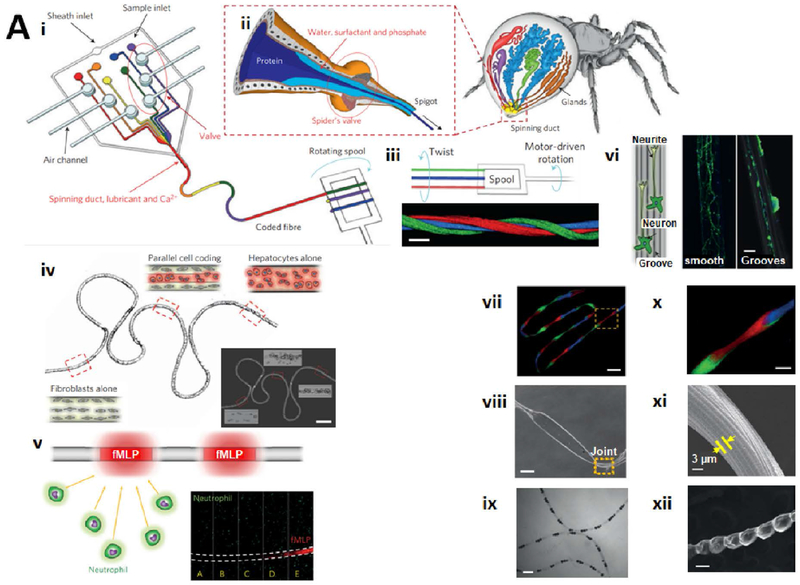
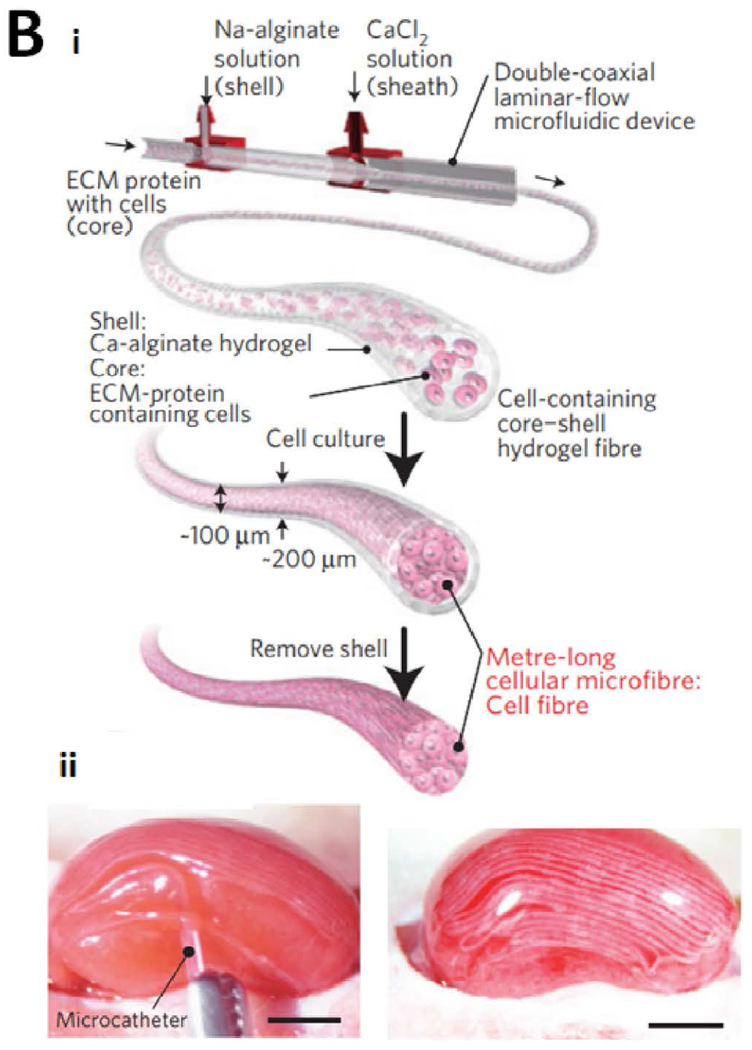
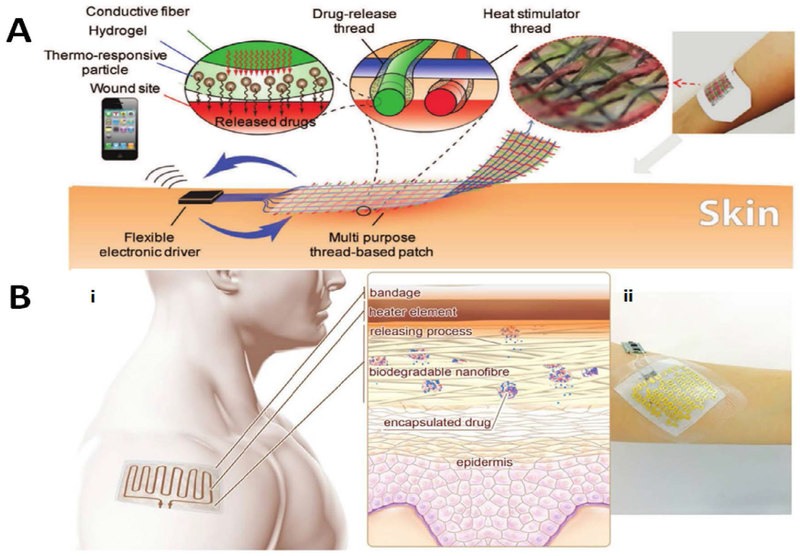
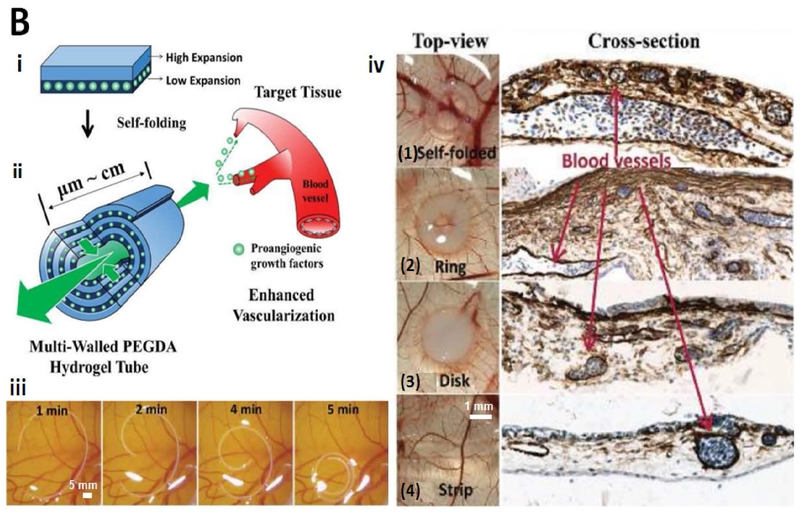
Abstract
Advances in biomaterial synthesis and fabrication, stem cell biology, bioimaging, microsurgery procedures, and microscale technologies have made minimally invasive therapeutics a viable tool in regenerative medicine. Therapeutics, herein defined as cells, biomaterials, biomolecules, and their combinations, can be delivered in a minimally invasive way to regenerate different tissues in the body, such as bone, cartilage, pancreas, cardiac, skeletal muscle, liver, skin, and neural tissues. Sophisticated methods of tracking, sensing, and stimulation of therapeutics in vivo using nanobiomaterials and soft bioelectronic devices provide great opportunities to further develop minimally invasive and regenerative therapeutics (MIRET). In general, minimally invasive delivery methods offer high yield with low risk of complications and reduced costs compared to conventional delivery methods. Here, we review minimally invasive approaches for delivering regenerative therapeutics into the body. The use of MIRET to treat different tissues and organs is described. Although some clinical trials have been done using MIRET, it is hoped that such therapeutics find wider applications to treat patients. Finally, we highlight some future perspective and challenges for this emerging field.
Keywords: Biomaterials, Biomolecules, Delivery, Minimally invasive, Scaffolds, Tissue regeneration
1. Introduction
The world’s aging population is growing at a high rate. Currently, people aged 65 and older represent 8.5% of the world’s population and this is expected to reach 17% by 2050 [1]. In the US alone, the number of individuals older than 65 is estimated to be 83.7 million by 2050. As the people’s age goes up, most of their organs naturally undergo reduced function or deterioration. In addition, different diseases or accidents can affect people’s life leading to tissue loss and organ failure. Different therapies have been proposed to address these problems with marginal success. For instance, the use of pharmacological therapies and drugs have not been efficient to treat some diseases, such as Alzheimer’s disease (AD) and ischemic heart conditions. Surgical treatment of vertebral fractures and use of bone cements have been associated with limited success. Joint replacement for osteoarthritis has been adopted; however, this treatment still suffers from complications. Organ transplantation has also been utilized to replace organs in end-stage failure; however, there is a shortage of organs available for the transplantation. In addition, immunosuppression is needed to prevent organ rejection, which is associated with complications, such as the development of infections and malignancies. Therefore, new therapeutic solutions are required to tackle these problems.
Different regenerative therapeutics have been explored to address the challenges associated with age-related, disease, and shortage conditions of tissues and organs (Figure 1). These regenerative therapeutics are based on using biomaterials, cells, bioactive molecules, or their combinations. However, invasive implantation of these therapeutics in the body is associated with various problems and risks. Minimally invasive procedures provide advantages for the delivery of regenerative therapeutics, such as reduced trauma, fast recovery, and reduced risk of complications. The combination of minimally invasive procedures and regenerative therapeutics has recently been introduced for treatment of different diseases, such as myocardial infarction (MI) [2], joint problems [3], and intervertebral disc (IVD) injuries [4]. The administration of therapeutics can be done using natural orifices, natural passages (e.g., intravascular), natural spaces (e.g., intraperitoneal), or tissue-specific routes (e.g., intrathecal, intraventricular, and intra-articular) (Figure 2). Minimally invasive and regenerative therapeutics (MIRET) can also be equipped with image-guided delivery, intra-operative monitoring, and post-treatment tracking of cells or biomaterials. Taken together, the field of MIRET is emerging as a potentially powerful therapeutics modality.
Figure 1. Applications of minimally invasive therapeutics to regenerate different parts of the body.
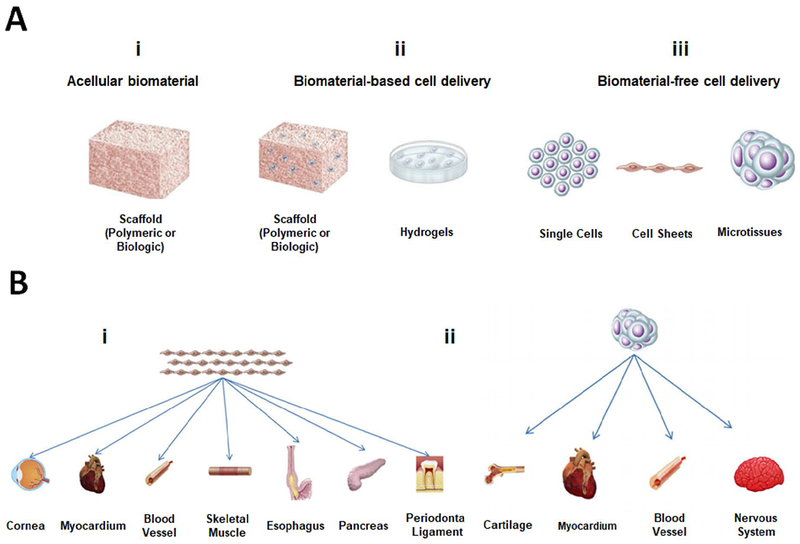
(A) Different MIRET including (i) acellular biomaterials, (ii) biomaterial-based cell delivery, and (iii) biomaterial-free cell delivery. (B) Biomaterial-free cell delivery approaches to regenerate different parts of the body. A and B are reproduced with permission from [376].
Figure 2. Minimally invasive approaches to deliver regenerative therapeutics into the (i) brain, (ii) heart, (iii) liver, (iv) IVD, and (v) knee joint.
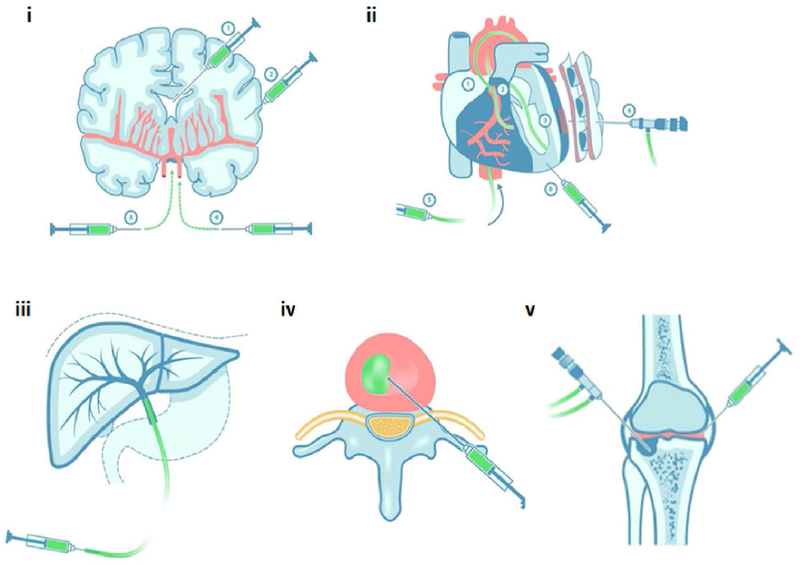
Minimally invasive and regenerative therapy can be achieved using direct injection, endoscope, or catheter. Redrawn and modified from [377].
In this review, we describe minimally invasive procedures for the delivery of regenerative therapeutics. Following that, applications of MIRET to treat different tissues and organs are discussed. Finally, challenges and future perspectives to further develop materials and technologies used in minimally invasive therapeutics are described.
2. Technologies and materials in delivering and monitoring regenerative therapeutics
2.1. Needles
Needles have been utilized as medical tools for centuries. Needles are commonly used with a syringe to deliver a substance to the body through a sharp tip in a minimally invasive manner. Needles have been used for the delivery of various therapeutic agents including cells [5], genes [6], proteins [7], and peptides [8]. Cell delivery through needles at clinically relevant injection rates may cause cell damage because of cell membrane disruption [9]. Therefore, cell delivery using needles should be optimized to protect cells during the injection. Small-diameter needles apply destructive shear stress on cells. High pressure gradient during the injection and several shear and normal forces [10] may also injure cells. The pressures dropped from 178.65±12.16 kPa for Newtonian fluids (e.g., phosphate buffered saline (PBS)) to 406.72±19.66 kPa for viscoelastic gels [11]. On the other hand, cells in large-diameter needles may sediment after a while and this affects cell viability. Needles with a size of 16–22 G are generally considered suitable for cell delivery [12]. It was shown that curved needles featuring small-to-large gradient in length prevented the backflow and enhanced cell retention at the site of injection by 3-fold when they were used in the endocardial delivery system [13].
Needles have also been utilized to deliver cell-laden biomaterials into the body. Cells encapsulated in hydrogels showed higher viability after injection via needles compared to bare cells due to protective role of hydrogels as shown for different cells in 1 wt% alginate [14]. For injectable cell-containing biomaterials, shear-thinning behavior of biomaterials is crucial to maintain cell viability and function after passing through needles [11, 15]. Shear-thinning of a material is defined as the material’s ability to decrease its viscosity with increasing shear [16]. Biomaterials that are shear-thinning are desirable as injectable cell-laden biomaterials because these materials can be extruded upon shear application in needles and then rapidly recover their original strength upon cessation of mechanical load [17]. High viscosity of cell-laden biomaterials and improper fluid pumping through needles can cause needle clogging and uneven injection flow. The concentration of cell suspension and its volume are also important determinants of achieving optimal flow through needles [17]. Electrostatic interaction between needle and cell-laden biomaterial together with surface roughness of needles can induce cell adhesion to the needle. One attempt to solve these hurdles includes the use of protein coating on the internal needle surface [18].
2.2. Catheters
Catheters are thin and relatively flexible tubes inserted into a cavity, duct, or vessel in the body to drain the body’s fluids or to deliver therapeutic agents [19]. The word “catheter” has an ancient Greek origin “kathiénai”, which means “to send down” or “to thrust into” [20]. Catheters have been used over centuries. The invention of rubbers made a significant development in using catheters. The first Foley catheter made from latex was introduced in 1930s [21] and since then it has widely been utilized in clinical settings. Today, catheters are mostly made of silicon, polyurethane, polyethylene, and thermoplastic elastomers based on their applications [22]. An ideal material for making catheters should have high resistance to kinking, good structural integrity, suitable rigidity for easy insertion, low bacterial adhesion, low thrombogenicity, biocompatibility, long-term stability, minimal irritation, and inertness to therapeutics. Catheters represent an important tool in minimally invasive delivery procedures allowing quick recovery time after catheterization. They have widely been used for the delivery of stem cells (SCs) and hydrogel-based regenerative therapeutics [23].
Catheters with the aid of sensing technologies can be used for real-time sensing and monitoring of flow, temperature, pressure, and biomolecules in the body [24]. For example, Li et al. [25] fabricated flexible biosensors mounted on catheters (2 to 5 French) for monitoring the cardiovascular system . The flexible biosensors were easily rolled up around the catheters for potential clinical applications. Further integration of soft electronics and biorobotics into catheters for their remote control and maintenance would be helpful for successful use of catheters in delivering regenerative therapeutics. With the aid of imaging techniques (e.g., ultrasound), catheters can also be equipped for real-time imaging of the body. This capability allows guiding catheters precisely to the target site. For example, Nissen et al. [26] fabricated catheters (diameter 1.83 mm) capable of ultrasound imaging of blood vessels . The catheters were able to measure vessels with diameters higher than 5.7 mm and cross-sectional areas more than 29.6 mm2. However, the navigation of catheters through complex routes and curvatures remains a limiting factor and warrants further development in the catheter design and fabrication. Moreover, the most important issue associated with the using catheters is infection. The use of antimicrobial-impregnated catheters is an effective method in preventing the infection [27]. However, these catheters are still at early stages of development.
2.3. Microrobot devices
Advances in robotics have revolutionized the ability of humans to interact with and manipulate the surroundings [28]. Robotic technologies have been utilized in medicine and significantly improved the healthcare. In particular, microrobots have found many biomedical applications particularly in minimally invasive diagnostic and therapeutic procedures [29]. Microrobots are in submillimeter sizes and they can be run using small-scale and off-board powering methods, such as bacterial actuators [30], piezoelectric actuators [31], and swimming tail actuators [32].
Microrobots can be employed for localized delivery of biological and chemical substances in a minimally invasive manner. Microrobots can also deliver various forms of energy, such as radiation and heat at target sites in the body. Therefore, potential applications of microrobots would be in targeted drug/gene delivery or in situ cancer treatment [33]. Information from specific locations in the body can also be obtained and transduced using microrobots in different ways, such as visible light, ultrasound, and radio [34].
Microrobots have shown a great promise to serve as smart cell/biomaterial delivery vehicles. For example, Kim et al. [35] fabricated magnetically manipulated microrobots using lithography and used them to transport human embryonic kidney cells. Srivastava et al. [36] introduced a dual-action microrobot called medibot capable of performing single-cell surgery and drug delivery in situ. Camptothecin was used as the drug model to deliver to HeLa cells and the drug-loaded cells were tracked over three days of culture. Microrobots were utilized to precisely destruct cancer cells among living cells. Recently, biodegradable microrobots have been fabricated allowing safe removal of microrobots from the body [37]. However, there are still some challenges regarding broad use of microrobots in minimally invasive delivery of therapeutics. The size of developed microrobots is still large to access major parts of the body through the vascular network [38]. In addition, mechanical parts of microrobots are rigid and may harm cells and tissues in the body. It is hoped that the integration of advanced biomaterials, nanoscale technologies, and robotic science would lead to the development of advanced and highly capable microrobots for minimally invasive delivery of regenerative therapeutics [39].
2.4. Nanoparticles
As discussed by the National Research Council, image guidance has remarkably improved the targeting and monitoring of MIRET [40]. Nanoparticles (NPs) can be used as carriers or imaging probes for regenerative therapeutics [41, 42]. In particular, magnetic NPs (MNPs) combined with therapeutics can be guided to the target site using magnetic forces [42]. Magnetic resonance imaging (MRI) with the aid of MNPs can provide imaging guidance for minimally invasive procedures [40]. MRI provides great advantages for monitoring, guiding, and controlling minimally invasive and therapeutic interventions, which include excellent tissue discrimination, high indication of nerves and blood vessels, submillimeter resolution, multiplanar and three-dimensional (3D) image acquisition [40]. For example, Chertok et al. [43] used iron oxide NPs (12 mg Fe/kg) for minimally invasive delivery of drugs to brain tumors (i.e., orthotopic 9L-gliosarcomas). MRI (magnetic field density of 0.4 T for 30 min) was utilized for non-invasive monitoring of the NPs. The magnetic NPs were administered intravenously, which avoided the potential toxicity of NPs due to their accumulation. In another study, polyethylene glycol (PEG) was functionalized using iron oxide NP-reduced graphene oxide and used for image-guided photothermal therapy of tumors in a mouse model over seven days [44]. This nanocomposite material provided strong supermagnetic property and high physiological stability. An MRI-based trajectory guide system also enabled the delivery of viral vectors in gene therapy [45]. MRI has also been upgraded to an intraoperative and real-time (RT)-MRI targeting-guided delivery system [46]. This system provided valuable guidance, accurate targeting, monitoring, and visualization during an intracerebral cell delivery [46] (Figure 3A).
Figure 3. Minimally invasive and remotely controllable delivery of drugs to the brain.
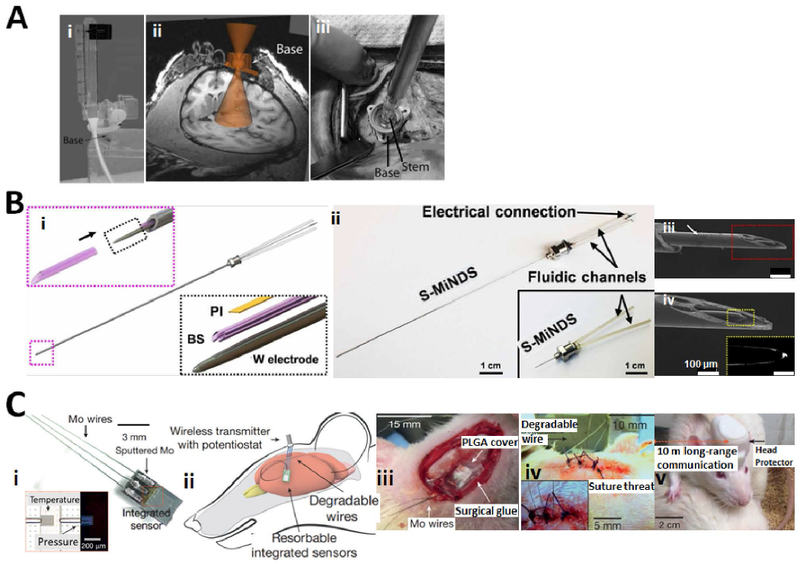
(A) (i) Photograph of the device base with remote introducer and guiding insert. (ii) Placement of the base on the skull was guided by a cone of projection using MRI. (iii) Surgical demonstration of device application on the primate’s skull using laser calibration rod inserted in the stem. Three self-tapping screws were used to secure the base in the place. Reproduced with permission from [46]. (B) (i) Main components of miniaturized drug delivery system (S-MiNDS) with enlarged components (tungsten (W) electrode and polyimide (PI) template) and borosilicate (BS) aligner tip that aligns with the key device components following arrow direction. (ii) S-MiNDS images with the fluidic channels and electrical connection. (iii, iv) Scanning electron microscopy (SEM) images of the tip of the device including magnified image of BS aligner tip in red rectangular and W electrode in the yellow rectangular. Reproduced with permission from [190]. (C) (i) Bioresorbable sensors integrated with dissolvable metal connects and biodegradable wires. Inset: optical micrograph of serpentine silicon nano-membrane of the sensing parts. Temperature sensor is not on the air cavity while the pressure sensor is on the edge of air cavity. (ii) Biodegradable sensors intracranially implanted in rat and connected to an external wireless data-transmission unit. (iii) Demonstration of an implanted bioresorbable sensor in the rat. A thin film of PLGA with 80-μm thickness and a dissolvable surgical glue were used to seal the craniectomy. (iv) A sutured mouse and (v) a freely moving mouse with implanted biosensor. Reproduced with permission from [362].
SC labeling and tracking can be performed using MRI with supermagnetic iron oxide NPs (SMIONs) [47], optical imaging with gold NPs [48], fluorescence imaging with quantum dots [49], polymeric NPs [50], and silica NPs [51]. These NPs with magnetic or optical properties enable real-time tracking of intracellular processes. Although these NPs have not been approved for SC tracking in clinical settings, advances in nanotechnology, bioimaging, and chemical functionalization would bring the molecular and cellular imaging closer to the clinic. Direct SC labeling can be accomplished for early SC detection with fluorescence, MRI, single photon emission computed tomography (SPECT), and position emission tomography (PET). Indirect SC labeling approaches include the use of reporter gene imaging with the aid of fluorescence, bioluminescence, SPECT, PET, and MRI imaging [52]. Near-infrared (NIR) fluorescence imaging is another technology for cell tracking by the aid of IR fluorescent protein/gene labeling [53]. The latter technique enabled the detection of injected labeled mesenchymal SCs (MSCs) in mice [54]. Current limitations of these imaging technologies are stable labeling, in vivo biocompatibility, and long-term tracking performance. However, these technologies have provided great opportunities for further development of MIRET.
2.5. Electrosprayed and electrospun materials
Electrospraying, also known as electrohydrodynamic spraying is a technique in which an electrical force is used to atomize a liquid, producing highly-charged, non-aggregating small droplets [55]. The size of droplets may be as low as several nanometers. This technique has been used to produce a range of nanomaterials, including nanoparticles [56], nano-thin films, and MEMS (Microelectromechanical Systems). The applications of biomaterials produced through electrospraying encompass drug delivery [57], biofunctional films and patches [58], and the functionalization of biomedical scaffolds [59]. Another method to produce biomaterials for minimally invasive therapeutics is electrospinning in which thin fibers of a desirable polymeric material are produce via applying an electrical force to a viscous polymeric solution/melt under flow [60]. Electrospinning has found a wide range of applications in developing scaffolds for tissue engineering [61] and drug release [62], fibrous dressings, and medical textiles [63] [64] [65].
2.6. Implantable and degradable microfluidic devices
The administration of a certain chemical compound to human body for therapeutic purposes is achieved mainly by using conventional drug delivery preparations such as oral administration, inhalation or injection. However, these methods have their own limitations. Thus, implantable and degradable microfluidic devices can be very useful for use in minimally invasive drug delivery particularly for controlled profile release of drugs [66]. For example, Jeong et al. introduced ultrathin, flexible optofluidic neural probe systems using polydimethylsiloxane (PDMS)-based microfluidics allowing wireless control of pharmacological delivery and stimulating optically the deep brain of freely moving animals [67]. The PDMS probes could be long-term in vivo durable and biocompatible and potentially applicable for chronic implantation. In addition, degradable and flexible microfluidic probes have been investigated for minimizing tissue damage when implanted. For instance, bio-dissolvable polymers such as poly(lactide-co-glycolide) (PLGA) [68], silk [69], or maltose [70] have been employed for temporally stiffening probes, which eventually will enable minimally invasive and targeted drug delivery to end organs.
The technologies and materials discussed above have greatly helped in delivering and monitoring regenerative therapeutics, which are introduced into the body in a minimally invasive way. In what follows, we divided these therapeutics into three main categories (i.e., cells, biomolecules, and biomaterials) and discussed their applications to regenerate different tissues in the body.
3. Applications of MIRET
3.1. Heart
The heart has a long waiting list in transplantation, which makes it as the third needed organ after the liver and kidney [71]. Heart diseases and failure are a leading cause of morbidity and mortality in the world. MIRET have been used for the treatment of various cardiac conditions including MI, cardiovascular diseases, and heart failure. Different regenerative therapeutics including cells, biomaterials, and their combinations have been used for heart regeneration and repair as described below.
3.1.1. Cells
Cell therapy has been investigated as a promising approach to restore cardiac function in patients with heart diseases or failure. This approach involves the transplantation of cardiac progenitor cells into the heart to enhance the regeneration process [72]. Cardiac progenitor cells can be delivered via transcardial injection, transepicardial infusion, and intracoronary endovascular infusion into the heart [73] (Figure 4). Despite initial enthusiasm with cell therapy products, their therapeutic efficacy in the clinic has been disappointing, mainly due to the loss of implanted cells and lack of adhesion to the host’ tissue. Major meta-analyses have reached contradictory results regarding the efficacy of cell therapy for the heart [74]. However, some clinical trials, such as intracoronary infusion of bone marrow-derived cells resulted in some improvement in the heart function [52]. The latter study included 3000 patients for phase III and suggested the suitability of these cells in decreasing the mortality in the patients.
Figure 4. Dual-antibody-conjugated SMIONs for targeted cell delivery to the injured myocardium.
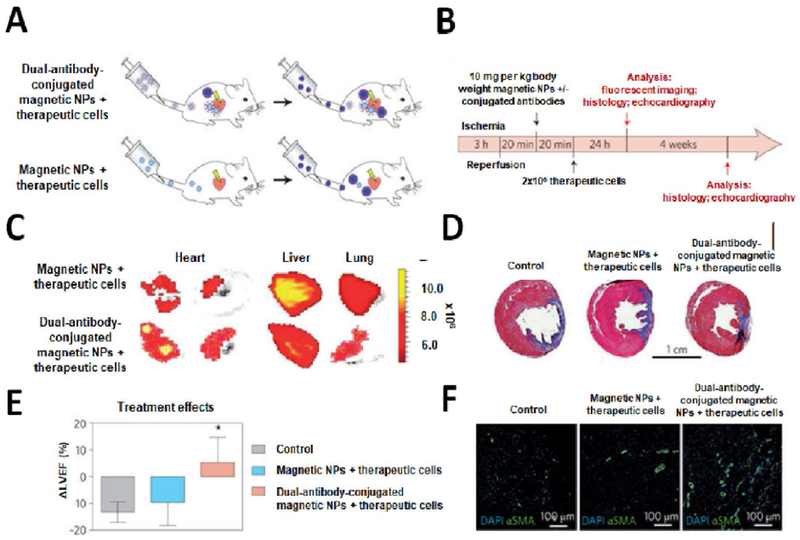
(A, B) Schematics and timeline of the delivery of NPs and cells with and without conjugated antibody. (C) Fluorescent imaging of rat’s organs 24 hrs after cell injection, showing the effect of incorporating antibodies in magnetic NPs on decreasing off-target cell distribution. Rats which received the dual-antibody-conjugated magnetic NPs have more targeted accumulation in their organs compared to those injected with regular magnetic NPs. (D) The whole heart section imaging (trichrome staining) four weeks after reperfusion showed a prominent depletion of scar size (blue) and significant increase in viable (red) tissues in animals, which received dual-antibody-conjugated SMIONs compared to control sample and regular magnetic NPs. (E) Echocardiography results four weeks after reperfusion confirmed that left ventricular ejection fraction in the targeted-NP group was significantly improved compared to the control and regular NPs groups. (F) Confocal microscopic images: NP-targeted cell therapies can greatly improve the angiogenesis compared to the control and regular NP groups. Blue denotes DAPI for cell nuclei and green represents alpha smooth muscle actin (α-SMA) for smooth muscle cells. Reproduced with permission from [378].
Different SCs have been used in cardiac regeneration and repair. Cardiomyogenic potential of adipose tissue-derived SCs (ADSCs) was shown and reviewed elsewhere [75]. ADSCs can be differentiated into cardiac cells and used in cardiac cell therapy. Another type of SCs used in cardiac regeneration is cardiosphere-derived cells (CDCs) that was utilized in one clinical trial to treat ventricular dysfunction [76]. The cells were delivered via catheters to arteries in an infarcted site. The results of six-month follow-up confirmed the safety of the procedure. There was a significant reduction in the scar tissue mass in the CDC-treated group relative to the control group. Furthermore, viable heart mass and tissue contractility were significantly increased. The mass of the scar (inversely related to viable left ventricular mass) in the treated patients was decreased by 8.4 and 12.9 g after 6- and 9-month post-treatment. Achieving such a successful clinical translation may be limited by 1) poor cell engraftment and electromechanical coupling to the native tissue, 2) lack of accurate and safe in vivo cell monitoring, 3) inability to produce patient-specific, mature, and functional cardiomyocytes in large numbers, and 4) risk of arrhythmias. Transplanted cells may also have paracrine effect on tissue regeneration by positively influencing restorative processes in the native tissues, such as neovascularization, myocardial protection, cardiac remodeling, and cell differentiation.
3.1.2. Biomolecules
Cells secret biomolecules for certain activities, such as cellular adhesion, proliferation, and differentiation. These biomolecules have usually short half-life with local function [77]. In particular, biomolecules have been accepted as a regenerative therapy in heart regeneration. For instance, a cocktail of growth factors (basic fibroblast growth factor (bFGF)-2 (2 µg), insulin-like growth factor-1 (IGF-1) (1 µg), hepatocyte growth factor (HGF) (2 µg), and stromal cell-derived factor-1α (0.6 µg)) was administered intraperitoneally to rats with some observed benefits in relation to cardiac function, infarct size, and vascularization [78]. However, new materials and technologies are needed to increase the half-life of growth factors and to preserve their bioactivity. Until then, the routine clinical use of biomolecules is largely hindered [79]. Recently, exosomes (EXs) were used in cardiac repair because of their cardioregenerative characteristic [80]. In particular, the EXs as secreted membrane vesicles provided key functions for intercellular and tissue-level communication. Currently, there is growing evidence from animal studies on the EX potential for the treatment of heart diseases [81]. EXs can potentially be utilized to stimulate the endogenous regenerative process of heart in a minimally invasive manner. For example, cardiac SC-derived EXs were injected into acute MI hearts of rodents and pigs, and reduced scar size and improved cardiac function were observed [82].
Minimally invasive delivery of biomolecules is also applicable for cardiac tissue regeneration in situ. For instance, Hu et al. [83] demonstrated that intramyocardial injection of embryonic transcription factor (T-box 18) in pig models was efficient to convert cardiomyocytes to pacemaker cells. The pacemaker cells were physiologically active indicating successful implantation of the gene therapy approach in vivo. The pacemaker cells were active from day 2 to day 14 post-implantation without using electronic pacemakers. Minimally invasive gene therapy approaches can also be extended to treat cardiovascular diseases [84]. For example, adeno-associated viral vectors have widely been used as a cardiotropic, efficient, and safe tool to genetically treat cardiovascular diseases [85]. In the first clinical trial, these vectors were delivered for nine patients using intracoronary injection in a relatively simple and minimally invasive way [86]. Although the number of patients in this study was small, early results showed no sign of safety risk (Several patients showed improvements in symptomatic, functional, and left ventricular function/remodeling parameters in 12-month follow-up.). The latter study opened up the way for more studies in clinically relevant gene therapy approaches for cardiovascular diseases.
3.1.3. Biomaterials
Biomaterials have been used for cardiac repair through direct injection into the heart at specific locations or serving as patches on the myocardium to provide mechanical support [87]. Hydrogels derived from decellularized extracellular matrix (ECM) were delivered through catheters and they enhanced endogenous cardiomyocytes and preserved the cardiac function in MI rat models [88]. Hydrogels have also been used to reverse fibrotic changes in the damaged heart [89]. Intra-myocardial injection of some biomaterials to treat heart failure has reached phase II clinical trials [90]. Recently, an injectable hyaluronic acid (HA) hydrogel was used for local and sustained delivery of micro-RNA (miR-302/367) to enhance proliferation and function of cardiomyocytes after ischemic injury [91]. In particular, the gels decreased end-systolic (50%) and cardiac end-diastolic (39%), and increased fractional shortening (64%) and ejection fraction (32%) of the heart four weeks after the injection compared to controls. Injectable hydrogels as the cardiac patches were also shown to enhance cardiac endogenous capacity and function [92]. These injectable hydrogels include collagen, Matrigel®, fibrin, HA, alginate, chitosan, PEG-based materials, and acrylamides.
Degradation times of injectable biomaterials differ according to several factors that include the type of the material, molecular weight [93], processing technique, crosslinking and environment. For example, photo crosslinking gelatin methacryloyl (GelMA) hydrogel with 15% concentration was degraded over eight weeks when incubated in collagenase solution [94].
Biomaterials as structurally designed scaffolds have been utilized to provide mechanical support to infarcted tissues [95]. Moreover, the scaffolds have shown to increase angiogenesis by providing topographical cues and reduce cardiac dilation and fibrosis [96]. Another interesting application of scaffolds in cardiac regeneration is in situ tissue engineering by which the scaffolds induce the regeneration based on the host’s tissue response [97]. The natural immune response to the scaffolds can further be controlled through the integrin-mediated interactions of scaffolds with the native tissues [98]. Minimally invasive approaches have been used to deliver scaffolds for in situ tissue engineering. For instance, engineered heart valves were implanted through catheters and showed rapid in vivo modeling and long-term functionality (sufficient coaptation and leaflet motion with minor paravalvular leakage) in sheep models after 24 weeks [99]. The latter study may provide an alternative solution for currently used prosthetic devices, which require replacement and care on regular basis.
3.1.4. Combinatorial therapy
Combinatorial therapies in cardiac tissue regeneration aim to use the advantages of cells, biomolecules, and biomaterials in the regeneration process. Some combinatorial therapy studies have already reached to the clinic. For example, the efficacy of combined autologous cardiac SCs and bFGF for the treatment of ischemic cardiomyopathy and heart failure was evaluated in a clinical study called ALCADIA (Autologous Human Cardiac-Derived Stem Cell to Treat Ischemic Cardiomyopathy) [100]. The results showed that the cell therapy was safe and the bFGF had positive effects on the tissue recovery. Because of small size of the study, a solid conclusion on the efficacy of approach could not be derived. Biomaterials can also be utilized to induce proliferation and differentiation of transplanted cells [101]. For example, the use of poly(glycolide-co-caprolactone) scaffolds seeded with rat bone marrow-derived mononuclear cells promoted migration and differentiation of cardiomyocytes and neovascularization in a rat MI model [102]. The end diastolic left ventricular thickness in rats treated with the cell-laden scaffolds was 8.6±0.6 mm compared to the sham-operated group (11.1±0.5 mm). It is important to achieve the synchronous beating of transplanted biomaterial-cardiomyocyte patches with the host tissue [103]. Injectable biomaterials with clinically relevant sizes were recently developed using 3D printing and they were seeded with cardiomyocytes derived from human induced pluripotent stem cells (iPSCs). The cell-laden biomaterial was used for the treatment of MI in mice and then in porcine model. Successful cell survival and engraftment and significant reduction in the infarct size were obtained [104]. Although, there are lots of benefits using embryonic stem cells or induced pluripotent stem cells for minimally invasive and regenerative therapeutics, one critical issue in the clinical application of these cells is the risk of teratoma formation [105]. Other patches were also produced using fibrin scaffolds and iPSC-derived cardiomyocytes, smooth muscle, and endothelial cells. The patches were then used for the treatment of MI in pigs leading to significant reduction in infarct size and improved cardiac function [106]. The engraftment rate was 10.9±1.8% after four weeks of the implantation. The released EXs from cardiac patches provided cytoprotective characteristics for cells.
Sequential combinatory therapy is another approach which has been pursued to promote myocardial repair. In angiogenesis, early release of vascular endothelial growth factor (VEGF) is required to trigger neovessel formation. Subsequently, the addition of platelet-derived growth factor (PDGF) BB stabilizes the neovessels. In an in vivo study on rat MI model, sequential delivery of VEGF and PDGF was accomplished by embedding VEGF in fibrin gel and PDGF in a heparin-based coacervate that is distributed in the same fibrin gel. The therapy was delivered by intramyocardial injection and resulted in improved cardiac function, angiogenesis and reduced fibrosis and inflammation compared to control groups [107]. Another example is the controlled and sequential delivery of IGF-1 and HGF was enabled by injectable affinity-binding alginate and used to treat MI in rats. This therapy can potentially induce endogenous regeneration of cardiac muscle [108].
Shape-memory biomaterials offer great potential to make minimally invasive scaffolds in cardiac tissue engineering. Such scaffolds can be structurally diminished for transplantation in a minimally invasive way and then return to their original shape at desired location upon applying external or internal stimuli. Recently, an elastomeric polymer was used to make flexible and shape-memory scaffolds for cardiac tissue regeneration [2]. Cardiac patches made of this scaffold (dimensions: 1 cm by 1 cm and thickness: 100 µm) were able to fold into small tubes and then recover their original shape upon releasing from the tubes (Figure 5). It was shown that cardiac function was significantly improved in MI rats after six weeks following the use of these patches. Thoracoscopic delivery of patches with human SC-derived cardiomyocytes on the epicardium of porcine models also led to the replacement of scar tissues and improvement in the cardiac function. The shape-memory property of scaffolds was due to their lattice structure. Therefore, other scaffold materials can be used to make such injectable and smart cardiac patches.
Figure 5. Shape-memory scaffolds for minimally invasive delivery of cardiac patches.
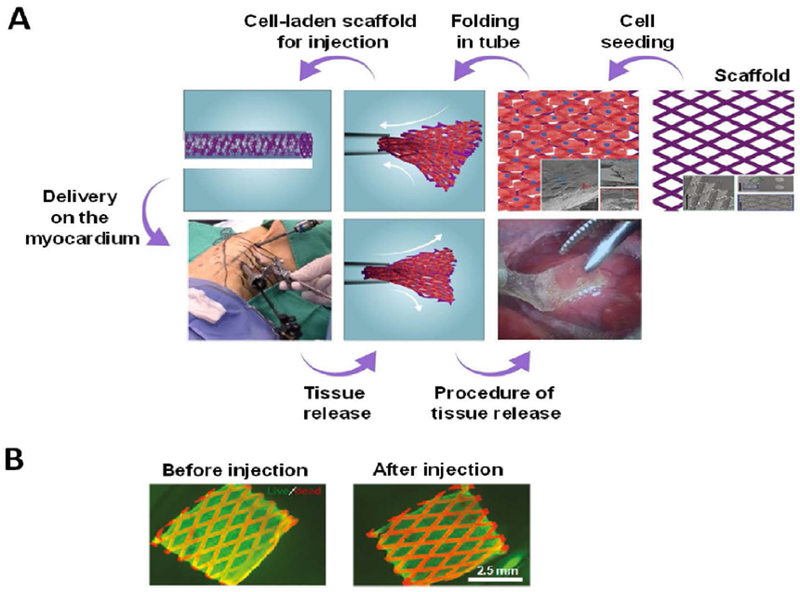
(A) An illustration demonstrating shape-memory scaffold for delivering cardiomyocytes to the epicardium using thoracoscopy. The cardiac patch is first loaded into the throacoscope. Then, upon releasing it recovered its shape and extended out to sit on the heart. (B) Photographs showing fluorescence images of live (green) and dead (red) neonatal rat cardiomyocytes on the patch before and after injection. Pictures were reproduced with permission from [2].
3.2. Spinal cord
The spinal cord is composed of a fragile, long, and tube-like structure that starts from the brain stem and goes down through the vertebral canal. The spinal cord consists of nerves that exchange messages between the brain and the rest of the body. Spinal cord injury (SCI) results from trauma to the spinal cord and it is associated with sudden loss of motor, sensory, and autonomic functions distal to the site of injury [109]. There are three million people affected by traumatic SCI worldwide and ~180,000 new cases are reported annually [110]. Due to the complex pathophysiology of spinal cord, various treatment methods resulted only in marginal success. Common regenerative therapies use cells, biomolecules, biomaterials or their combinations. The integration of minimally invasive approaches with these regenerative therapeutics may provide safe, efficient, and delicate treatments of SCI as discussed below.
3.2.1. Cells
Cell therapy has emerged as a potential approach to promote SCI repair. The purpose of using cells in the treatment of SCI is to: 1) provide trophic support [111], 2) make immunomodulation and neurotrophic protection [112], and 3) induce tissue regeneration [113]. Neural stem cells, Schwann cells, olfactory ensheathing cells, MSCs, and oligodendrocyte precursor cells (OPCs) have been used in SCI cell therapy [114]. Interestingly, MSCs can release some soluble factors that can activate resident progenitor cells for spinal cord regeneration [115]. A clinical study documented that MSCs administrated through intravenous or intrathecal injection into 25 SCI patients partially restored the autonomic function of nerves within 12 months after the injection [116]. Therapeutic efficiency of MSCs, directly injected into the spinal cord was also shown in 28 patients with chronic SCI [117]. The motor performance of the spinal cord was improved in some patients. Recently, there has been interest in dental pulp cells in SCI repair because they contain neural cells. These cells were able to induce functional recovery for the spinal cord in a rodent model [118]. Drug-loaded NPs were conjugated with cells to stimulate transplanted cells (~10-fold increase in the therapeutic efficiency of the cells) [119]. Hybrid cell-NPs can be delivered using a minimally invasive and single-stage process called electrospraying [120] by which the drug distribution and load efficiency can be precisely controlled.
In general, common problems of cell therapy for SCI treatment include, 1) cell migration [121], 2) dissemination [122], 3) uncontrolled proliferation [123], 4) aberrant axonal growth leading to allodynia [124], 5) formation of ectopic SC colonies [125], 6) differentiation into unwanted cells, such as astroglial cells [126] or neutrophils [126] leading to the failure of functional recovery, and 7) teratoma or tumor development [126]. Although some cell-based therapies to treat SCI, which include the use of MSCs [127] , CNS SCs [128], spinal cord-derived neural stem cells (NSCs) [129], and OPCs [130] have reached clinical trials, efficacy and long-term outcome of the clinical trials have to be confirmed before moving to large-scale clinical applications [114]. There is also little information regarding mechanisms by which cells induce repair and improve the function of the spinal cord [131]. In particular, minimally invasive approaches in SCI repair should be further developed and adapted for large-scale, accurate, and automated applications in the clinic.
3.2.2. Biomolecules
Biomolecules play a crucial role in mimicking the natural environment of neural system. Studies have shown that soluble factors derived from astrocytes, inflammatory, and ependymal cells have pro-regenerative characteristics including neuroregeneration and self-repair after SCI [132]. Therefore, manipulating the local microenvironment of these cells may stimulate self-repair of the spinal cord [133]. Some biomolecules have shown great promise in SCI treatment including anti-regeneration inhibitors such as Rho-associated protein kinase inhibitors (Rho-ROCKIs) [134] and granulocyte colony-stimulating factor [135]. In clinical trials, Rho-ROCKI in fibrin gel was delivered to the epidural space through intrathecal injection. The forty-eight patients with acute SCI treated with the Rho-ROCKI resulted in long-term improvement of motor function with no significant adverse effects [136]. The motor score was improved for treated patients to 27.3±13.3 compared to the baseline group (1.8±5.1) after one year of treatment. Following the latter study, a phase III clinical trial was initiated in 2016 [137]. A randomized design is required to demonstrate the efficacy of the latter clinical study. In another study, intrathecal injection of conditioned medium of bone marrow-derived mesenchymal stem cells (BMSCs) into SCI rat models was found to increase axonal regeneration and improved locomotor recovery. The results were more effective than using individual growth factors [111]. Fractalkine (a chemokine to recruit reparative monocytes) was also used to regenerate nerves in rats [138]. The significant effect of fractalkine was confirmed using electrophysiological and histological analyses. Further advances in molecular and cellular biology would enable us to recognize more effective biomolecules in SCI repair [139].
3.2.3. Biomaterials
The role of biomaterials in SCI treatment may involve filling cavities resulting from SCI [140], bridging spinal cord cut-ends to guide axonal growth [141], and delivering therapeutic agents or cells [142] (Figure 6). The use of acellular biomaterials to treat SCI avoids complexities associated with cells [143]. These biomaterials include hydrogels [144], nanostructured composites [145], and nanofibers [146]. PEG hydrogels were found to promote and guide axonal regeneration and hence they were considered as promising biomaterials for SCI repair in a minimally invasive manner [147]. Polymeric nanocomposites (HA and methylcellulose) together PLGA NPs were injected into the intrathecal space of injured spinal cord in rats [148]. The PLGA NPs were utilized for sustained drug delivery to the SCI. The biomaterial showed no inflammatory response after 28 days post-implantation. Nanofibers can be used for the treatment of SCI and recently it was demonstrated that nanofibers containing a laminin motif influenced neurogenesis both in vitro and in vivo [149]. A high laminin concentration (0.25 % w/v) provided higher cell viability that a low concentration (0.125 % w/v). Until now, biomaterials that reached clinical trials for SCI treatment were mostly utilized as drug delivery matrices where the implantation of biomaterials through intrathecal injection combined with surgical decompression is a commonly used technique in the clinic [150].
Figure 6. Different biomaterial designs to promote recovery after SCI.
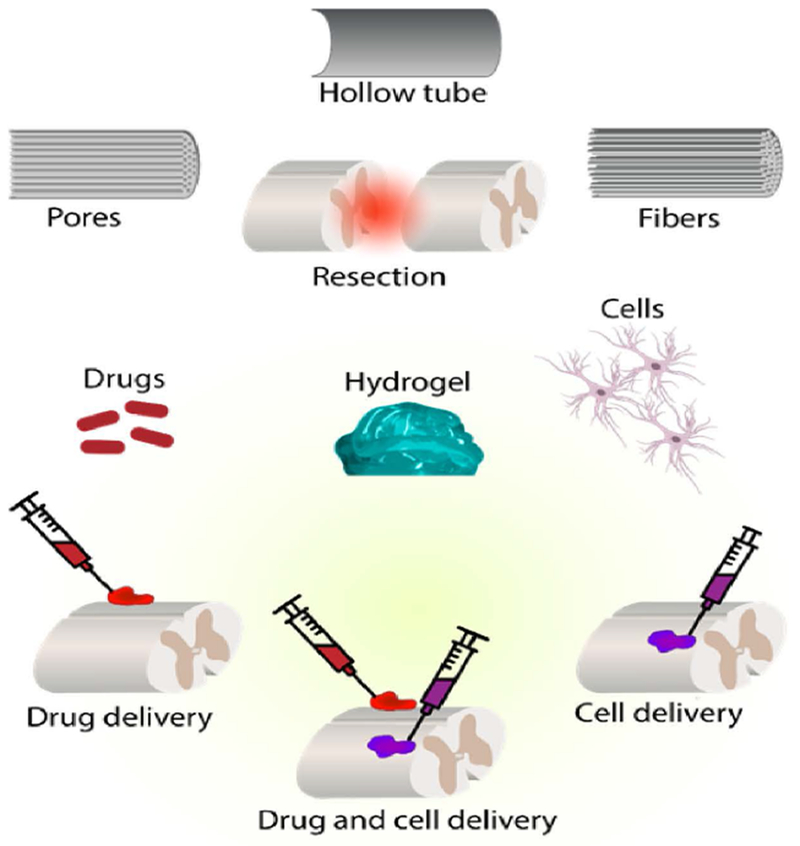
Tubular scaffolds are beneficial for resection or full transection in injuries. Injuries can also be occupied with fibers or pores for cell growth. Hydrogels with drugs or cells can fill the defect area. Soft hydrogels can be intrathecally injected as MIRET.
Electroconductive and magnetic NPs can be used to guide neurite growth in vitro and in vivo [151] and cellular organization and differentiation [152]. For example, rod-shaped SMION (0.0046 %v)-containing gels were shown to guide nerve extension parallel to aligned rods despite low gel concentration (3 %w/v) [153]. Gold NPs can be bonded to neurons for optical excitation [154]. This bioconjugation provides an exciting opportunity to monitor and stimulate neural cells in a non-invasive and remote manner. For instance, pulsed infrared light was used for neural stimulation by increasing the neural responsiveness using plasmonic gold nanorods [155]. The neural tissues were stimulated for up to 0.956 J/cm2 without any significant thermal damage. Gold NPs can have additional therapeutic effects on neural growth and differentiation. For example, gold NPs incorporated into electrospun poly(ε-caprolactone) (PCL)/gelatin scaffolds enhanced axonal elongation and network formation of PC12 cells in vitro [156]. The efficiency and fate of NPs combined with biomaterials in SCI repair should be further investigated prior to their clinical applications.
3.2.4. Combinatorial therapy
SCI represents a complex problem because of multiple mechanisms of growth inhibition and degeneration after injury. Therefore, a combinatorial SCI repair utilizing cells, biomolecules, and biomaterials would be useful to tailor treatment strategy for a specific injury. Some combinatorial approaches have shown promising results in SCI treatment [157]. One of the most robust corticospinal axonal regeneration was reported to occur in severe SCI in rats treated with fibrin containing NSCs and growth factors [158]. The axons grew in large numbers at a rate of 1–2 mm per day. In another work, astrocytes were transfected with plasmids encoding nerve growth factor (NGF) and encapsulated in collagen. The cell viability was more than 90% in the gels. After injecting the compound to rat dorsal root ganglia, a significant enhancement in the axonal growth was observed [159]. A recent work reported that an artificial neural network (a scaffold combined with Schwann cells and adult SCs) integrated with the host tissue and served as a neuronal relay for signal transfer in defected spine [160]. In general, biomaterials have been used to protect loaded cells from the local SCI environment, improve their survival, and maintain them in situ to have their therapeutic function [161, 162]. As a result, injected cells with biomaterials have more therapeutic effect than cells alone [161].
Different injectable cell-laden hydrogels have been proposed for SCI treatment including HA hydrogel and NSCs [163], self-assembling peptide gel and NSCs [164], polyurethane-based gel and bone marrow stromal cells [165], laminin/collagen gel and Schwann cells [166], and Matrigel® and Schwann cells [167]. These therapeutics have shown promising results with respect to neurotrophic factor expression, optimized post-traumatic milieu, improved cell survival after implantation, neurite extension, SC differentiation to neurons, and directed linear axonal regeneration. A multitude of biomolecules can also be encapsulated in gels and released in a controllable manner to increase the regeneration of damaged spine cords [168]. Mechanical properties of hydrogels should be close to those of the native ECM of spinal cord to enhance axonal ingrowth. Injectable nanofibers with bone marrow-derived bioactive motifs and human endometrial-derived stromal cells were also used to induce neural regeneration in rats [169]. Higher axonal regeneration and myelination were demonstrated in the implanted group compared to the control group. In another work, embryonic stem cell-derived OPCs with ciliary neurotrophic factor were injected into rats leading to significant improvement in locomotor function of the spinal cord [170]. The soluble factor enhanced the survival of cells over four times compared to control group. In a clinical study, BMSCs in chitosan-laminin scaffolds were injected into 14 patients with SCI. There was improvement in motor, sensory, and neurological functions of the spinal cord after the operation. However, the improvement was limited, not enabling patients to stand up and extend their knees while walking unaided [171]. The latter study confirmed that further investigations are required on combinatorial therapy in SCI repair prior to clinical trials.
3.3. Brain
Traumatic brain injury causes sudden damage to the brain and it is almost irreparable because neural tissues have poor regenerative capability [172]. Minimally invasive therapeutics have been used to regenerate the brain following stroke (hemorrhagic or ischemic [173]), trauma [174], Parkinson’s disease (PD) [175], AD [176], Huntington [177], and amyotrophic lateral sclerosis [178]. Conventional systemic administration is insufficient in the regenerative treatment of brain conditions [179], because of their poor penetrability of the blood brain barrier (BBB) and short plasma half-life, the risk of an off-target exposure in the brain and less control on effects of therapeutic agents [180]. MRI-guided focused ultrasound can be used in several indications including the disruption of BBB [181, 182]. Direct delivery of SCs to target area was performed by using MRI-guided focused ultrasound [181]. Direct delivery of injectable regenerative therapeutics to the lesion can be performed through intracerebral, endovascular (catheter) or ventricular delivery [183, 184].
Ventricular system contains cerebrospinal fluid that enables growth factors and cells to be delivered to most of areas of the brain [185]. This route enables uneven distribution of administered drug with sharp concentration gradient. Hence, drug delivery systems targeting specific regions of the brain are pursued [186]. In clinical trials, mostly stem cells are transplanted through custom‐designed cannula needles, syringes, or catheters in the presence of MRI‐guided delivery to the target tissue [187]. Development of image-guided brain surgeries has provided controlled delivery of therapeutics [188]. In an in vivo study, prelabeled NSCs with superparamagnetic iron oxide and RT-MRI were used to guide the injection cannula to the putamen and monitor the delivery [187]. Needle blockage due to precipitation and aggregation of cells in cannulas and infusion lines can be avoided with the use of intraoperative RT-IMRI [46].
Microdevices have also been utilized for remote delivery of therapeutics. These devices are capable of regulating therapeutic concentrations in the brain [189]. For instance, a novel miniaturized system was recently developed for drug delivery to the brain [190] (Figure 3B). This implantable and remotely controllable device was successfully tested in small and large animals. The drugs were delivered at flow rates of up to 10 µl/hr through the device. Such advanced systems would be useful to deliver therapeutics in a minimally invasive and precise manner. Different MIRET for brain repair are discussed below.
3.3.1. Cells
Cell therapy has widely been used to regenerate neural tissues in the brain. Some cell therapy approaches have reached clinical trials. For instance, prelabeled NSCs with SMIONs were injected to the putamen and the delivery was monitored using RT-MRI [187]. Allogeneic BMSCs (delivered intravenously and intrathecally to the brain) are in phase I and II clinical trials for treatment of PD [191, 192]. A phase I clinical trial for patients with AD using human umbilical cord blood-derived MSCs was recently completed and the safety and efficacy of cell therapy was confirmed [193]. Autologous bone marrow mononuclear cells were also applied intrathecally in a phase I clinical trial to treat traumatic brain injury [194]. Safety and clinical efficiency of modified BMSCs for stroke treatment were proven in phases I and II clinical studies [195]. The use of human NSCs for PD’s patients is in clinical trials to evaluate their safety and efficiency [196, 197]. Intracerebral transplantation of NSCs to treat ischemic stroke is in a phase I clinical trial [198]. Intracerebral microinjection of NSC-derived neurons has also been used to treat PD in phases I and II clinical trials [199]. These procedures have shown great promise to be included into the mainstream of clinical practice in near future.
3.3.2. Biomolecules
Biomolecules have extensively been explored for brain repair. The regeneration capability of biomolecules is mainly based on endogenously stimulating repair mechanisms and targeting inhibitory factors [200]. The biomolecules can be delivered in a minimally invasive way using microneedles and catheters. For example, glial cell line-derived neurotrophic factor was directly delivered into the putamen of five PD’s patients using catheters in a phase I clinical trial [184]. There were no significant side effects after one year of treatment. Moreover, improvements in the off-medication motor sub-score and activities of daily living sub-score were observed (39% and 61%, respectively). A carrier-mediated transport and receptor-mediated transcytosis system enabled the transport of neurotherapeutic agents [201]. Focused ultrasound also helped to improve the accuracy of biomolecule delivery [202]. EXs represent another class of biomolecules were also used to deliver short interfering RNA to the mouse’s brain [203]. Dendritic cells were used for the EX production. The therapeutic efficiency of EXs was measured using mRNA and protein knockdown of beta-secretase 1 (BACE1, 60% and 62%, respectively), a therapeutic target in AD. In another work, EXs derived from MSCs (100 µg EXs injected through the tail vein) were shown to improve the recovery of traumatic brain injury in rats after 35 days [204]. A systemic injection of EXs also led to recovery after traumatic brain injury in rats [205]. EXs promoted the neurovascular remodeling and increased neuroinflammation in the injured brain at 14–35 days post injection.
3.3.3. Biomaterials
The most commonly used biomaterials in brain tissue repair are hydrogels. Hydrogels can be utilized as injectable materials with in situ solidification after their injection [206]. For example, self-assembled nanopeptide hydrogels with tunable mechanical properties facilitated angiogenesis and neurogenesis after they were injected into brain lesions in zebrafish [207]. Moreover, hydrogels have been used to release biomolecules that favor cellular ingrowth, angiogenesis, and axonogenesis [208]. For instance, an injectable gelatin-hydroxylphenylpropionic acid (gelatin -HPA) hydrogel was used for lesion filling to improve endogenous regenerative responses. Dextran sulfate/chitosan polyelectrolyte complex NPs (PCNs) were synthesized to encapsulate stromal cell-derived factor-1 for sustained release over four weeks. The gelatin-HPA/SDF-1[α]-PCN matrix provided tunable gelation and in situ covalent crosslinking after injection into brain lesions in a rat model of intracerebral hemorrhage and the results showed tissue recovery [209]. The matrix also persisted for a minimum of two weeks after injection. With incubating gelatin-HPA hydrogel in PBS containing type I collagenase, hydrogels performed lower degradation rate with higher crosslinking degrees. A thermosensitive and injectable hydrogel was also developed to deliver activin B into the striatum of a PD’s mouse model [210]. The results showed significant improvement in the brain function over five weeks. One challenge associated with these hydrogels is the lack of controllable on/off dosing that may prevent them from real-time interaction with neural network activity. This may be addressed by using a layer-by-layer constructed hydrogel [211] or injectable bioelectronics [212] by which the delivery procedure is managed using different doses of biomolecules in the hydrogel layers or using the electronic system.
3.3.4. Combinatorial therapy
Combinatorial therapy has been used in brain tissue engineering and repair to enhance the efficiency or function of individual therapies [213]. For example, injectable hydrogels made of hydrazone crosslinked HA-polyvinyl alcohol (PVA) and alginate-PVA with mechanical properties close to those of the brain were successfully developed and used for 3D neural cell culture [214]. The mechanical properties of hydrogels were a key factor to significantly enhance the therapeutic efficiency of cells. A combination of gingival SC-derived neuronal cells and 3D bioconjugated and injectable hydrogels was developed to support cell survival and proliferation [215]. Another interesting class of combinatorial therapies is self-healing and injectable polysaccharide-based hydrogels that were used for NSC delivery to treat neurological diseases. These hydrogels supported proliferation of NSCs and also favored their neuronal differentiation (Figure 7A) [216]. The hydrogels at the density of 0.02 g/mL supported the cell growth and differentiation for up to nine days in culture. Cells cultured on electrospun fibers were also utilized to treat brain defects. For example, electrospun tyrosine-derived polycarbonate was seeded with neuronal cells and then injected into the brain tissue of mice (Figure 7B). The survival and engraftment of neurons into the striatum after transplantation were increased (~3.5-fold and ~38-fold improvements in proliferation and function in vitro and in survival in vivo after, respectively). Moreover, the fibers supported neurite outgrowth and survival ex vivo [217]. Brain tissue regeneration can also be achieved with the combination of hydrogels, SCs, and growth factors [210]. In one study, collagen-based hydrogels loaded with glial cell-derived neurotrophic factor (GDNF) were used for intra-striatal transplantation in a PD’s rat model. The results demonstrated enhancement in the survival and function of encapsulated dopaminergic neurons and an increase in their capacity for striatal re-innervation [218].
Figure 7. Injectable cell-laden hydrogels in brain repair.
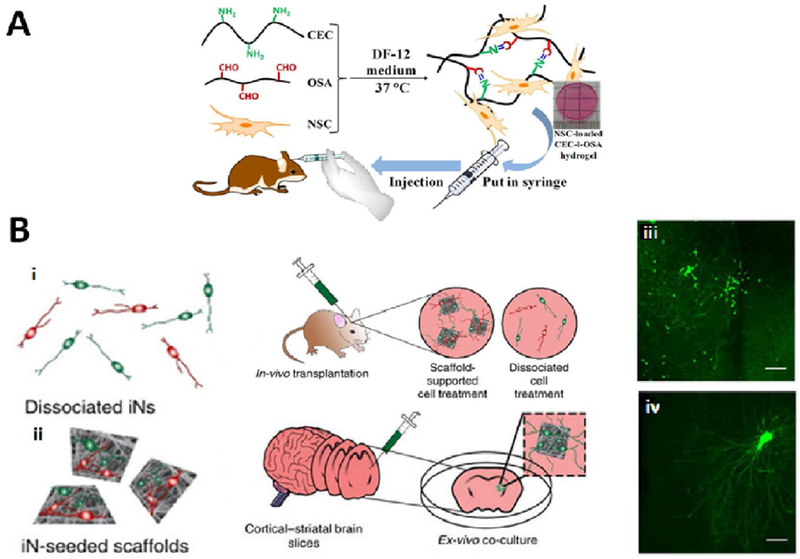
(A) Injectable self-healing hydrogels made of N-carboxyethyl chitosan (CEC) and oxidized sodium alginate (OSA) carrying NSCs for transplantation. The cell-laden hydrogels were cultured in DF-12 medium at 37 ºC. and then injected using needles into the lesion cavity. Reproduced with permission from [216]. (B) Electrospun scaffolds support induced neuronal cells (iNs) outgrowth and survival in vivo and ex vivo. (i, ii) The scaffolds seeded with the iNs were injected into the mouse striatum and onto the mouse pup brain slices. Compared to only green fluorescent protein-labelled iNs (iii), transplanted scaffold-supported iNs (iv) significantly enhanced the neurite length when injected onto the mouse brain slices ex vivo (n=8 brain slices for each transplantation mode). Reproduced with permission form [217].
3.4. Bone
Although bone grafts have widely been used in the clinic to augment bone regeneration and repair, engineered bone tissues may provide a better solution because of facile fabrication, wide accessibility, and the use of patients’ own cells that eliminates the organ rejection [219]. MIRET can benefit bone regeneration in different aspects. One important area of interest is the treatment of vertebral compression fractures and associated kyphosis (forward bending) back deformity. For this purpose, various injectable therapeutics have been investigated including biomaterials (such as bone cement, osteoconductive ceramic biomaterials, and more recently hydrogels) and SCs for bone regeneration [220–222]. Regenerative procedures in craniomaxillofacial (CMF) surgery and dentistry (e.g., periodontal regeneration [223, 224]) can also benefit from the application of MIRET. In particular, such therapeutics can be used to reconstruct the complex CMF skeleton for which pre-manufactured implants are not always suitable to fit desired shape and to provide appropriate contour. In this section, we will discuss applications of MIRET in bone regeneration and repair.
3.4.1. Biomaterials
Biomaterials have widely been utilized in bone tissue engineering. For example, injectable biomaterials were used in percutaneous vertebroplasty and kyphoplasty to treat vertebral compression fractures in which the biomaterial was injected via cannula under guided real-time X-ray fluoroscopy [225, 226]. Although this method relieved the pain and restored the kyphotic angle [226], cement leakage [225], exothermic reaction in polymethylmethacrylate (PMMA) (injectable biomaterial) curing, cardiopulmonary toxicity, and low bioactivity and mechanical properties of PMMA were major concerns. Other biomaterials including carbonated apatite, bioactive PMMA, and calcium phosphate have been investigated to enhance compressive strength and bioactivity of injectable PMMA [227]. A recent study introduced bioactive, glass-based, and injectable bone cement [228]. Compared to conventional cement made of PMMA and calcium phosphate cement (CPC), this bioactive cement led to improved bone regeneration in critical-sized bone defects in a rabbit model. Other bone cements were also developed including magnesium phosphates; however, they suffer from insufficient degradation, failure to heal large defects, and lack of injectability. Thus, they were combined with other osteoconductive yet flexible and biodegradable bone cements, such as hybrid HA and fibrin gels [221]. Magnesium ammonium phosphate hexahydrate (struvite) implants with different porosities were also found to increase new bone formation in both unloaded and loaded bone defects [229].
Some biomaterials, such as calcium sulfate cement (CSC) have been clinically used for vertebroplasty of compression fractures. However, radiological examination showed disc vacuum formation. There was also cement leakage, non-uniformity, and rapid CSC resorption [230]. Therefore, more studies are needed to assess long-term effects of injected material on adjacent vertebrae [231]. It was reported that chitosan (reinforcing agent) and mannitol (pore-forming agent) added into the CPC composite increased flexural strength and porosity of the material to support osteoblast viability and proliferation in vitro [232]. Processing parameters of injectable CPCs can also be adjusted to achieve high injectability, macroporosity, and strength [233]. An injectable and thermoresponsive methylcellulose hydrogel containing calcium phosphate NPs was also studied and the results showed an improved bone regeneration in vivo [234]. In another in vitro study, silica nanopowder was incorporated into chitosan-tripolyphosphate microparticles and it was shown that adding silica to the microparticles enhanced osteoblast growth and proliferation [235]. Microparticles suspended in PBS solution containing lysozymes with pH 5.1 degraded in 15 weeks and the degradation rate was slower in physiological pH [236].
3.4.2. Combinatorial therapy
Some minimally invasive therapeutics for bone regeneration comprise of a combination of biomaterials with cells. The development of injectable SC-containing biomaterials has been attracted much attention as a minimally invasive and regenerative therapy to treat bone defects [237]. In one study, human BMSCs were encapsulated in fibrin/alginate hydrogels and the mixture was used as an injectable bone therapy [237] which induced osseous bridge with high new bone area fraction in an in vivo study with rats. MSCs encapsulated in alginate/fibrin microbeads were also used as injectable matrices for bone tissue regeneration [238]. In another study, biofunctionalized macroporous CPCs with human iPSCs, BMSCs, and umbilical cord MSCs were utilized to treat critical-size rat cranial bone defects and a robust bone regeneration was observed [239]. The cell viability was ~90% on the CPC scaffolds. The fractions of bone area were 30.4±5.8% and 11.0±6.3% for treated and control animals, respectively. IPSC-derived osteogenic cells were also encapsulated in alginate/fibrin microbeads and then dispersed in CPC and implanted in rats. After injection, high cell viability inside the microbeads, bone regeneration, and scaffold resorption were observed [220]. The fractions of new bone were 35.7±5.1% and 18.3±3.2% for treated animals with MSCs and gels and MSCs only, respectively.
Sequential delivery was also applied to improve the bone regeneration. In tissue repair process, macrophages exhibit a pro-inflammatory phenotype (M1) and initiate angiogenesis at early stages and perform a pro-healing phenotype (M2) for vessel maturation at later sequence. A sequential delivery was proposed by physically adsorbing interferon-gamma (IFNg) onto the scaffolds for short release to promote the M1 phenotype; and, attaching interleukin-4 (IL4) to the scaffolds via biotin-streptavidin binding for sustained release (released over six days) to promote the M2 phenotype. Bone scaffold was implanted subcutaneously on Murine model showing better vascularization in scaffolds releasing IFNg compared to controls, improving angiogenesis and healing [240].
The combination of biomaterials and biomolecules has been reported as a minimally invasive therapy in bone regeneration. For example, the regeneration capability of injectable biomaterials (e.g., calcium phosphate/gelatin composite microparticles) with transforming growth factor-β1 was investigated in rabbit femoral defects. It was found that the growth factor resulted in no benefit in terms of mechanical strength. However, the growth factor led to significant increase in bone response and composite degradation 12 weeks after the implantation [222]. Injectable HA-based hydrogels containing hydroxyapatite (HAp) NPs and bone morphogenetic protein-2 (BMP2) were also shown to make bone formation in rat models [241]. In combined biomolecule-biomaterial therapy, it is important to select proper biomaterial carrier to ensure controlled release of loaded biomolecule and to avoid complications [242]. In a clinical trial, a platelet-rich fibrin implant was delivered using catheters for bone regeneration in a minimally invasive manner. Six out of eleven patients who received the dental implants showed positive results [223].
Some studies have combined cells, biomolecules, biomaterials and proposed them as a minimally invasive and regenerative therapy in bone regeneration. In one study, bone formation was observed in rats when a combined therapy of brin glue, biodegradable tricalcium phosphate, and MSCs was used [243]. In another study, a composite of nano-HAp and platelet-rich fibrin granules encapsulating MSCs was used for the treatment of rabbit calvarial large bone defects and enhanced bone regeneration was reported [244].
3.5. Cartilage
Articular cartilage has limited self-regenerative capacity after injury. The gold standard in articular cartilage repair is to perforate the subchondral bone plate [245], facilitating BMSC migration to the lesion, and potentially improving cartilage regeneration. However, this strategy was not able to provide a reliable and long-term treatment for cartilage microfractures [246]. Large variations in clinical outcome and doubts about the effectiveness of conventional cartilage repair methods have been reported [247]. Novel MIRET may provide effective therapeutic strategy for cartilage repair as described below.
3.5.1. Cells
A common cell therapy method for cartilage repair is the use of autologous chondrocyte implantation [248, 249, 250], which is based on chondrocyte expansion in vitro [251]. However, rapid cell proliferation may result in the formation of fibrocartilage with inferior mechanical properties [191, 252]. A current trend in healing injured cartilage is mainly focused on regenerative potential of SCs, which can be stimulated to differentiate to chondrocytes [245]. For instance, intra-articular injection of MSCs as a minimally invasive and regenerative approach has been used in cartilage repair. Intra-articular injection of human MSC-derived EXs also resulted in regeneration of osteochondral defects in rat models [253]. The animals were treated with 100 µg EXs for a period of 12 weeks. Immunohistochemistry and histological analyses and scoring at six and 12 weeks post-surgery confirmed positive effect of EXs on the regeneration process. For accurate SC delivery and preventing extra-articular and off-target injections, online monitoring has been conducted using anatomical landmark-guided delivery, fluoroscopically guided techniques, and ultrasound [248].
Some clinical trials are still going on in cartilage repair using minimally invasive SC therapy. For instance, ADSCs were injected into knees of 18 patients and followed up for six months. A dose-escalation trial was conducted in the first phase of the study to assess the clinical efficacy. Interestingly, the group injected with the lowest dose of ADSCs had a statistically significant improvement in the cartilage repair [254]. In another study, six patients with eight arthritic knees suffering from pain were injected with adipose-derived stromal vascular cells and followed up for 12 months. This minimally invasive and regenerative procedure was found to be safe and led to lower pain without any sign of infection or other adverse effects. All patients gained full function and showed significant improvement compared to the baseline [250]. A longer follow-up trial, up to five years, was reported using BMSCs injected intra-articularly in four patients. Walking time, stair climbing, gelling pain, patella crepitus, flexion contracture, and pain visual analogue score were improved within six months followed by a gradual deterioration. After five years, all SC-treated patients had better performance than the baseline [251]. In another work, larger trial groups (30 patients) with knee osteoarthritis were treated with similar intra-articular injection of allogeneic BMSCs and followed up for 12 months [255]. Significant improvement was observed in the cell-treated patients. Earlier studies have also focused on using ADSCs [256], allogeneic BMSCs [196], BMSCs [257], autologous peripheral blood progenitors [258], and infrapatellar fat pad-derived MSCs [259].
3.5.2. Biomolecules
Key biomolecules in cartilage regeneration include the MSC secretome, such as cytokines, growth factors, and ECM molecules, which have been isolated as membranes vesicles (EXs) with diameters ~ 50–100 nm. EX-based therapy has recently emerged as an effective method in treating a variety of cartilage-related diseases [260]. EXs may be considered as vehicles containing a broad range of biomolecules, which have been used as a minimally-invasive therapy for cartilage repair and regeneration [261]. Cosenza et al. [262] showed that the direct intra-articular injection of EXs derived from mouse bone marrow MSC was equally effective as MSC injection in preventing the development of collagenase-mediated OA in mice. Interestingly, in an immunocompetent rat model, MSC-derived EXs (from human embryonic stem cells) enhanced the repair of critical-sized osteochondral defects in the femoral groove when they underwent intra-articular injections on a weekly basis [263].
3.5.3. Combinatorial therapy
Biomaterials have been combined with cells in cartilage repair. Biomaterials for cartilage repair provide not only mechanical support for cartilage defects but also induce growth, spreading, and differentiation of cells [264]. To develop an injectable scaffold for the treatment of cartilage, the use of MSCs with PLGA was evaluated in vitro and in the subcutis of nude mice [265]. The majority of the cells were alive for up to 21 days. The cells in the PLGA secreted 2.36±0.422 µg/mL and 8.47±0.871 µg/mL of glycosaminoglycan at days 7 and 21 of culture, respectively. Hydrogels can also be employed with varying zonal structures for the treatment of osteochondral defects [266]. For the treatment of chondral and osteochondral defects, accurate size and shape of defects can only be defined after debridement. Thus, methods such as in situ 3D bioprinting or hydrogel application in a minimally invasive way are the most suited procedures to provide custom-tailored treatments. For instance, intra-articular injection of SC-derived EXs with a hydrogel glue used as a tissue patch for articular cartilage repair [267]. The tissue patch demonstrated ease of handling, biocompatibility, and most importantly integration to the native tissue.
3.6. Intervertebral disc
The IVDs link the vertebral bodies together. The IVD is responsible for weight bearing, motion, and flexibility of the spine. Degenerative or injured IVDs cause back pain and may need surgical interventions to treat them [268]. The use of MIRET has avoided major surgical interventions as explained in the following sections.
3.6.1. Cells
IVD injuries can be repaired using minimally invasive cell therapy under imaging guidance. For example, BMSCs were injected into the IVD nucleus pulposus of 10 patients with lumbar IVD degeneration and intact annulus fibrosus. The patients’ conditions were improved after the treatment [269]. Another study using injectable MSCs confirmed short-term preservation of injected cells within the IVDs [270]. Other cell types including autologous nucleus pulposus cells (NPCs), disc chondrocytes, auricular cartilage-derived chondrocytes, allogeneic NPCs, and xenogeneic NPCs have also been used for IVD treatment [271]. In general, complications associated with cell-based therapies include low cell viability and cell leakage. Some cell-based clinical studies have shown promising outcomes [272]. For example, autologous bone marrow concentrated cells were injected into the intradiscal space of 26 patients. The patients who were injected with more colony-forming units of marrow aspirate had substantially more pain relief [273]. Phase I and II clinical trials using intradiscal injection of allogenic MSCs in saline were completed [273]. In the phase I clinical trial, one-year follow-up showed significant relief of pain and disability and profound improvement in the disc quality of patients. The following treatment with single intradiscal injection of allogeneic mesenchymal precursors (phase II clinical trial) provided a promising outcome (in terms of pain and function) after 36 months [274]. In another study, autologous MSCs were percutaneously injected into patients with degenerative IVD. Most of them experienced pain relief and improvement in disability and disc hydration [269]. Similar results were observed with the use of autologous disc-derived chondrocytes [275].
3.6.2. Biomolecules
Bioactive molecules are typically used in an early degenerative disc to repair the disturbed homeostasis [276]. In vivo studies using bioactive factors, such as growth and differentiation factor 5 (GDF-5), osteogenic protein 1 (OP-1, homologous to BMP-7) have shown promising outcome [277]. Besides individual growth factors, the injection of platelet-rich plasma (PRP) as a therapeutic cocktail of many biomolecules, such as growth factors from the platelet cytoplasm-originated α-granules have been effective in the IVD regeneration [278]. The use of PRP has shown promises in hindering the degeneration of IVD in preclinical studies. Autologous PRP injection in rabbits with IVD degeneration restored disc height and signal intensity [279]. Intradiscal PRP injection into the center of the nucleus pulposus with the aid of fluoroscopic guidance was employed in a clinical trial on 14 patients with discogenic low back pain. During 10 months follow up, mean T2 values did not notably change and the pain scores profoundly decreased at one month and sustained over follow up period [280]. Other clinical studies also performed promising potentials for using PRP [281]; however, more studies are needed to optimize the therapeutic effect of this treatment including randomized controlled clinical studies [282]. Injection of growth factors can be also used for IVD degeneration. The safety and tolerability of intradiscal recombinant human growth and differentiation factor-5 for the treatment of early stage lumbar disc degeneration was evaluated in phases I and II clinical trial [283]. Other biomolecules such as PDGF, IGF-1, bFGF, BMP and beta transforming growth factors were employed for in vivo studies on different animal models to slow down discal degenerative process [284].
3.6.3. Biomaterials
Biomaterials have been developed to treat IVD disorders in a minimally invasive manner. For instance, an injectable implant composed of hydrolyzed polyacrylonitrile was implanted into degenerative discs [285]. In general, injectable biomaterials reduce procedural complexities in IVD treatment and offer in situ fixation and filling of disc defects [286]. In a clinical study, a hydrogel (silk and elastin) was injected into the nucleus void in patients suffering from disc herniation. After two years, it was found that the pain was relieved and the implant was stable. The safety of the injection and pressurized discal fill with a vented needle was confirmed and this strategy seemed to work better than microdiscectomy alone[287].
An injectable and cross-linkable hydrogel comprising silk fibroin and polyurethane with cyclic stability, strong axial compressive modulus, and fatigue-resistive properties was developed for nucleus replacement and its biocompatibility was confirmed in rabbits [288]. Future hydrogels should mimic biological and physical properties of the native nucleus pulposus to promote IVD regeneration.
3.6.4. Combinatorial therapy
Cell-biomaterial therapeutic strategies have shown promising results in IVD repair. The use of biomaterials prevents off-target cell escape in regenerative therapies [288, 289]. Fibrin was investigated for minimally invasive delivery of cells and it showed poor mechanical properties and leakage [290]. However, the use of PLGA with cells was found to enhance cell proliferation in a canine model [291]. Bioadhesives can reduce the risk of sliding in implanted gels. Clinical trials using MSCs or ADSCs suspended in PRP for treating degenerative disc diseases are ongoing [285]. In a clinical study, juvenile chondrocytes with fibrin carrier were percutaneously injected into 15 patients for the treatment of lumbar spondylosis. Ten patients had significant improvement, and eight out of nine patients with the posterior annular tears had significant resolution [292].
3.7. Skeletal muscle
Skeletal muscle tissues are comprised of muscle fiber bundles that are formed by cylindrical, long, and multinucleated cellular structures called myotubes [293]. Skeletal muscle tissues have a robust regenerative capacity; however, they lose their functionality under compromised conditions, such as tumor ablation, traumatic injury, and muscular dystrophy [294]. Skeletal muscle tissue engineering strategies using cells, soluble factors, and biomaterials have shown great promise in muscle tissue repair and recovery [295]. Electrical and mechanical stimulation have also improved the maturation of engineered muscle tissues [296]. There are some studies on using MIRET in skeletal muscle tissue regeneration. For example, Kim et al.[297] used collagen-based hydrogels with neonatal rat myoblasts as a regenerative therapy to treat post-glossectomy defects in rats. The animals that received the cell-laden hydrogel injections had a significant increase in the muscle weight and volume compared to control animals without any injection. Some evidence of neurotization and neovascularization were also reported in the injected group. Nakamura et al. [298]. used MSC-derived EXs to accelerate skeletal muscle regeneration. The EXs promoted angiogenesis and myogenesis in vitro and muscle regeneration in a mouse injury model. In another study, Wang et al.[299] reported that an injectable and shape-memory alginate scaffold was able to regenerate injured tibialis anterior muscles in mice. Growth factors and myoblasts were then added to the injected hydrogel in situ. In our recent study, Salehi et al. [300] showed that ultrathin PLGA nanoribbons can be used in minimally invasive delivery of skeletal muscle cells by using needles, and the differentiation of embedded myoblasts into myotubes was demonstrated. The cells were aligned (>74%), maintained their viability (>80%), and produced long myotubes (> 400 µm in length) on the nanoribbons. These studies demonstrate great promise of MIRET in skeletal muscle tissue regeneration for pre-clinical and clinical applications.
3.8. Abdominal organs
3.8.1. Pancreas
The pancreas is an important organ, which has two main functions of regulating blood sugar (endocrine function) and digesting food (exocrine function) [301]. When pancreas fails (e.g., in diabetic patients), control of blood sugar is lost and treatment is needed. Although pancreatic islet transplantation is a common treatment for type I diabetes, maintaining the blood sugar level remains a challenge and administration of an immunosuppressant is required [302]. Therefore, research has been focused on SCs as a source for β-cells [303]. The SC therapy is not yet approved for wide application in the clinic; however, more than 100 clinical trials around the world have been reported, mostly in phase II. Although different SC types have been investigated, the most clinical trials have used MSCs. Intravenously delivered autologous MSCs in 20 patients preserved β-cell function after one year without adverse effects [233, 304]. However, half of patients treated with hematopoietic SCs showed an adverse immunological response after four years [305]. Islet transplantation has also been carried out by implantation into the omentum [241, 306] or to the gastrointestinal mucosa [307] in a minimally invasive approach using laparoscopy.
Biomaterials have been used to protect β-cells from harsh native microenvironment and to deliver them in a facile way. Cell microencapsulation in alginate was recently proposed as an effective approach to overcome the host’s immune response. Human SC-derived β-cells in the hydrogel were implanted intraperitoneally in mice without accompanying immunosuppression to correct blood sugar [308]. More recently, nylon helical threads were developed to deliver large number of cells. The threads were covered with an adherent islet-containing alginate. The threads were implanted under mice renal capsule and proved to be capable of correcting induced diabetes in immuno-competent mice [309]. The thread scalability and retrievability using laparoscopic procedure were also demonstrated in dogs. This device can be used as an off-the-shelf product and cell encapsulation in the threads can be performed in the clinic. Similarly, β-cells loaded in collagen fibers were shown to secret insulin. The β-cell-laden fibers implanted in the renal subcapsular space of diabetic mice led to normalization of blood glucose and the hyperglycemia returned after their removal (Figure 8A, 8B) [310, 311].
Figure 8. Fabrication and implantation of microfibers for minimally invasive delivery of therapeutics.
(A) (i) Fiber fabrication using microfluidic system. The fibers were continuously extruded, gelated, and collected on the rotating spool. (ii) Schematic of silk-spinning by spiders. Proteins are produced by the silk gland, and in the spinning duct, protein solutions are solidified while spider’s valve controls the flow. (iii) Schematic of producing multiple twisted fibers. The bottom is fluorescence image of twisted fibers stained with red, green, and blue. (iv) Schematic of periodically mixed coded fiber comprised of hepatocytes, fibroblasts or parallel hepatocytes and fibroblasts. The inset shows the optical image of cells in a periodically coded fiber of which micrographs of embedded cells in three parts of the fiber are shown. (v) Schematic of fiber that was periodically coded with fMLP to mediate neutrophil migration. The fluorescence micrograph reveals the boundary between two parts of a fiber (neutrophil is green and fMLP is red). (vi) Schematic illustration of neuron aligning on grooved fiber. (vii, x) Fluorescence micrographs of neurons on grooved fibers (green is neurofilament and blue is cell nucleus). Fluorescence micrographs show a serially coded fiber and its magnified image. (viii, xi) Micrographs of tapered fiber having grooved surface. (ix, xii) Light microscopy and SEM images of air bubbles coded fiber. Scale-bars: iii: 500 μm, iv: 1 mm, vi: 50 μm, vii, viii, x: 300 μm, ix: 1 mm, xi: 5 μm and xii: 50 μm). Reproduced with permission from [311]. (B) Cell-laden meter-long microfibers implanted under the renal capsule. (i) A double coaxial microfluidic device was used to form long cell-containing core-shell microfibers in which the core contains cells in the gelated ECM-protein and second layer is alginate gelated in second connectors of microfluidic system with coflowing calcium solution. The cells in the gelated ECM protein migrate and form a long cellular microfiber with cell-to-cell contacts. The Ca-alginate shell can be selectively dissolved using an enzymatic reaction. (ii) Images show 20 cm-long primary islet cell microfibers injecting with a microcatheter into the subrenal capsular space of a mouse during (left) and after (right) the implantation. Scale bars: 2 mm. Reproduced with permission from [328].
3.8.2. Urinary bladder
The urinary bladder is a muscular organ with hallow-like structure, which stores and evacuates urine. Bladder malfunction can occur in diverse conditions, such as SCI, neurogenic bladder injury, chronic and infection inflammation, and exstrophy [312]. Significant reduction in bladder capacity and compliance need to be augmented using surgical procedures [313]. MIRET have been used in urinary bladder augmentation in pre-clinical and clinical studies. For instance, Calvano et al. [314] employed laparoscopic augmentation cystoplasty as a minimally invasive approach for ureteral reconstruction in porcine models using a collagen-based biomaterial. All implanted animals survived 7 weeks after the implantation and the ureter function was recovered. Shalhav et al. [315] also utilized a minimally invasive laparoscopic technique to replace ureter and to deliver a biodegradable graft in a large animal model. Farahat et el. [316] used endoscopic urethroplasty to deliver an intestinal submucosal patch to treat urethral strictures in 10 patients. The patches were introduced using silicon catheters. Three months follow-up confirmed that the most cases recovered the functionality of urethra. Interestingly, the MIRET reduced time and complexity of normal endoscopic surgery. Endoscopic urethroplasty was also employed for treatment of iatrogenic and inflammatory strictures of the bulbar urethra in 9 patients [317]. MIRET have also been utilized to treat neurogenic bladder in some clinical trials [318]. However, more clinical trials are required to improve the efficiency and safety of MIRET in the clinic.
3.8.3. Liver
The liver plays an essential role in the body with multiple functions including the synthesis of cholesterol, triglycerides, proteins, glycogen, blood clotting factors, and bile production [319]. Liver tissue regeneration is a well-orchestrated and complex procedure. There are currently different cell types that can be used for liver regeneration, including xenogeneic cells and SC-derived hepatocytes. Liver progenitor cells were utilized in several clinical trials, but there are still challenges to be addressed before they can be used as a widespread clinical therapy [320]. In particular, safety, tumorigenicity, and xenozoonosis of xenogeneic cells are still a major concern. Hepatocytes delivered in a minimally invasive manner suffer from limited engraftment and lack of long-term efficacy [321]. In mice, human iPSC-derived hepatic cells were shown to repopulate cirrhotic liver and function [322]. An important potential source for hepatocytes can also be the discarded marginal livers [323]. However, clinical safety and efficacy of these cells need further investigation.
Engineered liver tissues can also be implanted in a minimally invasive way. In one work, engineered nucleating seeds of liver (6 mm diameter) were fabricated from hepatocytes, endothelial cells, and fibroblasts in fibrin and were shown to grow and expand by up to 50-fold in 11 weeks upon implantation in the mesentery of nude mice with induced liver injury [324]. They were able to perform liver function, including drug metabolism. Hepatic buds can also be produced by mixing human-iPSC-derived cells, BMSCs, and human umbilical vein endothelial cells (HUVECs) [325]. However, the risk of tumorigenicity is a concern. Hepatocyte-laden fibers were also developed using hepatocyte-laden alginate and chitosan/alginate [326] or hepatocyte- and fibroblast-laden alginate [327] for minimally invasive delivery of hepatic constructs. The alginate removal resulted in scaffold-free micro-organoids (1 mm long and ~50 mm diameter) with high cell viability of ~80% over 30 days (Figure 8B). Significantly enhanced liver function was observed for up to 90 days compared to monolayer culture and single cell type cultivation in hydrogel fibers [327]. Hepatic cell- and fibroblast-laden fibers were also shown to result in albumin production two times more than hepatocyte-fiber monocultures [328].
3.9. Eye
The eye is a delicate organ and its proper function is required for visualization. MIRET have been used to regenerate lens and retina tissues. For example, Lin et al. [329] used a minimally invasive capsulorhexis surgery recruiting stem cells to regenerate lens in vivo. Jafar Mazumder et al. [330] synthesized thermogelling and cell-adhesive HA/poly(N-isopropylacrylamide) biomaterials as potential MIRET in retinal repair. The cells were suspended in the biomaterial and injected into the subretinal space as a potential treatment for retinal diseases, such as diabetic retinopathy or age-related macular degeneration. Successful grafting of cell-laden biomaterial was shown with minimal host response. A widely used non-invasive therapy for eye treatment is photodynamic therapy (PDT) using light and photosensitizing material [331]. PDT is a treatment modality by which exogenous photosensitizers are activated in target cells using laser light. This photoactivation induces singlet oxygen or other reactive oxygen species in the cells leading to cell necrosis and/or apoptosis [332]. Chan et al. used PDT to treat acute central serous chorioretinopathy in patients in a one-year and randomized clinical trial [333]. Forty-three patients received indocyanine green angiography-guided PDT with verteporfin and twenty-one patients received placebo. The verteporfin-treated patients were subjected to PDT for 10 min using laser after the drug infusion. The results showed that the PDT with verteporfin is a more effective therapy in treating central serous chorioretinopathy compared to placebo. PDT has also been proposed as a cost-effective approach to treat age-related macular degeneration [334]. Taken together, further works need to be done to reveal potential applications of MIRET in eye regeneration and repair.
3.10. Skin
The skin covers the outermost surface of the body and it serves as a protective barrier. Regenerative skin therapeutics can usually be applied on top of skin lesion in a non-invasive manner [32]. The use of skin grafts as drug development models have comprehensively been discussed elsewhere [32]. There are some skin-related therapeutics, which require to cross the skin in a minimally invasive manner. For instance, microneedles have been recently developed and proposed for the treatment of chronic wounds [335]. In addition to increased drug dosage passed through the stratum corneum [336, 337], the use of microneedles is associated with minimal tissue damage and rapid healing [338]. Skin regeneration (especially in compromised wounds) can be promoted with drugs and growth factors delivered by microneedles [339]. Microneedles were also successfully used to deliver triamcinolone acetonide for the treatment of alopecia areata [340]. Steroids, minoxidil and PRP were administered via microneedles leading to stimulated hair follicle growth [341]. Microneedles have also been used for skin rejuvenation in which repeatedly puncturing the skin stimulates natural skin healing via the production of new collagen and elastin [335]. A clinical trial reported that significantly increased level of collagen and tropoelastin from baseline was obtained after six microneedling sessions resulting in skin tightening [342]. In a recent review, results of various clinical studies of microneedle application in skin regeneration were discussed [338]. In one clinical study, microneedle arrays (150-μm long with a tip radius of 1-μm) were used and shown to be painless, causing no skin damage or irritation [343]. Microneedles were also considered to be useful in transdermal drug delivery [337]. Recently, wearable thread-based patches using thermoresponsive hydrogels were developed for transdermal drug delivery [344, 345] (Figure 9). The threads were connected to a microcontroller that wirelessly communicated with a mobile phone for remotely controlled drug release upon heating [344].
Figure 9. Multifunctional thread-based dressing for transdermal drug delivery.
(A) Thermo-responsive particles were included in hydrogels and coated on flexible thread-based heater. Individual coated threads were woven together into fabrics and connected to a flexible microcontroller that individually powered them up and wirelessly communicated with mobile phone. Upon heating, thermos-responsive particles activated and drug was released. Reproduced with permission from [344]. (B) Drug delivery system as a bandage integrating heater and electronics. (i) The integrated flexible heater raised the temperature to release the payload of nanocarriers embedded in the nanofibers of the mesh. (ii) A typical wearable bandage with the miniaturized electronics. Reproduced with permission from [345].
PDT can also be used to deliver drugs to skin. For instance, Rossetti et al. used PDT to deliver a phthalocyanine derivative (i.e., zinc phthalocyanine tetrasulfonate) to skin [346]. This method can be explored for drug delivery for skin regenerative purposes.
MIRET can also be used to treat skin diseases, such as skin-related fistulas, skin cancer, acne, and skin ulcers. For instance, human-derived MSCs were shown to be efficacious and safe to treat perianal fistulas [347]. Note that antibacterial photodynamic inactivation is a common technique to treat acne and skin ulcers [348]; however, regenerative approaches are still a viable option. A multitude of scaffolds have been fabricated and commercialized for skin tissue regeneration [349]. Stem cells, growth factors, and drugs have also been incorporated into these scaffolds to further enhance the regeneration process. Moreover, biofabrication technologies have been utilized to include topographical cues in the scaffolds mimicking the structure of native skin tissues [350]. For instance, nanofiber scaffolds were fabricated using electrospinning technique and showed improved skin regenerative capability compared to scaffolds without any topographical cues. In particular, adhesion, proliferation, and migration of fibroblasts were increased on the electrospun nanofibers [351].
Sequential drug delivery system can be used for the treatment non-healing infected wounds. In a study, vancomycin (antibacterial) was linked to the injectable chitosan/HA hydrogel for first stage release, and VEGF were encapsulated into PLGA microspheres for subsequent delivery. Independent profile release of vancomycin (pH-dependent) and VEGF (depends on the pore size of PLGA microspheres) led to inhibition of bacteria growth and acceleration in vein endothelial cell proliferation in vitro and in vivo [352].
4. Current challenges and future perspectives
MIRET have already showed great applications in regeneration of different tissues and organs in the body. Progress in MIRET owes to great advances made in the fields of synthesis and fabrication of biomaterials, stem cell biology, microfabrication technologies, biosensors, monitoring, and delivery tools. These fields have significantly developed in recent years and that can benefit regenerative therapeutics to be delivered in a safe, controllable, and minimally invasive way. Scientific discussion and collaboration among experts in the aforementioned fields would be helpful to enhance the efficiency and widespread applications of MIRET. Proper integration of various technologies and biomaterials is a key factor for successful implantation of MIRET in the future (Figure 10). 3D printing technologies have recently been introduced into the biomedical field [353]. A 3D bioprinted GelMA hydrogel that embeds hiPSCs derived hepatic cells with HUVECs and ADSCs in a hexagonal system was developed and showed enhanced phenotype and function over weeks of culture as compared to 2D monolayer culture and a 3D hepatic progenitor cell-only model [354]. Combining advanced regenerative strategies and tools such as 3D bioprinting will enable to achieve better outcome and may help to reduce the need for transplantation. Vascularity of constructed tissue and its integration into the body upon subsequent implantation remains a big challenge which can be facilitated using bioprinting of endothelial cells, smooth muscle cells/stem cells with hollow fibers in dynamic microfluidic systems. MIRET can largely use the advantages of these technologies to fabricate clinically relevant and custom-designed structures of biomaterials or cell-laden constructs. Recently, a handheld device was developed for 3D bioprinting of cell-laden hydrogels in a direct-write and manual fashion [355]. Such devices can be helpful in minimally invasive implantation of regenerative therapeutics. To this end, printing nozzles should be minimized and be flexible to directly print biomaterials or cell-laden structures in vivo. The printed structures will harness the natural microenvironment in the body to continue their growth or maturation in situ. In particular, hydrogels with self-healing and shear thinning properties [356] are suitable candidates for 3D bioprinting of complex tissues at high resolution in the body.
Figure 10. Evolution of personalized and regenerative therapy.
(A) An illustration showing important advances made in different MIRET disciplines with future outlook. (B) Schematic showing the evolution of using cells, biomaterials, and other elements in regenerative therapy. More possibilities will be brought up in the future by integrating more technologies and multimodal MIRET.
The use of smart scaffolds with the ability to curve, fold, or roll-up in a dynamic and controllable manner will be useful for four-dimensional (4D) biofabrication of novel regenerative therapeutics that can be implanted in minimally invasive way (Figure 11A) [357]. Examples include the self-folding of PEG with different molecular weights and concentrations (Figure 11B) [358] and cellular origami [359]. These materials can be engineered to respond to stimuli in a controllable and minimally invasive manner in various tissue regeneration applications. For instance, it is feasible to develop a cellularized, multifunctional, and endovascular shape-memory polymer that can be folded and used for the treatment of diseased tubular structures. Smart scaffolds can also be designed and fabricated to release drugs and growth factors. For example, stimuli-responsive polymers may enable on-demand release in response to changes in pH, O2 level, and temperature [360]. The combination of stimuli-responsive materials in making MIRET may benefit the efficacy and function of regenerative therapeutics. A combined stimuli-responsive strategy (internal or external) may help in overcoming problems with using one method in particular, such as premature or burst release of biomolecules.
Figure 11. Four-dimensional (4D) biofabrication and implantation of cell-laden biomaterials.
(A) (i) 4D bioprinting of cell-laden self-folding hydrogel-based tubes using methacrylated alginate (AA-MA) or HA-MA on different substrates (glass or polystyrene (PS)). Green light (530 nm) was used for mild drying of structures. Instant folding into tubes was obtained upon immersion of crosslinked films in water, phosphate-buffered saline (PBS), or cell culture media. (ii) The tube responsiveness (cartoons (upper panel) and representative photographs (lower panel)) in water (1), same tube immersed in CaCl2 solution (2), which led to an additional crosslinking of alginate with Ca2+ ions and complete unfolding of the tube, and folded tube immersed in EDTA solution (3), where ethylenediaminetetraacetic acid (EDTA) bound the Ca2+ ions from the alginate, leading to refolding of the film into a tube [379]. (B) (i) Schematic of multi-walled PEG hydrogel tube encapsulating growth factors in uniaxial direction in low expansion layer providing sustained release of agents for improving neovascularization. (ii) Vascular endothelial growth factor (VEGF)-releasing multi-walled hydrogel tube to enhance vascularization (ii). (iii) Optical images of self-folding process for a bi-layered PEG hydrogel band (1 mm width and 20 mm length) on chicken chorioallantoic membrane (CAM). (iv) Optical images of vascular networks from top-view and microscopic histological cross-sections of CAMs that were stained with a marker for α-SMA. All hydrogel shapes were loaded with 60 ng of VEGF. Concentrations of VEGF for tube, ring, disk and strips hydrogels were 7.5, 7.5, 5.0 and 15.0 μg ml−1, respectively. Reproduced with permission from [358].
Biosensors have been utilized to monitor regenerative therapeutics in specific parts of the body [361]. For example, wireless and bioresorbable sensors were developed and implanted into deep areas of the brain [362] (Figure 3C). The sensors were capable of measuring intracranial pressure and temperature in mice. This sensor was dissolved within 30 hours. In vitro tests revealed the sensor adaptability to measure fluid flow, motion, pH or thermal characteristics with possible extensions to measure biomolecular-binding events in the deep brain. Biosensors can also be used to monitor the behavior and function of implanted cells in vivo and thereby to reveal the molecular mechanism of cell behavior and function, which requires their superior stability, high selectivity, and conjugation to cell biomarkers [363]. The use of flexible, biodegradable, and injectable siosensors will enable long-term monitoring of MIRET in dedicated organs, such as heart and brain [364]. Wearable wireless devices can also be used to amplify signals from biosensors for precision drug delivery [365].
Although some MIRET have reached clinical trials (Table 1), these therapeutics are not a major part of routine clinical therapy despite encouraging results in many studies. More and large-scale clinical trials are needed to prove the safety and efficacy of MIRET. These clinical trials will pave the way for regulatory approval and commercialization of therapeutics. To this end, procedures should be developed for large-scale production, preservation, and transport of regenerative therapeutics. Proper training of technicians, nurses, and doctors in clinics is important to successfully deliver regenerative therapeutics in a minimally invasive manner. We hope that these therapeutics would find wide applications in the clinic in the near future.
Table 1.
Examples of clinical trials of MIRET are presented showing the type of MIRET therapy, indication (disease/condition), phase of the trial and outcome. Various types of MERITs were provided, including the use of intracoronary cardiosphere-derived cells (CDCs), intramyocardial injection of different types of biomaterials such as hyaluronan (HA), poly ethylene glycol (PEG)-based materials for the treatment of myocardial infarction (MI), the use of mesenchymal stem cells (MSCs) and human central nervous system (CNS) stem cells, or Rho-associated protein kinase inhibitor (Rho-ROCKI) for the treatment of spinal cord injury (SCI), bone marrow derived MSCs (BMSCs), for the treatment of Parkinson’s disease (PD), and adipose tissue derived stromal cells (ADSCs) for the treatment of osteoarthritis or Intradiscal injection of recombinant human growth and differentiation factor-5 (rhGDF-5) for the treatment of degenerative intervertebral disc.
| No. | MIRET | Disease/condition | Clinical trial stage | Outcome | Ref. |
|---|---|---|---|---|---|
| 1 | Intracoronary infusion of bone marrow-derived cells | MI | Phase III | Some improvement in the heart function | [366] |
| 2 | Intracoronary CDCs | MI | Phase I | Reduction in scar mass, increases in viable heart mass and regional contractility, and regional systolic wall thickening | [367] |
| 3 | Intra-myocardial injection of some biomaterials, including hydrogels include collagen, Matrigel®, fibrin, HA, alginate, chitosan, PEG-based materials, and acrylamides. | MI | Phase II | Results depended on the case. Some cardiac function improvement was achieved. | [90] |
| 4 | MSCs | SCI | Phase I and II | Ongoing | [127] |
| 5 | Human CNS stem cell transplantation | SCI | Phase II | Terminated | [128] |
| 6 | Human spinal cord-derived neural stem cell transplantation | Chronic SCI | Phase I | Not reported | [129] |
| 7 | Rho-ROCKI in fibrin gel delivered to the epidural space through intrathecal injection | SCI | Phases I and II | The forty-eight patients with acute SCI treated with the Rho-ROCKI resulted in long-term improvement of motor function with no significant adverse effects. Following the latter study, a phase III clinical trial was initiated in 2016. | [136, 137] |
| 8 | Allogeneic BMSCs (delivered intravenously and intrathecally to the brain) | PD | Phase I and II | Treatment of PD | [368] |
| 9 | Human umbilical cord blood-derived MSCs | AD | Phase I | The safety and efficacy of cell therapy was confirmed | [369] |
| 10 | Autologous bone marrow mononuclear cells applied intrathecally | Traumatic brain injury | Phase I | Not reported | [370] |
| 11 | BMSCs | Stroke | Phases I and II | Significant improvement from baseline (mean) was reported for: (1) European Stroke Scale, (2) National Institutes of Health Stroke Scale, (3) Fugl-Meyer total score, and (4) Fugl-Meyer motor function total score | [195] |
| 12 | Human neural stem cell delivery | PD | Phases II and III | Not reported | [371] |
| 13 | Intracerebral transplantation of neural stem cells | Ischemic stroke | Phase I | Not reported | [372] |
| 14 | Transplantation of neural stem cell-derived neurons | PD | Phases I and II | Not reported | [373] |
| 15 | Direct brain infusion of glial cell line-derived neurotrophic factor | PD | Phase I | Improvement in the off-medication motor sub-score as well as in the activities of daily living sub-score | [184] |
| 16 | Platelet-rich fibrin dental implant for guided bone regeneration | Partially or completely edentulous healthy adults | Not indicated | Successful clinical application with no significant adverse events | [223] |
| 17 | ADSCs | Severe osteoarthritis of the knee | Phase I | The group injected with the lowest dose of cells had a statistically significant improvement in the cartilage repair. | [254] |
| 18 | ADSCs | Osteoarthritis in the human knee joint | Phase I | All patients gained full function and showed significant improvement compared to the baseline. | [250] |
| 19 | MSCs | Knee osteoarthritis | Phase I | After five years, all SC-treated patients had better performance than the baseline. | [251] |
| 20 | Intra-articular injection of allogeneic BMSCs | Knee osteoarthritis | Phases I and II | Significant improvement was observed in the cell-treated patients. | [255] |
| 21 | Intradiscal injection of allogenic MSCs in saline | Degenerative disc disease | Phases I and II | Significant relief of pain and disability and profound improvement in the disc quality of patients. | [374] |
| 22 | Intradiscal injection of allogeneic mesenchymal precursors | Degenerative disc disease | Phase II | Promising outcome (in terms of pain and function) after 36 months | [274] |
| 23 | Percutaneously injection of MSCs | Degenerative disc disease | Phase I | Improvement in disability and disc hydration | [269, 275] |
| 24 | Allogeneic chondrocytes | Lumbar spondylosis with mechanical low-back pain | Phase I | Ten patients had significant improvement, and eight out of nine patients with the posterior annular tears had significant resolution. | [292] |
| 25 | Autologous MSCs | Type 1 Diabetes | Not indicated | Preserved β-cell function after one year without adverse effects. | [304, 375] |
| 26 | Autologous nonmyeloablative hematopoietic stem cell transplantation | Type 1 Diabetes | Not indicated | Half of patients treated showed adverse immunological response after four years | [305] |
| 27 | Various regenerative medicine strategies | Neurogenic bladder | Not indicated | Clinical improvement based on urodynamics studies | [318] |
| 28 | Liver progenitor cell therapy | Various types of liver damage | Not indicated | Safety, tumorigenicity, and xenozoonosis of xenogeneic cells are still a major concern. | [320] |
| 29 | Microneedling for facial rejuvenation | Facial issues | Not indicated | Significant increase in the level of collagen and tropoelastin | [342] |
| 30 | Intradiscal rhGDF-5 | Degenerative disc disease | Phases I and II | Not reported | [283] |
5. Conclusions
MIRET have been evolved as a distinct entity as a result of interplay and alliance of different research fields, including biomaterial synthesis and fabrication, stem cell biology, microsurgery procedures, bioimaging, and microscale technologies. Nowadays, cells, biomaterials, biomolecules, and their combinations can be delivered in a minimally invasive way to regenerate different tissues and organs in the body with high yield and low risk, complications, and cost. Advanced approaches of monitoring, delivery, and stimulation of administered therapeutics in vivo using nanobiomaterials and soft bioelectronic devices provide great opportunities to further develop MIRET. Although some clinical studies have been done using MIRET, it is envisioned that such therapeutics would find wider applications as an efficient and translational therapy in the clinic.
Acknowledgements
The authors have no competing interests. The authors acknowledge funding from the National Institutes of Health (EB021857, AR066193, AR057837, CA214411, HL137193, EB024403, EB023052, EB022403, and EB021857), and Air Force Office of Sponsored Research under award #FA9550–15-1–0273.
References
- [1].He W, Goodkind D, Kowal P, An Aging World: 2015, International Population Report No. P95/16–1, United States Census Bureau, Washington, DC, USA: 2016. [Google Scholar]
- [2].Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li R-K, Caldarone CA, Radisic M, Nature Materials 2017, 16, 1038. [DOI] [PubMed] [Google Scholar]
- [3].Krettek C, Schandelmaier P, Nliclau T, Tscherne H, Injury 1997, 28, A20. [DOI] [PubMed] [Google Scholar]
- [4].Mayer HM, Wiechert K, Korge A, Qose I, “Minimally invasive total disc replacement: surgical technique and preliminary clinical results”, Berlin, Heidelberg, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC, Tissue Engineering Part A 2011, 18, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nikol S, Huehns TY, Krausz E, Armeanu S, Engelmann MG, Winder D, Salmons B, Hoefling B, Gene Therapy 1999, 6, 737. [DOI] [PubMed] [Google Scholar]
- [7].Sullivan SP, Murthy N, Prausnitz MR, Advanced Materials 2008, 20, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang S, Qiu Y, Gao Y, Acta Pharmaceutica Sinica B 2014, 4, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yan C, Mackay ME, Czymmek K, Nagarkar RP, Schneider JP, Pochan DJ, Langmuir 2012, 28, 6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amer MH, White LJ, Shakesheff KM, The Journal of pharmacy and pharmacology 2015, 67, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grellier M, Bareille R, Bourget C, Amedee J, J Tissue Eng Regen Med 2009, 3, 302. [DOI] [PubMed] [Google Scholar]; Amer MH, Rose FRAJ, Shakesheff KM, Modo M, White LJ, npj Regenerative Medicine 2017, 2, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC, Tissue Engineering Part A 2012, 18, 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Potts MB, Silvestrini MT, Lim DA, Surgical Neurology International 2013, 4, S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Behfar A, Latere J-P, Bartunek J, Homsy C, Daro D, Crespo-Diaz RJ, Stalboerger PG, Steenwinckel V, Seron A, Redfield MM, Terzic A, Circulation. Cardiovascular interventions 2013, 6, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bidarra SJ, Barrias CC, Granja PL, Acta biomaterialia 2014, 10, 1646. [DOI] [PubMed] [Google Scholar]
- [15].Wagner MA, Marks WH, Bhatia SK, 2014, 24, 151. [DOI] [PubMed] [Google Scholar]
- [16].Ahadian S, Savoji H, Khademhosseini A, CHEMICAL ENGINEERING PROGRESS 2018, 114, 56. [Google Scholar]
- [17].Chen MH, Wang LL, Chung JJ, Kim Y-H, Atluri P, Burdick JA, ACS Biomaterials Science & Engineering 2017, 3, 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O’Cearbhaill ED, Ng KS, Karp JM, Mayo Clinic Proceedings 2014, 89, 259. [DOI] [PubMed] [Google Scholar]
- [19].Johnson JR, Kuskowski MA, Wilt TJ, Annals of Internal Medicine 2006, 144, 116. [DOI] [PubMed] [Google Scholar]
- [20].Feneley RCL, Hopley IB, Wells PNT, Journal of Medical Engineering & Technology 2015, 39, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foley FEB, The Journal of Urology 1937, 38, 134. [Google Scholar]
- [22].Tingey KG, The Journal of the Association for Vascular Access 2000, 5, 14. [Google Scholar]
- [23].Martens TP, Godier AFG, Parks JJ, Wan LQ, Koeckert MS, Eng GM, Hudson BI, Sherman W, Vunjak-Novakovic G, Cell transplantation 2009, 18, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].French PJ, Tanase D, Goosen JFL, Sensors Update 2003, 13, 107. [Google Scholar]
- [25].Li C, Han J, Ahn CH, Biosensors and Bioelectronics 2007, 22, 1988. [DOI] [PubMed] [Google Scholar]
- [26].Nissen SE, Grines CL, Gurley JC, Sublett K, Haynie D, Diaz C, Booth DC, DeMaria AN, Circulation 1990, 81, 660. [DOI] [PubMed] [Google Scholar]
- [27].Trautner BW, Darouiche RO, Archives of internal medicine 2004, 164, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moustris GP, Hiridis SC, Deliparaschos KM, Konstantinidis KM, The International Journal of Medical Robotics and Computer Assisted Surgery 2011, 7, 375. [DOI] [PubMed] [Google Scholar]
- [29].Nelson BJ, Kaliakatsos IK, Abbott JJ, Annual Review of Biomedical Engineering 2010, 12, 55. [DOI] [PubMed] [Google Scholar]
- [30].Park SJ, Park S-H, Cho S, Kim D-M, Lee Y, Ko SY, Hong Y, Choy HE, Min J-J, Park J-O, Park S, Scientific Reports 2013, 3, 3394. [DOI] [PMC free article] [PubMed] [Google Scholar]; Park SJ, Lee YK, Cho S, Uthaman S, Park IK, Min JJ, Ko SY, Park JO, Park S, Biotechnology and bioengineering 2015, 112, 769. [DOI] [PubMed] [Google Scholar]
- [31].Karpelson M, Wei G-Y, Wood RJ, Sensors and actuators A: Physical 2012, 176, 78. [Google Scholar]
- [32].Mohammadi Mohammad H, Heidary Araghi B, Beydaghi V, Geraili A, Moradi F, Jafari P, Janmaleki M, Valente Karolina P, Akbari M, Sanati‐Nezhad A, Advanced Healthcare Materials 2016, 5, 2459. [DOI] [PubMed] [Google Scholar]
- [33].Qiu F, Fujita S, Mhanna R, Zhang L, Simona BR, Nelson BJ, Advanced Functional Materials 2015, 25, 1666. [Google Scholar]
- [34].Patra D, Sengupta S, Duan W, Zhang H, Pavlick R, Sen A, Nanoscale 2013, 5, 1273. [DOI] [PubMed] [Google Scholar]
- [35].Kim S, Qiu F, Kim S, Ghanbari A, Moon C, Zhang L, Nelson BJ, Choi H, Advanced Materials 2013, 25, 5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Srivastava SK, Medina-Sánchez M, Koch B, Schmidt OG, Advanced Materials 2016, 28, 832. [DOI] [PubMed] [Google Scholar]
- [37].TirgarBahnamiri P, Bagheri-Khoulenjani S, Medical Hypotheses 2017, 102, 56. [DOI] [PubMed] [Google Scholar]
- [38].Alemzadeh H, Raman J, Leveson N, Kalbarczyk Z, Iyer RK, PLOS ONE 2016, 11, e0151470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li J, Esteban-Fernández de Ávila B, Gao W, Zhang L, Wang J, Science Robotics 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Council NR, Mathematics and Physics of Emerging Biomedical Imaging, The National Academies Press, Washington, DC: 1996. [PubMed] [Google Scholar]
- [41].Darvishi S, Souissi M, Kharaziha M, Karimzadeh F, Sahara R, Ahadian S, Electrochimica Acta 2018, 261, 275 [Google Scholar]; Veiseh O, Gunn JW, Zhang M, Advanced Drug Delivery Reviews 2010, 62, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reddy LH, Arias JL, Nicolas J, Couvreur P, Chemical Reviews 2012, 112, 5818. [DOI] [PubMed] [Google Scholar]
- [43].Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC, Biomaterials 2008, 29, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kai Y, Lilei H, Xingxing M, Shuoqi Y, Liang C, Xiaoze S, Changhui L, Yonggang L, Zhuang L, Advanced Materials 2012, 24, 1868.22378564 [Google Scholar]
- [45].Ren J, Chen YI, Liu CH, Chen PC, Prentice H, Wu JY, Liu PK, Gene Therapy 2015, 23, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vermilyea SC, Lu J, Olsen M, Guthrie S, Tao Y, Fekete EM, Riedel MK, Brunner K, Boettcher C, Bondarenko V, Brodsky E, Block WF, Alexander A, Zhang S-C, Emborg ME, Cell Transplantation 2017, 26, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jing X.-h., Yang L, Duan X.-j., Xie B, Chen W, Li Z, Tan H.-b., Joint Bone Spine 2008, 75, 432. [DOI] [PubMed] [Google Scholar]
- [48].Nam SY, Ricles LM, Suggs LJ, Emelianov SY, PLOS ONE 2012, 7, e37267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hsieh S-C, Wang F-F, Lin C-S, Chen Y-J, Hung S-C, Wang Y-J, Biomaterials 2006, 27, 1656. [DOI] [PubMed] [Google Scholar]
- [50].Yang F, Cho S-W, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim B-S, Langer R, Anderson DG, Proceedings of the National Academy of Sciences 2010, 107, 3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chung T-H, Wu S-H, Yao M, Lu C-W, Lin Y-S, Hung Y, Mou C-Y, Chen Y-C, Huang D-M, Biomaterials 2007, 28, 2959. [DOI] [PubMed] [Google Scholar]
- [52].Fu Y, Kraitchman DL, Expert review of cardiovascular therapy 2010, 8, 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Owens EA, Henary M, El Fakhri G, Choi HS, Accounts of Chemical Research 2016, 49, 1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim SM, Jeong CH, Woo JS, Ryu CH, Lee J-H, Jeun S-S, International Journal of Nanomedicine 2016, 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jaworek A, Sobczyk AT, Journal of Electrostatics 2008, 66, 197. [Google Scholar]
- [56].Jaworek A, Powder Technology 2007, 176, 18. [Google Scholar]
- [57].Bock N, Dargaville TR, Woodruff MA, Progress in Polymer Science 2012, 37, 1510 [Google Scholar]; Bock N, Woodruff MA, Hutmacher DW, Dargaville TR, Polymers 2011, 3, 131. [Google Scholar]
- [58].Ahire JJ, Dicks LMT, Current Microbiology 2016, 73, 236. [DOI] [PubMed] [Google Scholar]
- [59].Guarino V, Altobelli R, Cirillo V, Cummaro A, Ambrosio L, Polymers for Advanced Technologies 2015, 26, 1359. [Google Scholar]
- [60].Soares RMD, Siqueira NM, Prabhakaram MP, Ramakrishna S, Materials Science and Engineering: C 2018. [DOI] [PubMed] [Google Scholar]
- [61].Ashammakhi N, Ndreu A, Piras A, Nikkola L, Sindelar T, Ylikauppila H, Harlin A, Gomes ME, Neves N, Chiellini E, Journal of nanoscience and nanotechnology 2007, 7, 862. [DOI] [PubMed] [Google Scholar]
- [62].Ashammakhi N, Wimpenny I, Nikkola L, Yang Y, Journal of biomedical nanotechnology 2009, 5, 1. [DOI] [PubMed] [Google Scholar]
- [63].Venugopal J, Zhang YZ, Ramakrishna S, Proceedings of the Institution of Mechanical Engineers, Part N: Journal of Nanoengineering and Nanosystems 2004, 218, 35. [Google Scholar]
- [64].Mouriño V, in Applications of Nanocomposite Materials in Drug Delivery, (Eds: Inamuddin, Asiri AM, Mohammad A), Woodhead Publishing, 2018, 491. [Google Scholar]
- [65].Yang G, Li X, He Y, Ma J, Ni G, Zhou S, Progress in Polymer Science 2018, 81, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McCall JG, Jeong J-W, Current Opinion in Pharmacology 2017, 36, 78. [DOI] [PubMed] [Google Scholar]
- [67].Jeong J-W, McCall Jordan G., Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim Joo Y., Jang K-I, Shi Y, Hong Daniel Y., Liu Y, Schmitz Gavin P., Xia L, He Z, Gamble P, Ray Wilson Z., Huang Y, Bruchas Michael R., Rogers John A., Cell 2015, 162, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Park SI, Shin G, McCall JG, Al-Hasani R, Norris A, Xia L, Brenner DS, Noh KN, Bang SY, Bhatti DL, Jang K-I, Kang S-K, Mickle AD, Dussor G, Price TJ, Gereau RW, Bruchas MR, Rogers JA, Proceedings of the National Academy of Sciences 2016, 113, E8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lecomte A, Castagnola V, Descamps E, Dahan L, Blatché MC, Dinis TM, Leclerc E, Egles C, Bergaud C, Journal of Micromechanics and Microengineering 2015, 25, 125003. [Google Scholar]
- [70].Zhuolin X, Shih-Cheng Y, Ning X, Tao S, Wei Mong T, Songsong Z, Lun-De L, Nitish VT, Chengkuo L, Journal of Micromechanics and Microengineering 2014, 24, 065015. [Google Scholar]
- [71].Maher B, Nature 2013, 499, 20. [DOI] [PubMed] [Google Scholar]
- [72].Le TYL, Chong JJH, Cell death discovery 2016, 2, 16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Segers VFM, Lee RT, Nature 2008, 451, 937. [DOI] [PubMed] [Google Scholar]
- [74].Cambria E, Pasqualini FS, Wolint P, Günter J, Steiger J, Bopp A, Hoerstrup SP, Emmert MY, npj Regenerative Medicine 2017, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bayes-Genis A, Vives J, Roura S, Mirabel C. m., Gálvez-Montón C, Stem Cell Research & Therapy 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E, The Lancet 2012, 379, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tallawi M, Rosellini E, Barbani N, Cascone MG, Rai R, Saint-Pierre G, Boccaccini AR, Journal of the Royal Society Interface 2015, 12, 20150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hwang H, Kloner R, Journal of Cardiovascular Pharmacology 2011, 57, 282. [DOI] [PubMed] [Google Scholar]
- [79].Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H, The American Journal of Pathology 2002, 160, 2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bei Y, Das S, Rodosthenous RS, Holvoet P, Vanhaverbeke M, Monteiro MC, Monteiro VVS, Radosinska J, Bartekova M, Jansen F, Li Q, Rajasingh J, Xiao J, Theranostics 2017, 7, 4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Janita AM, Christien MB, Volkmar F, Martina S, Christof S, Stem Cells International 2017. [Google Scholar]
- [82].Lewis FC, Kumar SD, Ellison-Hughes GM, Pharmacol Res. 2017. [DOI] [PubMed] [Google Scholar]
- [83].Hu Y-F, Dawkins JF, Cho HC, Marbán E, Cingolani E, Science translational medicine 2014, 6, 245ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hammoudi N, Ishikawa K, Hajjar RJ, Current opinion in cardiology 2015, 30, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mason D, Chen Y-Z, Krishnan HV, Sant S, Journal of Controlled Release 2015, 215, 101. [DOI] [PubMed] [Google Scholar]
- [86].Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ, Journal of cardiac failure 2009, 15, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Conant G, Ahadian S, Zhao Y, Radisic M, Scientific reports 2017, 7, 11807. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Science translational medicine 2013, 5, 173ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Journal of the American College of Cardiology 2012, 59, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deddens JC, Sadeghi AH, Hjortnaes J, van Laake LW, Buijsrogge BM, Doevendans PA, Khademhosseini A, Sluijte JPG, Advanced Healthcare Materials 2017, 6, 1600571. [DOI] [PubMed] [Google Scholar]
- [90].Zhu Y, Matsumura Y, Wagner WR, Biomaterials 2017, 129, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA, Nat Biomed Eng 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Ramakrishna S, International Journal of Nanomedicine 2012, 7, 5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kong HJ, Kaigler D, Kim K, Mooney DJ, Biomacromolecules 2004, 5, 1720. [DOI] [PubMed] [Google Scholar]
- [94].Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, Ng KW, Dokmeci MR, Ghaemmaghami AM, Khademhosseini A, Advanced healthcare materials 2016, 5, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ahadian S, Huyer LD, Estili M, Yee B, Smith N, Xu Z, Sun Y, Radisic M, Acta biomaterialia 2017, 52, 81. [DOI] [PubMed] [Google Scholar]; Ahadian S, Rahal R, Ramón‐Azcón J, Obregón R, Hasan A, Tissue Engineering for Artificial Organs: Regenerative Medicine, Smart Diagnostics and Personalized Medicine, 35. [Google Scholar]
- [96].Mahdavi GA, Ahmadi TSH, Masoud S, Yunes P, Amirhossein S, Journal of Cellular Biochemistry 2017, 118, 2454.28128477 [Google Scholar]
- [97].Wissing TB, Bonito V, Bouten CVC, Smits A, NPJ Regen Med 2017, 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Cha BH, Shin SR, Leijten J, Li YC, Singh S, Liu JC, Annabi N, Abdi R, Dokmeci MR, Vrana NE, Ghaemmaghami AM, Khademhosseini A, Advanced Healthcare Materials 2017, 6, n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Driessen-Mol A, Emmert MY, Dijkman PE, Frese L, Sanders B, Weber B, Cesarovic N, Sidler M, Leenders J, Jenni R, Journal of the American College of Cardiology 2014, 63, 1320. [DOI] [PubMed] [Google Scholar]
- [100].Takehara N, Nagata M, Ogata T, Nakamura T, Matoba S, Gojo S, Sawada T, Yaku H, Matsubara H, American Heart Association 2012. Circulation 2012. [Google Scholar]
- [101].Kaiser NJ, Coulombe KLK, Biomedical materials (Bristol, England) 2015, 10, 034003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Piao H, Piao S, Kwon J-S, Sohn J-H, Lee Y-S, Bae J-W, Hwang K-K, Kim D-W, Kim B-S, Jeon O, Park Y-B, Cho M-C, Biomaterials 2007, 28, 641. [DOI] [PubMed] [Google Scholar]
- [103].Moorthi A, Tyan Y-C, Chung T-W, Biomaterials Science 2017, 5, 1976. [DOI] [PubMed] [Google Scholar]
- [104].Gao L, Kupfer M, Jung J, Yang L, Zhang P, Da Sie Y, Tran Q, Ajeti V, Freeman B, Fast V, Campagnola P, Ogle B, Zhang J, Circulation Research 2017, 120, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kooreman NG, Wu JC, Journal of The Royal Society Interface 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J, Circulation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Awada HK, Johnson NR, Wang Y, Journal of Controlled Release 2015, 207, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ruvinov E, Leor J, Cohen S, Biomaterials 2011, 32, 565. [DOI] [PubMed] [Google Scholar]
- [109].Hagen EM, World Journal of Orthopedics 2015, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lee B, Cripps R, Fitzharris M, Wing P, Spinal cord 2014, 52, 110. [DOI] [PubMed] [Google Scholar]
- [111].Kanekiyo K, Wakabayashi T, Nakano N, Yamada Y, Tamachi M, Suzuki Y, Fukushima M, Saito F, Abe S, Tsukagoshi C, Miyamoto C, Ide C, J Neurotrauma 2018, 35, 521. [DOI] [PubMed] [Google Scholar]
- [112].Sufang H, Bin W, Xing L, Zhifeng X, Jin H, Yannan Z, Yongxiang F, Yanyun Y, Bing C, Jianwu D, Journal of Biomedical Materials Research Part A 2016, 104, 1759.26990583 [Google Scholar]
- [113].Oh SK, Jeon SR, Korean Journal of Neurotrauma 2016, 12, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W, Nature Neuroscience 2017, 20, 637. [DOI] [PubMed] [Google Scholar]
- [115].Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A, Methods 2016, 99, 62. [DOI] [PubMed] [Google Scholar]
- [116].Yao L, He C, Zhao Y, Wang J, Tang M, Li J, Wu Y, Ao L, Hu X, Neural regeneration research 2013, 8, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhu H, Poon W, Liu Y, Leung GK-K, Wong Y, Feng Y, Ng SCP, Tsang KS, Sun DTF, Yeung DK, Cell transplantation 2016, 25, 1925. [DOI] [PubMed] [Google Scholar]
- [118].Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M, The Journal of Clinical Investigation 2012, 122, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ, Nature medicine 2010, 16, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Tian L, Prabhakaran MP, Ramakrishna S, Regenerative biomaterials 2015, 2, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Steward O, Sharp Kelli G., Matsudaira Yee K, Cell 2014, 156, 385. [DOI] [PubMed] [Google Scholar]
- [122].Tuszynski Mark H., Wang Y, Graham L, Gao M, Wu D, Brock J, Blesch A, Rosenzweig Ephron S., t Leif A., Zheng B, Conner James M., Marsala M, Lu P, Cell 2014, 156, 388. [DOI] [PubMed] [Google Scholar]
- [123].Willerth SM, Sakiyama-Elbert SE, Advanced drug delivery reviews 2008, 60, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hofstetter CP, Holmström NAV, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L, Nature Neuroscience 2005, 8, 346. [DOI] [PubMed] [Google Scholar]
- [125].Steward O, Sharp KG, Yee KM, Hatch MN, Bonner JF, The Journal of Neuroscience 2014, 34, 14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Nguyen HX, Hooshmand MJ, Saiwai H, Maddox J, Salehi A, Lakatos A, Nishi RA, Salazar D, Uchida N, Anderson AJ, The Journal of Neuroscience 2017, 37, 9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127]. https://ClinicalTrials.gov/show/NCT02917291; , https://ClinicalTrials.gov/show/NCT02481440; , https://ClinicalTrials.gov/show/NCT03003364.
- [128]. https://ClinicalTrials.gov/show/NCT02163876.
- [129]. https://ClinicalTrials.gov/show/NCT01772810.
- [130].Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, Cummings BJ, Stem Cell Reports 2017, 8, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chhabra HS, Sarda K, Advanced Drug Delivery Reviews 2017, 120, 41. [DOI] [PubMed] [Google Scholar]
- [132].Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV, Nature 2016, 532, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Horner PJ, Gage FH, Nature 2000, 407, 963. [DOI] [PubMed] [Google Scholar]
- [134].Forgione N, Fehlings MG, World Neurosurgery 2014, 82, e535. [DOI] [PubMed] [Google Scholar]
- [135].Khorasanizadeh M, Eskian M, Vaccaro AR, Rahimi-Movaghar V, CNS Drugs 2017, 31, 911. [DOI] [PubMed] [Google Scholar]
- [136].Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L, J Neurotrauma 2011, 28, 787. [DOI] [PubMed] [Google Scholar]
- [137].Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG, Neurosurgery 2017, 80, S9. [DOI] [PubMed] [Google Scholar]
- [138].Mokarram N, Dymanus K, Srinivasan A, Lyon JG, Tipton J, Chu J, English AW, Bellamkonda RV, Proceedings of the National Academy of Sciences of the United States of America 2017, 114, E5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hickey K, Stabenfeldt SE, Biomedical Materials 2018, 13, 044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Perale G, Rossi F, Santoro M, Peviani M, Papa S, Llupi D, Torriani P, Micotti E, Previdi S, Cervo L, Sundström E, Boccaccini AR, Masi M, Forloni G, Veglianese P, Journal of Controlled Release 2012, 159, 271. [DOI] [PubMed] [Google Scholar]
- [141].Ziemba AM, Gilbert RJ, Frontiers in Pharmacology 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kazuto F, Hiroshi T, Hisakazu M, Peptide Science 2016, 106, 47626501895 [Google Scholar]; Vismara I, Papa S, Rossi F, Forloni G, Veglianese P, Trends in Molecular Medicine 2017, 23, 831. [DOI] [PubMed] [Google Scholar]
- [142].Ozer H, Bozkurt H, Bozkurt G, International Journal of Neuroscience 2018. [DOI] [PubMed] [Google Scholar]
- [143].Slotkin JR, Pritchard CD, Luque B, Ye J, Layer RT, Lawrence MS, O’Shea TM, Roy RR, Zhong H, Vollenweider I, Edgerton VR, Courtine G, Woodard EJ, Langer R, Biomaterials 2017, 123, 63. [DOI] [PubMed] [Google Scholar]
- [144].Perale G, Rossi F, Sundstrom E, Bacchiega S, Masi M, Forloni G, Veglianese P, ACS Chemical Neuroscience 2011, 2, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gelain F, Panseri S, Antonini S, Cunha C, Donega M, Lowery J, Taraballi F, Cerri G, Montagna M, Baldissera F, Vescovi A, ACS Nano 2011, 5, 227. [DOI] [PubMed] [Google Scholar]
- [146].Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA, The Journal of neuroscience : the official journal of the Society for Neuroscience 2008, 28, 3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kong X.-b., Tang Q.-y., Chen X.-y., Tu Y, Sun S.-z., Sun Z.-l., Neural Regeneration Research 2017, 12, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Baumann MD, Kang CE, Tator CH, Shoichet MS, Biomaterials 2010, 31, 7631. [DOI] [PubMed] [Google Scholar]
- [149].Tavakol S, Mousavi SMM, Tavakol B, Hoveizi E, Ai J, Sorkhabadi SMR, Mol Neurobiol 2017, 54, 2483. [DOI] [PubMed] [Google Scholar]
- [150].Fehlings MG, Perrin RG, Spine 2006, 31, S28. [DOI] [PubMed] [Google Scholar]
- [151].Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, De Laporte L, Small 2017, 13, 1702207. [DOI] [PubMed] [Google Scholar]
- [152].Du V, Luciani N, Richard S, Mary G, Gay C, Mazuel F, Reffay M, Menasché P, Agbulut O, Wilhelm C, Nature Communications 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Rose JC, Cámara-Torres M, Rahimi K, Köhler J, Möller M, De Laporte L, Nano Lett. 2017, 17, 3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Carvalho-de-Souza JL, Treger JS, Dang B, Kent SBH, Pepperberg DR, Bezanilla F, Neuron 2015, 86, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Eom K, Kim J, Choi JM, Kang T, Chang JW, Byun KM, Jun SB, Kim SJ, Small 2014, 10, 3853. [DOI] [PubMed] [Google Scholar]
- [156].Baranes K, Shevach M, Shefi O, Dvir T, Nano letters 2016, 16, 2916. [DOI] [PubMed] [Google Scholar]
- [157].Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L, Stem Cell Research & Therapy 2014, 5, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH, Cell 2012, 150, 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Berndt M, Li Y, Seyedhassantehrani N, Yao L, ACS Biomaterials Science & Engineering 2017, 3, 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lin X-Y, Lai B-Q, Zeng X, Che M-T, Ling E-A, Wu W, Zeng Y-S, Cell Transplantation 2016, 25, 1425. [DOI] [PubMed] [Google Scholar]
- [161].Caron I, Rossi F, Papa S, Aloe R, Sculco M, Mauri E, Sacchetti A, Erba E, Panini N, Parazzi V, Barilani M, Forloni G, Perale G, Lazzari L, Veglianese P, Biomaterials 2016, 75, 135. [DOI] [PubMed] [Google Scholar]
- [162].Liu J, Chen J, Liu B, Yang C, Xie D, Zheng X, Xu S, Chen T, Wang L, Zhang Z, Bai X, Jin D, Journal of the neurological sciences 2013, 325, 127. [DOI] [PubMed] [Google Scholar]
- [163].Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS, Biomaterials 2013, 34, 3775. [DOI] [PubMed] [Google Scholar]
- [164].Zweckberger K, Ahuja CS, Liu Y, Wang J, Fehlings MG, Acta Biomaterialia 2016, 42, 77. [DOI] [PubMed] [Google Scholar]
- [165].Ritfeld GJ, Rauck B, Novosat TL, Park D, Patel P, Roos RAC, Wang Y, Oudega M, Biomaterials 2014, 35, 1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Patel V, Joseph G, Patel A, Patel S, Bustin D, Mawson D, Tuesta LM, Puentes R, Ghosh M, Pearse DD, J Neurotrauma 2010, 27, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Williams RR, Henao M, Pearse DD, Bunge MB, Cell transplantation 2015, 24, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Macaya D, Spector M, Biomedical materials 2012, 7, 012001. [DOI] [PubMed] [Google Scholar]
- [169].Tavakol S, Saber R, Hoveizi E, Aligholi H, Ai J, Rezayat SM, Mol Neurobiol 2016, 53, 3298. [DOI] [PubMed] [Google Scholar]
- [170].Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, DeVries WH, Shields CB, Magnuson DSK, Xu X, Kim DH, Whittemore SR, The Journal of neuroscience : the official journal of the Society for Neuroscience 2010, 30, 2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Amr SM, Gouda A, Koptan WT, Galal AA, Abdel-Fattah DS, Rashed LA, Atta HM, Abdel-Aziz MT, J Spinal Cord Med 2014, 37, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Cheng T-Y, Chen M-H, Chang W-H, Huang M-Y, Wang T-W, Biomaterials 2013, 34, 2005. [DOI] [PubMed] [Google Scholar]
- [173].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O, Nature Medicine 2002, 8, 963. [DOI] [PubMed] [Google Scholar]
- [174].Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M, Cell Transplantation 2002, 11, 275. [PubMed] [Google Scholar]
- [175].Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L, Nature 2011, 480, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Felsenstein KM, Candelario KM, Steindler DA, Borchelt DR, Translational research : the journal of laboratory and clinical medicine 2014, 163, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tartaglione AM, Popoli P, Calamandrei G, Neuroscience & Biobehavioral Reviews 2017, 77. [DOI] [PubMed] [Google Scholar]
- [178].Staff N, Madigan N, Morris J, Jentoft M, Sorenson E, Butler G, Gastineau D, Dietz A, Windebank A, Neurology 2016, 87, 2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Nagahara AH, Tuszynski MH, Nature Reviews Drug Discovery 2011, 10, 209. [DOI] [PubMed] [Google Scholar]
- [180].Pardridge WM, Fluids and barriers of the CNS 2011, 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Alison B, Carlos AA-G, Milan G, Jessica FJ, Isabelle A, Kullervo H, PLoS One 2011, 6, e27877.22114718 [Google Scholar]
- [182].Ding H, Wu F, Nair MP, Drug discovery today 2013, 18, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Jessberger S, Transfusion Medicine and Hemotherapy 2016, 43, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].O’Sullivan K, Hotton GR, Brooks DJ, Gill SS, Bunnage M, Svendsen CN, Patel NK, McCarter R, Heywood P, Nature Medicine 2003, 9, 589. [DOI] [PubMed] [Google Scholar]
- [185].Blasberg RG, Patlak C, Fenstermacher JD, Journal of Pharmacology and Experimental Therapeutics 1975, 195, 73. [PubMed] [Google Scholar]
- [186].Blakeley J, Current Neurology and Neuroscience Reports 2008, 8, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Malloy KE, Li J, Choudhury GR, Torres A, Gupta S, Kantorak C, Goble T, Fox PT, Clarke GD, Daadi MM, STEM CELLS Translational Medicine 2017, 6, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tuominen J, Yrjänä SK, Katisko JP, Heikkilä J, Koivukangas J, in Intraoperative Imaging in Neurosurgery, Springer, 2003, 115. [DOI] [PubMed] [Google Scholar]
- [189].Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung Y-S, Olumolade O, Small SA, Morrison B, Konofagou EE, Physics in medicine and biology 2012, 57, N65. [DOI] [PMC free article] [PubMed] [Google Scholar]; Spieth S, Schumacher A, Kallenbach C, Messner S, Zengerle R, Journal of Micromechanics and Microengineering. 2012, 22, 065020. [Google Scholar]
- [190].Dagdeviren C, Ramadi KB, Joe P, Spencer K, Schwerdt HN, Shimazu H, Delcasso S, Amemori K.-i., Nunez-Lopez C, Graybiel AM, Cima MJ, Langer R, Science Translational Medicine 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Duan L, Liang Y, Ma B, Zhu W, Wang D, American Journal of Translational Research 2015, 7, 2127. [PMC free article] [PubMed] [Google Scholar]
- [192].Daniel JH, Jerry CH, Kyriacos AA, Science 2012, 338, 917.23161992 [Google Scholar]
- [193].Sun Y, Greet B, Burkland D, John M, Razavi M, Babakhani A, “Wirelessly powered implantable pacemaker with on-chip antenna”, 2017. [Google Scholar]
- [194].Berner A, Henkel J, Woodruff MA, Steck R, Nerlich M, Schuetz MA, Hutmacher DW, STEM CELLS Translational Medicine 2015, 4, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Steinberg G, Kondziolka D, Wechsler L, Lunsford L, Coburn M, Billigen J, Kim A, Johnson J, Bates D, King B, Case C, McGrogan M, Yankee E, Schwartz N, Stroke 2016, 47, 1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Vangsness JCT, Farr n. J., Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M, The Journal of bone and joint surgery. American volume 2014, 96, 90. [DOI] [PubMed] [Google Scholar]
- [197].Betjes MGH, Nature reviews. Nephrology 2011, 7, 257. [DOI] [PubMed] [Google Scholar]
- [198].O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S, AJIC: American Journal of Infection Control 2011, 39, S34. [DOI] [PubMed] [Google Scholar]
- [199].Vergidis P, Patel R, Infectious Disease Clinics of North America 2012, 26, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Donaghue IE, Tam R, Sefton MV, Shoichet MS, Journal of Controlled Release 2014, 190, 219. [DOI] [PubMed] [Google Scholar]
- [201].Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, Bumbaca D, Gadkar K, Hoyte K, Luk W, Lu Y, Ernst JA, Scearce-Levie K, Couch JA, Dennis MS, Watts RJ, Science translational medicine 2014, 6, 261ra154 [DOI] [PubMed] [Google Scholar]; Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, Loetscher H, Ghosh A, Freskgård P-O, Neuron 2014, 81, 49. [DOI] [PubMed] [Google Scholar]
- [202].Burgess A, Hynynen K, Advances in Experimental Medicine and Biology 2016, 880, 293. [DOI] [PubMed] [Google Scholar]; Poon C, McMahon D, Hynynen K, Neuropharmacology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, Nature Biotechnology 2011, 29, 341. [DOI] [PubMed] [Google Scholar]
- [204].Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y, Journal of Neurosurgery 2015, 122, 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y, Neurochemistry International 2017, 111, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Nih LR, Carmichael ST, Segura T, Current opinion in biotechnology 2016, 40, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Tzu-Wei W, Kai-Chieh C, Liang-Hsin C, Shih-Yung L, Chia-Wei Y, Yung-Jen C, Nanoscale 2017, 9, 16281.29046917 [Google Scholar]
- [208].Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS, Biomaterials 2009, 30, 4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Lim TC, ProQuest Dissertations Publishing, 2014. [Google Scholar]
- [210].Sun W, Motta A, Shi Y, Seekamp A, Schmidt H, Gorb SN, Migliaresi C, Fuchs S, Biomedical materials (Bristol, England) 2016, 11, 035009. [DOI] [PubMed] [Google Scholar]
- [211].Lynam D, Peterson C, Maloney R, Shahriari D, Garrison A, Saleh S, Mehrotra S, Chan C, Sakamoto J, Carbohydrate polymers 2014, 103, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Xie C, Liu J, Fu T-M, Dai X, Zhou W, Lieber CM, Nature materials 2015, 14, 1286. [DOI] [PubMed] [Google Scholar]
- [213].Willerth SM, Sakiyama-Elbert SE, Advanced drug delivery reviews 2007, 59, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Karvinen J, Joki T, Ylä-Outinen L, Koivisto JT, Narkilahti S, Kellomäki M, Reactive and Functional Polymers 2018, 124, 29. [Google Scholar]
- [215].Rao SR, Subbarayan R, Dinesh MG, Arumugam G, Raja STK, Experimental & Molecular Medicine 2016, 48, e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q, Scientific Reports 2016, 6, 37841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Aaron LC, Neal KB, Nicola LF, Apoorva H, Stephen C, Jennifer CM, Ronald PH, Kenneth P, Marius W, Joachim K, Zhiping PP, Prabhas VM, Nature Communications 2016, 7, 10862. [Google Scholar]
- [218].Niamh M, Abhay P, Eilís D, Scientific Reports (Nature Publisher Group) 2017, 7, 1. [Google Scholar]
- [219].Amini AR, Laurencin CT, Nukavarapu SP, Critical Reviews™ in Biomedical Engineering 2012, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Wang P, Song Y, Weir MD, Sun J, Zhao L, Simon CG, Xu HHK, Dental materials : official publication of the Academy of Dental Materials 2016, 32, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Mikael PE, Kim HS, Nukavarapu SP, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2017. [DOI] [PubMed] [Google Scholar]
- [222].Link DP, van den Dolder J, van den Beucken JJ, Wolke JG, Mikos AG, Jansen JA, Biomaterials 2008, 29, 675. [DOI] [PubMed] [Google Scholar]
- [223].Kfir E, Kfir V, Eliav E, Kaluski E, The Journal of oral implantology 2007, 33, 205. [DOI] [PubMed] [Google Scholar]
- [224].Blokhuis TJ, Injury 2009, 40, S11. [DOI] [PubMed] [Google Scholar]
- [225].Hulme PA, Krebs J, Ferguson SJ, Berlemann U, Spine 2006, 31, 1983. [DOI] [PubMed] [Google Scholar]
- [226].Chen F, Xia Y-H, Cao W-Z, Shan WEI, Gao Y, Feng BO, Wang D, Oncology Letters 2016, 11, 1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Manson NA, Phillips FM, Instructional course lectures 2007, 56, 273. [PubMed] [Google Scholar]
- [228].Tengjiao z., Huihui R, Ailing L, Bingchuan L, Caiyun c., Yanmei D, Yun T, Dong Q, Scientific Reports (Nature Publisher Group) 2017, 7, 1. [Google Scholar]
- [229].Kanter B, Vikman A, Brückner T, Schamel M, Gbureck U, Ignatius A, Acta Biomaterialia 2018. [DOI] [PubMed] [Google Scholar]
- [230].Bu BX, Wang MJ, Liu WF, Wang YS, Tan HL, Orthopaedics & traumatology, surgery & research : OTSR 2015, 101, 227. [DOI] [PubMed] [Google Scholar]
- [231].Spivak JM, Johnson MG, Journal of the American Academy of Orthopaedic Surgeons 2005, 13, 6. [DOI] [PubMed] [Google Scholar]
- [232].Xu HHK, Simon CG, Biomaterials 2005, 26, 1337. [DOI] [PubMed] [Google Scholar]
- [233].Xu HHK, Weir MD, Burguera EF, Fraser AM, Biomaterials 2006, 27, 4279. [DOI] [PubMed] [Google Scholar]
- [234].Kim MH, Kim BS, Int J Biol Macromol. 2018, 109, 57. [DOI] [PubMed] [Google Scholar]
- [235].Gaihre B, Lecka-Czernik B, Jayasuriya AC, Journal of Biomaterials Applications 2018, 32, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Mantripragada VP, Jayasuriya AC, Materials Science and Engineering: C 2014, 42, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Song Y, Zhang C, Wang P, Wang L, Bao C, Weir MD, Reynolds MA, Ren K, Zhao L, Xu HHK, Mater Sci Eng C Mater Biol Appl. 2017, 75, 895. [DOI] [PubMed] [Google Scholar]
- [238].Zhou H, Xu HHK, Biomaterials 2011, 32, 7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Wang P, Liu X, Zhao L, Weir MD, Sun J, Chen W, Man Y, Xu HHK, Acta biomaterialia 2015, 18, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Biomaterials 2015, 37, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Martínez‐Sanz E, Varghese OP, Kisiel M, Engstrand T, Reich KM, Bohner M, Jonsson KB, Kohler T, Müller R, Ossipov DA, Hilborn J, Journal of Tissue Engineering and Regenerative Medicine 2012, 6, s23. [DOI] [PubMed] [Google Scholar]
- [242].Ashammakhi N, J Craniofac Surg 2018, 29, 125. [DOI] [PubMed] [Google Scholar]
- [243].Yamada Y, Seong Boo J, Ozawa R, Nagasaka T, Okazaki Y, Hata K.-i., Ueda M, Journal of Cranio-Maxillofacial Surgery 2003, 31, 27. [DOI] [PubMed] [Google Scholar]
- [244].Wang X, Li G, Guo J, Yang L, Liu Y, Sun Q, Li R, Yu W, Experimental and Therapeutic Medicine 2017, 13, 1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Lee W. Y.-w., Wang B, Journal of Orthopaedic Translation 2017, 9, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S, World journal of orthopedics 2014, 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Musumeci G, Loreto C, Carnazza ML, Martinez G, Knee Surgery, Sports Traumatology, Arthroscopy 2011, 19, 307. [DOI] [PubMed] [Google Scholar]
- [248].Gilliland CA, Salazar LD, Borchers JR, The Physician and Sportsmedicine 2011, 39, 121. [DOI] [PubMed] [Google Scholar]; Sage W, Pickup L, Smith TO, Denton ERE, Toms AP, Rheumatology (Oxford, England) 2013, 52, 743. [DOI] [PubMed] [Google Scholar]
- [249].Rutten MJ, Collins JM, Maresch BJ, Smeets JHR, Janssen CM, Kiemeney LALM, Jager GJ, European Radiology 2009, 19, 722. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C, STEM CELLS Translational Medicine 2016, 5, 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Fodor PB, Paulseth SG, Aesthetic surgery journal / the American Society for Aesthetic Plastic surgery 2016, 36, 229. [DOI] [PubMed] [Google Scholar]
- [251].Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B, International Journal of Rheumatic Diseases 2016, 19, 219. [DOI] [PubMed] [Google Scholar]
- [252].Mingjie W, Zhiguo Y, Ning M, Chunxiang H, Weimin G, Gengyi Z, Yu Z, Mingxue C, Shuang G, Jiang P, Aiyuan W, Yu W, Xiang S, Wenjing X, Shibi L, Shuyun L, Quanyi G, Stem Cells International 2017, 2017, 1. [Google Scholar]
- [253].Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS, Osteoarthritis and Cartilage 2016, 24, 2135. [DOI] [PubMed] [Google Scholar]
- [254].Yves-Marie P, Lars R, Rosanna F, Oliver P, Christophe D, Frank B, Luc S, Louis C, Sandrine F, Philippe B, Danièle N, François C, Catherine C, Gina L, Joachim S, Daniel H, Sophie D, Ulrich N, Christian J, STEM CELLS Translational Medicine 2016, 5, 847.27217345 [Google Scholar]
- [255].Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J, Transplantation 2015, 99, 1681. [DOI] [PubMed] [Google Scholar]
- [256].Hyunchul JC, Gil LY, Hyoung SW, Hyang K, Won CJ, Cheol JE, Eun KJ, Hackjoon S, Sun SJ, Seob SI, Chan RJ, Sohee O, Sup YK, STEM CELLS 2014, 32, 1254.24449146 [Google Scholar]
- [257].Wong KL, Lee KBL, Tai BC, Law P, Lee EH, Hui JHP, Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 2020. [DOI] [PubMed] [Google Scholar]; Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García-Sancho J, Transplantation Journal 2013, 95, 1535. [DOI] [PubMed] [Google Scholar]; Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B, International Journal of Rheumatic Diseases 2011, 14, 211. [DOI] [PubMed] [Google Scholar]
- [258].Saw K-Y, Anz A, Siew-Yoke Jee C, Merican S, Ching-Soong Ng R, Roohi SA, Ragavanaidu K, Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 684. [DOI] [PubMed] [Google Scholar]; Saw K-Y, Anz A, Merican S, Tay Y-G, Ragavanaidu K, Jee CSY, McGuire DA, Arthroscopy: The Journal of Arthroscopic and Related Surgery 2011, 27, 493. [DOI] [PubMed] [Google Scholar]
- [259].Koh Y-G, Jo S-B, Kwon O-R, Suh D-S, Lee S-W, Park S-H, Choi Y-J, Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 748. [DOI] [PubMed] [Google Scholar]; Koh Y-G, Choi Y-J, The Knee 2012, 19, 902. [DOI] [PubMed] [Google Scholar]
- [260].Toh WS, Lai RC, Hui JHP, Lim SK, “MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment”, presented at Seminars in cell & developmental biology, 2017. [DOI] [PubMed] [Google Scholar]
- [261].De Bari C, Roelofs AJ, Current opinion in pharmacology 2018, 40, 74. [DOI] [PubMed] [Google Scholar]
- [262].Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D, Scientific reports 2017, 7, 16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Zhang S, Chu W, Lai R, Lim S, Hui J, Toh W, Osteoarthritis and cartilage 2016, 24, 2135. [DOI] [PubMed] [Google Scholar]; Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS, Biomaterials 2018, 156, 16. [DOI] [PubMed] [Google Scholar]
- [264].Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M, Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2013, 29, 174. [DOI] [PubMed] [Google Scholar]; Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, Restrepo A, Shive MS, The Journal of Bone and Joint Surgery. American Volume 2013, 95, 1640. [DOI] [PubMed] [Google Scholar]; Kon E, Roffi A, Filardo G, Tesei G, Marcacci M, Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 2015, 31, 767. [DOI] [PubMed] [Google Scholar]
- [265].Solouk A, Mirzadeh H, Amanpour S, Progress in Biomaterials 2014, 3, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Klein TJ, Rizzi SC, Reichert JC, Georgi N, Malda J, Schuurman W, Crawford RW, Hutmacher DW, Macromolecular Bioscience 2009, 9, 1049. [DOI] [PubMed] [Google Scholar]; Xuetao S, Jianhua Z, Yihua Z, Lei L, Hongkai W, Advanced Healthcare Materials 2013, 2, 846. [DOI] [PubMed] [Google Scholar]
- [267].Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L, Nanoscale 2017, 9, 4430. [DOI] [PubMed] [Google Scholar]
- [268].Raj PP, Pain Practice 2008, 8, 18. [DOI] [PubMed] [Google Scholar]
- [269].Orozco L, Soler R, Morera C, Alberca M, Sanchez A, Garcia-Sancho J, Transplantation 2011, 92, 822. [DOI] [PubMed] [Google Scholar]
- [270].Prologo JD, Pirasteh A, Tenley N, Yuan L, Corn D, Hart D, Love Z, Lazarus HM, Lee Z, Journal of vascular and interventional radiology : JVIR 2012, 23, 1084. [DOI] [PubMed] [Google Scholar]
- [271].David O, Tony G, Peter G, Jeffrey VR, Graham J, Stem Cells International 2015, 2015, 1. [Google Scholar]
- [272].Sakai D, Schol J, Journal of Orthopaedic Translation 2017, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Pettine KA, Murphy MB, Suzuki RK, Sand TT, STEM CELLS 2015, 33, 146. [DOI] [PubMed] [Google Scholar]
- [274].GlobeNewswire, Durable Three-Year Outcomes In Degenerative Disc Disease After a Single Injection of Mesoblast’s Cell Therapy, https://globenewswire.com/news-release/2017/03/15/937833/0/en/Durable-Three-Year-Outcomes-In-Degenerative-Disc-Disease-After-a-Single-Injection-of-Mesoblast-s-Cell-Therapy.html (accessed: March 2017).
- [275].Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ, Biomolecular engineering 2007, 24, 5. [DOI] [PubMed] [Google Scholar]
- [276].Sakai D, Grad S, Advanced drug delivery reviews 2015, 84, 159. [DOI] [PubMed] [Google Scholar]
- [277].Masuda K, European Spine Journal 2008, 17, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang H, Wang L, Park JB, Park P, Yang VC, Hollister SJ, La Marca F, Lin C-Y, Arthritis research & therapy 2009, 11, R172. [DOI] [PMC free article] [PubMed] [Google Scholar]; Than KD, Rahman SU, Wang L, Khan A, Kyere KA, Than TT, Miyata Y, Park Y-S, La Marca F, Kim HM, The Spine Journal 2014, 14, 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Wang S-Z, Rui Y-F, Tan Q, Wang C, Arthritis research & therapy 2013, 15, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Gui K, Ren W, Yu Y, Li X, Dong J, Yin W, Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 2015, 21, 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Akeda K, Ohishi K, Masuda K, Bae WC, Takegami N, Yamada J, Nakamura T, Sakakibara T, Kasai Y, Sudo A, Asian Spine Journal 2017, 11, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, LaSalle EE, Nguyen JT, Solomon JL, Lutz GE, PM&R 2016, 8, 1. [DOI] [PubMed] [Google Scholar]; Levi D, Horn S, Tyszko S, Levin J, Hecht-Leavitt C, Walko E, Pain Medicine 2016, 17, 1010. [DOI] [PubMed] [Google Scholar]
- [282].Monfett M, Harrison J, Boachie-Adjei K, Lutz G, International Orthopaedics 2016, 40, 1321. [DOI] [PubMed] [Google Scholar]
- [283]. https://ClinicalTrials.gov/show/NCT00813813.
- [284].Paglia DN, Singh H, Karukonda T, Drissi H, Moss IL, Spine 2016, 41, E449. [DOI] [PubMed] [Google Scholar]; Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM, Spine (Phila Pa 1976) 2007, 32, 1174. [DOI] [PubMed] [Google Scholar]; Walsh AJ, Bradford DS, Lotz JC, Spine 2004, 29, 156. [DOI] [PubMed] [Google Scholar]; Hou Y, Shi G, Shi J, Xu G, Guo Y, Xu P, International Orthopaedics 2016, 40, 1143. [DOI] [PubMed] [Google Scholar]
- [285].Pennicooke B, Moriguchi Y, Hussain I, Bonssar L, Härtl R, Cureus 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Bowles RD, Setton LA, Biomaterials 2017, 129, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Berlemann U, Schwarzenbach O, European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2009, 18, 1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Jingen H, Yang L, Ling C, Kwabena Gyabaah O-A, Gewen X, Feilong H, Junjie B, Xiangjin L, Yiping H, Scientific Reports (Nature Publisher Group) 2017, 7, 1. [Google Scholar]
- [289].Melrose J, Regenerative Medicine 2016, 11, 705. [DOI] [PubMed] [Google Scholar]
- [290].Colombini A, Ceriani C, Banfi G, Brayda-Bruno M, Moretti M, Tissue Engineering Part B: Reviews 2014, 20, 713. [DOI] [PubMed] [Google Scholar]
- [291].Di-Ke R, Hongkui X, Chao Z, Chaofeng W, Chen X, Chao L, Qing H, Vol. 16, Mary Ann Liebert, Inc, New Rochelle: 2010, 2381. [Google Scholar]
- [292].Coric D, Pettine K, Sumich A, Boltes MO, Journal of Neurosurgery: Spine 2013, 18, 85. [DOI] [PubMed] [Google Scholar]
- [293].Ostrovidov S, Hosseini V, Ahadian S, Fujie T, Parthiban SP, Ramalingam M, Bae H, Kaji H, Khademhosseini A, Tissue Engineering Part B: Reviews 2014, 20, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Qazi TH, Mooney DJ, Pumberger M, Geißler S, Duda GN, Biomaterials 2015, 53, 502. [DOI] [PubMed] [Google Scholar]
- [295].Ahadian S, Sadeghian RB, Yaginuma S, Ramón-Azcón J, Nashimoto Y, Liang X, Bae H, Nakajima K, Shiku H, Matsue T, Biomaterials science 2015, 3, 1449. [DOI] [PubMed] [Google Scholar]
- [296].Ahadian S, Ostrovidov S, Hosseini V, Kaji H, Ramalingam M, Bae H, Khademhosseini A, Organogenesis 2013, 9, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Kim J, Hadlock T, Cheney M, Varvares M, Marler J, Archives of facial plastic surgery 2003, 5, 403. [DOI] [PubMed] [Google Scholar]
- [298].Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M, FEBS Letters 2015, 589, 1257. [DOI] [PubMed] [Google Scholar]
- [299].Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H, Molecular Therapy 2014, 22, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Salehi S, Ostrovidov S, Ebrahimi M, Sadeghian RB, Liang X, Nakajima K, Bae H, Fujie T, Khademhosseini A, ACS Biomaterials Science & Engineering 2017, 3, 579. [DOI] [PubMed] [Google Scholar]
- [301].Das SLM, Kennedy JIC, Murphy R, Phillips ARJ, Windsor JA, Petrov MS, World Journal of Gastroenterology: WJG 2014, 20, 17196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Edmond AR, Breay WP, Peter AS, David B, Eman A, Norman MK, Jonathan RTL, Shapiro AMJ, Diabetes 2005, 54, 206015983207 [Google Scholar]; Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV, The New England Journal of Medicine 2000, 343, 230. [DOI] [PubMed] [Google Scholar]
- [303].Gangaram-Panday ST, Faas MM, de Vos P, Trends in Molecular Medicine 2007, 13, 164. [DOI] [PubMed] [Google Scholar]; Bonner-Weir S, Aguayo-Mazzucato C, Nature Reviews Endocrinology 2010, 6, 139. [DOI] [PubMed] [Google Scholar]; Bouwens L, Houbracken I, Mfopou JK, Nature reviews. Endocrinology 2013, 9, 598. [DOI] [PubMed] [Google Scholar]
- [304].Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K, Diabetes 2015, 64, 587. [DOI] [PubMed] [Google Scholar]
- [305].D’Addio F, Valderrama Vasquez A, Ben Nasr M, Franek E, Zhu D, Li L, Ning G, Snarski E, Fiorina P, Diabetes 2014, 63, 3041. [DOI] [PubMed] [Google Scholar]
- [306].Kriz J, Vilk G, Mazzuca DM, Toleikis PM, Foster PJ, White DJG, The American Journal of Surgery 2012, 203, 793. [DOI] [PubMed] [Google Scholar]; Schmidt C, Nature Biotechnology 2017, 35, 8. [DOI] [PubMed] [Google Scholar]
- [307].Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K-I, Nagasaka T, Tissue Engineering 2004, 10, 955. [DOI] [PubMed] [Google Scholar]; Ginebra MP, Espanol M, Montufar EB, Perez RA, Mestres G, Acta Biomaterialia 2010, 6, 2863. [DOI] [PubMed] [Google Scholar]
- [308].Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Nature Medicine 2016, 22, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Wang B, Liu W, Xing D, Li R, Lv C, Li Y, Yan X, Ke Y, Xu Y, Du Y, Lin J, Scientific Reports (Nature Publisher Group) 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Shin S-J, Park J-Y, Lee J-Y, Park H, Park Y-D, Lee K-B, Whang C-M, Lee S-H, Langmuir 2007, 23, 9104. [DOI] [PubMed] [Google Scholar]
- [311].Kang E, Jeong GS, Choi YY, Lee KH, Khademhosseini A, Lee S-H, Nature Materials 2011, 10, 877. [DOI] [PubMed] [Google Scholar]
- [312].Sahai A, Khan MS, Arya M, John J, Singh R, Patel HRH, Expert opinion on pharmacotherapy 2006, 7, 509. [DOI] [PubMed] [Google Scholar]
- [313].Husmann DA, Rathbun SR, Journal of pediatric urology 2008, 4, 381. [DOI] [PubMed] [Google Scholar]
- [314].Calvano CJ, Moran ME, Parekh A, Desai PJ, Cisek LJ, Journal of endourology 2000, 14, 213. [DOI] [PubMed] [Google Scholar]
- [315].Shalhav AL, Elbahnasy AM, Bercowsky E, Kovacs G, Brewer A, Maxwell KL, McDougall EM, Clayman RV, Journal of endourology 1999, 13, 241. [DOI] [PubMed] [Google Scholar]
- [316].Farahat YA, Elbahnasy AM, El-Gamal OM, Ramadan AR, El-Abd SA, Taha MR, Journal of endourology 2009, 23, 2001. [DOI] [PubMed] [Google Scholar]
- [317].le Roux PJ, The Journal of Urology 2005, 173, 140. [DOI] [PubMed] [Google Scholar]
- [318].Yoo JJ, Olson J, Atala A, Kim B, International neurourology journal 2011, 15, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Vella SJ, Beattie P, Cademartiri R, Laromaine A, Martinez AW, Phillips ST, Mirica KA, Whitesides GM, Analytical chemistry 2012, 84, 2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Riehle KJ, Dan YY, Campbell JS, Fausto N, Journal of Gastroenterology and Hepatology 2011, 26, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Yanagi Y, Nakayama K, Taguchi T, Enosawa S, Tamura T, Yoshimaru K, Matsuura T, Hayashida M, Kohashi K, Oda Y, Yamaza T, Kobayashi E, Scientific Reports 2017, 7, 14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Liu H, Kim Y, Sharkis S, Marchionni L, Jang Y-Y, Science Translational Medicine 2011, 3, 82ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Izamis M-L, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, Uygun K, PLOS ONE 2013, 8, e69758. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uygun BE, Izamis M-L, Jaramillo M, Chen Y, Price G, Ozer S, Yarmush ML, Artificial organs 2017, 41, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Stevens KR, Scull MA, Ramanan V, Fortin CL, Chaturvedi RR, Knouse KA, Xiao JW, Fung C, Mirabella T, Chen AX, McCue MG, Yang MT, Fleming HE, Chung K, de Jong YP, Chen CS, Rice CM, Bhatia SN, Science Translational Medicine 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Takanori T, Keisuke S, Masahiro E, Hiroyuki K, Masaki K, Takunori O, Ran-Ran Z, Yasuharu U, Yun-Wen Z, Naoto K, Shinsuke A, Yasuhisa A, Hideki T, Nature 2013, 499, 481.23823721 [Google Scholar]
- [326].Lee BR, Lee KH, Kang E, Kim D-S, Lee S-H, Biomicrofluidics 2011, 5, 022208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, Seki M, Biomaterials 2012, 33, 8304. [DOI] [PubMed] [Google Scholar]
- [328].Onoe H, Okitsu T, Itou A, Kato-Negishi M, Gojo R, Kiriya D, Sato K, Miura S, Iwanaga S, Kuribayashi-Shigetomi K, Matsunaga YT, Shimoyama Y, Takeuchi S, Nature Materials 2013, 12, 584. [DOI] [PubMed] [Google Scholar]
- [329].Lin H, Ouyang H, Zhu J, Huang S, Liu Z, Chen S, Cao G, Li G, Signer RAJ, Xu Y, Nature 2016, 531, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Mazumder MAJ, Fitzpatrick SD, Muirhead B, Sheardown H, Journal of Biomedical Materials Research Part A 2012, 100A, 1877. [DOI] [PubMed] [Google Scholar]
- [331].Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U, Graefe’s Archive for Clinical and Experimental Ophthalmology 2002, 240, 748. [DOI] [PubMed] [Google Scholar]
- [332].Huang P, Lin J, Wang X, Wang Z, Zhang C, He M, Wang K, Chen F, Li Z, Shen G, Advanced Materials 2012, 24, 5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Chan W-M, Lai TYY, Lai RYK, Liu DTL, Lam DSC, Ophthalmology 2008, 115, 1756. [DOI] [PubMed] [Google Scholar]
- [334].Sharma S, Brown GC, Brown MM, Hollands H, Shah GK, Ophthalmology 2001, 108, 2051. [DOI] [PubMed] [Google Scholar]
- [335].Singh A, Yadav S, Indian dermatology online journal 2016, 7, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Donnelly RF, Singh TRR, Woolfson AD, Drug Delivery 2010, 17, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Kalluri H, Banga AK, Journal of Drug Delivery Science and Technology 2009, 19, 303. [Google Scholar]
- [338].Ogundele M, Okafor HK, Journal of Pharmaceutical Research International 2017, 1. [Google Scholar]
- [339].Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF, Materials Science & Engineering R 2016, 104, 1 [Google Scholar]; Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, Juzeniene A, Iani V, McCarthy HO, Moan J, Journal of Controlled Release 2008, 129, 154. [DOI] [PubMed] [Google Scholar]
- [340].Chandrashekar B, Yepuri V, Mysore V, Journal of cutaneous and aesthetic surgery 2014, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Fertig RM, Gamret AC, Cervantes J, Tosti A, Journal of the European Academy of Dermatology and Venereology 2017. [DOI] [PubMed] [Google Scholar]
- [342].El‐Domyati M, Barakat M, Awad S, Medhat W, El‐Fakahany H, Farag H, International Journal of Dermatology 2015, 54, 1361. [DOI] [PubMed] [Google Scholar]
- [343].Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR, Anesthesia and Analgesia 2001, 92, 502. [DOI] [PubMed] [Google Scholar]
- [344].Mostafalu P, Kiaee G, Giatsidis G, Khalilpour A, Nabavinia M, Dokmeci MR, Sonkusale S, Orgill DP, Tamayol A, Khademhosseini A, Adv Funct Mater 2017. [Google Scholar]
- [345].Ali T, Alireza Hassani N, Pooria M, Ali KY, Mattia C, Musab A, Mohamed Shaaban A.-w., Zeynab Izadi N, Shahrzad L, Mohsen A, Nasim A, Seok Hyun Y, Adnan M, Mehmet RD, Ali K, Scientific Reports (Nature Publisher Group) 2017, 7, 1. [Google Scholar]
- [346].Rossetti FC, Lopes LB, Carollo ARH, Thomazini JA, Tedesco AC, Bentley MVLB, Journal of controlled release 2011, 155, 400. [DOI] [PubMed] [Google Scholar]
- [347].Ferrer L, Kimbrel EA, Lam A, Falk EB, Zewe C, Juopperi T, Lanza R, Hoffman A, Regenerative medicine 2016, 11, 33. [DOI] [PubMed] [Google Scholar]
- [348].Maisch T, Szeimies R-M, Jori G, Abels C, Photochemical & Photobiological Sciences 2004, 3, 907. [DOI] [PubMed] [Google Scholar]
- [349].Yildirimer L, Thanh NTK, Seifalian AM, Trends in Biotechnology 2012, 30, 638. [DOI] [PubMed] [Google Scholar]
- [350].Pereira RF, Barrias CC, Granja PL, Bartolo PJ, Nanomedicine 2013, 8, 603. [DOI] [PubMed] [Google Scholar]
- [351].Norouzi M, Boroujeni Samaneh M, Omidvarkordshouli N, Soleimani M, Advanced Healthcare Materials 2015, 4, 1114. [DOI] [PubMed] [Google Scholar]
- [352].Huang J, Ren J, Chen G, Li Z, Liu Y, Wang G, Wu X, Materials Science and Engineering: C 2018, 89, 213. [DOI] [PubMed] [Google Scholar]
- [353].N. BN; Ashammakhi F; Kaarela O, J Craniofac Surg 2018 [DOI] [PubMed] [Google Scholar]; Obregón R, Ramón-Azcón J, Ahadian S, Shiku H, Bae H, Ramalingam M, Matsue T, Journal of nanoscience and nanotechnology 2014, 14, 487. [DOI] [PubMed] [Google Scholar]
- [354].Xuanyi M, Xin Q, Wei Z, Yi-Shuan L, Suli Y, Hong Z, Justin L, Pengrui W, Cheuk Sun Edwin L, Fabian Z, Gen-Sheng F, Farah S, Shu C, Shaochen C, Proceedings of the National Academy of Sciences 2016, 113, 2206. [Google Scholar]
- [355].Highley CB, Rodell CB, Burdick JA, Advanced Materials 2015, 27, 5075. [DOI] [PubMed] [Google Scholar]
- [356].Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A, Olsen BD, ACS nano 2014, 8, 9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Ahadian S, Khademhosseini A, Regenerative Biomaterials 2018, 5, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Baek K, Jeong JH, Shkumatov A, Bashir R, Kong H, Advanced Materials 2013, 25, 5568. [DOI] [PubMed] [Google Scholar]
- [359].Kuribayashi-Shigetomi K, Onoe H, Takeuchi S, PLOS ONE 2012, 7, e51085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Wei M, Gao Y, Li X, Serpe MJ, Polymer Chemistry 2017, 8, 127. [Google Scholar]
- [361].Yoo YK, Lee J, Kim J, Kim G, Kim S, Kim J, Chun H, Lee JH, Lee CJ, Hwang KS, Scientific reports 2016, 6, 33694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Seung-Kyun K, Rory KM, Suk-Won H, Seung Min L, Daniel VH, Neil AK, Jiho S, Paul G, Huanyu C, Sooyoun Y, Zhuangjian L, Jordan GM, Manu S, Hanze Y, Jeonghyun K, Gayoung P, Webb RC, Chi Hwan L, Sangjin C, Dae Seung W, Amit DG, Bharat V, Albert HK, Kyung-Mi L, Jianjun C, Younggang H, Sang Hoon L, Paul VB, Wilson ZR, John AR, Nature 2016, 530, 71.26779949 [Google Scholar]
- [363].Wegner KD, Hildebrandt N, Chemical Society Reviews 2015, 44, 4792. [DOI] [PubMed] [Google Scholar]
- [364].Hong G, Fu T-M, Zhou T, Schuhmann TG, Huang J, Lieber CM, Nano letters 2015, 15, 6979. [DOI] [PubMed] [Google Scholar]
- [365].Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, Shin K, Choi SH, Hyeon T, Kim D-H, Science advances 2017, 3, e1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366]. https://ClinicalTrials.gov/show/NCT01569178.
- [367].Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Marbán E, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, The Lancet 2012, 379, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368]. https://ClinicalTrials.gov/show/NCT01446614.
- [369]. https://ClinicalTrials.gov/show/NCT01297218.
- [370]. https://ClinicalTrials.gov/show/NCT02028104.
- [371].https://ClinicalTrials.gov/show/NCT03128450; , https://ClinicalTrials.gov/show/NCT02452723.
- [372]. https://ClinicalTrials.gov/show/NCT03296618.
- [373]. https://ClinicalTrials.gov/show/NCT03309514.
- [374]. https://ClinicalTrials.gov/show/NCT01860417.
- [375]. https://ClinicalTrials.gov/show/NCT01068951.
- [376].Kelm JM, Fussenegger M, Advanced drug delivery reviews 2010, 62, 753. [DOI] [PubMed] [Google Scholar]
- [377].O’Cearbhaill ED, Ng KS, Karp JM, “Emerging medical devices for minimally invasive cell therapy”, 2014. [DOI] [PubMed] [Google Scholar]
- [378].Mahmoudi M, Yu M, Serpooshan V, Wu JC, Langer R, Lee RT, Karp JM, Farokhzad OC, Nature nanotechnology 2017, 12, 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Alina K, Ridge M, Georgi S, G. CT, Leonid I, Advanced Materials 2017, 29, 1703443. [Google Scholar]