Abstract
CNS injuries, such as traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), and cerebral ischemic stroke, are important causes of death and long-term disability worldwide. As an important class of pervasive genes involved in many pathophysiological processes, long non-coding RNAs (lncRNAs) have received attention in the past decades. Multiple studies indicate that lncRNAs are abundant in the CNS and have a key role in brain function as well as many neurological disorders, especially in CNS injuries. Several investigations have deciphered that regulation of lncRNAs exert pro-angiogenesis, anti-apoptosis, and anti-inflammation effects in CNS injury via different molecules and pathways, including microRNA (miRNA), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B (PI3K/AKT), Notch, and p53. Thus, lncRNAs show great promise as molecular targets in CNS injuries. In this article, we provide an updated review of the current state of our knowledge about the relationship between lncRNAs and CNS injuries, highlighting the specific roles of lncRNAs in CNS injuries.
Keywords: CNS injuries, long non-coding RNAs, MALAT1, MEG3, downstream molecules
Main Text
CNS injuries are one of the leading causes of disability and death in modern society, resulting in high medical costs.1 CNS injuries usually include traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH), and cerebral ischemic stroke.2 The pathological processes of CNS injuries include a series of neurological events, such as inflammation, oxidative stress, apoptosis, autophagy, and blood-brain barrier (BBB) disruption, and eventually result in neuronal cell death in the brain.3 Despite the efforts on searching effective methods, patients suffering with severe CNS injuries always end up with poor prognosis. Therefore, new and effective strategies of treatment are urgently needed to reduce the heavy disease and economic burden.
Long non-coding RNAs (lncRNAs) are known as non-coding RNA transcripts greater than 200 nucleotides.4 Although lncRNAs were primarily considered as simply transcriptional by-products, accumulated evidence suggests that lncRNAs participate in regulation of various physiological and pathophysiological processes, such as immunity, inflammation, cell differentiation, proliferation, and survival, by modulating the stability and nuclear retention of their target genes.5 lncRNAs regulate gene expression at epigenetic, transcriptional, post-transcriptional, and chromatin remodeling levels,6, 7 and they can activate or inhibit the expression of target genes by directly binding to the target genes or recruiting transcription factors (Figure 1).8 Studies have shown that the dysregulation of lncRNAs was implicated in many human diseases.9, 10, 11, 12, 13 Moreover, the expression profiles of lncRNAs were associated with specific neuroanatomical regions, cell types, and developmental processes, suggesting a potential functional role of lncRNAs in the nervous system.14, 15, 16 The altered expression of lncRNAs was involved in brain development and functional diversification and contributed to diverse neurological disorders such as CNS injuries.17, 18 In addition, many gain- and loss-of-function approaches found that lncRNAs played an important role in CNS injury-induced secondary brain damage.19 In this regard, highlighting the potentially functional roles of lncRNAs in CNS injuries is important. In the present study, we provide an overview of lncRNA functions in CNS injuries and the associated molecular mechanisms.
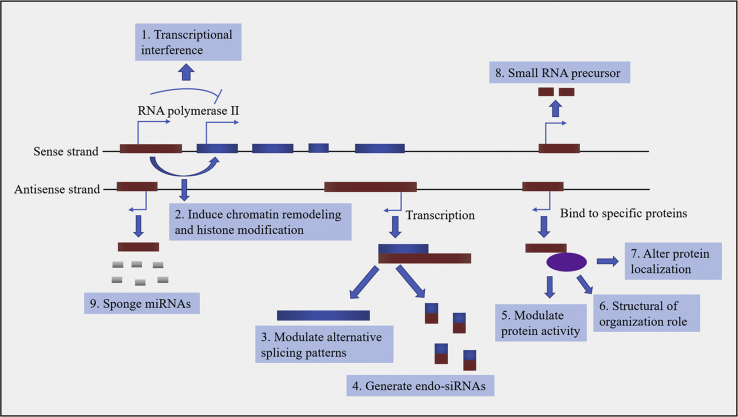
Figure 1.
Paradigm for the Functions of lncRNAs
lncRNAs (red-brown) can negatively or positively regulate the expression of the coding gene (navy blue) by transcriptional interference (1) or by inducing chromatin remodeling and histone modifications (2). Moreover, transcription of lncRNAs by antisense transcripts can hybridize to their specific coding gene (navy blue), generating alternative splicing (3), or various endo-siRNAs (4). Furthermore, by interacting with specific proteins (purple), lncRNAs can modulate protein activity (5), form cellular substructures (6), or alter protein localization (7). In addition, lncRNAs can be processed to produce small RNAs, such as miRNAs (8). Finally, as miRNA sponges, lncRNAs can influence the endogenous RNAs.
lncRNAs in CNS Injuries
During studies assessing the functions of lncRNAs, it was revealed that lncRNAs may act as potential onco- or tumor-suppressor RNAs in numerous cancer types.20 This observation led to investigations into the potential role of lncRNAs in various models. Recently, the roles of lncRNAs in CNS injuries were elucidated. Numbers of aberrantly expressed lncRNAs were identified using techniques such as microarray or RNA sequencing (RNA-seq).21, 22 Specifically, lncRNAs such as metastasis associated lung adenocarcinoma transcript 1 (MALAT1), maternally expressed gene 3 (MEG3), brain-derived neurotrophic factor antisense RNA (BDNF-AS), nuclear enriched abundant transcript 1 (NEAT1), growth arrest-specific transcript 5 (GAS5), and CAMK2D-associated transcript 1 (C2dat1) were found to affect secondary brain injury in CNS injury models (Table 1).
Table 1.
The Functions and Molecular Targets of lncRNAs in CNS Injuries
| lncRNAs | Models | Animals and/or Cells | Expression | Beneficial Functions of the Regulation of lncRNAs | Molecular Targets |
|---|---|---|---|---|---|
| ANRIL | MACO | rats, HUVECs | increased | promote angiogenesis, decrease infarction and inflammation | NF-κB |
| BDNF-AS | H/R injury | HCNs, human astrocytes | increased | increase MMP, ameliorate apoptosis | PI3K/AKT |
| C2dat1 | I/R injury | mice, mouse neurons | increased | promote neuronal survival, decrease inflammation | NF-κB |
| FosDT | MCAO | rats | increased | ameliorate motor deficits, reduce infarct volume | REST |
| GAS5 | HIBD | rats, rat neurons | increased | reduce brain infarct size, improve neurological function recovery | miR-23a |
| Gm4419 | TBI | mouse astrocytes | increased | decrease inflammation, improve neurological deficits | miR-4661 |
| OGD/R injury | rat microglial cells | increased | reduce neuroinflammation | NF-κB | |
| H19 | OGD/R injury | mice | increased | attenuate neurological deficits and inflammation | – |
| I/R injury | rats, SH-SY5Y cells | increased | decrease cell death and autophagy | – | |
| MALAT1 | OGD/R injury | mice, mouse neurons | increased | attenuate autophagy, protect BBB function | miR-30a |
| BMECs | increased | promote autophagy, inhibit cell death and apoptosis | miR-26b, PI3K/AKT | ||
| MEG3 | hypoxic injury | PC12 cells | increased | attenuate cell injury and apoptosis | miR-147 |
| MCAO | mice, rats, HMECs | increased | ameliorate brain lesion, promote angiogenesis | Notch, miR-181b | |
| SAH | rats, rat neurons | increased | decrease cell death and apoptosis | PI3K/AKT | |
| OGD/R injury | HT22 cells | increased | improve neurological function, attenuate apoptosis | miR-181b | |
| N1LR | I/R injury | rats | increased | enhance proliferation, inhibit apoptosis, protect BBB function | p53 |
| OGD/R injury | N2a cells | ||||
| NEAT1 | TBI | rats | increased | improve neurological function, reduce cell death | – |
| NKILA | ICH | rats, rat neurons | decreased | induce autophagy, decrease inflammation | NF-κB |
| RMST | OGD/R injury | mouse neurons | increased | reduce brain infarct size, improve neurological function | – |
| MCAO | mice | ||||
| SNHG1 | OGD/R injury | BMECs | increased | promote cell survival and angiogenesis | miR-199a |
| TUG1 | MACO | rats | increased | decrease cell apoptosis, improve BBB function | miR-9 |
| OGD/R injury | rat neurons |
lncRNAs, long non-coding RNAs; ANRIL, antisense non-coding RNA in the INK4 locus; MCAO, middle cerebral artery occlusion; HUVECs, human umbilical vein endothelial cells; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; BDNF-AS, brain-derived neurotrophic factor antisense RNA; H/R, hypoxia-reoxygenation; HCNs, human cortical neurons; MMP, mitochondrial membrane potential; PI3K/AKT, phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B; C2dat1, CaMK2D-associated transcript 1; I/R, ischemia-reperfusion; FosDT, Fos downstream transcript; GAS5, growth arrest-specific transcript 5; HIBD, hypoxic/ischemic brain damage; miRNAs, microRNAs; OGD/R, oxygen-glucose deprivation/reoxygenation; TBI, traumatic brain injury; MALAT1, metastasis-associate lung adenocarcinoma transcript 1; BMECs, brain microvascular endothelial cells; MEG3, maternally expressed gene 3; HMECs, human microvascular endothelial cells; SAH, subarachnoid hemorrhage; NEAT1, nuclear enriched abundant transcript 1; ICH, intracerebral hemorrhage; RMST, rhabdomyosarcoma 2-associated transcript; SNHG1, small nucleolar RNA host gene 1; TUG1, taurine-upregulated gene 1.
MALAT1
MALAT1, a well conserved, stable, and abundant lncRNA (6.5 kb), was initially considered to be upregulated in solid tumors and associated with cancer cell proliferation, metastasis, survival, and recurrence.23 However, growing evidence indicates that MALAT1 has a special role in regeneration after brain injury. It was abundantly expressed in vascular endothelial cells, skeletal muscle, and cardiomyocytes and participated in the pathological inflammatory processes, proliferation, differentiation, myogenesis, and angiogenesis.24 It has been shown that MALAT1 could protect brain microvascular endothelial cells (BMECs) from injury caused by oxygen-glucose deprivation-reoxygenation by serving as an autophagy inducer.25 In addition, the role of MALAT1 in endothelial cell damage and hyperglycemia-induced inflammation has been previously reported.26
MEG3
MEG3 is an ∼1.6 kb imprinted gene belonging to the imprinted DLK1-MEG3 locus located at chromosome 14q32.3 DLK1 locus in humans. There are 12 different MEG3 gene transcripts generated by alternative splicing, and the MEG3 gene encodes a non-coding RNA of approximately 1700 nucleotides.27 MEG3 was first identified as a lncRNA with the function of a tumor suppressor.28 Subsequent results revealed that MEG3 may be involved in cellular remodeling and necessary for the neurons to resist injuries.29 Altered expression of MEG3 was observed to mediate ischemic neuronal death by activating p53 both in vitro and in vivo.30 Moreover, downregulation of MEG3 could protect against ischemic damage and enhance neurobehavioral outcomes.29 Furthermore, MEG3 functioned as a competing endogenous RNA for miR-181b to regulate 12/15-LOX expression in middle cerebral artery occlusion-induced ischemic infarct of brain nerve cells.31
BDNF-AS
BDNF is a member of the neurotrophin family of growth factors. It is abundantly expressed during embryonic development and contributes to the development of the nervous system by synchronizing neuronal and glial maturation as well as participating in axonal and dendritic differentiation.32 In the brain, BDNF acts on neurons in the hippocampus, cortex, and basal forebrain—areas vital to learning, memory, and higher thinking, supporting neuronal growth, differentiation, plasticity, and survival.33 Thus, upregulation of BDNF is thought to have beneficial effects on neurological disorders.34 BDNF-AS is one type of lncRNA transcribed by RNA polymerase II without an open reading frame.35 Chromatin immunoprecipitation results showed that BDNF-AS reduced the localization of EZH2 and H3K27me3 in the BDNF promoter region and inhibited the expression of BDNF, thereby affecting the growth and differentiation of neural cells. Inhibition of BDNF-AS led to increased protein levels of BDNF and induced neuronal growth and differentiation.35 Besides, BDNF-AS was identified to be significantly upregulated in patients with cerebral infarction, whereas BDNF-AS small interfering RNA (siRNA) suppressed hypoxia-reoxygenation (H/R)-induced neurotoxicity through activation of the BDNF.36 In addition, a recent study has declared that BDNF-AS knockdown was a novel method to prevent neurotoxicity in mouse embryonic neural stem cell (ESC)-derived neurons.37
NEAT1
NEAT1 is an ∼3.2 kb novel nuclear lncRNA. It localizes to specific nuclear structures called paraspeckles and is essential for the formation and maintenance of paraspeckles.38 Paraspeckles are formed by the binding of paraspeckle protein (PSP)1, PSP2, and p54nrb to the NEAT1 transcriptional start site. The functions of paraspeckles are to mediate the development of corpus luteum and mammary gland as well as modulate cytoplasmic proteins and RNA functions by migrating to the cytoplasm.39 Recently, NEAT1 has been proposed to be distributed in many subcellular regions where no paraspeckles were observed,40 suggesting that NEAT1 may have other functions besides paraspeckle formation. The effects of NEAT1 on brain injuries were also investigated. For example, it has been shown that NEAT1 was downregulated in cortical neurons in response to neuronal activity.41 Moreover, NEAT1 promoted brain injury in septic mice by positively regulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), while si-NEAT1 transfection could reduce this injury.42 In addition, NEAT1 has been shown to play a role in protecting cells subjected to lethal harm from undergoing early apoptosis after TBI, suggesting that NEAT1 potentially exerted a regulatory effect on cell apoptosis.43
GAS5
GAS5 is a lncRNA that hosts a number of small nucleolar RNAs (snoRNAs) within its introns. So it was originally considered that the biological functions of GAS5 were mediated by introns.20 However, recent studies indicate that GAS5 was increased during growth arrest induced by serum starvation or the lack of growth factors.44 Moreover, upregulation of GAS5 could cause a slower cell cycle and an increase in cell apoptosis.45 These results suggeste that GAS5 plays a key role in normal growth arrest and apoptosis. Therefore, GAS5 may also participate in numerous novel and unexpected cellular functions without introns. Recently, the functions of GAS5 in the neural system were discovered. In a previous study, highly expressed GAS5 was observed in rat hippocampus after TBI by microarray and quantitative real-time PCR analysis, suggesting that GAS5 might be involved in TBI pathological processes.46 Further studies confirmed the pro-apoptotic effect of GAS5 by downregulation of miR-335 and upregulation of Rasa1 in a TBI cell model.47 Besides, GAS5 was shown to be a promising therapeutic target for the treatment of hypoxic/ischemic brain damage (HIBD).48
C2dat1
C2dat1 is a sense lncRNA that overlaps with introns 13–15 and exon 14 of the CaMK2D gene in the genome.49 It was first discovered in a lncRNA array analysis of a rat middle cerebral artery occlusion (MCAO) model in 2016.49 C2dat1 was mainly located in the nucleus of N2a cells, and the inhibition of C2dat1 led to suppression of CaMK2D mRNA and protein.50 These results indicated that C2dat1 may interact with CaMK2D directly. It has been shown that C2dat1 was upregulated in murine ischemia/reperfusion (I/R) models and in mouse neuronal cells upon ischemia oxygen-glucose deprivation/reoxygenation (OGD/R). C2dat1 could regulate the expression of CAMK2D/CaMKIIδ in response to OGD/R and C2dat1-induced CaMKIIδ expression, further promoting neuronal survival by activating the NF-κB signaling pathway.49
Other lncRNAs in CNS Injuries
Besides the six well-studied lncRNAs, there were also other lncRNAs that have been explored in CNS injury models, such as BC048612,51 Uc.173,52 Gm4419,53 HOTAIR,54 N1LR,55 and RMST.56 All these lncRNAs played important roles in CNS injuries by affecting secondary brain damage.
The Function of lncRNAs in CNS Injuries
Regulation of lncRNA NEAT1, and MALAT1 was first reported to exhibit neuroprotection on CNS injuries in 2014.57 Subsequently, a large number of studies have demonstrated that regulation of lncRNAs played a protective role in CNS injuries. The neuroprotection of lncRNAs was attributed to their improvement of cognitive function, promotion of angiogenesis, inhibition of apoptosis and inflammation, regulation of autophagy, and protection of BBB function (Table 2).
Table 2.
Mechanisms of Regulation of lncRNAs in CNS Injuries
| Mechanisms | Factors | Associated Molecules |
|---|---|---|
| Improve cognitive function | reduce neuronal loss in the hippocampus and cortex | – |
| Promote angiogenesis | induce endothelial proliferation, migration and augment vasopermeability | VEGF |
| Decrease apoptosis | reduce cellular blebbing, chromosomal DNA fragmentation, and formation of apoptotic bodies | p53, Bcl-2, Bax, caspase-3 |
| Suppress inflammation | decrease inflammatory factors and attenuate inflammatory response | NF-κB, TNF-α, IL-1β, IL-6, ICAM-1 |
| Affect autophagy | increase the expression of LC3-II and promote the formation of autophagosome | Beclin-1, LC3 |
| Preserve BBB function | reduce endothelial cell markers and tight junction protein loss | GSTα3, GPx |
lncRNAs, long non-coding RNAs; VEGF, vascular endothelial growth factor; Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; ICAM-1, intercellular adhesion molecule 1; LC3, microtubule-associated protein light chain 3; BBB, blood-brain barrier; GSTα3, glutathione S transferase alpha 3; GPx, glutathione peroxidase.
Cognitive Function
Cognitive function impairment is a critical factor involved in the pathogenesis of CNS injuries, which may lead to defects in memory, attention, language, executive function, and social communication.58, 59, 60, 61 Cognitive function impairment is related to poor long-term outcome in the areas of independent living, return-to-work, and community integration.62 Thus, treatment of cognitive function impairment is logical therapeutic targets, and could significantly improve recovery rates and decrease sequelae.
The effects of lncRNAs on cognition after CNS injuries have been explored by many studies. Previous reports have indicated that intracerebroventricular injection of RMST short hairpin RNA (shRNA) significantly decreased brain RMST expression, improved neurological function, and reduced brain infarct size in a mouse MCAO-induced ischemic stroke model.56 Furthermore, treatment with exosomes containing lncRNA MALAT1 significantly recovered the function of motor behavior in a rat TBI model.63 In addition, it has been shown that knockdown of lncRNA Fos downstream transcript (FosDT) improved the motor recovery in a rat ischemic brain injury model, as measured by rotarod test, beam walk test, and adhesive removal test.64 The precise mechanisms underlying how lncRNAs regulated cognitive function were unclear. It has been well established that cognitive dysfunction involved selective neuronal loss in the hippocampus and cortex.65 Therefore, regulation of lncRNAs may also improve cognitive function by intervening with these pathological processes. However, this is just our hypothesis, and further studies are needed to explain it.
Angiogenesis
Angiogenesis is defined as the formation of new blood vessels from pre-existing vessels.66 Angiogenesis plays an important role in mediating functional recovery after CNS injuries, and various pharmacological drugs have been shown to improve functional outcome after CNS injuries by promoting angiogenesis.67 Angiogenesis could supply oxygen and nutrients to brain, alleviate brain ischemia, and accelerate the structural remodeling of injured brain, ultimately promoting the recovery of neurological function.68 Therefore, promoting angiogenesis is a therapeutic strategy for the treatment of CNS injuries. Angiogenesis is regulated by many vascular growth factors, including vascular endothelial growth factor (VEGF). VEGF can induce endothelial proliferation and migration and augment vasopermeability, making it a significant target for promoting angiogenesis.67
Since angiogenesis is a benefit for the damage of brain tissues after CNS injuries, it can be supposed that regulation of lncRNAs may attenuate brain damage in response to CNS injuries by promoting angiogenesis. Consistent with this hypothesis, Wang et al.69 proposed that upregulation of lncRNA SNHG1 promoted the angiogenesis of BMECs by activation of VEGF in an OGD/R-induced ischemic stroke model. In another study, it was shown that knockdown of lncRNA MEG3 increased the formation of blood vessels after focal ischemia, as analyzed by micro-CT scanning, indicating that MEG3 downregulation could promote angiogenesis.29 Because angiogenesis is one of the major factors determining nerve function recovery after CNS injuries, facilitating angiogenesis by targeting lncRNAs could be a vital therapeutic strategy.
Apoptosis
Apoptosis is a very tightly programmed cell death (PCD) that plays a crucial role in normal cell turnover, proper development, and function of the immune system, hormone-dependent atrophy, embryonic development, and chemical-induced cell death.70 Insufficient apoptosis can cause autoimmunity or cancer, while accelerated apoptosis can cause neurodegenerative diseases or ischemic damage.71 The characteristic morphology changes of apoptosis cells include blebbing, cell shrinkage, nuclear fragmentation, chromosomal DNA fragmentation, and formation of apoptotic bodies.72 In the physiological state, apoptosis is a highly regulated and controlled process that confers advantages during an organism’s life cycle. However, if apoptosis occurs in CNS injuries, it may lead to secondary brain injury and exacerbate the damage of CNS injuries.73 The functions of lncRNAs in CNS injury-induced neuronal apoptosis have been studied. The results obtained by Wu et al.55 demonstrated that overexpression of lncRNA N1LR significantly reduced neuronal apoptosis and neural cell loss in I/R-induced mouse brains, as evaluated by Annexin V/PI staining. In addition, Liang et al.74 showed that, in a rat SAH model, lncRNA MEG3 overexpression upregulated the expressions of Bcl-2-associated X protein (Bax), p53, and cleaved caspase-3 and downregulated the expression of Bcl-2, suggesting that MEG3 could increase cell apoptosis. In another study, conducted by Zhong et al.,75 it was found that inhibition of lncRNA BDNF-AS was associated with decreased apoptosis in the brain following ischemic injury, as measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining. These results manifestly demonstrate that regulation of lncRNAs could reduce neuronal apoptosis in models of CNS injuries.
So far, studies have only examined the role of lncRNAs on apoptosis after CNS injuries in general. However, apoptosis can be divided into two pathways: the mitochondria-dependent pathway (the intrinsic pathway) and the death receptor-dependent pathway (the extrinsic pathway). The mitochondria-dependent pathway is one of the most important pathways of apoptosis, which is regulated by the B cell lymphoma-2 (Bcl-2) family proteins, including pro-apoptotic proteins (e.g., Bax, Bak, Bid, and Bad) and anti-apoptotic proteins (e.g., Bcl-2, Bcl-x, Bcl-xL, and Bcl-w). Mitochondrial damage induces the release of cytochrome c from mitochondrion through alteration of mitochondrial membrane permeability, resulting in the activation of caspase-3 and subsequent apoptosis. On the contrary, the death receptor-dependent pathway is initiated by the binding of Fas ligand to Fas receptor and the binding of tumor necrosis factor (TNF) ligand to TNF receptor, resulting in the activation of caspase-8. Once caspase-8 is activated, the execution phase of apoptosis is triggered.76 Therefore, the apoptotic pathway that is associated with the effects of lncRNAs on apoptosis remains unclear and further studies are needed to clarify it.
Inflammation
Inflammation plays a critical role in the secondary brain injury after CNS injuries.77 Under normal conditions, inflammation is a cellular and molecular process that aims to combat invading pathogens, restore damaged cells, and preserve the health of the tissue.78 However, under pathological conditions such as CNS injuries, excessive inflammation could function as a reactionary system to further aggravate brain damage, leading to BBB breakdown, cerebral edema, microglial and astrocytic activation, peripheral leukocyte migration, recruitment, and the release of inflammatory cytokines, such as TNF-α/β, interleukin-1β (IL-1β), -6, -10, and intercellular adhesion molecule 1 (ICAM-1).79 These cytokines, in turn, recruit additional blood-borne neutrophils and monocytes into the injured brain, thus expanding the inflammatory cascade and exacerbating the brain injury.80
Numerous reports have demonstrated that lncRNAs exert a central effect in inflammation caused by CNS injuries. The role of lncRNAs in CNS injury-induced inflammation was first described by Zhang et al.81 in 2017. They found that the pro-inflammatory factors such as E-selectin, MCP-1, and IL-6 were significantly increased in an in vivo ischemic stroke model. Silencing of lncRNA MALAT1 by GapmeR further increased the levels of these pro-inflammatory factors, suggesting that MALAT1 has anti-inflammatory effects in ischemic stroke.81 Furthermore, in an in vitro model of ischemic stroke, high levels of lncRNA Gm4419 elevated the expression of TNF-α, IL-1β, and IL-6, resulting in more aggressive inflammation in astrocytes following OGD/R damage.53 In addition, in TBI models, overexpression of lncRNA NEAT1 reduced the production of IL-1β, TNF-α, and nitrite both in vivo and in vitro, suggesting that NEAT1 had an anti-inflammatory effect.82 The underlying mechanisms of the lncRNA-mediated inflammatory reaction are complicated. Studies have indicated that the NF-κB might be the key target, and we will discuss the detailed mechanisms in the following sections. Since CNS injury-triggered inflammatory reaction causes damage not only in brain, but also in other organs, lncRNAs may be a promising target for anti-inflammatory therapy after CNS injuries.
Autophagy
Autophagy is a self-catabolic process whereby cells remove cytosolic proteins and organelles and degrade themselves in a stressed or nutrient-deprived state.83 During autophagy, the double membrane vesicle, known as autophagosome, first encloses cytoplasmic constituents such as damaged mitochondrion, endosomes, and proteins. Autophagosome then fuses with lysosome to form autolysosome, and the cytoplasmic components are degraded or recycled by acidic lysosomal hydrolases. The process of cargo delivery by autophagosome and degradation by autolysosome is named autophagy flux.84 One of the most important functions of autophagy is to maintain the metabolism essential for cell survival in nutrient deprivation or other stress situations. However, dysfunction of autophagy is involved in multiple diseases, including CNS injuries, and extensive activation of autophagy can lead to cell death (type II PCD).85, 86 Indeed, the role of autophagy in CNS injuries is indefinite; inhibition of autophagy can either attenuate or promote brain damage. Luo et al.87 found that suppression of autophagy by 3-methyladenine (3-MA) or bafliomycin A1 (BafA1) reduced TBI-induced cell death and apoptosis, indicating that autophagy promoted apoptosis in TBI. Conversely, Wang et al.88 reported that inhibition of autophagy by 3-MA increased brain edema and BBB disfunction and aggravated neurological deficits in SAH, suggesting that autophagy played a beneficial role in SAH.
There were also studies demonstrating that regulation of lncRNAs could affect autophagy in CNS injuries. However, the roles of lncRNA-modulated autophagy in CNS injuries, especially in cerebral ischemic stroke, were also controversial. Li et al.25 have shown that upregulation of lncRNA MALAT1 protected BMECs against OGD/R-induced BMEC death by activation of autophagy, suggesting a protective role of MALAT1 and autophagy in ischemic stroke. Interestingly, in another study conducted by Guo et al.,89 they revealed that downregulation of lncRNA MALAT1 attenuated OGD/R-induced neuronal cell death by suppression of autophagy, suggesting a detrimental role of MALAT1 and autophagy in ischemic stroke. The discrepancies may be due to the different cell types used in these two studies. Although the lncRNA and ischemic stroke model were the same, the cells used in the Li et al.25 experiments were BMECs, while the cells used in Guo et al.89 experiments were cerebral cortex neurons. In conclusion, in combination with the previous reports, we thought that, depending on different CNS injury models and cell types, the lncRNAs, autophagy and cell death may have inhibitory, additive, or even synergistic effects.
BBB Function
BBB is a highly specialized, semi-permeable barrier that separates the circulating blood from the brain and extracellular fluid in CNS.90 Structurally, BBB is formed by brain endothelial cells with tight junction (TJ), and it allows the passage of water, lipid-soluble molecules, glucose, and amino acids, which are important to brain homeostasis and neural functions.91 Disruption of BBB is a key secondary injury process following CNS injuries.92, 93, 94 Many pathological conditions, such as TJ breakdown, endothelial cell death, and decreased coupling between TJ proteins and cytoskeletons, may lead to the damage of BBB.95 In an in vitro model of cerebral ischemic stroke, Li et al.25 found that lncRNA MALAT1 protected BBB against OGD/R -induced injury by promoting the survival of BMECs. Furthermore, another in vitro study confirmed the protective effects of lncRNA on BBB in ischemic stroke.96
Downstream Molecules of lncRNAs in CNS Injuries
The specific mechanisms mediating the functions of lncRNAs in CNS injuries have yet to be fully explained; a number of downstream molecules of lncRNAs have been proposed that may illustrate their biological effects (Figure 2).
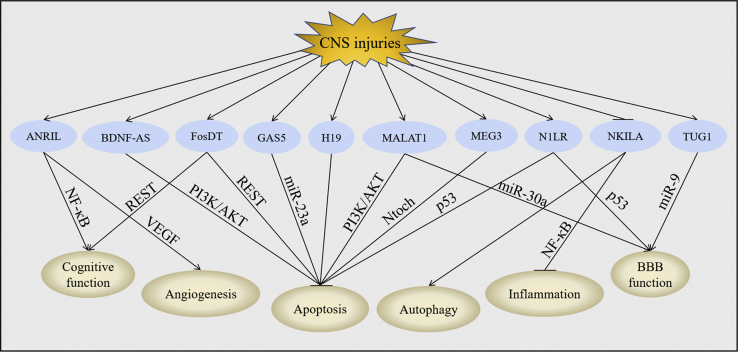
Figure 2.
Downstream Molecules of lncRNAs in CNS Injuries
CNS injuries upregulated the expression of ANRIL, BDNF-AS, FosDT, GAS5, H19, MALAT1, MEG3, N1LR, and TUG1, but they downregulated the expression of NKILA. Changes of these lncRNAs further leads to the modulation of miRNAs, activation of VEGF and Notch, and inhibition of NF-κB, REST, PI3K/AKT, and p53. Regulation of these downstream molecules subsequently improved cognitive function, promoted angiogenesis, activated autophagy, protected BBB function, and suppressed apoptosis and inflammation post-CNS injuries.
MicroRNA (miRNA)
miRNA is a small non-coding RNA molecule approximately 22 nucleotides in length.97 Briefly, the formation of a miRNA begins with the transcription of a primary miRNA (pri-miRNA) that harbors the microRNA. The pri-miRNA is then processed by a microprocessor complex to form a precursor miRNA (pre-miRNAs) in the nucleus. The pre-miRNA is then transported into the cytoplasm from the nucleus by exportin-5. In the cytoplasm, the pre-miRNA is cleaved to a single-stranded miRNA by the RNase III-like endonuclease Dicer. Subsequently, each stranded miRNA is incorporated into an Argonaute protein containing RNA-induced silencing complex (RISC). The RISC-loaded miRNA contains a seed region that recognizes complementary sequences at the 3′ untranslated region (UTR) of mRNA, resulting in negative regulation, such as transcript degradation or post-translational suppression.98, 99, 100
miRNAs are present and stable in many mammalian cell types and appear to target nearly 60% of genes in humans.97 They control multiple cellular processes, including cell proliferation and differentiation, cell survival, immune responses, angiogenesis, and inflammation.101 Therefore, due to the simple structure and the ability to modulate cellular functions, miRNAs have raised the research climax for developing potential miRNA-based therapies. An expanding body of evidence has revealed that lncRNAs could regulate the expression and function of miRNAs in CNS injuries. It has been shown that silencing of lncRNA GAS5 protected against hypoxia/ischemia-induced neonatal brain injury by activation of miR-23a.48 Moreover, lncRNA MALAT1 could serve as an endogenous sponge to downregulate miR-26b expression by directly binding to miR-26b, thus promoting BMEC survival under the OGD/R condition.25 Furthermore, downregulation of lncRNA MEG3 decreased apoptosis of neural cells after hypoxic damage by targeting miR-147.96
How lncRNAs regulate miRNAs has been fully clarified. lncRNAs may act as miRNA sponges or antagomirs, also known as competing endogenous RNAs (ceRNAs), to identify cytoplasmic miRNAs and sequester miRNAs away from mRNAs, thereby regulating the expression of the miRNA target protein. In addition to their function as ceRNAs, lncRNAs also compete with miRNAs for binding to target mRNAs. Furthermore, some lncRNAs are processed to generate certain miRNAs. Finally, lncRNAs can act as platforms for transportation of miRNAs to mRNAs, and even as platforms for transporting outside of cells or between cells.100, 102, 103, 104
NF-κB
NF-κB is a family of transcription factors that play important roles in inflammation.105 NF-κB is abundant in the brain neurons and exhibits diverse functions. It can protect neurons against injuries and regulate neuron inflammation by controlling the transcription of genes such as cytokines, chemokines, and inflammatory transcription factors.106 Several CNS diseases are associated with NF-κB activated by inflammatory mediators. A number of negative mediators have been proposed to regulate NF-κB. Among them, the IkB is considered a major brake in NF-kB signaling.107 Under physiological conditions, NF-κB is inactive and sequestered in the cytoplasm by binding to its inhibitory kinase IkB. However, under pathologic conditions, such as CNS injuries, IkB can be phosphorylated by IkB kinases (IKKs), leading to the degradation of IkB and subsequent activation of NF-κB.108 Activation of NF-κB has been shown to be central to the pathophysiology of inflammatory response triggered by lncRNAs. In ischemic stroke models, the inflammatory response reflexed by the levels of TNF-α, IL-1β, IL-6, and their mediator, NF-κB, were suppressed by knockdown of lncRNA Gm441953 or C2dat1.49 Furthermore, in a rat ICH model, the activity of NF-κB and the expression of pro-inflammatory cytokines were much more prominent in lncRNA NKILA knockdown groups than that in sham groups.109 Therefore, lncRNAs are significant for the regulation of neuroinflammation in response to CNS injuries by activation of NF-κB in the brain.
How lncRNAs regulate NF-κB has not been well characterized, but there are reports showing that lncRNAs could directly interact with NF-κB or its transcripts. For example, lncRNA NKILA, which locates primarily in the cytoplasm, could inhibit both basal and cytokine-stimulated NF-κB. This inhibition is mediated by the interaction between NKILA and the p65 subunit of NF-κB, leading to the binding of NKILA with NF-κB/IkB to form a stable NKILA/NF-κB/IkB complex. Subsequently, the phosphorylation site of IkB is masked, thus suppressing IKK-induced IkB phosphorylation and NF-κB activation.110 In addition, some lncRNAs regulated NF-κB by interfering with the signaling components upstream of NF-κB, which affects the phosphorylation of IKK and IkB. For example, knockdown of lncRNA C2dat1 could downregulate CaMKIIδ expression, suppress IKK phosphorylation, and inhibit NF-κB activity.111 Besides, knockdown of lncRNA Gm4419 inhibited the phosphorylation of IkB by physically associating with IkB, resulting in decreased nucleus NF-κB levels.53
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase/Protein Kinase B (PI3K/AKT) Pathway
The PI3K/AKT pathway is a highly conserved, tightly controlled signaling cascade that plays an essential role in the regulation of cell proliferation, differentiation, metabolism, and survival in both physiological and pathological conditions.112 The PI3K/AKT pathway is activated by various receptors, including receptor tyrosine kinases (RTKs) and G-protein-coupled receptors (GPCRs). Stimulation of RTKs or GPCRs leads to the activation of class I PI3Ks which phosphorylate phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3). Subsequently, PIP3 recruits AKT to the plasma membrane and phosphorylates AKT on its serine 472 site and threonine 308 kinase site. The phosphorylated AKT is involved in its downstream mTORC1-mediated response to biogenesis of ribosomes and proteins.113, 114, 115 This activity can be reversed by the PI3K antagonist phosphatase and tensin homolog (PTEN) through dephosphorylation of PIP3 to PIP2. Notably, the PI3K/AKT pathway has a critical role in neuronal survival in a variety of CNS injuries, and regulation of this pathway has the potential to treat brain injury.116
Recently, multiple lncRNAs were found to provide protection in CNS injury models by activation of the PI3K/AKT pathway. Xin et al.117 found that overexpression of lncRNA MALAT1 decreased cell apoptosis by activation of PI3K and phosphorylation of AKT in cerebral I/R injury. Furthermore, Zhong et al.36 indicated that knockdown of lncRNA BDNF-AS attenuated H/R-induced mitochondrial membrane potential (MMP) decrease and cell apoptosis through the activation of the PI3K/AKT pathway. Moreover, Liang et al.74 revealed that lncRNA MEG3 aggravated SAH-induced neuronal cell apoptosis via inhibiting the PI3K/AKT pathway. These data suggest that the PI3K/AKT pathway may be a potential therapeutic target for lncRNAs in CNS injuries.
But how lncRNAs regulate the PI3K/AKT pathway in CNS injuries is uncertain. There have been several explanations, and these explanations are consistently related to miRNA-mediated PTEN. That means lncRNAs first regulate miRNAs, which further modulate PTEN and the downstream PI3K/AKT pathway. For instance, by inhibiting the expression of miR-103, lncRNA GAS5 significantly enhanced the expression of PTEN, which negatively regulated the PI3K/AKT pathway, to promote cancer cell apoptosis.118 In another case, lncRNA HULC activated the PI3K/AKT pathway by inhibiting the miR-15a-PTEN axis in liver cancer.119 In addition, downregulation of lncRNA fer-1-like family member 4 (FER1L4) could result in a reduction of PTEN expression by freeing miR-106a-5p, thus activating the PI3K/AKT pathway.120 So, by combination with previous literature, it can be supposed that lncRNAs may also mediate the PI3K/AKT pathway by modulation of the miRNA-PTEN axis in CNS injury models. However, this is just our speculation, and further studies are needed to confirm it.
Notch Pathway
The Notch signaling pathway belongs to an evolutionarily conserved signal transduction system and plays critical roles in cell proliferation, differentiation, apoptosis, and homeostasis of multicellular organisms. It also influences synaptic plasticity as well as learning and memory in the adult brain.121 Binding of Notch receptors to ligands activates Notch intracellular cytoplasmic domain (NICD), which translocates to the nucleus and induces transcription of Notch target genes, such as hairy and enhancer of split-1 (Hes-1) and Hes-related with YRPW motif-1 (Hey-1).122 Current evidence has highlighted a key role of the Notch pathway in CNS injuries. The Notch pathway is activated in endothelial cells in response to ischemia and determines the formation of native arterial collateral networks. Inhibition of Notch pathway has been shown to impair reparative angiogenesis after ischemia.123 lncRNAs could also facilitate Notch to regulate angiogenesis in ischemic stroke. It has been suggested that the protein levels of NICD, Hes-1, and Hey-1 were decreased by overexpression of MEG3 but increased by knockdown of MEG3 in the ischemic brain. Furthermore, inhibition of Notch by N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycinetbutylester (DAPT) impaired MEG3-induced angiogenic migration and sprouting.29
The mechanisms of how lncRNAs regulate Notch pathway have not been explained in detail. It has been shown that lncRNA steroid receptor RNA activator (SRA) could activate the Notch pathway by functioning together with DEAD box protein 5 (Ddx5). Ddx5 localized to recombination signal-binding protein J (RBP-J)-binding sites of the Notch target genes Hes-1 and pre T cell antigen receptor alpha (preTCRα). Upregulation of SRA promoted the activity of Ddx5 and increased the expression of Hes-1 and preTCRα, thereby activating the Notch pathway.124 However, the mechanisms by which other lncRNAs regulate the Notch pathway are unclear, which is an interesting aspect worth exploring.
There are four structurally related Notch receptors (Notch1–4) that bind transmembrane ligands of the Jagged (Jagged-1 and Jagged-2) or the Delta-like (DLL1, DLL3, and DLL4) families. Binding of Notch receptors to ligands induces separation of the extracellular receptor subunit from the transmembrane subunit.125 Although the Notch receptors are structurally similar, each member appears to exert a specific biological role. Notch1 mutation can cause vascular malformations, Notch2 mutation can cause vascular and renal defects, and Notch3 mutation can cause cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) syndrome. Despite that, Notch4 is dispensable for embryonic development; some of its functions overlap with those of Notch1.126 Since there has been reports showing that Notch can be regulated by lncRNA MEG3 in ischemic stroke, further studies are needed to confirm whether other lncRNAs could regulate Notch in CNS injury models and which Notch receptor is regulated by lncRNAs.
p53
The p53 protein is a pleiotropic transcription factor that plays a significant role in the determination of cell fate under several conditions.127 Upon cellular stress, such as DNA damage, oxidative stress, and nerve growth factor (NGF) withdrawal, p53 binds to a consensus response element (p53RE) in the promoter of p53 target genes, such as the pro-apoptotic genes Bax and PUMA, cell-cycle arrest, and DNA-repair gene p21, thus leading to their transcription activation and triggering cell-cycle arrest, promoting apoptosis, regulating differentiation, and altering cellular lifespan.128, 129, 130 In addition, evidence has suggested that p53 contributes to direct neurons toward a specific phenotype in critical conditions, such as during development and following cellular damage. It has been reported that ischemic injury was strongly correlated with an increase in p53 activity127 and that the activation of p53 in response to ischemic injury was regulated by lncRNA. Wu et al.55 implied that lncRNA N1LR protected the brain from ischemic stroke-induced apoptosis by suppressing p53 phosphorylation. lncRNAs have been shown to regulate p53 by affecting p53 mRNA stability and impacting transcription of p53 target genes. For example, N1LR could serve as an inhibitor to prevent the p53 protein from being phosphorylated on serine 15, thereby inhibiting the activity of p53.55
Other Aspects of lncRNA Research in CNS Injuries
CNS injuries are a diverse group of disorders that involve many characteristics. There are various pathological processes responsible for the secondary damage of CNS injuries, including glutamate excitotoxicity, inflammation, oxidative stress, and apoptosis. Although the functions of lncRNAs on CNS injury-induced cognitive function, oxidative stress, apoptosis, inflammation, autophagy, and BBB disruption have been widely described, its role in oxidative stress and excitotoxicity have not been illustrated so far.
Oxidative Stress
Oxidative stress is an event caused by the imbalance between the biological systems, leading to the generation of free radicals such as reactive oxygen species (ROS) and the systems responsible for the removal of free radicals, also known as the enzymatic and non-enzymatic antioxidant cellular defense systems. The excessive generation of free radicals due to excitotoxicity or depletion of the antioxidant system induces toxic effects, protein oxidation, DNA redox imbalance, and mitochondrial electron transport chain (METC) inhibition, resulting in oxidative stress damage.131, 132 Recently, there has been growing evidence demonstrating that oxidative stress plays a crucial role in the development of cerebral edema, inflammation, and apoptosis and contributes to secondary neuronal damage in CNS injuries.133, 134, 135
There were also studies showing that lncRNAs could regulate oxidative stress. Zhang et al.136 indicated that lncRNA FOXD3-AS1 could regulate oxidative stress-induced apoptosis via sponging miRNA-150 in human lung epithelial cells. Moreover, Gao et al.137 proposed that lncRNA MT1DP could aggravate cadmium-induced oxidative stress by interaction with miR-365 and suppression of nuclear factor erythroid 2-related factor 2 (Nrf2) in human hepatocellular carcinoma cell line HepG2. In addition, downregulation of lncRNA LINC00963 inhibited oxidative stress by activation of the forkhead box O (FoxO) signaling pathway in chronic renal failure.138 Therefore, further studies are needed to estimate whether lncRNAs could affect oxidative stress in CNS injuries.
Excitotoxicity
Excitotoxicity occurs early after CNS injuries and plays an important role in apoptosis, necrosis, and autophagy.139 Excitotoxicity begins with the abnormal influx of Na+ and efflux of K+, contributing to the stopping or slowing of ATP production. The decreased ATP levels, in response to Na+/K+ imbalance, further reduces the reuptake of glutamate, which is the main excitatory neurotransmitter in the brain. Glutamate can mediate excitotoxicity by permitting the Ca2+ influx into cells via the activation of N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), and kainate receptors. Cellular accumulation of Ca2+ produces a series of cellular events (excitotoxicity) that lead to mitochondrial failure and apoptosis.140, 141, 142, 143 Therefore, glutamate is considered the main contributor that triggers excitotoxic cell damage, and a variety of experimental and clinical studies support the views that CNS injuries induce excitotoxicity by increase of glutamate.144, 145
The functions of lncRNAs in excitotoxicity have also been well established. It has been shown that silencing of lncRNA BDNF-AS attenuated Aβ25–35-induced neurotoxicity in Alzheimer’s disease (AD).146 Besides, lncRNA Gadd45a has been suggested to be associated with sevoflurane-induced neurotoxicity in rat neural stem cells.147 Furthermore, lncRNA SNHG1 could promote α-synuclein aggregation and excitotoxicity by targeting the miR-15b-5p/SIAH1 axis in Parkinson’s disease (PD).148 Thus, it can be speculated that lncRNAs may also participate in CNS injury-induced excitotoxicity. However, further studies are required to verify it.
Conclusions
lncRNAs play a key role in CNS injuries and are involved in a number of cellular and molecular processes of CNS injuries. In this review, we describe the functions of lncRNAs as well as some downstream moleculars of lncRNAs in CNS injuries. These observations make lncRNAs more attractive therapeutic targets for developing new therapeutic strategies to achieve better outcomes for patients suffering from CNS injuries. It is certain that further studies are needed to clarify the specific mechanisms of lncRNAs in CNS injuries. Finally, targeting lncRNAs may hold promise for clinical therapies.
Author Contributions
L.Z. conducted the literature review and initial draft of the manuscript. H.W. provided overall supervision and edited the manuscript. Both L.Z. and H.W. read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81672503 and 81702484).
References
- 1.Wang Y., Tan H., Hui X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. BioMed Res. Int. 2018;2018:7848901. doi: 10.1155/2018/7848901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H., Young W., Chen L., Feng S., Zoubi Z.M.A., Sharma H.S., Saberi H., Moviglia G.A., He X., Muresanu D.F. Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017) Cell Transplant. 2018;27:310–324. doi: 10.1177/0963689717746999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boguszewska-Czubara A., Budzynska B., Skalicka-Wozniak K., Kurzepa J. Perspectives and new aspects of metalloproteinases’ inhibitors in therapy of CNS disorders: from chemistry to medicine. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666180514111500. Published online May 13, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Roberts T.C., Morris K.V., Weinberg M.S. Perspectives on the mechanism of transcriptional regulation by long non-coding RNAs. Epigenetics. 2014;9:13–20. doi: 10.4161/epi.26700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee K.T., Nam J.W. Post-transcriptional and translational regulation of mRNA-like long non-coding RNAs by microRNAs in early developmental stages of zebrafish embryos. BMB Rep. 2017;50:226–231. doi: 10.5483/BMBRep.2017.50.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bali K.K., Kuner R. Noncoding RNAs: key molecules in understanding and treating pain. Trends Mol. Med. 2014;20:437–448. doi: 10.1016/j.molmed.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z., Li X., Chen C., Li S., Shen J., Tse G., Chan M.T.V., Wu W.K.K. Long non-coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2018;51:e12483. doi: 10.1111/cpr.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharap A., Nakka V.P., Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P., Zuo X., Deng H., Liu X., Liu L., Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res. Bull. 2013;97:69–80. doi: 10.1016/j.brainresbull.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt A.M., Chang H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grammatikakis I., Panda A.C., Abdelmohsen K., Gorospe M. Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging. Aging (Albany N.Y.) 2014;6:992–1009. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booton R., Lindsay M.A. Emerging role of MicroRNAs and long noncoding RNAs in respiratory disease. Chest. 2014;146:193–204. doi: 10.1378/chest.13-2736. [DOI] [PubMed] [Google Scholar]
- 14.Ramos A.D., Diaz A., Nellore A., Delgado R.N., Park K.Y., Gonzales-Roybal G., Oldham M.C., Song J.S., Lim D.A. Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell. 2013;12:616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer T.R., Dinger M.E., Sunkin S.M., Mehler M.F., Mattick J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S.H., Kwon J.T., Kim J., Jeong J., Kim J., Lee S., Cho C. Profiling of testis-specific long noncoding RNAs in mice. BMC Genomics. 2018;19:539. doi: 10.1186/s12864-018-4931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi I.A., Mehler M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spadaro P.A., Bredy T.W. Emerging role of non-coding RNA in neural plasticity, cognitive function, and neuropsychiatric disorders. Front. Genet. 2012;3:132. doi: 10.3389/fgene.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He D., Wang J., Lu Y., Deng Y., Zhao C., Xu L., Chen Y., Hu Y.C., Zhou W., Lu Q.R. lncRNA Functional Networks in Oligodendrocytes Reveal Stage-Specific Myelination Control by an lncOL1/Suz12 Complex in the CNS. Neuron. 2017;93:362–378. doi: 10.1016/j.neuron.2016.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi X., Sun M., Liu H., Yao Y., Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Dykstra-Aiello C., Jickling G.C., Ander B.P., Shroff N., Zhan X., Liu D., Hull H., Orantia M., Stamova B.S., Sharp F.R. Altered Expression of Long Noncoding RNAs in Blood After Ischemic Stroke and Proximity to Putative Stroke Risk Loci. Stroke. 2016;47:2896–2903. doi: 10.1161/STROKEAHA.116.013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J., Yuan L., Zhang X., Hamblin M.H., Zhu T., Meng F., Li Y., Chen Y.E., Yin K.J. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. Exp. Neurol. 2016;277:162–170. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J., Xu W., Du X., Hou J. MALAT1 silencing suppresses prostate cancer progression by upregulating miR-1 and downregulating KRAS. OncoTargets Ther. 2018;11:3461–3473. doi: 10.2147/OTT.S164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao Y., Peng C., Li J., Wu D., Wang X. LncRNA MALAT1 is neuroprotective in a rat model of spinal cord ischemia-reperfusion injury through miR-204 regulation. Curr. Neurovasc. Res. 2018;15:211–219. doi: 10.2174/1567202615666180712153150. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Li J., Tang N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience. 2017;354:1–10. doi: 10.1016/j.neuroscience.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Puthanveetil P., Chen S., Feng B., Gautam A., Chakrabarti S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell. Mol. Med. 2015;19:1418–1425. doi: 10.1111/jcmm.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondal T., Subhash S., Vaid R., Enroth S., Uday S., Reinius B., Mitra S., Mohammed A., James A.R., Hoberg E. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nat. Commun. 2015;6:7743. doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji L., Li X. Long noncoding RNA MEG3 is a tumor suppressor in choriocarcinoma by upregulation of microRNA-211. J Cell Physiol. 2019 doi: 10.1002/jcp.28853. Published online May 23, 2019. [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Li Q., Zhang K.S., Hu B., Niu X., Zhou S.M., Li S.G., Luo Y.P., Wang Y., Deng Z.F. Downregulation of the Long Non-Coding RNA Meg3 Promotes Angiogenesis After Ischemic Brain Injury by Activating Notch Signaling. Mol. Neurobiol. 2017;54:8179–8190. doi: 10.1007/s12035-016-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao M.H., Szeto V., Yang B.B., Zhu S.Z., Sun H.S., Feng Z.P. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018;9:281. doi: 10.1038/s41419-018-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Hou L., Huang W., Gao Y., Lv X., Tang J. The Mechanism of Long Non-coding RNA MEG3 for Neurons Apoptosis Caused by Hypoxia: Mediated by miR-181b-12/15-LOX Signaling Pathway. Front. Cell. Neurosci. 2016;10:201. doi: 10.3389/fncel.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marler K.J., Suetterlin P., Dopplapudi A., Rubikaite A., Adnan J., Maiorano N.A., Lowe A.S., Thompson I.D., Pathania M., Bordey A. BDNF promotes axon branching of retinal ganglion cells via miRNA-132 and p250GAP. J. Neurosci. 2014;34:969–979. doi: 10.1523/JNEUROSCI.1910-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song J.H., Yu J.T., Tan L. Brain-Derived Neurotrophic Factor in Alzheimer’s Disease: Risk, Mechanisms, and Therapy. Mol. Neurobiol. 2015;52:1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- 34.Sona C., Kumar A., Dogra S., Kumar B.A., Umrao D., Yadav P.N. Docosahexaenoic acid modulates brain-derived neurotrophic factor via GPR40 in the brain and alleviates diabesity-associated learning and memory deficits in mice. Neurobiol. Dis. 2018;118:94–107. doi: 10.1016/j.nbd.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Modarresi F., Faghihi M.A., Lopez-Toledano M.A., Fatemi R.P., Magistri M., Brothers S.P., van der Brug M.P., Wahlestedt C. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat. Biotechnol. 2012;30:453–459. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong J.B., Li X., Zhong S.M., Liu J.D., Chen C.B., Wu X.Y. Knockdown of long noncoding antisense RNA brain-derived neurotrophic factor attenuates hypoxia/reoxygenation-induced nerve cell apoptosis through the BDNF-TrkB-PI3K/Akt signaling pathway. Neuroreport. 2017;28:910–916. doi: 10.1097/WNR.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X., Lin C., Li Y., Ye J., Zhou J., Guo P. Long noncoding RNA BDNF-AS regulates ketamine-induced neurotoxicity in neural stem cell derived neurons. Biomed. Pharmacother. 2016;82:722–728. doi: 10.1016/j.biopha.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 38.Liu K., Mao X., Chen Y., Li T., Ton H. Regulatory role of long non-coding RNAs during reproductive disease. Am. J. Transl. Res. 2018;10:1–12. [PMC free article] [PubMed] [Google Scholar]
- 39.Yu X., Li Z., Zheng H., Chan M.T., Wu W.K. NEAT1: A novel cancer-related long non-coding RNA. Cell Prolif. 2017;50:50. doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa S., Naganuma T., Shioi G., Hirose T. Paraspeckles are subpopulation-specific nuclear bodies that are not essential in mice. J. Cell Biol. 2011;193:31–39. doi: 10.1083/jcb.201011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry G., Briggs J.A., Hwang D.W., Nayler S.P., Fortuna P.R., Jonkhout N., Dachet F., Maag J.L., Mestdagh P., Singh E.M. The long non-coding RNA NEAT1 is responsive to neuronal activity and is associated with hyperexcitability states. Sci. Rep. 2017;7:40127. doi: 10.1038/srep40127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W.Q., Wang Y.J., Zheng Y., Chen X. Effects of long non-coding RNA NEAT1 on sepsis-induced brain injury in mice via NF-κB. Eur. Rev. Med. Pharmacol. Sci. 2019;23:3933–3939. doi: 10.26355/eurrev_201905_17822. [DOI] [PubMed] [Google Scholar]
- 43.Hirose T., Virnicchi G., Tanigawa A., Naganuma T., Li R., Kimura H., Yokoi T., Nakagawa S., Bénard M., Fox A.H., Pierron G. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell. 2014;25:169–183. doi: 10.1091/mbc.E13-09-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simion V., Haemmig S., Feinberg M.W. LncRNAs in vascular biology and disease. Vascul. Pharmacol. 2018;114:145–156. doi: 10.1016/j.vph.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X., Sun M., Liu H., Yao Y., Kong R., Chen F., Song Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol. Carcinog. 2015;54(Suppl 1):E1–E12. doi: 10.1002/mc.22120. [DOI] [PubMed] [Google Scholar]
- 46.Wang C.F., Zhao C.C., Weng W.J., Lei J., Lin Y., Mao Q., Gao G.Y., Feng J.F., Jiang J.Y. Alteration in Long Non-Coding RNA Expression after Traumatic Brain Injury in Rats. J. Neurotrauma. 2017;34:2100–2108. doi: 10.1089/neu.2016.4642. [DOI] [PubMed] [Google Scholar]
- 47.Dai X., Yi M., Wang D., Chen Y., Xu X. Changqin NO. 1 inhibits neuronal apoptosis via suppressing GAS5 expression in a traumatic brain injury mice model. Biol. Chem. 2019;400:753–763. doi: 10.1515/hsz-2018-0340. [DOI] [PubMed] [Google Scholar]
- 48.Zhao R.B., Zhu L.H., Shu J.P., Qiao L.X., Xia Z.K. GAS5 silencing protects against hypoxia/ischemia-induced neonatal brain injury. Biochem. Biophys. Res. Commun. 2018;497:285–291. doi: 10.1016/j.bbrc.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 49.Xu Q., Deng F., Xing Z., Wu Z., Cen B., Xu S., Zhao Z., Nepomuceno R., Bhuiyan M.I., Sun D. Long non-coding RNA C2dat1 regulates CaMKIIδ expression to promote neuronal survival through the NF-κB signaling pathway following cerebral ischemia. Cell Death Dis. 2016;7:e2173. doi: 10.1038/cddis.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia D., Niu Y., Li D., Liu Z. lncRNA C2dat1 Promotes Cell Proliferation, Migration, and Invasion by Targeting miR-34a-5p in Osteosarcoma Cells. Oncol. Res. 2018;26:753–764. doi: 10.3727/096504017X15024946480113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Kaur P., Tan J.R., Karolina D.S., Sepramaniam S., Armugam A., Wong P.T., Jeyaseelan K. A long non-coding RNA, BC048612 and a microRNA, miR-203 coordinate the gene expression of neuronal growth regulator 1 (NEGR1) adhesion protein. Biochim. Biophys. Acta. 2016;1863:533–543. doi: 10.1016/j.bbamcr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Nan A., Zhou X., Chen L., Liu M., Zhang N., Zhang L., Luo Y., Liu Z., Dai L., Jiang Y. A transcribed ultraconserved noncoding RNA, Uc.173, is a key molecule for the inhibition of lead-induced neuronal apoptosis. Oncotarget. 2016;7:112–124. doi: 10.18632/oncotarget.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen Y., Yu Y., Fu X. LncRNA Gm4419 contributes to OGD/R injury of cerebral microglial cells via IκB phosphorylation and NF-κB activation. Biochem. Biophys. Res. Commun. 2017;487:923–929. doi: 10.1016/j.bbrc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Wang J.Y., Feng Y., Fu Y.H., Liu G.L. Effect of Sevoflurane Anesthesia on Brain Is Mediated by lncRNA HOTAIR. J. Mol. Neurosci. 2018;64:346–351. doi: 10.1007/s12031-018-1029-y. [DOI] [PubMed] [Google Scholar]
- 55.Wu Z., Wu P., Zuo X., Yu N., Qin Y., Xu Q., He S., Cen B., Liao W., Ji A. LncRNA-N1LR Enhances Neuroprotection Against Ischemic Stroke Probably by Inhibiting p53 Phosphorylation. Mol. Neurobiol. 2017;54:7670–7685. doi: 10.1007/s12035-016-0246-z. [DOI] [PubMed] [Google Scholar]
- 56.Hou X.X., Cheng H. Long non-coding RNA RMST silencing protects against middle cerebral artery occlusion (MCAO)-induced ischemic stroke. Biochem. Biophys. Res. Commun. 2018;495:2602–2608. doi: 10.1016/j.bbrc.2017.12.087. [DOI] [PubMed] [Google Scholar]
- 57.Tajiri N., Acosta S.A., Shahaduzzaman M., Ishikawa H., Shinozuka K., Pabon M., Hernandez-Ontiveros D., Kim D.W., Metcalf C., Staples M. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J. Neurosci. 2014;34:313–326. doi: 10.1523/JNEUROSCI.2425-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood R.L., Rutterford N.A. Demographic and cognitive predictors of long-term psychosocial outcome following traumatic brain injury. J. Int. Neuropsychol. Soc. 2006;12:350–358. doi: 10.1017/s1355617706060498. [DOI] [PubMed] [Google Scholar]
- 59.Shin S.S., Dixon C.E. Alterations in Cholinergic Pathways and Therapeutic Strategies Targeting Cholinergic System after Traumatic Brain Injury. J. Neurotrauma. 2015;32:1429–1440. doi: 10.1089/neu.2014.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardenas D.D., McLean A., Jr., Farrell-Roberts L., Baker L., Brooke M., Haselkorn J. Oral physostigmine and impaired memory in adults with brain injury. Brain Inj. 1994;8:579–587. doi: 10.3109/02699059409151010. [DOI] [PubMed] [Google Scholar]
- 61.McLean A., Jr., Stanton K.M., Cardenas D.D., Bergerud D.B. Memory training combined with the use of oral physostigmine. Brain Inj. 1987;1:145–159. doi: 10.3109/02699058709034453. [DOI] [PubMed] [Google Scholar]
- 62.Benedictus M.R., Spikman J.M., van der Naalt J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch. Phys. Med. Rehabil. 2010;91:1436–1441. doi: 10.1016/j.apmr.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 63.Patel N.A., Moss L.D., Lee J.Y., Tajiri N., Acosta S., Hudson C., Parag S., Cooper D.R., Borlongan C.V., Bickford P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflammation. 2018;15:204. doi: 10.1186/s12974-018-1240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta S.L., Kim T., Vemuganti R. Long Noncoding RNA FosDT Promotes Ischemic Brain Injury by Interacting with REST-Associated Chromatin-Modifying Proteins. J. Neurosci. 2015;35:16443–16449. doi: 10.1523/JNEUROSCI.2943-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z., Ji C., Wu L., Qiu J., Li Q., Shao Z., Chen G. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS ONE. 2014;9:e97685. doi: 10.1371/journal.pone.0097685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu H., Jiang H., Lu D., Qu C., Xiong Y., Zhou D., Chopp M., Mahmood A. Induction of angiogenesis and modulation of vascular endothelial growth factor receptor-2 by simvastatin after traumatic brain injury. Neurosurgery. 2011;68:1363–1371, discussion 1371. doi: 10.1227/NEU.0b013e31820c06b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salehi A., Zhang J.H., Obenaus A. Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 2017;37:2320–2339. doi: 10.1177/0271678X17701460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han Y., Seyfried D., Meng Y., Yang D., Schultz L., Chopp M., Seyfried D. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2018;131:1–11. doi: 10.3171/2018.2.JNS171475. [DOI] [PubMed] [Google Scholar]
- 69.Wang Z., Wang R., Wang K., Liu X. Upregulated long noncoding RNA Snhg1 promotes the angiogenesis of brain microvascular endothelial cells after oxygen-glucose deprivation treatment by targeting miR-199a. Can. J. Physiol. Pharmacol. 2018;96:909–915. doi: 10.1139/cjpp-2018-0107. [DOI] [PubMed] [Google Scholar]
- 70.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassan M., Watari H., AbuAlmaaty A., Ohba Y., Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Grosse J., Warnke E., Wehland M., Pietsch J., Pohl F., Wise P., Magnusson N.E., Eilles C., Grimm D. Mechanisms of apoptosis in irradiated and sunitinib-treated follicular thyroid cancer cells. Apoptosis. 2014;19:480–490. doi: 10.1007/s10495-013-0937-0. [DOI] [PubMed] [Google Scholar]
- 73.Hiebert J.B., Shen Q., Thimmesch A.R., Pierce J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015;350:132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 74.Liang Z., Chi Y.J., Lin G.Q., Xiao L.F., Su G.L., Yang L.M. LncRNA MEG3 participates in neuronal cell injury induced by subarachnoid hemorrhage via inhibiting the Pi3k/Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2824–2831. doi: 10.26355/eurrev_201805_14983. [DOI] [PubMed] [Google Scholar]
- 75.Xu L., Zhang Z., Xie T., Zhang X., Dai T. Inhibition of BDNF-AS Provides Neuroprotection for Retinal Ganglion Cells against Ischemic Injury. PLoS ONE. 2016;11:e0164941. doi: 10.1371/journal.pone.0164941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 77.Ghirnikar R.S., Lee Y.L., Eng L.F. Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem. Res. 1998;23:329–340. doi: 10.1023/a:1022453332560. [DOI] [PubMed] [Google Scholar]
- 78.Sorby-Adams A.J., Marcoionni A.M., Dempsey E.R., Woenig J.A., Turner R.J. The Role of Neurogenic Inflammation in Blood-Brain Barrier Disruption and Development of Cerebral Oedema Following Acute Central Nervous System (CNS) Injury. Int. J. Mol. Sci. 2017;18:18. doi: 10.3390/ijms18081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merrill J.E., Benveniste E.N. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 80.Corrigan F., Mander K.A., Leonard A.V., Vink R. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflammation. 2016;13:264. doi: 10.1186/s12974-016-0738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X., Tang X., Liu K., Hamblin M.H., Yin K.J. Long Noncoding RNA Malat1 Regulates Cerebrovascular Pathologies in Ischemic Stroke. J. Neurosci. 2017;37:1797–1806. doi: 10.1523/JNEUROSCI.3389-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong J., Jiang L., Huang Z., Zhang H., Cheng C., Liu H., He J., Wu J., Darwazeh R., Wu Y., Sun X. The long non-coding RNA Neat1 is an important mediator of the therapeutic effect of bexarotene on traumatic brain injury in mice. Brain Behav. Immun. 2017;65:183–194. doi: 10.1016/j.bbi.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogata M., Hino S., Saito A., Morikawa K., Kondo S., Kanemoto S., Murakami T., Taniguchi M., Tanii I., Yoshinaga K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Byun S., Lee E., Lee K.W. Therapeutic Implications of Autophagy Inducers in Immunological Disorders, Infection, and Cancer. Int. J. Mol. Sci. 2017;18:18. doi: 10.3390/ijms18091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lipinski M.M., Wu J., Faden A.I., Sarkar C. Function and Mechanisms of Autophagy in Brain and Spinal Cord Trauma. Antioxid. Redox Signal. 2015;23:565–577. doi: 10.1089/ars.2015.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo C.L., Li B.X., Li Q.Q., Chen X.P., Sun Y.X., Bao H.J., Dai D.K., Shen Y.W., Xu H.F., Ni H. Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. doi: 10.1016/j.neuroscience.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z., Shi X.Y., Yin J., Zuo G., Zhang J., Chen G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J. Mol. Neurosci. 2012;46:192–202. doi: 10.1007/s12031-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 89.Guo D., Ma J., Yan L., Li T., Li Z., Han X., Shui S. Down-Regulation of Lncrna MALAT1 Attenuates Neuronal Cell Death Through Suppressing Beclin1-Dependent Autophagy by Regulating Mir-30a in Cerebral Ischemic Stroke. Cell. Physiol. Biochem. 2017;43:182–194. doi: 10.1159/000480337. [DOI] [PubMed] [Google Scholar]
- 90.Blanchette M., Daneman R. Formation and maintenance of the BBB. Mech. Dev. 2015;138:8–16. doi: 10.1016/j.mod.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Jia W., Lu R., Martin T.A., Jiang W.G. The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review) Mol. Med. Rep. 2014;9:779–785. doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 92.Petty M.A., Lo E.H. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog. Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 93.DeWitt D.S., Prough D.S. Traumatic cerebral vascular injury: the effects of concussive brain injury on the cerebral vasculature. J. Neurotrauma. 2003;20:795–825. doi: 10.1089/089771503322385755. [DOI] [PubMed] [Google Scholar]
- 94.Oby E., Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 95.Lyeth B.G., Liu S., Hamm R.J. Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res. 1993;617:69–75. doi: 10.1016/0006-8993(93)90614-s. [DOI] [PubMed] [Google Scholar]
- 96.Han L., Dong Z., Liu N., Xie F., Wang N. Maternally Expressed Gene 3 (MEG3) Enhances PC12 Cell Hypoxia Injury by Targeting MiR-147. Cell. Physiol. Biochem. 2017;43:2457–2469. doi: 10.1159/000484452. [DOI] [PubMed] [Google Scholar]
- 97.Strub G.M., Perkins J.A. MicroRNAs for the pediatric otolaryngologist. Int. J. Pediatr. Otorhinolaryngol. 2018;112:195–207. doi: 10.1016/j.ijporl.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 98.Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jalali S., Bhartiya D., Lalwani M.K., Sivasubbu S., Scaria V. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS ONE. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoon J.H., Abdelmohsen K., Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin. Cell Dev. Biol. 2014;34:9–14. doi: 10.1016/j.semcdb.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Igaz P., Igaz I., Nagy Z., Nyírő G., Szabó P.M., Falus A., Patócs A., Rácz K. MicroRNAs in adrenal tumors: relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2015;72:417–428. doi: 10.1007/s00018-014-1752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paneru B., Ali A., Al-Tobasei R., Kenney B., Salem M. Crosstalk among lncRNAs, microRNAs and mRNAs in the muscle ‘degradome’ of rainbow trout. Sci. Rep. 2018;8:8416. doi: 10.1038/s41598-018-26753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li D.S., Ainiwaer J.L., Sheyhiding I., Zhang Z., Zhang L.W. Identification of key long non-coding RNAs as competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2285–2295. [PubMed] [Google Scholar]
- 104.Paraskevopoulou M.D., Hatzigeorgiou A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 105.Kopitar-Jerala N. Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front. Mol. Neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Djordjevic J., Sabbir M.G., Albensi B.C. Traumatic Brain Injury as a Risk Factor for Alzheimer’s Disease: Is Inflammatory Signaling a Key Player? Curr. Alzheimer Res. 2016;13:730–738. doi: 10.2174/1567205013666160222110320. [DOI] [PubMed] [Google Scholar]
- 107.Shih R.H., Wang C.Y., Yang C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen F., Castranova V., Shi X., Demers L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 109.Jia J., Zhang M., Li Q., Zhou Q., Jiang Y. Long noncoding ribonucleic acid NKILA induces the endoplasmic reticulum stress/autophagy pathway and inhibits the nuclear factor-k-gene binding pathway in rats after intracerebral hemorrhage. J. Cell. Physiol. 2018;233:8839–8849. doi: 10.1002/jcp.26798. [DOI] [PubMed] [Google Scholar]
- 110.Liu B., Sun L., Liu Q., Gong C., Yao Y., Lv X., Lin L., Yao H., Su F., Li D. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Mao X., Su Z., Mookhtiar A.K. Long non-coding RNA: a versatile regulator of the nuclear factor-κB signalling circuit. Immunology. 2017;150:379–388. doi: 10.1111/imm.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsuda S., Nakagawa Y., Tsuji A., Kitagishi Y., Nakanishi A., Murai T. Implications of PI3K/AKT/PTEN Signaling on Superoxide Dismutases Expression and in the Pathogenesis of Alzheimer’s Disease. Diseases. 2018;6:6. doi: 10.3390/diseases6020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Keppler-Noreuil K.M., Parker V.E., Darling T.N., Martinez-Agosto J.A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. C. Semin. Med. Genet. 2016;172:402–421. doi: 10.1002/ajmg.c.31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weinberg M.A. RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anticancer Drugs. 2016;27:475–487. doi: 10.1097/CAD.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jha S.K., Jha N.K., Kar R., Ambasta R.K., Kumar P. p38 MAPK and PI3K/AKT Signalling Cascades inParkinson’s Disease. Int. J. Mol. Cell. Med. 2015;4:67–86. [PMC free article] [PubMed] [Google Scholar]
- 116.Endo H., Nito C., Kamada H., Yu F., Chan P.H. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–2146. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- 117.Xin J.W., Jiang Y.G. Long noncoding RNA MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and reoxygenation in human brain microvascular endothelial cells. Exp. Ther. Med. 2017;13:1225–1234. doi: 10.3892/etm.2017.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo C., Song W.Q., Sun P., Jin L., Dai H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015;22:100. doi: 10.1186/s12929-015-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xin X., Wu M., Meng Q., Wang C., Lu Y., Yang Y., Li X., Zheng Q., Pu H., Gui X. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol. Cancer. 2018;17:94. doi: 10.1186/s12943-018-0843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xia T., Chen S., Jiang Z., Shao Y., Jiang X., Li P., Xiao B., Guo J. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci. Rep. 2015;5:13445. doi: 10.1038/srep13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Back S.A. White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol. 2017;134:331–349. doi: 10.1007/s00401-017-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alberi L., Hoey S.E., Brai E., Scotti A.L., Marathe S. Notch signaling in the brain: in good and bad times. Ageing Res. Rev. 2013;12:801–814. doi: 10.1016/j.arr.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 123.Sawada M., Sawamoto K. Mechanisms of neurogenesis in the normal and injured adult brain. Keio J. Med. 2013;62:13–28. doi: 10.2302/kjm.2012-0005-re. [DOI] [PubMed] [Google Scholar]
- 124.Jung C., Mittler G., Oswald F., Borggrefe T. RNA helicase Ddx5 and the noncoding RNA SRA act as coactivators in the Notch signaling pathway. Biochim. Biophys. Acta. 2013;1833:1180–1189. doi: 10.1016/j.bbamcr.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 125.Hossain F., Majumder S., Ucar D.A., Rodriguez P.C., Golde T.E., Minter L.M., Osborne B.A., Miele L. Notch Signaling in Myeloid Cells as a Regulator of Tumor Immune Responses. Front. Immunol. 2018;9:1288. doi: 10.3389/fimmu.2018.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zanotti S., Canalis E. Notch Signaling and the Skeleton. Endocr. Rev. 2016;37:223–253. doi: 10.1210/er.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hong L.Z., Zhao X.Y., Zhang H.L. p53-mediated neuronal cell death in ischemic brain injury. Neurosci. Bull. 2010;26:232–240. doi: 10.1007/s12264-010-1111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vaseva A.V., Moll U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patodia S., Raivich G. Role of transcription factors in peripheral nerve regeneration. Front. Mol. Neurosci. 2012;5:8. doi: 10.3389/fnmol.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tedeschi A., Di Giovanni S. The non-apoptotic role of p53 in neuronal biology: enlightening the dark side of the moon. EMBO Rep. 2009;10:576–583. doi: 10.1038/embor.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pun P.B., Lu J., Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic. Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- 132.Hanafy K.A., Selim M.H. Antioxidant strategies in neurocritical care. Neurotherapeutics. 2012;9:44–55. doi: 10.1007/s13311-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li P., Stetler R.A., Leak R.K., Shi Y., Li Y., Yu W., Bennett M.V.L., Chen J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134(Pt B):208–217. doi: 10.1016/j.neuropharm.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodríguez-Rodríguez A., Egea-Guerrero J.J., Murillo-Cabezas F., Carrillo-Vico A. Oxidative stress in traumatic brain injury. Curr. Med. Chem. 2014;21:1201–1211. doi: 10.2174/0929867321666131217153310. [DOI] [PubMed] [Google Scholar]
- 135.Ma M.W., Wang J., Dhandapani K.M., Wang R., Brann D.W. NADPH oxidases in traumatic brain injury - Promising therapeutic targets? Redox Biol. 2018;16:285–293. doi: 10.1016/j.redox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang D., Lee H., Haspel J.A., Jin Y. Long noncoding RNA FOXD3-AS1 regulates oxidative stress-induced apoptosis via sponging microRNA-150. FASEB J. 2017;31:4472–4481. doi: 10.1096/fj.201700091R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gao M., Li C., Xu M., Liu Y., Cong M., Liu S. LncRNA MT1DP Aggravates Cadmium-Induced Oxidative Stress by Repressing the Function of Nrf2 and is Dependent on Interaction with miR-365. Adv. Sci. (Weinh.) 2018;5:1800087. doi: 10.1002/advs.201800087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen W., Zhang L., Zhou Z.Q., Ren Y.Q., Sun L.N., Man Y.L., Ma Z.W., Wang Z.K. Effects of Long Non-Coding RNA LINC00963 on Renal Interstitial Fibrosis and Oxidative Stress of Rats with Chronic Renal Failure via the Foxo Signaling Pathway. Cell. Physiol. Biochem. 2018;46:815–828. doi: 10.1159/000488739. [DOI] [PubMed] [Google Scholar]
- 139.Khatri N., Thakur M., Pareek V., Kumar S., Sharma S., Datusalia A.K. Oxidative stress: Major threat in traumatic brain injury. CNS Neurol. Disord. Drug Targets. 2018;17:689–695. doi: 10.2174/1871527317666180627120501. [DOI] [PubMed] [Google Scholar]
- 140.Kochanek P.M., Jackson T.C., Ferguson N.M., Carlson S.W., Simon D.W., Brockman E.C., Ji J., Bayır H., Poloyac S.M., Wagner A.K. Emerging therapies in traumatic brain injury. Semin. Neurol. 2015;35:83–100. doi: 10.1055/s-0035-1544237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin C., Londono I., Mallard C., Lodygensky G.A. New means to assess neonatal inflammatory brain injury. J. Neuroinflammation. 2015;12:180. doi: 10.1186/s12974-015-0397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sompol P., Norris C.M. Ca2+, Astrocyte Activation and Calcineurin/NFAT Signaling in Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2018;10:199. doi: 10.3389/fnagi.2018.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Movsas T.Z., Weiner R.L., Greenberg M.B., Holtzman D.M., Galindo R. Pretreatment with Human Chorionic Gonadotropin Protects the Neonatal Brain against the Effects of Hypoxic-Ischemic Injury. Front Pediatr. 2017;5:232. doi: 10.3389/fped.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang H., Zhang X., Zhang T., Chen L. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin. Chem. 2001;47:1458–1462. [PubMed] [Google Scholar]
- 145.Baker A.J., Moulton R.J., MacMillan V.H., Shedden P.M. Excitatory amino acids in cerebrospinal fluid following traumatic brain injury in humans. J. Neurosurg. 1993;79:369–372. doi: 10.3171/jns.1993.79.3.0369. [DOI] [PubMed] [Google Scholar]
- 146.Guo C.C., Jiao C.H., Gao Z.M. Silencing of LncRNA BDNF-AS attenuates Aβ25-35-induced neurotoxicity in PC12 cells by suppressing cell apoptosis and oxidative stress. Neurol. Res. 2018;40:795–804. doi: 10.1080/01616412.2018.1480921. [DOI] [PubMed] [Google Scholar]
- 147.Lu G., Xu H., Zhao W., Zhang J., Rao D., Xu S. Upregulation of long noncoding RNA Gadd45a is associated with sevoflurane-induced neurotoxicity in rat neural stem cells. Neuroreport. 2018;29:605–614. doi: 10.1097/WNR.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y., Lian Y.J., Ma Y.Q., Wu C.J., Zheng Y.K., Xie N.C. LncRNA SNHG1 promotes alpha-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology. 2017;68:212–221. doi: 10.1016/j.neuro.2017.12.001. [DOI] [PubMed] [Google Scholar]