medicine. Biofactors. 2017;43:760–769. doi: 10.1002/biof.1385. [DOI] [PubMed] [Google Scholar]
- Tang L.L., Ye K., Yang X.F., Zheng J.S. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J. Int. Med. Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- Tang X.N., Cairns B., Cairns N., Yenari M.A. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.N., Cairns B., Kim J.Y., Yenari M.A. NADPH oxidase in stroke and cerebrovascular disease. Neurol. Res. 2012;34:338–345. doi: 10.1179/1743132812Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.N., Zheng Z., Giffard R.G., Yenari M.A. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann. Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Baumer A.T., Vantler M., Bekhite M.M., Wartenberg M. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Trumbull K.A., McAllister D., Gandelman M.M., Fung W.Y., Lew T., Brennan L., Lopez N., Morre J. Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol. Dis. 2012;45:137–144. doi: 10.1016/j.nbd.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Yoshida L.S., Nishida S., Kobayashi T., Shimoyama T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 2004;72:3373–3382. doi: 10.1128/IAI.72.6.3373-3382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsal C., Oran M., Albayrak Y., Aktas C., Erboga M., Topcu B., Uygur R., Tulubas F. Neuroprotective effect of ebselen against intracerebroventricular streptozotocin-induced neuronal apoptosis and oxidative stress in rats. Toxicol. Ind. Health. 2016;32:730–740. doi: 10.1177/0748233713509429. [DOI] [PubMed] [Google Scholar]
- Valencia A., Sapp E., Kimm J.S., McClory H., Reeves P.B., Alexander J., Ansong K.A., Masso N. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington’s disease. Hum. Mol. Genet. 2013;22:1112–1131. doi: 10.1093/hmg/dds516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Van den Worm E., Beukelman C.J., Van den Berg A.J., Kroes B.H., Labadie R.P., Van Dijk H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur. J. Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- Wagner A.H., Kohler T., Ruckschloss U., Just I., Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler. Thromb. Vasc. Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- Wang M., Li Y., Ni C., Song G. Honokiol attenuates oligomeric amyloid beta1-42-Induced alzheimer’s disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor Kappa-B signaling pathway. Cell. Physiol. Biochem. 2017;43:69–81. doi: 10.1159/000480320. [DOI] [PubMed] [Google Scholar]
- Wang Q., Qian L., Chen S.H., Chu C.H., Wilson B., Oyarzabal E., Ali S., Robinson B. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson’s disease models. Brain. 2015;138:1247–1262. doi: 10.1093/brain/awv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Qin L., Liu B., Liu Y., Wilson B., Eling T.E., Langenbach R., Taniura S. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J. Neurochem. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wei X., Liu K., Zhang X., Yang F., Zhang H., He Y., Zhu T. NOX2 deficiency ameliorates cerebral injury through reduction of complexin II-mediated glutamate excitotoxicity in experimental stroke. Free Radic. Biol. Med. 2013;65:942–951. doi: 10.1016/j.freeradbiomed.2013.08.166. [DOI] [PubMed] [Google Scholar]
- Warner D.S., Sheng H., Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J. Exp. Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhang Y., Yang C., Wang Q., Zhuang Z., Sun Z. Neuroprotective effects of ebselen in traumatic brain injury model: involvement of nitric oxide and p38 mitogen-activated protein kinase signalling pathway. Clin. Exp. Pharmacol. Physiol. 2014;41:134–138. doi: 10.1111/1440-1681.12186. [DOI] [PubMed] [Google Scholar]
- Wilkinson B.L., Landreth G.E. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J. Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler K., Altenhoefer S.A., Kleikers P.W., Radermacher K.A., Kleinschnitz C. Schmidt HH VAS2870 is a pan-NADPH oxidase inhibitor. Cell. Mol. Life Sci. 2019;69:3159–3160. doi: 10.1007/s00018-012-1107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.C., Re D.B., Nagai M., Ischiropoulos H., Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Tan Y., Zheng Y., Du X., Liu Q. Ebselen ameliorates beta-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J. Biol. Inorg. Chem. 2017;22:851–865. doi: 10.1007/s00775-017-1463-2. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., Maeda S., Matsui T., Ueda S., Fukami K., Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta. 2012;1820:663–671. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Sano K., Takakura K., Saito I., Shinohara Y., Asano T., Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Zawada W.M., Mrak R.E., Biedermann J., Palmer Q.D., Gentleman S.M., Aboud O., Griffin W.S. Loss of angiotensin II receptor expression in dopamine neurons in Parkinson’s disease correlates with pathological progression and is accompanied by increases in Nox4- and 8-OH guanosine-related nucleic acid oxidation and caspase-3 activation. Acta Neuropathol. Commun. 2015;3:9. doi: 10.1186/s40478-015-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehendner C.M., Librizzi L., Hedrich J., Bauer N.M., Angamo E.A., de Curtis M., Luhmann H.J. Moderate hypoxia followed by reoxygenation results in blood-brain barrier breakdown via oxidative stress-dependent tight-junction protein disruption. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chan M.M., Andrews M.C., Mori T.A., Croft K.D., McKenzie K.U., Schyvens C.G., Whitworth J.A. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am. J. Hypertens. 2005;18:910–916. doi: 10.1016/j.amjhyper.2005.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.