Abstract
Oxysterols are 27-carbon oxidation products of cholesterol metabolism. Oxysterols possess several biological actions, including the promotion of cell death. Here, we examined the ability of 7-ketocholesterol (7-KC), cholestane-3β-5α-6β-triol (triol), and a mixture of 5α-cholestane-3β,6β-diol and 5α-cholestane-3β,6α-diol (diol) to promote cell death in a human breast cancer cell line (MDA-MB-231). We determined cell viability, after 24-h incubation with oxysterols. These oxysterols promoted apoptosis. At least part of the observed effects promoted by 7-KC and triol arose from an increase in the expression of the sonic hedgehog pathway mediator, smoothened. However, this increased expression was apparently independent of sonic hedgehog expression, which did not change. Moreover, these oxysterols led to increased expression of LXRα, which is involved in cellular cholesterol efflux, and the ATP-binding cassette transporters, ABCA1 and ABCG1. Diols did not affect these pathways. These results suggested that the sonic hedgehog and LXRα pathways might be involved in the apoptotic process promoted by 7-KC and triol.
Keywords: Oxysterol, Apoptosis, Cancer, ABC transporters, Sonic hedgehog, LXRα
Highlights
-
•
Oxysterols (7-KC, triol, and diol) promoted apoptosis in a human breast cell line.
-
•
Sonic hedgehog expression did not change as effect of 7-KC and triol.
-
•
Smoothened expression increased as effect of 7-KC and triol.
-
•
An increased expression of LXRα, ABCA1, and ABCG1 was also observed.
-
•
SMO and LXRα may be involved in the apoptotic process promoted by 7-KC and triol.
1. Introduction
Cholesterol is an essential component of cell membrane and also a precursor of steroid hormones and bile acids [1]. Oxysterols are a large family of 27-carbon oxidized derivatives of cholesterol and similarly, they are made of a steroid backbone and a side chain [2]. Endogenous oxysterols are formed either by auto-oxidation or by enzyme-mediated mechanisms [3], [4], [5]. Oxidation promotes the addition of hydroxyl, keto, hyperoxy, carbonyl, or epoxy groups to the cholesterol backbone, mostly at the C4–7 positions or at the C24, 25, and 27 positions of the lateral chain [6]. This process generates a large class of different oxysterols.
Bioactive lipids are endogenous lipid mediators that have functional actions. Several elements point to oxysterols as bioactive lipids since they are involved in a plethora of physiological and pathophysiological processes. In fact, several oxysterols are biologically active as regulatory molecules. For example, they regulate sterol and lipid metabolism, modulate signaling pathways, and influence cell proliferation and differentiation [6]. Consequently, oxysterols participate in a variety of pathophysiological processes, including atherosclerosis and cancer [7], [8], [9], [10], [11], [12]. Moreover, some oxysterols (e.g. 4β-hydroxycholesterol or 7α-hydroxycholestenone) are used as biomarkers of specific pathologies [12].
One particularly interesting effect of several oxysterols is the promotion of cell death. This effect has been observed in a number of cell lines [11], [13], [14], [15], [16], and it occurs through several mechanisms, including gene expression regulation [4], apoptosis, and autophagy induction [13], [17], [18]. Many cell types are sensitive to the cytostatic and cytotoxic effects of oxysterols, including several tumor cell lines [6], [19], [20], [21], [22].
These properties led us to investigate the potential use of oxysterols as chemotherapeutic agents in cancer [23]. Our results showed that 7-ketocholesterol (7-KC), a well-known oxysterol, had cytostatic and cytotoxic effects on melanoma, in vitro and in vivo. Therefore, we hypothesized that 7-KC cytotoxicity could be applied in cancer therapeutics. However, it has also been shown that the cytotoxic effect of oxysterols on cells varies according to both the type of oxysterol and the specific cell line [24]. This is not surprising considering the large number of molecules within this family and their complex metabolism [12]. Another reason is the large number of molecular targets identified for these bioactive lipids. From a functional point of view, the proteins that bind the oxysterols can be classified into receptors (nuclear and GPCRs) and regulatory or transport proteins [12].
Liver X receptors (LXR) were the first nuclear receptors described for oxysterols. LXR form a heterodimer with the retinoic X receptor (RXR). Following activation by oxysterols (LXR) or by retinoic acid (RXR), the heterodimer recruits co-activators proteins and can initiate transcriptional activity [12]. The genes targeted by the heterodimer LXR/RXR are mainly those involved in the reverse cholesterol transport, such as ATP-binding cassette transporters [25]. Importantly, not all the oxysterols behave as full LXR agonists; several of them actually behave as antagonists [12]. Among the cell membrane receptors known to bind oxysterols, the GPCR smoothened (SMO) was found to be activated by some oxysterols [26], [27].
Previously, we described the cytotoxic effect of 7-ketocholesterol, cholestane-triol, and cholestane-diol on several cell lines [24]. These oxysterols inhibited the S phase and stimulated the G0/G1 or G2/M phases. They also promoted apoptosis, as determined with Annexin V and propidium iodide assays. Here, we explore more deeply the apoptotic effect of these cholesterol oxides further by assessing their ability to affect Smoothened (SMO) and Sonic Hedgehog (SHh) expression as well as the expression of LXRα and ABCA1 and ABCG1 transporters, which could also be involved in the apoptotic process.
2. Materials and methods
2.1. Oxysterol solution preparation
7-ketocholesterol, cholestane-3β-5α-6β-triol, and a mixture of 5α-cholestane-3β,6β-diol and 5α-cholestane-3β,6α-diol were synthesized as described [24, Supplementary materials]. All other reagents and solvents were purchased from Sigma-Aldrich (MO, USA). All commercially available chemicals were used without purification. Stock solutions for each compound were prepared at a concentration of 1000 μM in absolute ethanol.
2.2. Cell culture and oxysterol treatment
A human mammary gland/breast cell line, derived from a metastatic site, (MDA-MB-231; ATCC HTB-26) was used in this study. Unless otherwise specified, reagents for culture procedures were purchased from Sigma. Culture contamination by mycoplasma was routinely tested with DAPI staining.
After thawing [28], cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Gibco, Karlsruhe, Germany), 100 U/mL penicillin, and 100 mg/mL streptomycin for 48 h, then plated at a density of 5 × 103 cells/cm3 in 96-well black flat bottom polystyrene microplates (Corning, MA). Cells were maintained at 37 °C under a humidified atmosphere with 5% CO2 [24].
Various concentrations of oxysterols (0–100 μM, 100 µL final volume) were added to cells and incubated for 24 h. Then, we tested cell viability and performed indirect immunofluorescence detection of SMO, SHh, LXRα, ABCA1, and ABCG1.
2.3. Cell viability assay
After 24-h treatment with oxysterols, cells were incubated with 0.1 μg/mL Hoechst 33342 (H1399- Molecular Probes, OR, USA) and 0.5 µL propidium iodide (PI) (P3566- Molecular Probes) for 15 min. An ImageXpress Micro high-content screening system (Molecular Devices, CA, USA) was used to determine the number of live and dead cells. Nine sites per well and three wells per treatment were evaluated. Cell Scoring MetaXpress software was used to analyze the number of cells and cell viability. For IC50 calculations, we evaluated survival data with a variable slope curve-fitting application provided in GraphPad Prism (GraphPad Software, CA, USA).
2.4. Indirect immunofluorescence detection of smoothened (SMO), sonic hedgehog (SHh), liver X receptor (LXRα), and ATP-binding cassette transporters (ABCA1 and ABCG1)
For all indirect immunofluorescence experiments, cells were plated at a density of 1.5 × 103 cells/cm2 in triplicate in 96-well black flat-bottom polystyrene microplates (3603- Corning). Cells were treated with different oxysterols for 24 h, and fixed in 4% paraformaldehyde (P-6148 Sigma Aldrich) for 2 h at 4 °C. After washing with DPBS, cells were permeabilized with 0.1% Triton X-100 solution (93443 Sigma Aldrich) at 4 °C for 15 min, followed by blocking with 5% BSA (A9418 Sigma Aldrich) for 40 min at room temperature. Cells were incubated overnight with the following antibodies: anti-SMO (1:100 dilution; NBP2–24543-Novus Biologicals, CO, USA); anti-SHh (1:400 dilution; ab53281- Abcam, Cambridge, UK); anti-LXRα (1:50 dilution; ab41902 Abcam); anti-ABCA1 (1:50 dilution; NB100–2068- Novus Biologicals); and anti-ABCG1 (1:50 dilution; H9619-M03- Abnova, Taipei, Taiwan). After incubating with anti-SMO and anti-SHh antibodies, cells were incubated for 1 h more with the anti-rabbit secondary antibody, AlexaFluor 488 (1:1000 dilution; A-11008- Molecular Probes, OR, USA). Cells incubated with antibodies against LXRα, ABCA1, and ABCG1 were incubated for 1 h with the anti-mouse secondary antibody, R-phycoerythrin (1:1000 dilution; P852- Molecular Probes). The fluorescence intensities of SMO, SHh, LXRα, ABCA1, and ABCG1 labeling were determined with ImageXpress. Nine sites per well and 3 wells per treatment were evaluated. Data were analized with the cell scoring MetaXpress software.
2.5. Statistical analysis
Data are expressed as the mean ± SD of at least three independent experiments. Means were compared with the Mann-Whitney U test provided in GraphPad Prism (GraphPad Software, CA). P-values ≤ 0.05 were considered significant.
3. Results and discussion
Bioactive lipids are known to regulate several cellular processes [29], [30], [31], [32], including cell growth, proliferation, differentiation, and death [33], [34]. Among the bioactive lipids, oxysterols are potent, biologically active molecules, involved in several cell functions, including inhibiting cell proliferation and promoting cell death [35], [36]. The most studied oxysterols, at least in terms of toxicity, are 25-hydroxycholesterol, 7β-hydroxycholesterol, and 7-ketocholesterol. The cytotoxic effects of these oxysterols have been demonstrated in several cell lines [16], [22], [35], [37], [38], [39], [40], [41], [42].
Here, as expected, 7-KC, triol, and diol reduced the number of cells. As described previously, apoptosis was involved as a cause of cell death [24]. We explored the mechanisms of apoptosis promoted by 7-KC, triol, and diol by evaluating the effects of subtoxic doses (30 μM) on the sonic hedgehog (SHh) pathway and liver X receptor alpha (LXRα). SHh can cause different effects on cells at different concentrations. The SHh pathway is activated when SHh binds to its receptor, the transmembrane protein, Patched (PTCH) [43]. PTCH proteins prevent downstream signaling by attenuating Smoothened (SMO) activity [44]. However, when SHh binds to PTCH, it removes the repression of SMO, which then activates a signal transduction pathway in the cytoplasm [45]. Recently, it was shown that oxysterols could allosterically activate SMO by binding to its extracellular cysteine-rich domain [46].
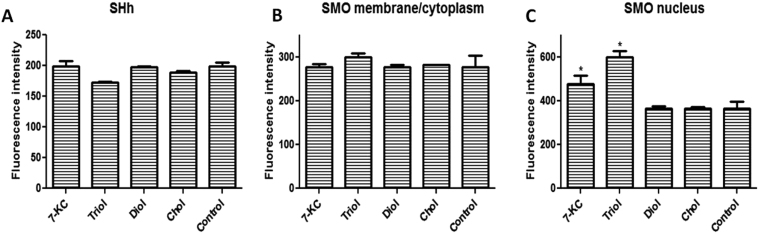
Here, we evaluated SHh with immunofluorescence. None of the oxysterols or cholesterol (as control) changed SHh protein expression (Fig. 1A). The effect of oxysterols on SMO was evaluated by assessing fluorescence intensity in the membrane/cytoplasm and in the nucleus. Cells expressed SMO protein. Neither oxysterols nor cholesterol changed SMO expression in the membrane/cytoplasm (Fig. 1B). On the other hand, SMO expression in the nucleus increased lines after treatment with 7-KC and triol (Fig. 1C). Cholesterol had no effect on nuclear SMO levels. Therefore, these oxysterols did not appear to act on SMO by changing SHh expression, but a possible direct action on SMO should be considered.
Fig. 1.
Immunofluorescence detection of sonic hedgehog (SHh) and smoothened (SMO) expression in MDA-MB-231 cell line after 24 h incubation with 30 µM oxysterols. A: SHh expression; B: SMO expression in the membrane/cytoplasm;C: SMO expression in the nucleus. The intensity of fluorescence was quantified with MetaXpress software. Abbreviations: 7KC: 7-ketocholesterol; triol: cholestan-3α-5β-6α-triol; diol: 5α-cholestane-3β,6β-diol/5α-cholestane-3β,6α-diol. Cholesterol was used as control. Data are presented as the mean ± SEM from three independent experiments performed in triplicate. *p < 0.005 compared to control.
LXRs are nuclear receptors with important roles in the transcriptional control of lipid metabolism. They were initially described as orphan receptors, but later, oxysterols were identified as their natural ligands. Activated LXRs form heterodimeric complexes with retinoic acid receptors (RXRs) [47]. LXRs exert important effects, including control of transcription factors and gene regulation. The genes targeted by LXR/RXR are mainly involved in cholesterol efflux from cells (reverse cholesterol transport) through the ATP-binding cassette transporters, ABCA1, ABCG5, ABCG8, and ABCG1 [12], [48]. It is well known that cholesterol metabolism is dysregulated in different malignant cells. LXRs have been described as having anticancer properties. They can regulate tumor growth in various cancer cell lines [49], [50], [51]. In the past few years, anti-proliferative effects of synthetic and natural LXR agonists have been observed in various types of human cancer, in vitro and in vivo: blastic plasmacytoid dendritic cell neoplasm [49], prostate cancer cells [52], melanoma [53], colon cancer cells [54], acute lymphoblastic leukemia [55], human lung cancer [56]. Therefore, LXR agonists have been considered as potential anti-cancer agents. It has been proposed that activation of LXR deprives cancer cell membranes of lipids essential for their growth, inhibiting cell proliferation, by stimulating cholesterol efflux via upregulation of ABCA1 and ABCG1 [48], [49]. However, whether these effects are related only to cholesterol efflux has not yet been elucidated.
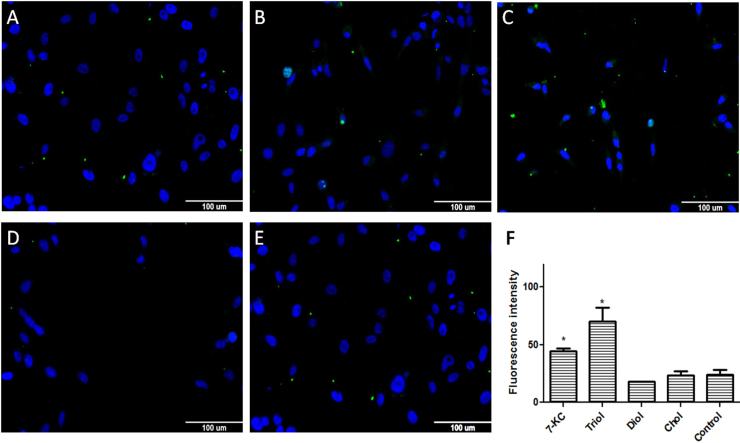
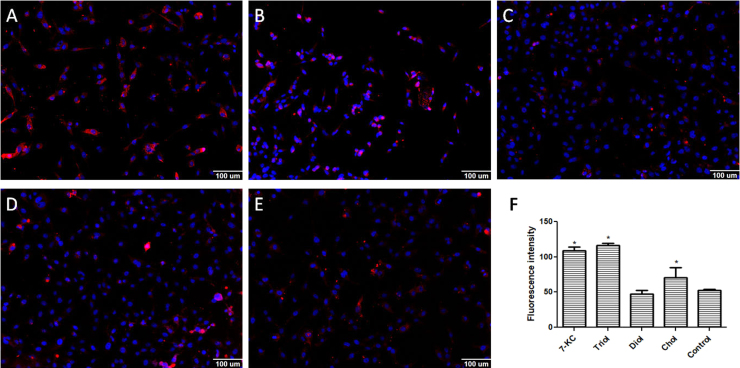
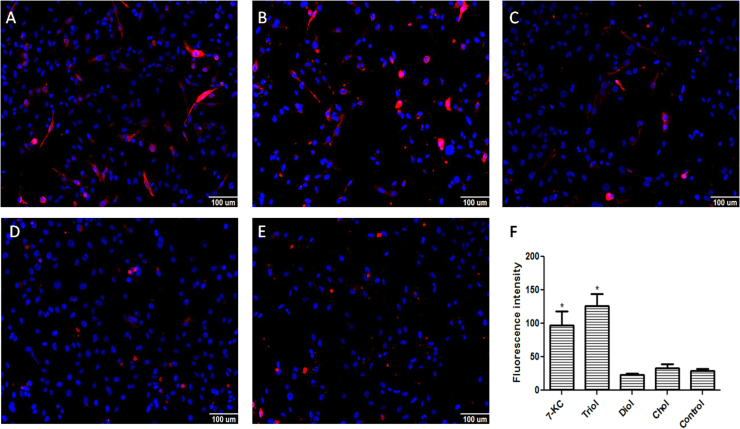
We evaluated the effect of 7-KC, triol, diol, and cholesterol (30 μM) on LXRα, ABCA1, and ABCG1 expression. LXRα fluorescence intensity increased when cells were treated with 7-KC and triol, but not with diol or cholesterol (Fig. 2). Based on these results, we tested ABCA1 and ABCG1 expression (Fig. 3, Fig. 4, respectively). Again, cells treated with 7-KC and triol showed elevated expression of ABCA1 and ABCG1 proteins, but no change was observed with diol treatment. Cholesterol caused small increases in ABCA1 expression, as expected, but no effect was observed on ABCG1 expression. Therefore, we hypothesized that 7-KC and triol might increase ABCA1 and ABCG1 expression levels by stimulating LXRα. This can, at least in part, contribute for the observed effects on cell proliferation and death. Besides, LXRα could also contribute to the process of apoptosis, as recently shown. In addition to the inhibition of cancer cell survival related to cholesterol deprivation, LXRs also control the expression of genes involved in many other processes [12]. Treatment with LXR agonists was described to be responsible for inducing intrinsic apoptotic cell death [49]. Increased apoptotic rates have been promoted by several mechanisms: up-regulation of the pro-apoptotic gene BAX and reduction of the anti-apoptotic gene BCL-2 expression [48], downregulation of AKT survival signaling [52], [56], caspase-3 pathway [53], caspase-1 dependent cell death induction [54], up-regulation of SOCS3 pathway [55]. It has also been suggested that ABCA1 and ABCG1 are required for the apoptotic clearance process, including appropriate phagocytosis of apoptotic cells [50], [51].
Fig. 2.
Immunofluorescence detection of LXRα expression in the MDA-MB-231 cell line after 24 h incubation with 30 µM of oxysterols. The intensity of fluorescence was quantified with MetaXpress software. Representative images of immunofluorescence after incubations with A: 7-ketocholesterol (7KC); B: cholestan-3α-5β-6α-triol (triol); C: 5α-cholestane-3β,6β-diol/5α-cholestane-3β,6α-diol (diol); or D: cholesterol (control); E: quantification of LXRα expression. Data are presented as the mean ± SEM from three independent experiments performed in triplicate. *p < 0.005 compared to control.
Fig. 3.
Immunofluorescence detection of ABCA1 expression in the MDA-MB-231 cell line after 24 h incubation with 30 µM of oxysterols. The intensity of fluorescence was quantified with MetaXpress software. Representative images of immunofluorescence after incubations with A: 7-ketocholesterol (7KC); B: cholestan-3α-5β-6α-triol (triol); C: 5α-cholestane-3β,6β-diol/5α-cholestane-3β,6α-diol (diol); or D: cholesterol (control); E: quantification of ABCA1 expression. Data are presented as the mean ± SEM from three independent experiments performed in triplicate. *p < 0.005 compared to control.
Fig. 4.
Immunofluorescence detection of ABCG1 expression in the MDA-MB-231 cell line after 24 h incubation with 30 µM of oxysterols. The intensity of fluorescence was quantified with MetaXpress software. Representative images of immunofluorescence after incubations with A: 7-ketocholesterol (7KC); B: cholestan-3α-5β-6α-triol (triol); C: 5α-cholestane-3β,6β-diol/5α-cholestane-3β,6α-diol (diol); or D: cholesterol (control); E: quantification of ABCG1 expression. Data are presented as the mean ± SEM from three independent experiments performed in triplicate. *p < 0.005 compared to control.
In conclusion, cytotoxic 7-ketocholesterol and cholestane-3β-5α-6β-triol could act on SMO and LXRα pathways to promote cell death. In view of these findings, their potential pharmacological utility merit further investigation.
CRediT authorship contribution statement
Debora Levy: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Thatiana Correa de Melo: Investigation, Data curation, Writing - review & editing. Beatriz A. Oliveira: Investigation, Data curation, Writing - review & editing. Jessica L. Paz: Investigation, Data curation, Writing - review & editing. Fabio A. de Freitas: Formal analysis, Writing - review & editing. Cadiele O. Reichert: Formal analysis, Writing - review & editing. Alessandro Rodrigues: Formal analysis, Writing - original draft, Writing - review & editing. Sergio P. Bydlowski: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Supervision.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Instituto Nacional de Ciência e Tecnologia – Fluidos Complexos (INCT-FCx); Instituto Nacional de Ciência e Tecnologia em Medicina Regenerativa (INCT-Regenera), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – 13/10073-5 and 16/21676-0), all from Brazil.
Acknowledgments
Competing financial interests
The authors declare no competing financial interests
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.12.008.
Appendix A. Transparency document
Supplementary material
References
- 1.Howe V., Sharpe L.J., Alexopoulos S.J. Cholesterol homeostasis: how do cells sense sterol excess? Chem. Phys. Lipids. 2016;199:170–178. doi: 10.1016/j.chemphyslip.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Jakobsson T., Treuter E., Gustafsson J.A. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol. Sci. 2012;33:394–404. doi: 10.1016/j.tips.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho J.F., Silva M.M., Moreira J.N. Selective cytotoxicity of oxysterols through structural modulation on rings A and B. Synthesis, in vitro evaluation, and SAR. J. Med. Chem. 2011;54:6375–6393. doi: 10.1021/jm200803d. [DOI] [PubMed] [Google Scholar]
- 4.Kulig W., Cwiklik L., Jurkiewicz P. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Zerbinati C., Iuliano L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017;111:151–155. doi: 10.1016/j.freeradbiomed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Kloudova A., Guengerich F.P., Soucek P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 2017;28:485–496. doi: 10.1016/j.tem.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lordan S., Mackrill J.J., O'Brien N.M. Oxysterols and mechanisms of apoptotic signaling: implications in the pathology of degenerative diseases. J. Nutr. Biochem. 2009;20:321–336. doi: 10.1016/j.jnutbio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho J.F., Silva M.M., Moreira J.N. Sterols as anticancer agents: synthesis of ring-B oxygenated steroids, cytotoxic profile, and comprehensive SAR analysis. J. Med. Chem. 2010;53:7632–7638. doi: 10.1021/jm1007769. [DOI] [PubMed] [Google Scholar]
- 9.Jusakul A., Yongvanit P., Loilome W. Mechanisms of oxysterol-induced carcinogenesis. Lipids Health Dis. 2011;10:44. doi: 10.1186/1476-511X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz J.L., Fernandes L.R., Levy D., Bydlowski S.P. Interrelationship between ATP-binding cassette transporters and oxysterols. Biochem. Pharmacol. 2013;86:80–88. doi: 10.1016/j.bcp.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Levy D., Ruiz J.L., Celestino A.T. Short-term effects of 7-ketocholesterol on human adipose tissue mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2014;446:720–725. doi: 10.1016/j.bbrc.2014.01.132. [DOI] [PubMed] [Google Scholar]
- 12.Mutemberezi V., Guillemot-Legris O., Muccioli G.G. Oxysterols: from cholesterol metabolites to key mediators. Prog. Lipid Res. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Nury T., Zarrouk A., Mackrill J.J. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7beta-hydroxycholesterol-, or 24(S)-hydroxycholesterol: protective effects of alpha-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3) Steroids. 2015;99:194–203. doi: 10.1016/j.steroids.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Berthier A., Lemaire-Ewing S., Prunet C. 7-Ketocholesterol-induced apoptosis. FEBS J. 2005;272:3093–3104. doi: 10.1111/j.1742-4658.2005.04723.x. [DOI] [PubMed] [Google Scholar]
- 15.Prunet C., Lemaire-Ewing S., Ménétrier F. Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol- and 7β-hydroxycholesterol-induced cell death: a morphological and biochemical study. J. Biochem. Mol. Toxicol. 2005;19:311–326. doi: 10.1002/jbt.20096. [DOI] [PubMed] [Google Scholar]
- 16.Silva S.F., Levy D., Ruiz J.L.M. Oxysterols in adipose tissue-derived mesenchymal stem cell proliferation and death. J. Steroid Biochem. Mol. Biol. 2017;169:164–175. doi: 10.1016/j.jsbmb.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Lizard G., Gueldry S., Sordet O. Glutathione is implied in the control of 7-ketocholesterol-induced apoptosis, which is associated with radical oxygen species production. FASEB J. 1998;12:1651–1663. doi: 10.1096/fasebj.12.15.1651. [DOI] [PubMed] [Google Scholar]
- 18.O'Callaghan Y.C., Woods J.A., O'Brien N.M. Comparative study of the cytotoxicity and apoptosis-inducing potential of commonly occurring oxysterols. Cell Biol. Toxicol. 2001;17:127–137. doi: 10.1023/a:1010914306375. [DOI] [PubMed] [Google Scholar]
- 19.Guardiola F., Codony R., Addis P.B. Biological effects of oxysterols: current status. Food Chem. Toxicol. 1996;34:193–211. doi: 10.1016/0278-6915(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 20.Silvente-Poirot S., Poirot M. Cholesterol epoxide hydrolase and cancer. Curr. Opin. Pharmacol. 2012;12:696–703. doi: 10.1016/j.coph.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Poirot M., Silvente-Poirot S. Cholesterol-5,6-epoxides: chemistry, biochemistry, metabolic fate and cancer. Biochimie. 2013;95:622–631. doi: 10.1016/j.biochi.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Rosa Fernandes L., Stern A.C.B., Cavaglieri Rd.C. 7-Ketocholesterol overcomes drug resistance in chronic myeloid leukemia cell lines beyond MDR1 mechanism. J. Proteom. 2017;151:12–23. doi: 10.1016/j.jprot.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Favero G.M., Paz J.L., Otake A.H. Cell internalization of 7-ketocholesterol-containing nanoemulsion through LDL receptor reduces melanoma growth in vitro and in vivo: a preliminary report. Oncotarget. 2018;9:14160–14174. doi: 10.18632/oncotarget.24389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D., Correa de Melo T., Ohira B.Y. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochem. Biophys. Res. Commun. 2018;505:1043–1049. doi: 10.1016/j.bbrc.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 26.Nachtergaele S., Mydock L.K., Krishnan K. Oxysterols are allosteric activators of the oncoprotein smoothened. Nat. Chem. Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers B.R., Sever N., Chong Y.C. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev. Cell. 2013;26:346–357. doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janz Fd.L., Debes Ad.A., Cavaglieri Rd.C. Evaluation of distinct freezing methods and cryoprotectants for human amniotic fluid stem cells cryopreservation. J. Biomed. Biotechnol. 2012;2012:1–10. doi: 10.1155/2012/649353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes A.A., Peranovich T.M., Maeda N.Y., Bydlowski S.P. Differential effects of enzymatic treatments on the storage and secretion of von Willebrand factor by human endothelial cells. Thromb. Res. 2001;101:291–297. doi: 10.1016/s0049-3848(00)00401-1. [DOI] [PubMed] [Google Scholar]
- 30.Bydlowski S.P., Yunker R.L., Rymaszewski Z., Subbiah M.T. Coffee extracts inhibit platelet aggregation in vivo and in vitro. Int. J. Vitam. Nutr. Res. 1987;57:217–223. [PubMed] [Google Scholar]
- 31.Sola B., Poirot M., de Medina P. Antiestrogen-binding site ligands induce autophagy in myeloma cells that proceeds through alteration of cholesterol metabolism. Oncotarget. 2013;4:911–922. doi: 10.18632/oncotarget.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bydlowski S.P., Yunker R.L., Subbiah M.T. Ontogeny of 6-keto-PGF1 alpha synthesis in rabbit aorta and the effect of premature weaning. Am. J. Physiol. -Heart Circ. Physiol. 1987;252:H14–H21. doi: 10.1152/ajpheart.1987.252.1.H14. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira A.K., Freitas V.M., Levy D. Anti-angiogenic and anti-metastatic activity of synthetic phosphoethanolamine. PLoS One. 2013;8:e57937. doi: 10.1371/journal.pone.0057937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C., Freter C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015;16:924–949. doi: 10.3390/ijms16010924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Weille J., Fabre C., Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem. Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Luu W., Sharpe L.J., Capell-Hattam I. Oxysterols: old tale, new twists. Annu. Rev. Pharmacol. Toxicol. 2016;56:447–467. doi: 10.1146/annurev-pharmtox-010715-103233. [DOI] [PubMed] [Google Scholar]
- 37.Nury T., Zarrouk A., Vejux A. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: impairment by alpha-tocopherol. Biochem. Biophys. Res. Commun. 2014;446:714–719. doi: 10.1016/j.bbrc.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 38.Pedruzzi E., Guichard C., Ollivier V. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poirot M., Silvente-Poirot S. Oxysterols and related sterols: implications in pharmacology and pathophysiology. Biochem. Pharmacol. 2013;86:1–2. doi: 10.1016/j.bcp.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Chang M.C., Chen Y.J., Liou E.J. 7-Ketocholesterol induces ATM/ATR, Chk1/Chk2, PI3K/Akt signalings, cytotoxicity and IL-8 production in endothelial cells. Oncotarget. 2016;7:74473–74483. doi: 10.18632/oncotarget.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy D., de Melo T.C., Ruiz J.L.M., Bydlowski S.P. Oxysterols and mesenchymal stem cell biology. Chem. Phys. Lipids. 2017;207:223–230. doi: 10.1016/j.chemphyslip.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Rosa-Fernandes L., Maselli L.M.F., Maeda N.Y. Outside-in, inside-out: proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chem. Phys. Lipids. 2017;207:231–238. doi: 10.1016/j.chemphyslip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Fattahi S., Langroudi M.P., Akhavan-Niaki H. Hedgehog signaling pathway: epigenetic regulation and role in disease and cancer development. J. Cell. Physiol. 2018;233:5726–5735. doi: 10.1002/jcp.26506. [DOI] [PubMed] [Google Scholar]
- 44.Carballo G.B., Honorato J.R., Farias de Lopes G.P., de Sampaio e Spohr T.C.L. Cell Commun. Signal. 2018;16:11. doi: 10.1186/s12964-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rimkus T.K., Carpenter R.L., Qasem S. Targeting the Sonic Hedgehog signaling pathway: review of smoothened and Gli inhibitors. Cancers. 2016;8:22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blassberg R., Jacob J. Lipid metabolism fattens up hedgehog signalling. BMC Biol. 2017;15:95. doi: 10.1186/s12915-017-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B., Tontonaz P. Liver X receptors in lipid signaling and membrane homeostasis. Nat. Rev. Endocrinol. 2018;14:452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El Roz A., Bard J.-M., Huvelin J.-M., Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 2012;32:3007–3013. [PubMed] [Google Scholar]
- 49.Ceroi A., Masson D., Roggy A. LXR agonist treatment of blastic plasmacytoid dendritic cell neoplasm restores cholesterol efflux and triggers apoptosis. Blood. 2016;128:2694–2707. doi: 10.1182/blood-2016-06-724807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fond A.M., Lee C.S., Schulman I.G., Kiss R.S., Ravichandran K.S. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. Clin. Investig. 2015;125:2748–2758. doi: 10.1172/JCI80300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Röszer T. Transcriptional control of apoptotic cell clearance by macrophage nuclear receptors. Apoptosis. 2017;22:284–294. doi: 10.1007/s10495-016-1310-x. [DOI] [PubMed] [Google Scholar]
- 52.Pommier A.J., Alves G., Viennois E. Liver X receptor activation down-regulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W., Jiang H., Zhang J. Liver X receptor activation induces apoptosis of melanoma cell through caspase pathway. Cancer Cell Int. 2014;14:16. doi: 10.1186/1475-2867-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derangère V., Chevriaux A., Courtaut F. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death Differ. 2014;21:1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang R., Liu Z., Li Y., Wu B. LXR agonist regulates the proliferation and apoptosis of human T-cell acute lymphoblastic leucemia cells via the SOCS3 pathway. Int. J. Biochem. Cell Biol. 2016;78:180–185. doi: 10.1016/j.biocel.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Liu S., Cao H., Chen D. LXR ligands induce apoptosis of EGFR-TKI-resistant human lung cancer cells in vitro by inhibiting Akt-NF-kB activation. Oncol. Lett. 2018;15:7168–7174. doi: 10.3892/ol.2018.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material