Abstract
While the liver demonstrates remarkable resilience during aging, there is growing evidence that it undergoes all the cellular hallmarks of aging, which increases the risk of liver and systemic disease. The aging process in the liver is driven by alterations of the genome and epigenome that contribute to dysregulation of mitochondrial function and nutrient sensing pathways, leading to cellular senescence and low-grade inflammation. These changes promote multiple phenotypic changes in all liver cells (hepatocytes, liver sinusoidal endothelial, hepatic stellate and Küpffer cells) and impairment of hepatic function. In particular, age-related changes in the liver sinusoidal endothelial cells are a significant but under-recognized risk factor for the development of age-related cardiometabolic disease.
Keywords: Endothelial, Hepatocyte, Genetic, Nutrient sensing pathways, Mitochondrial dysfunction, Senescence
Abbreviations: αSMA, alpha smooth muscle actin; AMPK, 5′ adenosine monophosphate-activated protein kinase; CR, caloric restriction; FOXO, forkhead box O; HSC, hepatic stellate cell; IGF-1, insulin like growth factor 1; IL-6, interleukin 6; IL-8, interleukin 8; KC, Küpffer cell; LSEC, liver sinusoidal endothelial cell; miR, microRNA; mTOR, mammalian target of rapamycin; NAD, nicotinamide adenine dinucleotide; NAFLD, non-alcoholic fatty liver disease; NO, nitric oxide; PDGF, platelet derived growth factor; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-α; ROS, reactive oxygen species; SIRT1, sirtuin 1; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor
Graphical Abstract
Highlights
-
•
Liver aging is driven by transcription and metabolic epigenome alterations.
-
•
This leads to cellular senescence and low-grade inflammation.
-
•
Hepatocyte, sinusoidal endothelial, stellate and Küpffer cells undergoes the hallmarks of aging.
-
•
Each cell type demonstrates phenotypical cellular changes with age.
1. Introduction
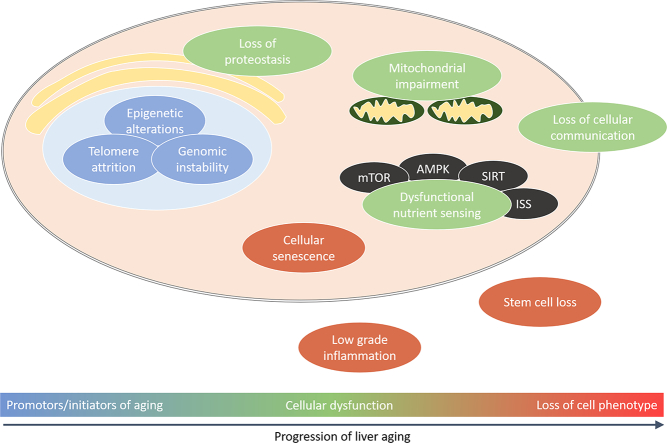
Aging leads to the progressive impairment of homeostasis at genomic, cellular, tissue and whole organism levels, which reduce survival and fertility while increasing the risk of disease and death. At the cellular level, aging is secondary to multiple processes that have been described as the ‘Hallmarks of Aging’: epigenetic alterations, genomic instability, telomere attrition, loss of proteostasis, dysregulation of nutrient sensing, altered intracellular communication, mitochondrial dysfunction, stem cell exhaustion, cellular senescence, inflammation and impaired adaption to stress [1]. Aging is more influential than any other risk factor for the development of chronic diseases such as neurodegeneration, cardiovascular disease, diabetes mellitus, osteoporosis and cancer; furthermore, there is considerable overlap between the Hallmarks of Aging and the pathogenic mechanisms for these diseases [2].
The liver is a complex metabolic organ that is essential for maintaining whole body homeostasis via regulation of energy metabolism, xenobiotic and endobiotic clearance, and molecular biosynthesis [3], therefore age-related changes in liver function contribute to systemic susceptibility to age-related diseases. For example, the liver regulates systemic energy metabolism via hepatic glucose and lipid homeostasis, steroid biosynthesis/degradation and insulin signaling [3]. Thus, the liver plays a key role in mediating the beneficial effects of nutritional interventions such as caloric restriction (CR) and protein restriction on aging and age-related disease. On the other hand, dysregulation of hepatic energy metabolism contributes to common age-related conditions such as insulin resistance, diabetes mellitus and non-alcoholic fatty liver disease (NAFLD) [4,5].
The Hallmarks of Aging impact directly on all the different types of liver cells: hepatocytes, liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs) and Küpffer cells (KCs). Most research on aging in the liver has focused on hepatocytes and there is substantial literature on the Hallmarks of Aging and these cells. Moreover, many studies of the aging liver have used liver tissue which is primarily hepatocytes. For the other three liver cell types, there is much less known about the effects of aging at the cellular level. Here, the effects of aging all liver cell types are reviewed, then integrated in a unified model at the organ level.
2. Hepatocytes
Hepatocytes are the parenchymal cells of the liver responsible for the majority of hepatic functions. Hepatocytes synthesize albumin, fibrinogen, and lipoproteins; regulate fatty acid and carbohydrate metabolism; synthesize cholesterol and bile salts; and contribute to drug and xenobiotic metabolism. These processes are regulated by targeted gene transcription, endoplasmic reticular protein synthesis, mitochondrial respiratory processes and autophagy [3,[6], [7], [8]]. Hepatocytes also control liver growth and repair via release of growth factors, particularly vascular endothelial growth factor (VEGF) (Fig. 1) [7].
Fig. 1.
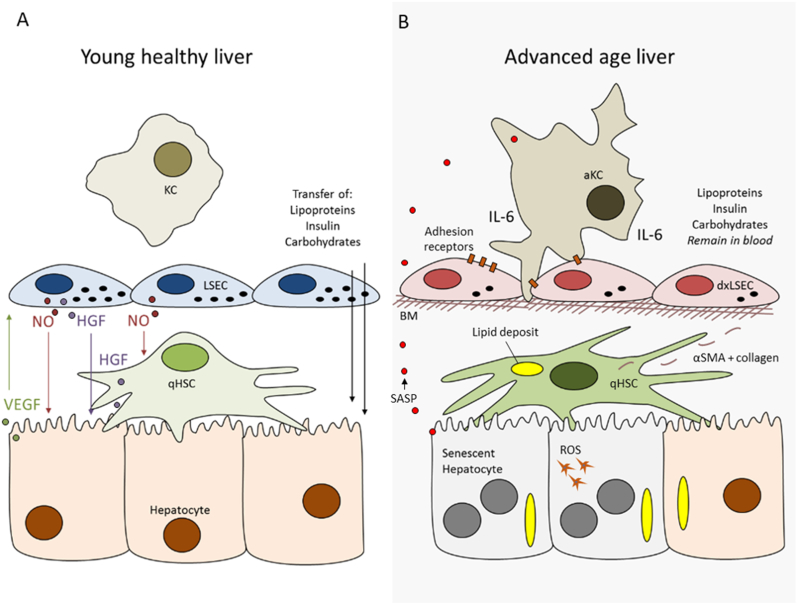
The aging liver. In the young liver solutes such as lipoproteins, insulin and carbohydrates are able to diffuse between the blood and hepatocytes, via the LSECs fenestrations. Intercellular communication via the release of vascular endothelial growth factor (VEGF) from hepatocytes and nitric oxide (NO) and hepatocyte growth factor (HGF) from LSECs and HSCs maintain the homeostatic phenotype of these liver cells. With age there are multiple changes to each cell type impairing the VEGF, NO and HGF dynamic. Hepatocytes demonstrate increased polyploidy and DNA damage, accumulation of lipofuscin, reduced mitochondrial oxidative capacity, increased oxidative stress and reactive oxygen species (ROS) and senescent cell accumulation with senescence associated secretory phenotype (SASP) (Section 2). SASP promotes recruitment of inflammatory cells. LSECs have reduced fenestrations, impaired angiocrine factor release and cellular autophagy as well as increased cell adhesion marker expression (Section 3). HSCs demonstrate phenotypical changes such as increased lipid loading, collagen and lamina production leading to basal membrane (BM) deposition, impaired vitamin A metabolism, promoting low-grade inflammation (Section 4). KCs accumulate within the liver with age and adhere to the adhesion markers expressed on LSECs. KCs contribute to the low-grade inflammation of the liver and drive interleukin 6 (IL-6) release but demonstrate impaired phagocytosis (Section 5). Abbreviations: a: activated; dx: dysfunction; q: quiescent.
With aging, there is evidence of genomic instability in hepatocytes. The number of hepatocytes decreases, associated with reduced rates of DNA synthesis and repair in addition to increased numbers of polyploid hepatocytes [9]. Oxidative stress leads to accumulation of products of lipid peroxidation and elevated nuclear DNA lesions such as 8-oxo-deoxyguanine [10]. There is dysregulation of senescence-associated signaling pathways such as p16, p21 and p53 [11]. In old mice, larger nuclei with high density DNA indicate an increase in the packing levels of condensed chromatins as suggested by increased extended chromatin fibres [12]. There is decreased expression of sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), and lower concentrations of nicotinamide adenine dinucleotide (NAD+) which lead to dysregulation of glycolysis, triglyceride synthesis, and lipid metabolism [13]. Sirtuin 6 expression also decreases and is associated with accumulation of DNA damage [13].
In a recent study it was shown that a subset of hepatocytes express high levels of telomerase [14]. This subset was further observed to promote repopulation of hepatocytes during normal homeostasis and following liver injury. Comparison between subtypes of hepatocytes using RNA sequencing demonstrated that metabolic activity is repressed and there is an acceleration of regenerative activity in hepatocytes with high telomerase expression.
Telomere shortening correlates with hepatocyte cell senescence and with the degree of hepatic cirrhosis and fibrosis. The extent to which telomere shortening contributes to senescent hepatocytes is debated [15,16] because hepatocytes are relatively resistant to replicative aging and the effects of telomere attrition [17]. Aging in hepatocytes is associated with various markers of cellular senescence such as increased heterochromatin protein 1β, elevated senescence-associated-β-galactosidase activity, p21, p16 and γ-H2AX [18]. Senescence may also be promoted by pathways independent of telomere shortening such as genomic damage, mitogens and other proliferation signals [19]. p53 is critical in cellular senescence in normal liver aging and models of DNA damage [20], and regulation of p53 is dependent on nutrient sensing pathways in NAFLD [21].
In hepatocytes age-associated DNA hyper-methylation has been reported for 18S and 28S ribosomal RNA genes in mice [22]. Specifically, negative correction between DNA methylation and hepatic glucokinase expression was also shown in old rats, where eleven CpG sites had age-related methylation in hepatic glucokinase promoter regions, this suggesting age-dependent susceptibility to hepatic insulin resistance and diabetes [23].
Impairment of metabolic pathways in the aging liver may be related to hepatocyte senescence. Senescent hepatocytes have altered expression of the Glut2 and Glut4 [24] as well as other genes involved in hepatic metabolism of glucose, lipids and proteins such as PI3K/Akt, MAPK, Jak/S, NF-κB, TGFβ, IGF1 and Ca/cAMP [25]. Aging hepatocytes have reduced mitochondrial enzymes (mitochondrial nitric oxide synthase, manganese superoxide dismutase, complexes I and IV) [26] and senescent hepatocytes release cytokines such as interleukin 6 (IL-6), tumor necrosis factor 1-α (TNFα) and interleukin 8 (IL-8) that contribute to age-related inflammation (referred to as the senescence associated secretory phenotype) (Fig. 1) [19,27]. Senescent hepatocytes have increased lipid droplet accumulation, decreased mitochondrial oxidizing capacity and increased production of reactive oxygen species (ROS) [28]. Similar findings are found in steatotic livers [29] providing an explanation for the increased incidence of NAFLD and non-alcoholic steatohepatitis in older people.
There are numerous age-related changes in mitochondria in hepatocytes. These include decreased mitochondrial biogenesis and autophagic degradation of mitochondria (mitophagy), dissociation of ATP synthase and increased accumulation of ROS leading to mitochondria DNA damage and impairment of respiratory chain complexes [4,[30], [31], [32]]. Age-dependent structural changes in the cristae and inner membrane of the mitochondria are observed, and mitochondria are often enlarged (‘mega-mitochondria’) [30]. In hepatocytes from Ercc1-deficient mice (a model of premature aging) abnormal mitochondrial morphology linked to mitochondrial dysfunction was observed [33]. These cells had reduced oxygen consumption, impaired mitochondrial membrane potential and proton leakage leading to elevated oxidative stress [33].
Autophagy is a key target of aging in the liver. There are three types of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy [8]. Macroautophagy is the most important for maintaining hepatic homeostasis [34]. The central role played by autophagy is proteolysis and hydrolysis of lipid stores and glycogen [8,35]. Homeostatic regulation of autophagy is dependent on energy sensing (upregulated during fasting or starvation), the circadian liver clock (cyclic patterns of cytochrome 1 upregulates gluconeogenesis), while high levels of ATP, insulin and free fatty acids downregulate autophagy [35]. Autophagy is suppressed in old livers [36] with a significant reduction in the number of hepatocytes that have autophagic vesicles in old mice [37]. The rate of autophagy-mediated proteolysis decreases with age [38], which possibly involves age-related impairment to the lipid kinase phosphatigylinositol-3-OH-kinase and p70 S6 kinase pathways [39]. The reduction in autophagy is promoted by changes in energy sensing pathways that occur with aging [40,41]. Age-associated decline in macroautophagy and chaperone-mediated autophagy lead to elevated levels of oxidized proteins and lipid peroxidation, protein misfolding and aggregation and in vivo elevation of alanine transaminase [40]. Chaperone-mediated autophagy is also impaired by aging that can lead to a gradual loss of proteostasis [42,43]. Overall, inadequate removal of damaged proteins leads to formation of protein aggregates such as lipofuscins, which are commonly observed in aged hepatocytes [[44], [45], [46]]. Subsequently, buildup of lipofuscins can increase the production of ROS that further inhibits autophagy [42,44].
Liver regeneration and repair is driven by a complex network of mitogenic growth factors, non-mitogenic cytokines, paracrine mediators and transcription factors [47]. With aging, there is a reduction in liver regeneration following partial hepatectomy [7]. No changes in VEGF expression are observed with age [48], however there is a reduced or absent activation of liver regeneration S-phase specific genes, DNA pol+, c-Myc, Cdc2 and Foxo1B and these are thought to contribute to reduced and delayed DNA replication and signaling cascades which control proliferation and repair of hepatocytes [7]. Age-associated oxidative stress inhibits activation of progenitor liver cells, thus causing further depletion of hepatocytes and liver mass [49].
3. Liver Endothelial Sinusoidal Cells
LSECs, the endothelial cells that line the hepatic sinusoids, have a number of important physiological roles, including facilitating bidirectional transfer of substrates between blood and hepatocytes, endocytosis of circulating proteins, regulation of immunotolerance, and maintaining sinusoidal microenvironment. With aging there are substantial changes in the structure and function of LSECs which impact on hepatic function and systemic risk of cardiometabolic disease. Given that old age is associated with changes in blood vessels in all other tissues and is a key contributor to many organ-level diseases, it is not surprising that aging influences LSECs [50].
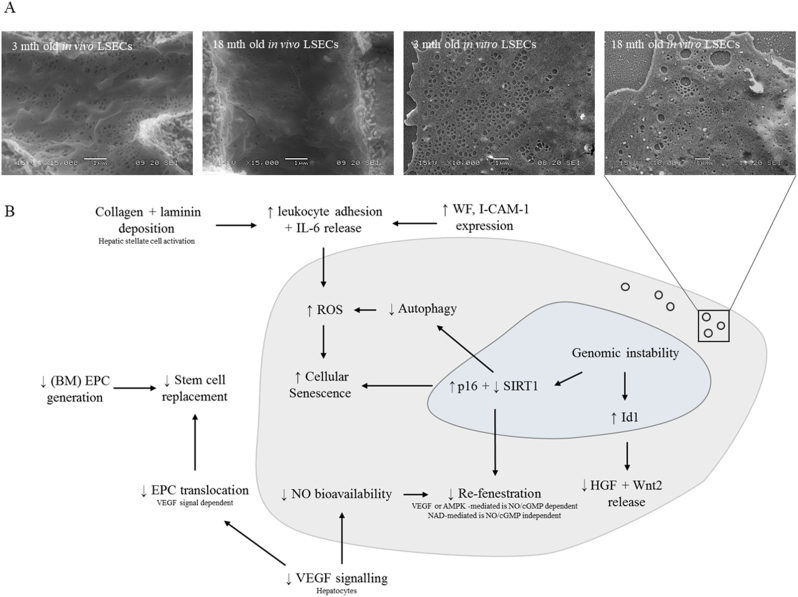
LSECs have a unique morphology that minimizes barriers for the exchange of substrates. LSECs are very thin and the cytoplasm is perforated by numerous nano-pores called fenestrations. Fenestrations have a diameter between 50 and 250 nm, and are mostly clustered into groups of 10–100 or more, called ‘sieve plates’ that occupy about 5% of the cell surface area. Fenestrations are dynamic structures and may vary in size and number in response to metabolites, cytokines, and oxygen concentration. In young animals, there is minimal basal lamina and collagen in the extravascular space (‘space of Disse’) again minimizing transport barriers. Pseudocapillarization is a term that has been used to describe the morphological changes that occur in LSEC with old age, which include: reduction in the number and size of fenestrations (Fig. 2a); thickening of the endothelium; deposition of basal lamina and collagen; altered expression of antigens such as von Willebrands factor, CD31 and collagen; and increased perisinusoidal staining with Masson's Trichome and Sirius Red (Fig. 1). This has been documented in several species including mice, rats, non-human primates and humans, and in mouse models of premature aging (ERCC1−/− mouse, Werner mouse) [29,45,51]. The loss of fenestrations has particular importance for susceptibility to cardiometabolic disease. Fenestrations are portals for the uptake of chylomicron remnants and insulin, and loss of fenestrations secondary to aging (and acutely following treatment with poloxamer 407) can lead to hyperlipidemia and hepatic insulin resistance by impairing the uptake of lipoproteins and insulin [52]. In addition, LSECs also play an essential role in the clearance of circulating macromolecules including collagen degradation products, hyaluronan and antibodies. With aging, this endocytotic activity, as measured by the uptake of formaldehyde-treated serum albumin is diminished [53].
Fig. 2.
Summary of changes that occur in the liver sinusoidal endothelial cell with aging. (a) loss of fenestrations and (b) pathways promoting ROS and cellular senescence in aging LSECs. Abbreviations: AMPK: 5′ adenosine monophosphate-activated protein kinase; BM: bone marrow; cGMP: cyclic guanosine monophosphate; EPC: endothelial progenitor cell; HGF: hepatocyte growth factor; ICAM-1: intercellular adhesion molecule 1; Id1: inhibitor of differentiation/DNA binding protein 1; IL-6: interleukin 6; NAD: nicotinamide adenine dinucleotide; NO: nitric oxide; ROS: reactive oxygen species; SIRT1: sirtuin 1; VEGF: vascular endothelial growth factor; WF: von Willebrand factor.
From the therapeutic perspective it has been proposed that reversal or prevention of age-related loss of fenestrations might improve systemic cardiometabolic health in old age. Fenestrations are regulated by mechanisms that influence the actin cytoskeleton and lipid rafts [54], and several agents that putatively act on this pathway were found to increase fenestrations in LSECs isolated from old mice, including 7-ketocholesterol, sildenafil, amlodipine, cytochalasin D, bosentan, 2.5-dimethoxy-4-iodoamphetamine and TNF-related apoptosis-inducing ligand. In addition, two drugs that delay aging by regulating the nutrient sensing pathways [55], nicotinamide mononucleotide and metformin [55,56] were also found to increase fenestrations in LSECs from old mice. This provides indirect evidence that aging is associated with dysregulated nutrient sensing pathways (one of the Hallmarks of Aging) in LSECs.
In LSECs, autophagy is important for cellular homeostasis. In mice and rat models of fibrosis induction the loss of endothelial autophagy reduces intrahepatic nitric oxide (NO) and impairs response to oxidative stress in LSECs and surrounding cells [57]. The release of NO from LSECs is critically importance for regulation of liver metabolism as it influences hepatic blood flow [58], glucose tolerance and fat content [59], maintenance of HSC quiescence, suppression of pro-fibrotic pathways [60] and prevention of stenosis [58].
With aging in LSEC (Fig. 2), there is a downregulation of the vasodilatory pathways (NO bioavailability, endothelial NO synthase protein expression, cyclic guanosine monophosphate, haem oxygenase-1) [61] and several angiocrine receptors (stabilin-2, CD32b and VEGF-R2). These changes are associated with increases in portal pressure and vascular resistance leading to reduced hepatic blood flow. There is also an increase intercellular adhesion molecule 1 expression in LSECs which leads to a substantial increase in leukocyte adhesion, further contributing to reduced sinusoidal blood flow [62]. Aging LSECs are in a moderate pro-inflammatory state as evidenced by increased CD68 positive cells and elevated expression of IL-6 [61] and there is upregulation of p16 and downregulation of SIRT1, which might influence cellular senescence [61]. In addition, there is reduced mRNA expression of Wnt2 and Hgf which are involved in regulation of hepatocyte regeneration [61].
Although the Hallmarks of Aging have been studied in endothelial cells more broadly [63], as yet there has not been any comprehensive investigation of these processes in LSECs specifically. Therefore, the effects of aging on mitochondrial function, epigenetics, oxidative stress and telomeres in LSECs remain largely unknown. Given the potential significance of the LSEC in maintaining cardiometabolic health and as a potential therapeutic target, this remains an important focus for future research.
4. Hepatic Stellate Cells
HSCs are pericytes located within the space of Disse that are involved in vitamin A and lipid storage, regulate extracellular matrix metabolism and possibly influence sinusoidal blood flow via contractile properties. When activated, HSCs lose their characteristic fat-filled vesicles and produce collagen, and this is a key initiating process in the development of hepatic fibrosis and cirrhosis. Therefore mechanisms underlying HSC activation have been a major focus of research into the pathogenesis of hepatic fibrosis [64], however there have only been a few studies investigating the effects of aging on HSCs [61,65,66].
Electron microscopy revealed that old age is associated with a marked increase in the number and size of lipid droplets in HSCs in mice and non-human primates, which can become so large that they protrude into the sinusoidal lumen and become visible by light microscopy as ‘signet ring cells’ [51,65]. There is an increase in the number of HSCs and a proportional increase in the number of cells that stain positive for the markers of HSC activation, alpha-smooth muscle actin (αSMA) and desmin.
It has been recently reported that in aging rats, there is increased expression of αSMA, collagen 1α1 and 1α2 and p-moesin [61]. These are markers of HSC activation and collagen deposition. Increased desmin protein expression and elevated HSC-related platelet derived growth factor (PDGF) receptor-β were also observed [61]. PDGF in the liver is a critical mitogen to drive HSC proliferation and migration. PDGFR-β is highly upregulated in activated HSCs [61]. In addition, this study in old rats also reported elevated patatin-like phospholipase domain-containing protein 3 and decreased cellular retinol-binding protein I expression in liver tissue consistent with changes in vitamin A metabolism. Mitochondrial superoxide expression was not significantly affected by aging [61]; however, in human liver tissue, telomere attrition has been reported [67].
There is some uncertainty about whether HSCs are activated in old age. On one hand, HSC activation in associated with loss of lipid droplets, which is not consistent with aging HSCs. On the other hand, there is altered expression of several proteins consistent with HSC activation [61]. In liver diseases, these two processes usually occur in parallel. It seems that in old age, there is dissociation between lipid droplet metabolism and HSC activation (Fig. 1).
5. Küpffer Cells
KCs are resident liver macrophages located within the lumen of the liver sinusoids. KCs produce soluble cell mediators such as TNFα and IL-6 as part of the innate immune response to infections and phagocytose macromolecules that are too large to be endocytosed by the LSECs. KC activation is seen in most types of liver disease. Although there have been many studies on aging and macrophages, there has not been much research reported on the effects of aging on KCs in the liver [61,[67], [68], [69], [70]].
The effects of aging on macrophages include reduced phagocytosis and autophagy and increased production of cytokines that contribute to the inflammatory phenotype of old age (‘inflammaging’). One of the first studies undertaken in rats showed that aging is associated with increased numbers of KCs which were basally activated in accordance of the ultrastructural morphology with reduced phagocytotic activity measured by the hepatic uptake of microspheres [68]. In another study, aging was associated with a redistribution of KCs into the lymphoid collections that are frequently seen in old rodent livers [69]. Recruitment of KC may be mediated by increased secretion of MCP-1 by aging hepatocytes [71]; however, MCP-1 is not essential for KC activation [72]. In old rats, there was an increase in the RNA expression of the inflammatory cytokine IL-6 in KCs which suggests that KCs might be one source of elevated IL-6 that is characteristic of old age [61]. Additionally, activation of KCs contributes to the pro-inflammatory state of the hepatic sinusoid. However there were no age-related changes in the expression of other markers of KC including TNFα, Mrc1, Arg1 or IL-10 [61].
6. The Liver and the Hallmarks of Aging
Many studies have examined the effects of aging on liver tissue rather than individual cell types. These studies primarily reflect changes in hepatocytes and have often reported the effects of aging on genetic and mitochondrial changes in the liver. Overall, age-related changes in liver tissue are similar to other tissues with respect to the Hallmarks of Aging. These are summarized below.
6.1. Genomic Instability
Genomic instability results from the accumulation of genetic damage throughout life promoted by exogenous factors and via DNA replication errors, hydrolytic reactions, oxidative stress and changes in gene transcription [10]. Mouse models of accelerated aging induced by mutation of DNA repair have shown that the aging liver accumulates genomic rearrangements [73]. Aging mice livers have chromosomal translocations and deletions of up to 66 megabases, possibly mediated by ROS [10]. The aging liver has increased incidence of polyploid hepatocytes with a reduced rate of DNA synthesis and repair [9]. As discussed in Section 2 this likely contributes to cellular metabolic dysfunction [10,74].
Genomic instability is thought to be a significant initiator of the aging phenotype in the liver. The promotion of genomic instability is further mediated by progressive DNA damage and epigenetic mutations as discussed below. These mutations have been identified as a marker that spans the progression between aging and diseased stages of the liver, with greater mutations observed in old, diseased livers [10]. It is a clear area of interest to identify pathways of genetic and epigenetic instability to target in aging (targeting CR pathways [75]), age-related disease [76] and liver diseases [77].
6.2. Telomere Attrition
Most studies of aging and telomeres have focused on leukocyte telomere length. However in a study of the effects of aging on mouse telomeres in different tissues it was found that telomeres in the liver decreased in length up until 25 months of age, along with similar changes in leukocytes, hippocampus, pituitary, retina, kidney, skeletal muscle and skin [78]. One study in humans found that telomere length in the liver was shortened in centenarians compared to newborns [79], while there was a reduction in the percentage of longer telomeres in livers of rats by 15 months of age [80].
6.3. Epigenetic Alterations
Dysregulation of the transcriptional networks and chromatin state that underpin gene expression is a crucial factor in aging [81]. There is a complex set of processes involved in regulating gene expression and influenced by aging including DNA methylation, transcription factors, histone marks, nucleosome positioning, and non-coding RNAs. Old age in most tissues is associated with reduced heterochromatin, characterized by loss of histone marks and nucleosome occupancy. Overall, there is hypomethylation of DNA within heterochromatin and hypermethylation of transcriptionally active regions [82,83].
In the liver, there are age-related changes in DNA methylation that correlate with chronological age [84,85]. In a study of human livers, aging was associated with a substantial change in DNA methylation at least until the age of 60 years, consistent with Horvath and Raj [86]s' epigenetic clock, a conserved pattern of age-related changes in DNA methylation. This was associated with changes in gene expression that included pathways involved with inflammation, metabolism and Wnt signaling [87]. Interventions that delay aging such as CR and rapamycin also influence epigenetic changes in the liver [88]. Specifically, CR acts on lipid biosynthesis genes and rapamycin on genes coding for growth factors and growth hormone receptors [89].
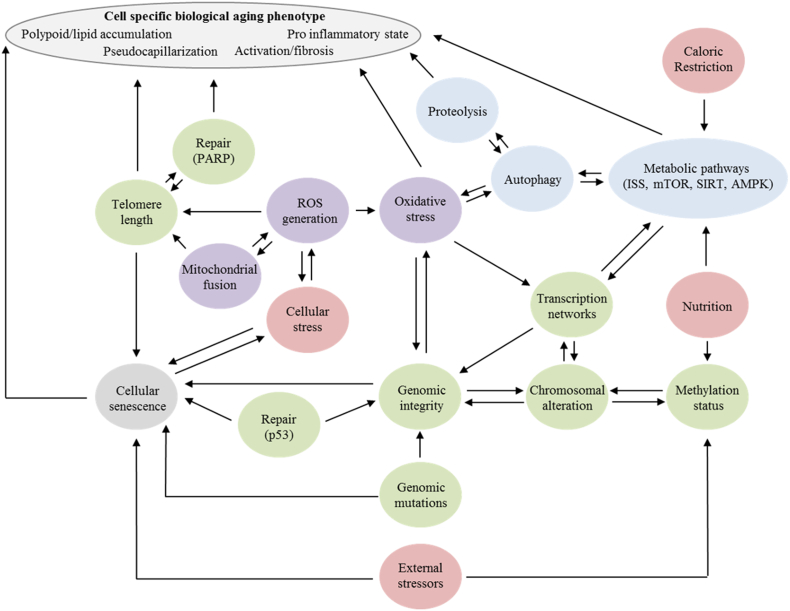
The aging mouse has many changes in the transcription network [83,90], including importantly alterations in Forkhead box O (FOXO), a key transcription factor implicated in aging [91]. FOXO influences NO regulation, hyperinsulinemia [92] and activation of HSCs [93] through its effects on downstream genes such as glucose 6-phosphatase, phosphoenolpyruvate carboxykinase, insulin-like growth factor-binding protein 1, PGC-1α, pyruvate Dehydrogenase Kinase 4, and hydroxymethylglutaryl-CoA synthase [94]. FOXO is regulated by NAD+/SIRT1, insulin like growth factor 1 (IGF-1), 5′ adenosine monophosphate-activated protein kinase (AMPK) and oxidative stress, all of which are influenced by aging (Fig. 3) [95].
Fig. 3.
Holistic model for integrating the hallmarks of aging within the liver. Interconnectome based on computational modeling of aging by Mc Auley and Mooney [151], Mc Auley and Mooney [152] and the data presented in this review. Green: nucleus related, red: external factors; Blue: metabolic related; Purple: mitochondrial related. Genomic stability is a key regulator of aging (Section 6.1) with methylation status (Section 6.3), genetic mutation (Section 6.3) and external stressors. Regulation of the transcription networks in both the nucleus and mitochondria occur by nutrient sensing pathways (Section 6.3) which are impaired with age (Section 6.6). Mitochondrial dysfunction (Section 6.7) leads to a shift in the balance of autophagy and oxidative stress in favor of stress contributing to the loss of proteostasis (Section 6.4) and impaired ER stress pathways (Section 6.5). In combination with telomere shortening (Section 6.2) there is a promotion of cellular senescence (Section 6.8). Abbreviations: AMPK: 5′ adenosine monophosphate-activated protein kinase; ISS: insulin/insulin like growth factor 1 signaling; mTOR: mammalian target of rapamycin; PARP: protein poly(ADP-ribose) polymerase; SIRT: sirtuin.
Nucleosome positioning is also critical for gene expression and most DNA-related processes. There are age-related changes in nucleosome occupancy in the mouse liver which contributes to metabolic dysfunction [96]. Nucleosome occupancy decreases with age as a result of reduced activity of histone chaperones and reduced production of core histone production and post-translational modifications [96].
As a consequence of these changes in regulation of transcription there are age-related changes in the liver transcriptome. In the mouse liver, these involve three main sets of interacting networks that include genes involved with inflammation, proliferative homeostasis (between cellular proliferation and death networks) and lipid metabolism (synthesis and oxidation) [90].
There are also changes in the expression on non-coding RNAs. For example, long-non coding RNAs including NEAT1, MEG3, Rian and Mirg are differentially expressed in the aging mouse liver [90]. Aging also effects the expression of some microRNAs (miR). These include: miR-146 which influences mitochondrial function and inflammation [97]; miR-146a, miR-376c and miR-411 which are associated with metabolic inflammation [98]; and miR-34a and miR-93 which target genes such as Spl, nrf-2, SIRT1, Mgst1 and influence cellular senescence and oxidative stress [99,100].
6.4. Loss of Proteostasis
Proteostasis is the synthesis, folding, trafficking and degradation of proteins [42]. Loss of proteostasis contributes to the burden of misfolded proteins (via endoplasmic reticulum stress [101]), oxidative stress and cellular damage within the liver [102]. The drivers of age-related loss of proteostasis loss are primarily epigenetic [103]. Defective autophagy, a key component of the proteostasis network, is a conserved feature of aging across tissues and organisms, and in the liver is secondary in part to defects in intracellular trafficking of lysosomes and autophagosomes [104]. In human livers, proteasome activity is well preserved with only subtle changes in proteasome subunit composition [105].
6.5. Response to ER Stress
In response to stress stimuli, ER signaling is promoted by heat shock proteins (HSPs). However, a marked decline of HSP70, HSP27 and HSP90 induction is observed in senescent and hepatocytes from old rats [106]. This decline in the availability and/or function of HSPs with age can then lead to accumulation of damaged proteins and loss of proteostasis. This damage can be further compounded by the decline of autophagic degradation with aging [44,107].
Accumulation of HSPs promotes the unfolded protein response (UPR), a highly conserved protective response that maintains cellular homeostasis Age-related deactivation of the UPR in the endoplasmic reticulum and mitochondria of hepatocytes is well documented, and has been shown to cause accumulation of misfolded proteins and increase in lipogenesis and lipotoxicity, amplification of ROS and inflammatory stress signaling, leading to an overall predisposition to age-related liver diseases such as steatosis and NAFLD [108]. Conversely, improvement of age-related UPR signaling pathways has been shown to induce activation of mitochondria leading to increased mitochondrial biogenesis and overall cell function [109].
6.6. Deregulated Nutrient Sensing
The nutrient sensing pathways mediate the effects of nutrition on aging and have a pivotal role in regulating the rate of aging. The major nutrient sensing pathways include the mammalian target of rapamycin (mTOR), sirtuins, AMPK and insulin/insulin-like growth factor signaling pathways [110,111]. The liver has the central role in regulating the systemic response to nutrition, therefore age-related changes in the nutrient sensing pathways in the liver may have substantial systemic effects.
Insulin and insulin like growth factor signaling pathways have an important role in energy metabolism and growth. Reduced IIS, enhanced insulin sensitivity and reduced plasma IGF-1 are associated with longevity, while the opposite are related to metabolic disorders that contribute to mortality [6,112]. The aging liver has reduced insulin sensitivity and contributes to impaired insulin clearance, in part secondary to age-related changes in the liver sinusoidal endothelium [52]. Growth hormone and IGF-I levels decline during normal aging and premature aging models resulting in insulin resistance, glucose intolerance, increased lipogenesis and lipid accumulation and fibrosis [113]. Metabolic pathways that contribute to insulin resistance are glycolysis (pyruvate dehydrogenase), lipogenesis (free fatty acid) and the citric acid cycle (TCA flux), which are impaired in the aging the liver and improved with CR [6,[114], [115], [116]].
AMPK signaling in many tissues declines with age, which impairs cellular homeostasis and mitochondrial function [117]. In the mouse liver, aging is associated with increased AMPK phosphorylation and activity. However, downstream pathways are not activated, and there is an absence of any response to hypoxia, indicating overall impairment of the AMPK pathway [118].
SIRT1 is a key orthologue of the class III histone deacetylases that are involved in the regulation of multiple transcription and protein targets relevant for aging (p53, FOXO, PGC-1α and NF-κB [119,120] influencing glucose homeostasis and lipid metabolism [115,121], FOXO [122], autophagy [123], inflammation [124], apoptosis [125], and cellular [126]). SIRT1 activity is dependent on NAD+ and has been shown to be critical to the beneficial effects of CR on longevity [114]. As the liver ages, SIRT1 is downregulated possibly secondary to repression by the CCAAT/enhancer-binding-protein/histone deacetylase1 complex [121]. Moreover, there are age-related reductions in liver NAD+ which further impairs SIRT1 function [61]. In a recent study, the liver was demonstrated to have age-related transcriptomic decline in NAD+ biosynthesis pathways, NAD scavenger cycle regulation and a decline in protein acetylation via changes in circadian regulated pathways [127]. This group showed that interventions such as CR promote activation of NAD+/SIRT1 pathways to reduce age-related hepatic acetyl-CoA metabolism.
mTOR regulates many processes implicated in aging including cell growth, protein synthesis, autophagy and insulin metabolism. Inhibition of mTOR with rapamycin delays aging and increases lifespan [128]. The effects of aging on mTOR activity in the liver is unclear with studies showing no change [129], increased activity [130] or decreased activity [131].
6.7. Mitochondrial Dysfunction
Many changes in mitochondria have been reported in the aging liver including mitochondrial DNA mutations [132], oxidative stress, impaired oxidative phosphorylation and structural changes [26]. Such changes reduce cellular bioenergetics and increase oxidative injury but also drive cells towards cellular senescence [133].
Computation modeling of ROS levels in cell cultures has shown that individual cells enter senescence at a time-invariant ROS level [133]. Separately, Passos, Nelson [134] proved that a drive towards a cellular senescence state involves ROS production from mitochondrial dysfunction. In relation to normal aging where ROS may be reduced and mitochondrial functionality maintained these models would agrees with Niemann, Johne [132]. Overall this data demonstrates that ROS regulations is highly important for mitochondrial dysfunction and for the transition towards cellular senescence with the drivers for the production of excess ROS are dependent on the interactions between epigenetic, nutrient sensing, cellular stress and proteostasis pathways [135].
6.8. Cellular Senescence
Cellular senescence describes irreversible cell cycle arrest and is thought to be predominantly driven by telomere attrition. It is associated with the secretion of inflammatory cytokines called the ‘senescence associated secretory phenotype’. Cell senescence-associated with telomere length shortening has been confirmed in livers of old human donors [136]. In addition to telomere shortening, changes to the nuclear size, DNA content, increased p21 [137,138], γ-H2AX [139] and β-galactosidase expression [140], formation of senescence-associated heterochromatin foci [141], genetic mutations of the telomerase enzyme complex [142] have all been confirmed to be features of senescence in human livers during normal aging and liver disease. In mice, the presence of increased senescent cells has been shown by the positive identification of (i) cellular markers [143,144]; (ii) senescence associated secretory phenotype [27], (iii) gene signatures of cellular senescence [145] and transcriptome phenotype markers [90]. While Cellular senescence has been implicated in age-related changes within the liver [144], and while the field remains contentious with multiple studies offering differing viewpoints White, Milholland [144], Aravinthan and Alexander [146], Morsiani, Bacalini [147]. White, Milholland [90] hepatocyte senescence and fat accumulation in aging mice was dependent of dietary intake [28]. It is generally accepted that hepatocytes and HSCs display a senescent phenotype with chronological age. The rate at which as cell can become senescent is thought to be dependent on the interplay of genetics and environmental factors such as toxin exposure, virus and of particular interest dietary influence with a recent study showing that hepatocyte senescence and fat accumulation in aging mice is dependent on dietary caloric and macronutrient intake [28].
6.9. Stem Cell Exhaustion
The effects of aging on liver progenitor cells has not been widely studied. Old age in mice was associated with reduced activation and proliferation of in situ liver progenitor cells following injury, but normal proliferative capacity of in vitro liver progenitor cells following isolation. It was concluded that impaired stem cell responses in the aged liver are secondary to inflammation rather than intrinsic deficits [49].
6.10. Altered Intercellular Communication
The function of the liver depends upon intercellular communication between hepatocytes, HSCs, KCs and LSECs using a wide array of substrates including cytokines such as TNFα, eicosanoids, NO, growth factors such as VEGF, extracellular matrix components and carbon monoxide [148]. There have been few studies on how these intercellular communications may change with aging in the liver. A recent study has reported that aging is associated with diminished synthesis of vasodilators and a proinflammatory state indicating dysregulation of the cells of the hepatic sinusoid [61]. It should also be noted that LSEC, which lies at the interface between blood and hepatocytes will have a pivotal role in regulating the interaction between the liver and circulating cytokines and growth factors, as well as the uptake of xenobiotics and endobiotics.
6.11. Aging Human Liver
The human liver demonstrates similar epigenetic, proteostasis, dysfunctional nutrient sensing, mitochondrial and senescent cell age related changes as to those observed in mice and rats. Recently the age-related changes in the human liver have been reviewed within a more clinical context [147]. Regarding associations between rodent models and humans, similar patterns of DNA methylation, histone modifications, CpG methylation, telomere shortening, and cellular senescence have been shown in aging human liver tissue [79,140,149,150]. DNA methylation has been shown to correlate with chronological aging in the human liver and accelerated methylation has been further linked to BMI and obesity [150]. Age-related changes in mitochondria, oxidative phosphorylation and electron transport genes have been demonstrated in the human liver in addition to changes in the expression of cell adhesion genes [150].
7. Summary and Outlook
The aging process in the liver is promoted by alterations in the genome and epigenome that contribute to dysregulation of mitochondrial function and nutrient sensing pathways. This leads to cellular senescence and low-grade inflammation facilitating multiple phenotypic changes in all liver cells (hepatocytes, liver sinusoidal endothelial, hepatic stellate and Küpffer cells) as shown in Fig. 1. The interconnectome between these pathways has been shown by the recent applications of -omics studies to investigate age-related changes that occur in the liver (Fig. 3). In 1985 one of the founding fathers of hepatology, Hans Popper (Sem Liver Disease) made the prescient statement that “the effect of age on the liver and of the liver on aging is full of promise if available methodologies are rigorously applied”. Since that time there has been a dramatic increase in technologies, particularly the various –omics, that have profoundly influenced our understanding of the aging liver. We can now start to integrate the effects of aging from its initiating events in the genome and epigenome through to whole organ changes.
Finally, of the cells of the liver, LSECs are a significant but under-recognized risk factor for the development of age-related cardiometabolic disease. We have highlighted that these cells are drastically changed during aging (Section 3 and Fig. 2) but may also act as a therapeutic target for age-related cardiometabolic diseases [115].
Declaration of Competing Interest
None.
Acknowledgements
The study was supported by the Australian National Health and Medical Research Council Projects #1141234; the Aging and Alzheimer's Research Foundation (a Division of the Medical Foundation of the University of Sydney); Sydney Medical School Foundation McKnight Bequest and The University of Sydney DVCR Research Equity Fellowship.
References
- 1.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17) doi: 10.1016/j.cub.2012.07.024. [R741-R52] [DOI] [PubMed] [Google Scholar]
- 3.Rui L. Energy metabolism in the liver. Compr Physiol. 2011;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H., Kisseleva T., Brenner D.A. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomini F., Rodella L.F., Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6(2):109. doi: 10.14336/AD.2014.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Z., Tas E., Yakar S., Muzumdar R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol Cell Endocrinol. 2017;455:115–130. doi: 10.1016/j.mce.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Timchenko N.A. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20(4):171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Yin X.M., Ding W.X., Gao W. Autophagy in the liver. Hepatology. 2008;47(5):1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 9.Basso A., Piantanelli L., Rossolini G., Roth G.S. Reduced DNA synthesis in primary cultures of hepatocytes from old mice is restored by thymus grafts. J Gerontol A Biol Sci Med Sci. 1998;53(2) doi: 10.1093/gerona/53a.2.b111. [B111-B6] [DOI] [PubMed] [Google Scholar]
- 10.Lebel M., de Souza-Pinto N.C., Bohr V.A. Metabolism, genomics, and DNA repair in the mouse aging liver. Curr Gerontol Geriatr Res. 2011;2011:15. doi: 10.1155/2011/859415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M.J., Chen F., Li J.X., Liu C.C., Zhang H.B., Xia Y. Reversal of hepatocyte senescence after continuous in vivo cell proliferation. Hepatology. 2014;60(1):349–361. doi: 10.1002/hep.27094. [DOI] [PubMed] [Google Scholar]
- 12.Moraes A.S., Guaraldo A.M.A., Mello M.L.S. Chromatin supraorganization and extensibility in mouse hepatocytes with development and aging. Cytometry A. 2007;71A(1):28–37. doi: 10.1002/cyto.a.20356. [DOI] [PubMed] [Google Scholar]
- 13.Ghiraldini F.G., Silva I.S., Mello M.L.S. Polyploidy and chromatin remodeling in hepatocytes from insulin-dependent diabetic and normoglycemic aged mice. Cytometry A. 2012;81A(9):755–764. doi: 10.1002/cyto.a.22102. [DOI] [PubMed] [Google Scholar]
- 14.Lin S., Nascimento E.M., Gajera C.R., Chen L., Neuhöfer P., Garbuzov A. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556(7700):244. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiemann S.U., Satyanarayana A., Tsahuridu M., Tillmann H.L., Zender L., Klempnauer J. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB. 2002;16(9):935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 16.Gokarn R., Solon-Biet S.M., Cogger V.C., Cooney G.J., Wahl D., McMahon A.C. Long-term dietary macronutrients and hepatic gene expression in aging mice. J Gerontol A Biol Sci Med Sci. 2018;73(12):1618–1625. doi: 10.1093/gerona/gly065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denchi E.L., Celli G., de Lange T. Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes Dev. 2006;20(19):2648–2653. doi: 10.1101/gad.1453606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine K.M., Skoien R., Bokil N.J., Melino M., Thomas G.P., Loo D. Senescent human hepatocytes express a unique secretory phenotype and promote macrophage migration. World J Gastroenterol. 2014;20(47):17851–17862. doi: 10.3748/wjg.v20.i47.17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Jurk D., Maddick M., Nelson G., Martin-Ruiz C., von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8(3):311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 21.Castro R.E., Ferreira D.M., Afonso M.B., Borralho P.M., Machado M.V., Cortez-Pinto H. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58(1):119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Swisshelm K., Disteche C.M., Thorvaldsen J., Nelson A., Salk D. Age-related increase in methylation of ribosomal genes and inactivation of chromosome-specific rRNA gene clusters in mouse. Mutat Res. 1990;237(3–4):131–146. doi: 10.1016/0921-8734(90)90019-n. [DOI] [PubMed] [Google Scholar]
- 23.Jiang M.H., Fei J., Lan M.S., Lu Z.P., Liu M., Fan W.W. Hypermethylation of hepatic Gck promoter in ageing rats contributes to diabetogenic potential. Diabetologia. 2008;51(8):1525. doi: 10.1007/s00125-008-1034-8. [DOI] [PubMed] [Google Scholar]
- 24.Aravinthan A., Challis B., Shannon N., Hoare M., Heaney J., Alexander G.J.M. Selective insulin resistance in hepatocyte senescence. Exp Cell Res. 2015;331(1):38–45. doi: 10.1016/j.yexcr.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Aravinthan A., Shannon N., Heaney J., Hoare M., Marshall A., Alexander G.J.M. The senescent hepatocyte gene signature in chronic liver disease. Exp Gerontol. 2014;60:37–45. doi: 10.1016/j.exger.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Navarro A., Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287(5) doi: 10.1152/ajpregu.00226.2004. [R1244-R9] [DOI] [PubMed] [Google Scholar]
- 27.Lasry A., Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol. 2015;36(4):217–228. doi: 10.1016/j.it.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21(5):739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Daum B., Walter A., Horst A., Osiewacz H.D., Kuhlbrandt W. Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. PNAS. 2013;110(38):15301–15306. doi: 10.1073/pnas.1305462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 32.Hagen T.M., Yowe D.L., Bartholomew J.C., Wehr C.M., Do K.L., Park J.-Y. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. PNAS. 1997;94(7):3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selfridge J., Hsia K.-T., Redhead N.J., Melton D.W. Correction of liver dysfunction in DNA repair-deficient mice with an ERCC1 transgene. Nucleic Acids Res. 2001;29(22):4541–4550. doi: 10.1093/nar/29.22.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takamura A., Komatsu M., Hara T., Sakamoto A., Kishi C., Waguri S. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madrigal-Matute J., Cuervo A.M. Regulation of liver metabolism by autophagy. Gastroenterology. 2016;150(2):328–339. doi: 10.1053/j.gastro.2015.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X., Hueckstaedt L.K., Ren J. Deficiency of insulin-like growth factor 1 attenuates aging-induced changes in hepatic function: role of autophagy. J Hepatol. 2013;59(2):308–317. doi: 10.1016/j.jhep.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Uddin M.N., Nishio N., Ito S., Suzuki H. Isobe K-i. Autophagic activity in thymus and liver during aging. Age. 2012;34(1):75–85. doi: 10.1007/s11357-011-9221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donati A., Paradiso C., Bergamini E., Cavallini G., Pollera M., Vittorini S. Age-related changes in the autophagic proteolysis of rat isolated liver cells: effects of antiaging dietary restrictions. J Gerontol A Biol Sci Med Sci. 2001;56(9) doi: 10.1093/gerona/56.9.b375. [B375-B83] [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Gorospe M., Kokkonen G.C., Boluyt M.O., Younes A., Mock Y.D. Impairments in both p70 S6 kinase and extracellular signal-regulated kinase signaling pathways contribute to the decline in proliferative capacity of aged hepatocytes. Exp Cell Res. 1998;240(1):40–48. doi: 10.1006/excr.1997.3931. [DOI] [PubMed] [Google Scholar]
- 40.Cuervo A.M., Dice J.F. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275(40):31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 41.Rubinsztein David C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 42.Schneider J.L., Villarroya J., Diaz-Carretero A., Patel B., Urbanska A.M., Thi M.M. Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell. 2015;14(2):249–264. doi: 10.1111/acel.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C., Cuervo A.M. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14(9):959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanlund J.M., Kregel K.C., Oberley T.D. Autophagy following heat stress: the role of aging and protein nitration. Autophagy. 2008;4(7):936–939. doi: 10.4161/auto.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cogger V.C., Svistounov D., Warren A., Zykova S., Melvin R.G., Solon-Biet S.M. Liver aging and pseudocapillarization in a Werner syndrome mouse model. J Gerontol A Biol Sci Med Sci. 2014;69(9):1076–1086. doi: 10.1093/gerona/glt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Couteur D.G., Cogger V.C., Markus A.M., Harvey P.J., Yin Z.L., Ansselin A.D. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33(3):537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 47.Michalopoulos G.K. Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65(4):1384–1392. doi: 10.1002/hep.28988. [DOI] [PubMed] [Google Scholar]
- 48.Cheluvappa R., Hilmer S.N., Kwun S.Y., Jamieson H.A., O'Reilly J.N., Muller M. The effect of old age on liver oxygenation and the hepatic expression of VEGF and VEGFR2. Exp Gerontol. 2007;42(10):1012–1019. doi: 10.1016/j.exger.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y., Wang X., Wang B., Zhou H., Dang S., Shi Y. Aging-associated oxidative stress inhibits liver progenitor cell activation in mice. Aging. 2017;9(5):1359–1374. doi: 10.18632/aging.101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Couteur D.G., Lakatta E.G. A vascular theory of aging. J Gerontol A Biol Sci Med Sci. 2010;65(10):1025–1027. doi: 10.1093/gerona/glq135. [DOI] [PubMed] [Google Scholar]
- 51.Cogger V.C., Warren A., Fraser R., Ngu M., McLean A.J., Le Couteur D.G. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003;38(10):1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Mohamad M., Mitchell S.J., Wu L.E., White M.Y., Cordwell S.J., Mach J. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell. 2016;15(4):706–715. doi: 10.1111/acel.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon-Santamaria J., Malovic I., Warren A., Oteiza A., Le Couteur D., Smedsrød B. Age-related changes in scavenger receptor–mediated endocytosis in rat liver sinusoidal endothelial cells. J Gerontol A Biol Sci Med Sci. 2010;65(9):951–960. doi: 10.1093/gerona/glq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cogger V.C., Roessner U., Warren A., Fraser R., Le Couteur D.G. A sieve-raft hypothesis for the regulation of endothelial fenestrations. Comput Struct Biotechnol J. 2013;8(11) doi: 10.5936/csbj.201308003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt N.J., Lockwood G., Warren A., Mao H., McCourt P., Le Couteur D.G. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2018;316(1) doi: 10.1152/ajpgi.00179.2018. [G144-G54] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunt N.J., Lockwood G., Kang S.W.S., Pulpitel T., Clark X., Mao H. Metformin induced attenuation of age-related pseudocapillarization in mouse liver endothelium. J Gerontol A Biol Sci Med Sci. 2019 [accepted] [Google Scholar]
- 57.Ruart M., Chavarria L., Campreciós G., Suárez-Herrera N., Montironi C., Guixé-Muntet S. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70(3):458–469. doi: 10.1016/j.jhep.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kus E., Jasiński K., Skórka T., Czyzynska-Cichon I., Chlopicki S. Short-term treatment with hepatoselective NO donor V-PYRRO/NO improves blood flow in hepatic microcirculation in liver steatosis in mice. Pharmacol Rep. 2018;70(3):463–469. doi: 10.1016/j.pharep.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017;66(1):212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 60.DeLeve L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61(5):1740–1746. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeso-Díaz R., Ortega-Ribera M., Fernández-Iglesias A., Hide D., Muñoz L., Hessheimer A.J. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018;17(6) doi: 10.1111/acel.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito Y., Sørensen K.K., Bethea N.W., Svistounov D., McCuskey M.K., Smedsrød B.H. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42(8):789–797. doi: 10.1016/j.exger.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donato Anthony J., Machin Daniel R., Lesniewski Lisa A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123(7):825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 65.Marcos R., Lopes C., Malhão F., Correia-Gomes C., Fonseca S., Lima M. Stereological assessment of sexual dimorphism in the rat liver reveals differences in hepatocytes and Kupffer cells but not hepatic stellate cells. J Anat. 2016;228(6):996–1005. doi: 10.1111/joa.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren A., Cogger V.C., Fraser R., DeLeve L.D., McCuskey R.S., Le Couteur D.G. The effects of old age on hepatic stellate cells. Curr Gerontol Geriatr Res. 2011;2011:7. doi: 10.1155/2011/439835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verma S., Tachtatzis P., Penrhyn-Lowe S., Scarpini C., Jurk D., Von Zglinicki T. Sustained telomere length in hepatocytes and cholangiocytes with increasing age in normal liver. Hepatology. 2012;56(4):1510–1520. doi: 10.1002/hep.25787. [DOI] [PubMed] [Google Scholar]
- 68.Hilmer S.N., Cogger V.C., Couteur D.G.L. Basal activity of Kupffer cells increases with old age. J Gerontol A Biol Sci Med Sci. 2007;62(9):973–978. doi: 10.1093/gerona/62.9.973. [DOI] [PubMed] [Google Scholar]
- 69.Stahl E.C., Haschak M.J., Popovich B., Brown B.N. Macrophages in the aging liver and age-related liver disease. Front Immunol. 2018;9:2795. doi: 10.3389/fimmu.2018.02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh P., Coskun Z.Z., Goode C., Dean A., Thompson-Snipes L., Darlington G. Lymphoid neogenesis and immune infiltration in aged liver. Hepatology. 2008;47(5):1680–1690. doi: 10.1002/hep.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stahl E., LoPresti S., Delgado E., Alencastro F., Wilkinson P., Duncan A.W. Age-induced hepatic steatosis and inflammation of murine livers is influenced by MCP-1. FASEB J. 2018;32(1_supplement) [150.5-.5] [Google Scholar]
- 72.Wyler S.L., Hennings D.L., D'Ingillo S.R., Lamb C.L., Horras C.J., Mitchell K.A. Monocyte chemoattractant protein (MCP)-1 is not required for Kupffer cell activation after partial hepatectomy. FASEB J. 2011;25(1_supplement) [1117.12-.12] [Google Scholar]
- 73.Gregg S.Q., Gutiérrez V., Robinson A.R., Woodell T., Nakao A., Ross M.A. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55(2):609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duncan A.W., editor. Seminars in cell & developmental biology. Elsevier; 2013. Aneuploidy, polyploidy and ploidy reversal in the liver. [DOI] [PubMed] [Google Scholar]
- 75.Li Y., Daniel M., TO Tollefsbol. Epigenetic regulation of caloric restriction in aging. BMC Med. 2011;9(1):98. doi: 10.1186/1741-7015-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunet A., Berger S.L. Epigenetics of aging and aging-related disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl_1):S17–S20. doi: 10.1093/gerona/glu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mann D.A. Epigenetics in liver disease. Hepatology. 2014;60(4):1418–1425. doi: 10.1002/hep.27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baek J.H., Son H., Jeong Y.-H., Park S.W., Kim H.J. Chronological aging standard curves of telomere length and mitochondrial DNA copy number in twelve tissues of C57BL/6 male mouse. Cells. 2019;8(3):247. doi: 10.3390/cells8030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takubo K., Nakamura K.-I., Izumiyama N., Furugori E., Sawabe M., Arai T. Telomere shortening with aging in human liver. J Gerontol A Biol Sci Med Sci. 2000;55(11) doi: 10.1093/gerona/55.11.b533. [B533-B6] [DOI] [PubMed] [Google Scholar]
- 80.Cherif H., Tarry J., Ozanne S., Hales C. Ageing and telomeres: a study into organ-and gender-specific telomere shortening. Nucleic Acids Res. 2003;31(5):1576–1583. doi: 10.1093/nar/gkg208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Booth L.N., Brunet A. The aging epigenome. Mol Cell. 2016;62(5):728–744. doi: 10.1016/j.molcel.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Benayoun B.A., Pollina E.A., Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16(10):593. doi: 10.1038/nrm4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou X., Sen I., Lin X.-X., Riedel C.G. Regulation of age-related decline by transcription factors and their crosstalk with the epigenome. Curr Genomics. 2018;19(6):464–482. doi: 10.2174/1389202919666180503125850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson R.F., Atzmon G., Gheorghe C., Liang H.Q., Lowes C., Greally J.M. Tissue-specific dysregulation of DNA methylation in aging. Aging Cell. 2010;9(4):506–518. doi: 10.1111/j.1474-9726.2010.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meer M.V., Podolskiy D.I., Tyshkovskiy A., Gladyshev V.N. A whole lifespan mouse multi-tissue DNA methylation clock. Elife. 2018;7 doi: 10.7554/eLife.40675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;1 doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 87.Bacalini M.G., Franceschi C., Gentilini D., Ravaioli F., Zhou X., Remondini D. Molecular aging of human liver: an epigenetic/transcriptomic signature. J Gerontol A Biol Sci Med Sci. 2018;74(1):1–8. doi: 10.1093/gerona/gly048. [DOI] [PubMed] [Google Scholar]
- 88.Wang T., Tsui B., Kreisberg J.F., Robertson N.A., Gross A.M., Yu M.K. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. doi: 10.1186/s13059-017-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hahn O., Stubbs T.M., Reik W., Grönke S., Beyer A., Partridge L. Hepatic gene body hypermethylation is a shared epigenetic signature of murine longevity. PLoS Genet. 2018;14(11) doi: 10.1371/journal.pgen.1007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White R.R., Milholland B., MacRae S.L., Lin M., Zheng D., Vijg J. Comprehensive transcriptional landscape of aging mouse liver. BMC Genomics. 2015;16(1):899. doi: 10.1186/s12864-015-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martins R., Lithgow G.J., Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15(2):196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsuchiya K., Accili D. Liver sinusoidal endothelial cells link hyperinsulinemia to hepatic insulin resistance. Diabetes. 2013;62(5):1478–1489. doi: 10.2337/db12-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu D., Yang C., Shen P., Chen L., Chen J., Sun X. rSjP40 suppresses hepatic stellate cell activation by promoting microRNA-155 expression and inhibiting STAT5 and FOXO3a expression. J Cell Mol Med. 2018;22(11):5486–5493. doi: 10.1111/jcmm.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barthel A., Schmoll D., Unterman T.G. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16(4):183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 96.Bochkis I.M., Przybylski D., Chen J., Regev A. Changes in nucleosome occupancy associated with metabolic alterations in aged mammalian liver. Cell Rep. 2014;9(3):996–1006. doi: 10.1016/j.celrep.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rippo M.R., Olivieri F., Monsurrò V., Prattichizzo F., Albertini M.C., Procopio A.D. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–163. doi: 10.1016/j.exger.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Victoria B., Lopez Y.O.N., Masternak M.M. MicroRNAs and the metabolic hallmarks of aging. Mol Cell Endocrinol. 2017;455:131–147. doi: 10.1016/j.mce.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh J.-E., Han J.-A., Hwang E.S. Downregulation of transcription factor, Sp1, during cellular senescence. Biochem Biophys Res Commun. 2007;353(1):86–91. doi: 10.1016/j.bbrc.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 100.Li N., Muthusamy S., Liang R., Sarojini H., Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech Ageing Dev. 2011;132(3):75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Wakabayashi S., Yoshida H. The essential biology of the endoplasmic reticulum stress response for structural and computational biologists. Comput Struct Biotechnol J. 2013;6(7) doi: 10.5936/csbj.201303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karunadharma P.P., Basisty N., Dai D.F., Chiao Y.A., Quarles E.K., Hsieh E.J. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell. 2015;14(4):547–557. doi: 10.1111/acel.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cellerino A., Ori A. What have we learned on aging from omics studies? Semin Cell Dev Biol. 2017;70:177–189. doi: 10.1016/j.semcdb.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 104.Bejarano E., Murray J.W., Wang X., Pampliega O., Yin D., Patel B. Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in aging. Aging Cell. 2018;17(4) doi: 10.1111/acel.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bellavista E., Martucci M., Vasuri F., Santoro A., Mishto M., Kloss A. Lifelong maintenance of composition, function and cellular/subcellular distribution of proteasomes in human liver. Mech Ageing Dev. 2014;141:26–34. doi: 10.1016/j.mad.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi R., Toyoda E., Aoki Y., Suzuki K.T., Goto S. Paradoxical increase of heat-shock response with age in a substrain of F344 rats: comparison between F344/DuCrj and F344/Jcl. Mech Ageing Dev. 2002;123(12):1605–1615. doi: 10.1016/s0047-6374(02)00096-9. [DOI] [PubMed] [Google Scholar]
- 107.Kwanten W.J., Vandewynckel Y.P., Martinet W., De Winter B.Y., Michielsen P.P., Van Hoof V.O. Hepatocellular autophagy modulates the unfolded protein response and fasting-induced steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G599–G609. doi: 10.1152/ajpgi.00418.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L., Wang H.-H. The essential functions of endoplasmic reticulum chaperones in hepatic lipid metabolism. Dig Liver Dis. 2016;48(7):709–716. doi: 10.1016/j.dld.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 109.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Canto C. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bitto A., Wang A.M., Bennett C.F., Kaeberlein M. Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb Perspect Med. 2015;5(11):a025114. doi: 10.1101/cshperspect.a025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fontana L., Partridge L., Longo V.D. Extending healthy life span—from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Heemst D. Insulin, IGF-1 and longevity. Aging Dis. 2010;1(2):147. [PMC free article] [PubMed] [Google Scholar]
- 113.Mariño G., Ugalde A.P., Fernández Á.F., Osorio F.G., Fueyo A., Freije J.M.P. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. PNAS. 2010;107(37):16268–16273. doi: 10.1073/pnas.1002696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haigis M.C., Sinclair D.A. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hunt N.J., McCourt P.A.G., Le Couteur D.G., Cogger V.C. Novel targets for delaying aging: the importance of the liver and advances in drug delivery. ADDR. 2018;135:39–49. doi: 10.1016/j.addr.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Willmes D.M., Birkenfeld A.L. The role of indy in metabolic regulation. Comput Struct Biotechnol J. 2013;6(7) doi: 10.5936/csbj.201303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reznick R.M., Zong H., Li J., Morino K., Moore I.K., Yu H.J. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5(2):151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mulligan J.D., Gonzalez A.A., Kumar R., Davis A.J., Saupe K.W. Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J Gerontol A Biol Sci Med Sci. 2005;60(1):21–27. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- 119.Anderson R.M., Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35(3):146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin J., Iakova P., Jiang Y., Medrano E.E., Timchenko N.A. The reduction of SIRT1 in livers of old mice leads to impaired body homeostasis and to inhibition of liver proliferation. Hepatology. 2011;54(3):989–998. doi: 10.1002/hep.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ganjam G.K., Dimova E.Y., Unterman T.G., Kietzmann T. FoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrol. J Biol Chem. 2009;284(45):30783–30797. doi: 10.1074/jbc.M109.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding S., Jiang J., Zhang G., Bu Y., Zhang G., Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramirez T., Li Y.M., Yin S., Xu M.J., Feng D., Zhou Z. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol. 2017;66(3):601–609. doi: 10.1016/j.jhep.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu M.H., Lin X.L., Li J., He J., Tan T.P., Wu S.J. Resveratrol induces apoptosis through modulation of the Akt/FoxO3a/Bim pathway in HepG2 cells. Mol Med Rep. 2016;13(2):1689–1694. doi: 10.3892/mmr.2015.4695. [DOI] [PubMed] [Google Scholar]
- 126.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169(1) doi: 10.1016/j.cell.2017.02.031. [132-47.e16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sato S., Solanas G., Peixoto F.O., Bee L., Symeonidi A., Schmidt M.S. Circadian reprogramming in the liver identifies metabolic pathways of aging. Cell. 2017;170(4) doi: 10.1016/j.cell.2017.07.042. [664–77. e11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baar E.L., Carbajal K.A., Ong I.M., Lamming D.W. Sex-and tissue-specific changes in mTOR signaling with age in C57 BL/6J mice. Aging Cell. 2016;15(1):155–166. doi: 10.1111/acel.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leontieva O.V., Paszkiewicz G.M., Blagosklonny M.V. Fasting levels of hepatic p-S6 are increased in old mice. Cell Cycle. 2014;13(17):2656–2659. doi: 10.4161/15384101.2014.949150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Houtkooper R.H., Argmann C., Houten S.M., Cantó C., Jeninga E.H., Andreux P.A. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niemann J., Johne C., Schröder S., Koch F., Ibrahim S.M., Schultz J. An mtDNA mutation accelerates liver aging by interfering with the ROS response and mitochondrial life cycle. Free Radic Biol Med. 2017;102:174–187. doi: 10.1016/j.freeradbiomed.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 133.Lawless C., Jurk D., Gillespie C.S., Shanley D., Saretzki G., von Zglinicki T. A stochastic step model of replicative senescence explains ROS production rate in ageing cell populations. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Passos J.F., Nelson G., Wang C., Richter T., Simillion C., Proctor C.J. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol. 2010;6(1):347. doi: 10.1038/msb.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giorgi C., Marchi S., Simoes I.C.M., Ren Z., Morciano G., Perrone M. Chapter six-mitochondria and reactive oxygen species in aging and age-related diseases. In: López-Otín C., Galluzzi L., editors. International review of cell and molecular biology. vol. 340. Academic Press; 2018. pp. 209–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Capri M., Olivieri F., Lanzarini C., Remondini D., Borelli V., Lazzarini R. Identification of miR-31-5p, miR-141-3p, miR-200c-3p, and GLT1 as human liver aging markers sensitive to donor–recipient age-mismatch in transplants. Aging Cell. 2017;16(2):262–272. doi: 10.1111/acel.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aravinthan A., Pietrosi G., Hoare M., Jupp J., Marshall A., Verrill C. Hepatocyte expression of the senescence marker p21 is linked to fibrosis and an adverse liver-related outcome in alcohol-related liver disease. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0072904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aini W., Miyagawa-Hayashino A., Ozeki M., Adeeb S., Hirata M., Tamaki K. Accelerated telomere reduction and hepatocyte senescence in tolerated human liver allografts. Transpl Immunol. 2014;31(2):55–59. doi: 10.1016/j.trim.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 139.Rey S., Quintavalle C., Burmeister K., Calabrese D., Schlageter M., Quagliata L. Liver damage and senescence increases in patients developing hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(8):1480–1486. doi: 10.1111/jgh.13717. [DOI] [PubMed] [Google Scholar]
- 140.Paradis V., Youssef N., Dargère D., Bâ N., Bonvoust F., Deschatrette J. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32(3):327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 141.Wang C., Chen W.-J., Wu Y.-F., You P., Zheng S.-Y., Liu C.-C. The extent of liver injury determines hepatocyte fate toward senescence or cancer. Cell Death Dis. 2018;9(5):575. doi: 10.1038/s41419-018-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Donati B., Valenti L. Telomeres, NAFLD and chronic liver disease. Int J Mol Sci. 2016;17(3):383. doi: 10.3390/ijms17030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ori A., Toyama B.H., Harris M.S., Bock T., Iskar M., Bork P. Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Syst. 2015;1(3):224–237. doi: 10.1016/j.cels.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White R.R., Milholland B., De Bruin A., Curran S., Laberge R.-M., Van Steeg H. Controlled induction of DNA double-strand breaks in the mouse liver induces features of tissue ageing. Nat Commun. 2015;6:6790. doi: 10.1038/ncomms7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hudgins A.D., Tazearslan C., Tare A., Zhu Y., Huffman D., Suh Y. Age-and tissue-specific expression of senescence biomarkers in mice. Front Genet. 2018;9:59. doi: 10.3389/fgene.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aravinthan A.D., Alexander G.J. Senescence in chronic liver disease: is the future in aging? J Hepatol. 2016;65(4):825–834. doi: 10.1016/j.jhep.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 147.Morsiani C., Bacalini M.G., Santoro A., Garagnani P., Collura S., D'Errico A. The peculiar aging of human liver: a geroscience perspective within transplant context. Ageing Res Rev. 2019;51:24–34. doi: 10.1016/j.arr.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 148.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161(III-XIII):1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 149.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):3156. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Horvath S., Erhart W., Brosch M., Ammerpohl O., von Schönfels W., Ahrens M. Obesity accelerates epigenetic aging of human liver. PNAS. 2014;111(43):15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mc Auley M., Mooney K. Chapter 7-using computational models to study aging. In: Ram J.L., Conn P.M., editors. Conn's handbook of models for human aging. 2nd ed. Academic Press; 2018. pp. 79–91. [Google Scholar]
- 152.Mc Auley M.T., Mooney K.M. Computationally modeling lipid metabolism and aging: a mini-review. Comput Struct Biotechnol J. 2015;13:38–46. doi: 10.1016/j.csbj.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]