Abstract
Purpose
Much concern was directed toward exploring the relationship between vitamin D and migraine. There is strong evidence that vitamin D supplementation can decrease frequency, severity, and duration of migraine headache attacks. The aim of this work was to investigate the difference in serum levels of 25 (OH)-vitamin D between patients with migraine and healthy controls, to determine the differences in headache characteristics according to vitamin D status, and to correlate serum 25 (OH)-vitamin D level with duration, frequency, and severity of migraine headache attacks.
Patients and methods
This is a case–control study conducted on 40 patients diagnosed with migraine and 40 healthy controls. History was taken from patients with migraine regarding headache characteristics. Migraine severity scale (MIGSEV) and Headache Impact Test-6 (HIT-6) were used for migraine assessment. Serum 25(OH)-vitamin D was measured for all patients and controls using enzyme-linked immunosorbent assay (ELISA).
Results
Patients with migraine had significantly lower 25(OH)-vitamin D serum level in comparison to controls (P-value=0.019). The incidence of aura, phonophobia/photophobia, autonomic manifestations, allodynia, and resistance to medications were significantly higher in migraineurs with vitamin D deficiency than those with normal vitamin D. There was a statistically significant negative correlation between 25(OH)-vitamin D serum level and attack duration in hours (P-value˂0.001), frequency of the attacks/month (P-value˂0.001), MIGSEV scale (P-value=0.001), and HIT-6 scale (P-value=0.001).
Conclusion
Patients with migraine had significant vitamin D deficiency compared to healthy controls. Such deficiency significantly affects headache characteristics, duration, frequency, and severity of headache attacks.
Keywords: MIGSEV, HIT-6, 25(OH)-vitamin D, ELISA
Introduction
Migraine and tension headache are the most common primary headache disorders that affect 80% of the people all over the world.1 Global estimation of migraine headache prevalence showed that migraine affects 1 in 10 people worldwide.2 It affects 6% of the men and18% of the women and has a peak incidence in ages between 25 and 55 years.3 A systematic analysis for the Global Burden of Disease (GBD) Study in 2015 considered migraine headache as the second largest contributor of disability-adjusted life years (DALYs) among all neurological disorders.4
Migraine attacks are characterized by being moderate-to-severe, pulsating, unilateral, associated with nausea, vomiting, phonophobia, and photophobia. The attack usually lasts from several hours to 2–3 days.5 About 25% of the patients with migraine perceive an aura, which is a transient disturbance in visual, sensory, language, or motor function preceding the migraine attack. Migraine headache is caused by the release of pain-producing inflammatory mediators around the cranial nerves and blood vessels. Such mediators induce vascular smooth muscle dysfunction.6,7
Options for migraine treatment remain unsatisfactory because of the reported lack of effectiveness and the significant side effects. Thus, efforts to identify more effective well-tolerated therapy for preventing migraine remain urgent.8
In recent years, vitamin D deficiency has been reported as a global public health problem. Prevalence of vitamin D deficiency ranged between 30% and 50% in normal populations.9,10 Despite high sun exposure in middle eastern countries, these countries are considered among the highly prevalent areas for vitamin D deficiency in the world.11
There is strong evidence supporting an association between vitamin D deficiency and chronic pain.12 Much concern was directed toward the presence of a possible relationship between vitamin D and migraine.13–15 Vitamin D was found to have a role in the pathways involved in the pathogenesis of migraine including pain sensitization, inflammation, and immune dysfunction.16,17 On the molecular level, Motaghi et al revealed that vitamin D receptor (VDR) gene polymorphisms may increase the risk for developing migraine without aura.18
Aim of this work
The aim of this work was to investigate the difference in the serum levels of 25-hydroxy vitamin D (25(OH) vit D) between patients with migraine headache and healthy controls. The secondary objectives were to determine the differences in headache characteristics according to vitamin D status and to correlate serum 25(OH)-vitamin D level with duration, frequency, and severity of migraine headache attacks.
Materials and methods
Study design
The present study is a case–control study. It was conducted on 40 patients diagnosed as having migraine headache with or without aura and 40 normal healthy controls. The patients were recruited in the period between October 2017 and October 2018 from the Neurology outpatient clinic and Pain clinic in Beni-Suef University Hospital, Egypt. A written informed consent was obtained from each participant in this study or from parent or legal guardian for participants under the age of 18 years. The study was conducted in accordance with the Declaration of Helsinki. The study was approved by local ethical committee in Faculty of Medicine, Beni-Suef University, Egypt. The committee’s reference number is FWA00015574.
Inclusion criteria
Selected patients fulfilled the criteria for diagnosis of migraine headache based on International Classification of Headache Disorders-II (ICHD-II) diagnostic criteria.7 The age of the selected patients ranged between 15 and 55 years.
Exclusion criteria
Patients in the migraine group were excluded from the study if they have secondary headache, bilateral papilledema, or magnetic resonance imaging (MRI) showing structural brain lesion. Patients with a history of concomitant medical or metabolic illness known to affect vitamin D level, such as infectious disorders, liver or kidney disease, gastrointestinal disease, cancer, sarcoidosis, or tuberculosis, were also excluded. Participants in both groups were excluded from the study if they consumed vitamin D supplements in the preceding 3 months (any dose); or if they were taking medications that could affect vitamin D serum level such as glucocorticoids, thiazide diuretics, or statins. Pregnant were also excluded from the study.
Participants of this study were subjected to the following
History taking regarding
The frequency of headache attacks/month, the duration headache attacks, the presence of aura, nausea/vomiting, phono/photophobia, autonomic manifestations, allodynia, and resistance to medications.
Migraine assessment
The Headache Impact Test-6 (HIT-6)
It consists of six items: pain, role functioning, social functioning, vitality, psychological distress, and cognitive functioning. The patient has to answer each of the six questions using one of the following five responses: “always”, “very often”, “sometimes”, “rarely”, or “never”. The total HIT-6 score ranges between 36 and 78, where a higher score of the test indicates a greater impact of migraine headache on daily activities.19,20
Migraine severity scale (MIGSEV)
It is used for assessment of migraine severity. It includes the following items: nausea, tolerability, disability in daily activities, and intensity of pain. It categorizes patients according to intensity of headache into three groups; mild, moderate, and severe.21
Laboratory assessment
Fasting early morning (5 mL) venous blood samples were collected from all included subjects in 6 mL plain tubes then centrifuged within 30 mins of collection. The serum samples were frozen at −20°C. Serum 25-hydroxyvitamin D [25(OH)D] was considered to be the most reliable indicator for assessment of vitamin D status, so it was measured using enzyme-linked immunosorbent assay (ELISA) using Stat Fax 303Plus equipment. Measuring range was 5–120 ng/mL. Results of serum vitamin D were classified into deficiency or insufficiency (serum vitamin D<30 ng/mL) and sufficiency (serum vitamin D≥30 ng/mL).
Statistical methods
The sample size calculation was done using G*Power version 3.1.9.2 Software based on a pilot study done preceding the present study. The probability of type I error (α) was 5%, and the power (1–β) was 80%. A total of 80 participants were required for the statistical significance (40 patients and 40 age- and sex-matched healthy controls). The data were coded and entered using: the statistical package for social science version 18 (SPSS v 18). Independent sample Student t-test was used for comparison between means of quantitative variables in migraine and control groups. Chi-square test was used for comparison between categorical data in migraine and control groups and also in migraine patients with normal vitamin D level and those with vitamin D deficiency. The Pearson correlation coefficient (r) was used to describe the degree of relationship between 25(OH)-vitamin D serum level and duration of migraine attack in hours, frequency of the attacks/month, MIGSEV scale and HIT-6 scale. The probability/significance value (P-value) ≥0.05 is not statistically significant and <0.05 is statistically significant.
Results
The mean age of patients in migraine group (n=40) was 32.18±7.47 years, whereas, the mean age of subjects in control group (n=40) was 28.8±8.25 years. In migraine group, 30% (n=12) of the patients were males and 70% (n=28) were females, whereas, in control group, 35% (n=14) of the subjects were males and 65% (n=26) were females. There was no statistically significant difference between both groups in either age (P-value=0.059) or sex (P-value=0.633) (Table 1).
Table 1.
Demographics of patients and control groups
| Migraine group (n=40) | Control group (n=40) | P-value | ||
|---|---|---|---|---|
| Age in years [mean (SD)] | 32.18 (7.47) | 28.8 (8.25) | 0.059 | |
| Sex | Male [n (%)] | 12 (30%) | 14 (35%) | 0.633 |
| Female [n (%)] | 28 (70%) | 26 (65%) | ||
Note: P-value≥0.05 (non significant).
The clinical characteristics of migraine regarding attack duration in hours, frequency of the attacks/month, side of migraine, aura, phonophobia/photophobia, autonomic manifestations, allodynia, resistance to medications, MIGSEV scale, and HIT-6 scale are demonstrated in Tables 2 and 3.
Table 2.
Clinical characteristics of migraine in patients group
| Patients (n=40) | ||
|---|---|---|
| Attack duration in hours [mean (SD)] | 14.23 (13.2) | |
| Frequency of the attacks/month | 7.58 (5.14) | |
| Side | Unilateral [n (%)] | 24 (60%) |
| Bilateral [n (%)] | 16 (40%) | |
| Aura | Present [n (%)] | 17 (42.5%) |
| Absent [n (%)] | 23 (57.5%) | |
|
Phonophobia/ Photophobia |
Present [n (%)] | 25 (62.5%) |
| Absent [n (%)] | 15 (37.5%) | |
| Autonomic manifestations | Present [n (%)] | 12 (30%) |
| Absent [n (%)] | 28 (70%) | |
| Allodynia | Present [n (%)] | 20 (50%) |
| Absent [n (%)] | 20 (50%) | |
| Resistance to medications | Resistant [n (%)] | 17 (42.5%) |
| Responsive [n (%)] | 23 (57.5%) | |
Table 3.
Scales for migraine assessment in patients group
| Patients (n=40) | ||
|---|---|---|
| MIGSEV scale [mean (SD)] | Intensity | 2.15 (0.83) |
| Disability | 1.65 (0.7) | |
| Tolerability | 1.55 (0.64) | |
| Nausea | 2.13 (0.85) | |
| Total score | 7.45 (2.58) | |
| HIT-6 scale [mean (SD)] | 60.05 (4.99) | |
Abbreviations: MIGSEV, migraine severity; HIT-6, The Headache Impact Test-6.
The mean value of 25(OH)-vitamin D serum level for patients in migraine group (n=40) was 32.11±18.93 ng/mL, whereas, the mean value of 25(OH)-vitamin D serum level for subjects in control group (n=40) was 41.86±17.52 ng/mL. There was a statistically significant difference between both groups (P-value=0.019) (Table 4). In migraine group, 35% of the patients (n=14) had normal vitamin D level (≥30 ng/mL) and 65% (n=26) had vitamin D deficiency (<30 ng/mL), whereas, in control group, 70% (n=28) of the subjects had normal vitamin D level (≥30 ng/mL) and 30% (n=12) had vitamin D deficiency (<30 ng/mL). There was a statistically significant difference between both groups (P-value =0.002) (Table 5).
Table 4.
25(OH)-vitamin D in migraine patients and control group
| Migraine group (n=40) | Control group (n=40) | P-value | |
|---|---|---|---|
| 25 (OH)-vitamin D in ng/mL | 32.11 (18.93) | 41.86 (17.52) | 0.019* |
Note: *P-value<0.05 (significant).
Table 5.
Frequency of vitamin D deficiency in migraine patients and control group
| Migraine group (n=40) | Control group (n=40) | P-value | |
|---|---|---|---|
| Subjects with normal vitamin D (≥30 ng/mL) [n (%)] | 14 (35%) | 28 (70%) | 0.002* |
| Subjects with vitamin D deficiency (<30 ng/mL) [n (%)] | 26 (65%) | 12 (30%) |
Note: *P-value<0.05 (significant).
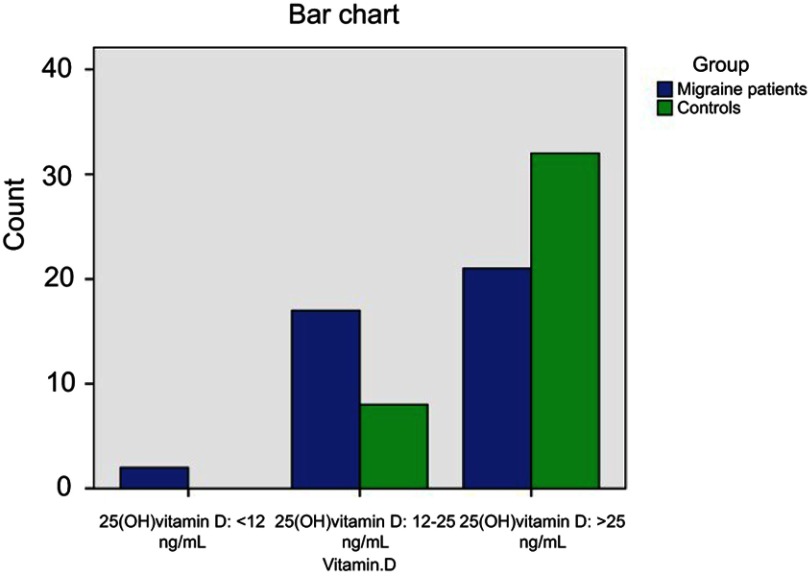
Migraine patients and controls were stratified according to 25(OH)-vitamin D serum level into subjects with severe vitamin D deficiency (<12 ng/mL), subjects with mild vitamin D deficiency (12–24 ng/mL) and subjects with optimal vitamin D (≥25 ng/mL). There was a statistically significant difference between migraine patients and controls (P-value =0.023) (Table 6, Figure 1).
Table 6.
Stratification of migraine patients and controls according to 25(OH)-vitamin D serum level
| Migraine group (n=40) | Control group (n=40) | P-value | |
|---|---|---|---|
| Subjects with 25 (OH) vitamin D <12 ng/mL [n (%)] | 2 (5%) | 0 (0%) | 0.023* |
| Subjects with 25 (OH) vitamin D 12–25 ng/mL [n (%)] | 17 (42.5%) | 8 (20%) | |
| Subjects with 25 (OH) vitamin D ≥25 ng/mL [n (%)] | 21 (52.5%) | 32 (80%) |
Note: *P-value<0.05 (significant).
Figure 1.
Stratification of migraine patients and controls according to 25(OH)-vitamin D serum level.
There was a statistically significant difference between migraine patients with normal vitamin D level and those with vitamin D deficiency regarding the presence of aura (χ2=3.913, P-value=0.048, Odds ratio=4.28, 95% CI=0.963–19.007), phonphobia/photophobia (χ2=6.593, P-value=0.01, Odds ratio=6, 95% CI=1.445–24.919), autonomic manifestations (χ2=5.358, P-value=0.021, Odds ratio=9.533, 95% CI=1.08–84.139), allodynia (χ2=3.956, P-value=0.047, Odds ratio=4, 95% CI=0.983–16.271), and resistance to medications (χ2=3.913, P-value=0.048, Odds ratio=4.28, 95% CI=0.963–19.007) (Table 7).
Table 7.
Effect of Vitamin D deficiency on clinical characteristics of migraine
| Migraine with normalvitamin D (n=14) | Migraine with vitamin Ddeficiency (n=26) | P-value | Odds ratio | 95% confidence interval | |||
|---|---|---|---|---|---|---|---|
| lower | Upper | ||||||
| Aura | Present [n (%)] | 3 (21.4%) | 14 (53.8%) | 0.048* | 4.28 | 0.963 | 19.007 |
| Absent [n (%)] | 11 (78.6%) | 12 (46.2%) | |||||
|
Phonophobia/ Photophobia |
Present [n (%)] | 5 (35.7%) | 20 (76.9%) | 0.01* | 6 | 1.445 | 24.919 |
| Absent [n (%)] | 9 (64.3%) | 6 (23.1%) | |||||
| Autonomic manifestations | Present [n (%)] | 1 (7.1%) | 11 (42.3%) | 0.021* | 9.533 | 1.08 | 84.139 |
| Absent [n (%)] | 13 (92.9%) | 15 (57.7%) | |||||
| Allodynia | Present [n (%)] | 4 (28.6%) | 16 (61.5%) | 0.047* | 4 | 0.983 | 16.271 |
| Absent [n (%)] | 10 (71.4%) | 10 (38.5%) | |||||
| Resistance to medications | Resistant [n (%)] | 3 (21.4%) | 14 (53.8%) | 0.048* | 4.28 | 0.963 | 19.007 |
| Responsive [n (%)] | 11 (78.6%) | 12 (46.2%) | |||||
Note: *P-value<0.05 (significant).
There was a statistically significant negative correlation between 25(OH)-vitamin D serum level and attack duration in hours (r coefficient=−0.552, P-value ˂0.001), frequency of the attacks/month (r coefficient=−0.629, P-value ˂0.001), MIGSEV scale (r coefficient=−0.492, P-value=0.001), and HIT-6 scale (r coefficient=−0.506, P-value=0.001) (Table 8).
Table 8.
Correlation between 25(OH)-vitamin D serum level and clinical characteristics of migraine
| 25 (OH)-vitamin D in ng/mL | |||
|---|---|---|---|
| (r) coefficient | P-value | ||
| Attack duration in hours | −0.552 | ˂0.001* | |
| Frequency of the attacks/month | −0.629 | ˂0.001* | |
| MIGSEV scale | Intensity | −0.497 | 0.001* |
| Disability | −0.427 | 0.006* | |
| Tolerability | −0.441 | 0.004* | |
| Nausea | −0.272 | 0.089 | |
| Total score | −0.492 | 0.001* | |
| HIT-6 scale | −0.506 | 0.001* | |
Notes: (r) using Pearson coefficient, P-value≥0.05 (non significant), *P-value<0.05 (significant).
Abbreviations: MIGSEV, migraine severity; HIT-6, The Headache Impact Test-6.
Discussion
The role of vitamin D is recently discussed as a key factor in neurovascular diseases.22,23 The causal relationship between vitamin D deficiency and migraine headache remains unknown mainly due to the small number of studies, contradictory results, and the lack of large randomized clinical trials that evaluate the beneficial effects of vitamin D supplementation in migraine headache.24
The objective of this work was to investigate the difference in the serum levels of 25(OH)-vitamin D between patients with migraine headache and healthy controls. The secondary objectives were to determine the differences in headache characteristics according to vitamin D status and to correlate serum 25 (OH)-vitamin D level with duration, frequency, and severity of migraine headache attacks.
The present study revealed that patients with migraine headache had significantly lower 25(OH)-vitamin D serum level in comparison to controls. The incidence of aura, phonophobia/photophobia, autonomic manifestations, allodynia, and resistance to medications were found to be significantly higher in migraineurs with vitamin D deficiency than those with normal vitamin D. There was a statistically significant negative correlation between 25(OH)-vitamin D serum level and attack duration in hours, frequency of the attacks/month, MIGSEV scale, and HIT-6 scale.
Such results were broadly consistent with a number of recent studies. Wheeler reported a significant decrease in vitamin D serum level in patients with chronic migraine in comparison to controls. In his study, he revealed that 14.8% of the patients with chronic migraine had vitamin D serum level below 20 ng/mL and 25.9% of the patients had vitamin D serum level between 20 and 30 ng/mL.13Additionally, Togha et al found that a serum vitamin D level between 50 and 100 ng/mL was associated with 80–83% lower odds of migraine headache than those with serum 25(OH)D levels below 20 ng/mL.25
A case report was done on two female patients with menstrual migraine suffering from vitamin D deficiency. With the consumption of vitamin D (1600–1200 IU per day) over 2 months treatment, there was a significant decrease in the frequency of migraine headache attacks in both patients.15Another study was done on postmenopausal patients with migraine and vitamin D deficiency. After supplementation of vitamin D, there was a significant reduction in the frequency and duration of migraine headache attacks.14
Similar findings were obtained by Gazerani et al who found that migraine patients on vitamin D supplement demonstrated a significant decrease in frequency and duration of migraine headache attacks as compared with those on placebo. However, migraine severity, pressure pain thresholds, and migraine-related symptoms, ie, aura, nausea, photo/phonophobia, and allodynia, showed no significant pattern of change across time for either groups (vitamin D versus and placebo).26
In contrast to our findings, Zandifar et al did not reveal any relationship between vitamin D plasma level and severity of the migraine headache attacks. Also, vitamin D plasma levels were not different among MIGSEV items.27
Similarly, a cross‑sectional study done on 11,614 participants revealed insignificant relationship between vitamin D deficiency and migraine. Surprisingly, there was a significant association between vitamin D deficiency and nonmigraine headache.28
Different mechanisms for the causal relationship between vitamin D deficiency and migraine were suggested. One of them was the reported low serum level of magnesium in patients with vitamin D deficiency. Interestingly, it has been demonstrated that there was a positive correlation between serum concentrations of 25(OH)-vitamin D and magnesium. Magnesium deficiency is known to play a role in the pathogenesis of migraine (especially menstrual migraine). After correction of magnesium deficiency, migraine headache characteristics significantly improved.29
Another explanation for the observed association between vitamin D deficiency and migraine may be the anti-inflammatory effects of vitamin D, which could affect neuroinflammation associated with migraine.30 Multiple studies showed that vitamin D, at physiologic levels, can suppress the production of proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6.31 Vitamin D also causes an increase in the production of the anti-inflammatory cytokine, interleukin-10.32
Additionally, vitamin D, in its active form 1,25(OH)2D, can inhibit the synthesis of inducible nitric oxide synthase, which generates nitric oxide (NO).33 NO stimulates the synthesis and release of Calcitonin gene-related peptide (CGRP) from trigeminal ganglion neurons, which in turn stimulates the release of NO. Thus, this might lead to a positive feedback loop that can enhance and maintain inflammatory processes within the trigeminal ganglion. This contributes to the sensitization of meningeal nociceptors during migraine.34
Taken together, these findings may explain the reported relationship between vitamin D deficiency and migraine, but the precise mechanism underlying the actual role of vitamin D in the pathogenesis of migraine remains to be elucidated.
Well-designed clinical trials should be conducted on a larger number of patients and for a longer duration to investigate effects of correcting vitamin D deficiency on the characteristics, frequency, severity, and duration of migraine headache attacks. Further researches should be also directed toward exploring the molecular and cellular mechanisms underlying the effect of vitamin D deficiency on increasing the odds of developing aura, phonophobia/photophobia, autonomic manifestations, allodynia, and resistance to medications in patients with migraine.
Conclusion
Patients with migraine have significantly lower 25(OH)-vitamin D serum level in comparison to controls and this raises awareness for the need for screening vitamin D status in patients with migraine. The incidence of aura, phonophobia/photophobia, autonomic manifestations, allodynia and resistance to medications were significantly higher in migraineurs with vitamin D deficiency than those with normal vitamin D. There was a statistically significant negative correlation between 25(OH)-vitamin D serum level and duration, frequency, and severity of migraine headache attacks.
Ethics approval and consent to participate
A written informed consent was obtained from each participant in this study or from parent or legal guardian for participants under the age of 18 years. The study was conducted in accordance with the Declaration of Helsinki and it was approved by local ethical committee in Faculty of Medicine, Beni-Suef University. The committee’s reference number is FWA00015574.
Data sharing statement
Authors report that the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
25(OH) vit D, 25 hydroxyvitamin D; CGRP, Calcitonin gene-related peptide; DALYs, disability-adjusted life years; ELISA, enzyme-linked immunosorbent assay; GBD, Global Burden of Disease; HIT-6, Headache Impact Test-6; ICHD-II, International Classification of Headache Disorders-II; MIGSEV, migraine severity; MRI, magnetic resonance imaging; NO, nitric oxide; SPSS v 18, Statistical Package for Social Science version 18; VDR, vitamin D receptor.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yoon MS, Katsarava Z, Obermann M, et al. Prevalence of primary headaches in Germany: results of the German headache consortium study. J Headache Pain. 2012;13:215–223. doi: 10.1007/s10194-012-0425-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. 2017;372:307–315. doi: 10.1016/j.jns.2016.11.071 [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF. Prevalence and impact of migraine. Neurol Clin. 1997;15(1):1–13. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet Neurol. 2017;16(11):877–897. doi: 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles A. The evolution of a migraine attack - a review of recent evidence. Headache. 2013;53(2):413–419. doi: 10.1111/head.12026 [DOI] [PubMed] [Google Scholar]
- 6.Steiner TJ. Lifting the burden: the global campaign to reduce the burden of headache worldwide. Aids for management of common headache disorders in primary care. J Headache Pain. 2007;8:S26–9. [PubMed] [Google Scholar]
- 7.Headache Classification Subcommittee of the International Headache Society. The International classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Supp 1):9–160. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53:644–655. doi: 10.1111/head.12055 [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677–681. doi: 10.1210/jc.2007-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr. 2011;29(2):149–155. doi: 10.3329/jhpn.v29i2.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mytton J, Frater AP, Oakley G, Murphy E, Barber MJ, Jahfar S. Vitamin D deficiency in multicultural primary care: a case series of 299 patients. Br J Gen Pract. 2007;57(540):577–579. [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. The association between vitamin D concentration and pain: a systematic review and meta-analysis. Public Health Nutr. 2018;21(11):2022–2037. doi: 10.1017/S1368980018000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler S. Vitamin D deficiency in chronic migraine. Headache. 2008;48:S52–S53. [Google Scholar]
- 14.Thys-Jacobs S. Alleviation of migraines with therapeutic vitamin D and calcium. Headache. 1994;34:590–592. doi: 10.1111/hed.1994.34.issue-10 [DOI] [PubMed] [Google Scholar]
- 15.Thys-Jacobs S. Vitamin D and calcium in menstrual migraine. Headache. 1994;34:544–546. doi: 10.1111/hed.1994.34.issue-9 [DOI] [PubMed] [Google Scholar]
- 16.Lukacs M, Tajti J, Fulop F, Toldi J, Edvinsson L, Vecsei L. Migraine, neurogenic inflammation, drug development – pharmacochemical aspects. Curr Med Chem. 2017;24:3649–3665. doi: 10.2174/0929867324666170712163437 [DOI] [PubMed] [Google Scholar]
- 17.Bruno PP, Carpino F, Carpino G, Zicari A. An overview on immune system and migraine. Eur Rev Med Pharmacol Sci. 2007;11:245–248. [PubMed] [Google Scholar]
- 18.Motaghi M, Haghjooy Javanmard S, Haghdoost F, et al. Relationship between vitamin D receptor gene polymorphisms and migraine without aura in an Iranian population. Biomed Res Int. 2013;2013:351942. doi: 10.1155/2013/351942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayliss MS, Dewey JE, Dunlap I, et al. A study of the feasibility of Internet administration of a computerized health survey: the headache impact test (HIT). Qual Life Res. 2003;12:953–961. doi: 10.1023/A:1026167214355 [DOI] [PubMed] [Google Scholar]
- 20.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–974. [DOI] [PubMed] [Google Scholar]
- 21.El Hasnaoui A, Vray M, Richard A, Nachit-Ouinekh F, Boureau F; MIGSEV Group. Assessing the severity of migraine: development of the MIGSEV scale. Headache. 2003;43(6):628–635. [DOI] [PubMed] [Google Scholar]
- 22.Asadi B, Khorvash F, Najaran A, Khorvash F. Cyproheptadine versus propranolol in the prevention of migraine headaches in children. Pak J Med Sci. 2012;28:309–311. [Google Scholar]
- 23.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29(6):415–422. doi: 10.1016/j.mam.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celikbilek A, Gocmen AY, Zararsiz G, et al. Serum levels of vitamin D, vitamin D-binding protein and vitamin D receptor in migraine patients from central Anatolia region. Int J Clin Pract. 2014;68:1272–1277. doi: 10.1111/ijcp.12456 [DOI] [PubMed] [Google Scholar]
- 25.Togha M, Razeghi Jahromi S, Ghorbani Z, Martami F, Seifishahpar M. Serum vitamin D status in a group of migraine patients compared with healthy controls: a case-control study. Headache. 2018;58(10):1530–1540. doi: 10.1111/head.13423 [DOI] [PubMed] [Google Scholar]
- 26.Gazerani P, Fuglsang R, Pedersen JG, et al. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D(3) supplementation in adult patients with migraine. Curr Med Res Opin. 2019 ;35(4):715-723. [DOI] [PubMed] [Google Scholar]
- 27.Zandifar A, Masjedi SS, Banihashemi M, et al. Vitamin D status in migraine patients: a case-control study. Biomed Res Int. 2014;2014:514782. doi: 10.1155/2014/514782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kjærgaard M, Eggen AE, Mathiesen EB, Jorde R. Association between headache and serum 25‑hydroxyvitamin D; the tromsø study: tromsø 6. Headache. 2012;52:1499–1505. doi: 10.1111/j.1526-4610.2012.02250.x [DOI] [PubMed] [Google Scholar]
- 29.vonLuckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence-based rationale? A systematic review. Headache. 2018;58:199–209. doi: 10.1111/head.13217 [DOI] [PubMed] [Google Scholar]
- 30.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11(1):29–36. doi: 10.1007/s11882-010-0161-8 [DOI] [PubMed] [Google Scholar]
- 33.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255–267. [DOI] [PubMed] [Google Scholar]
- 34.Messlinger K, Lennerz JK, Eberhardt M, Fischer MJ. CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache. 2012;52(9):1411–1427. doi: 10.1111/j.1526-4610.2012.02212.x [DOI] [PubMed] [Google Scholar]