Abstract
Background:The ketogenic diet (KD) is a high fat, low carbohydrate diet considered to be the treatment of choice for GLUT1deficiency syndrome, a metabolic disorder affecting the nervous system.
Aim:To present our experience in four patients with GLUT1 deficiency syndrome who were treated with KD.
Methods:Retrospective data from case series. Phenotypical features, mainly movement disorder and seizures, are being described for each patient. All four cases are currently following the Modified Atkins Diet.
Results:The response to ketogenic diet in our four patients was significant with improvement of movement disorder or seizures control.
Conclusion:The ketogenic diet is the treatment of choice for GLUT1deficiency syndrome. Recognition of the disorder is the key for appropriate management among clinicians. Diet compliance is an important issue in school age children.
Keywords:ketogenic diet, GLUT1 deficiency syndrome.
BACKGROUND
GLUT1 deficiency syndrome is a treatable metabolic disorder affecting the nervous system, caused by poor glucose transport at the cerebral level and clinically characterized by a variety of neurological signs and symptoms. The main biochemical feature is low CSF (cerebral spinal fluid) glucose level in conditions of normal blood glucose. The clinical picture is extremely variable, with uneven impairment, from epilepsy with seizures that are difficult to control with anti-epileptic drugs to complex movement disorder and developmental delay (1).
The disease was initially described by De Vivo et al., in 1991, in two patients with developmental delay, epileptic seizures and persistent low CSF/blood glucose ratios at repeated determinations (range 0.19–0.35). By comparative experimental studies in both patients and a control group, they suggested a possible genetic abnormality in the blood glucose transporter at the cerebral level. Both patients had seizures with early onset, during infancy. Ketogenic diet was initiated with favourable response on epileptic seizures and with better outcome on the long term for the patient in whom the diet was initiated earlier (2).
GLUT1 is a transmembrane glycoprotein, member of the GLUT family that facilitate blood glucose transport within the blood-brain barrier, and the gene associated to this family of transporters is SLC2A1, located on the short arm of chromosome1 (3).
Most mutations in the SLC2A1 gene are de novo mutations and transmission, in familial cases is autosomal dominant with complete penetrance (4).
MATERIAL AND METHODS
This is a retrospective study with data gathered from four patients diagnosed with GLUT1 deficiency syndrome and treated with ketogenic diet in our department. Signed consent form has been obtained from the families.
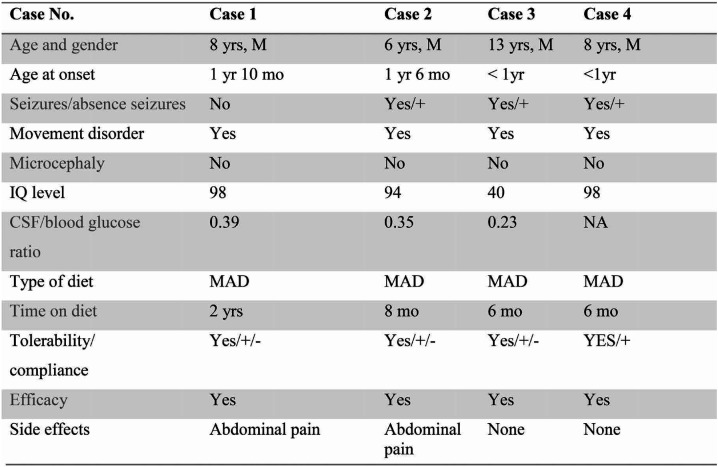
DESCRIPTION AND RESULTS (Table 1)
Case 1
Our first patient to be reported is an eight-year-old boy with no significant history related to pregnancy and delivery. He achieved independent walk at the age of one year and four months and since the age of one year and 10 month he started presenting episodes of gait disturbances, with falling, related to exercise, initially rare, 1-2 episodes per year. At the age of five, the episodes increased in frequency, one episode every two weeks, with variable duration, more prominent during febrile illnesses. Repeated evaluations and extensive investigations (blood, cerebral MRI, EEG, heart and ophthalmological exams) have not led to a conclusion. GLUT1 deficiency syndrome came up as differential diagnosis. A lumbar puncture has been performed, revealing a low level of CSF glucose, 37 mg/dL. The ratio CSF/blood glucose was 0.39, which was in line with the presumed aetiology. Genetic testing performed in a private laboratory confirmed the mutation in the SLC2A gene. At the age of diagnosis, clinical examination was within normal limits, except discrete motor inability (mother would describe his gait as shuffled). IQ level is 98. Ketogenic diet has been initiated since January 2016 with positive results. The level of ketosis is clearly correlating with the clinical picture. Mother is reporting rare episodes of “stiffening” or paroxysmal dyskinesia appearing after few days of non-ketosis. Compliance is an important issue, the child attending the mainstream school with good school performances.
Case 2
The second case is now a seven year-old boy with no significant personal history and normal milestones. Cases 1 and 2 are siblings. Mother recalls the onset of first clinical symptoms at the age of one year and six months, when the boy started to present rare, unexplained bursts of anger with aggressive behaviour towards others, followed by episodes of intense crying, with perioral cyanosis, considered to be breath holding spells occurring at the age of three. At the age of five, the boy presented an episode of gait disturbance, after exercising, similarly to his brother, and also continued with episodes of anger related to meals, which raised the suspicion of the same diagnosis in the younger brother. At the age of six, the boy started to present episodes of yawning, followed by loss of consciousness and falling. Although the family denied the diagnosis, postponing the laboratory work up, mother implemented a low carbohydrate diet and increased the lipids (with no specific plan), which made a difference in the clinical pictures. At the same age, while on summer holiday and related to high intake of carbohydrates, frequent episodes “absence like”, with drooling, head drop and urinary loss appeared, persistent for days. EEG during the episodes has revealed bilateral high amplitude delta waves. Lumbar puncture has been performed abroad, with a ratio of 0.35 CSF/blood glucose. Genetic testing is pending. No particular features, no microcephaly and normal clinical examination were found. IQ level is 94. He is now following the modified Atkins diet (aiming for 1:1 ratio) with visible results. Compliance is an issue as well, the boy attending the mainstream school. If the diet is omitted, he will have an “absence like” episode (“he walks into objects, not answering to questions, with urinary loss”). Mother is reporting rare episodes of abdominal pain.
Case 3
A 13-year-old boy, with no signifi- cant personal history, but with delayed cognitive and motor milestones, achieved independent walk at the age of two and expressive language after the age of two years and six months. He had history of a simple febrile convulsion at the age of one year and six months. Persistent gait disturbances, with difficulties climbing stairs, walking on heels and toes and ataxic elements have been revealed on repeated examinations. A kinetotherapy program has been initiated early. A MRI evaluation revealed ventriculomegaly. Early diagnosis was spastic paraparesis. Since the age of 10 he started presenting paroxysmal episodes with disturbed gait, tremor of one or both legs, sometimes followed by falling, for seconds, with remission if he stops walking. EEG has revealed persistent bilateral polyspike or spike and wave discharges with no clinical correspondent. Anticonvulsant medication has been tried with no benefit. A home video with paroxysmal movement disorder (considered to be paroxysmal diskinesia) has been very helpful in suggesting the diagnosis. The CSF/blood glucose ratio was 0.23. IQ level is 40. He is attending special school, with low school performances. Genetic testing performed through a research programme confirmed the mutation in the SLC2A gene (results unavailable). Modified Atkins Diet has been proposed since September 2016, but it has not been initiated until October 2017. At the beginning, parents regarded the diet more as a “punishment” for the child rather than a therapeutic option. Due to persistent episodes, with inability to walk for a long distance, the Modified Atkins Diet with 30 grams of carbohydrates daily has been initiated since October 2017 with good response. Mother said “it made a huge difference, her boy being now able to walk to school for a long distance”. Again, compliance is one of the main issues, mother describing some “bad days”, related to higher carbohydrates intakes, mainly at school. No side effects were reported so far.
Case 4
An eight-year-old boy with history of premature birth, at 34 weeks GA (gestational age), with good postnatal adaptation, but delayed cognitive and motor milestones, achieved independent walk at two years and two months, expressive language at the age of three years with specific therapy. First seizures were observed at the age of two, from awake, with arrest, some gestural automatisms, fixe gaze and blinking, variable as frequency and no response to standard anticonvulsant therapy, but some improvement with corticotherapy. He also had some gait disturbances with toe walking and ataxic gait exacerbated with effort. MRI revealed hippocampus asymmetry and EEG showed bilateral discharges suggestive for absence seizures. GLUT 1 diagnosis was established at the age of seven years and nine months. On assessment for KD he had almost daily seizures, predominantly on awakening. At six months on the diet with 20 grams of carbohydrates, the seizures are controlled and language, motricity and attention are improved too. Compliance is good and there are no reported side effects.
DISCUSSION
Up to 2007, J. Klepper identified 84 cases with GLUT1 deficiency syndrome reported in the literature. He analyzed the cases trying to define the classical and non-classical phenotype, with the presence of atypical forms. The author described the classical phenotype as characterized by epileptic seizures with onset during childhood, resistant to antiepileptic medication, movement disorder characterized by ataxia, dystonia and spasticity but also hypotonia. Development is delayed, especially on the cognitive and language side, and microcephaly can be an important sign in severely affected cases. The non-classical phenotype associates movement disorder and learning disability, and does not involve epileptic seizures (1).
According to this description, our patients may fall into one of these two categories: cases 2, 3 and 4 in the classical phenotype and case 1 in the non-classical one, although cases 1 and 3 are siblings. Microcephaly is not present in our patients, and intellectual level is lower in patient number 3. The boy was older than the others at the time of diagnosis.
Ketogenic diet, rich in fat and low in carbohydrates, remains the treatment of choice in this syndrome. In the absence of glucose, as the main source of energy for the brain, ketone bodies derived from lipid metabolism in the liver are used. While for epilepsy the diet mechanism is incompletely understood, in GLUT1 deficiency syndrome diet is essential for producing an alternative energy source for the brain (5). The ketogenic diet spectrum and its applicability have now been expanded with easier to tolerate and more permissive diets such as the modified Atkins diet or low glycemic index diet. In our patients, the modified Atkins diet has been proposed, with a carbohydrate intake of 20 grams daily, unrestricted proteins and increase in fat amount. None of the patients could follow the plan strictly, and around 30 grams/day were given daily. For cases 1 and 2, the mother is aiming for a 1:1 ratio between lipids and carbohydrates plus proteins, not always possible, but still with good results. The clinical picture is clearly correlating with the day with or without ketosis. For patient number 3, ketosis is difficult to achieve, but clear improvement is reported. Better compliance is reported in patient number 4.
A more recent article proposed a more strict diet in more severe, age-specific phenotypes. Thus, a diet with an increased lipid ratio of 4:1 or 3:1 is proposed for preschoolers, school children, adolescents and adults with severe phenotype, and the modified Atkins diet is proposed in milder phenotypes in school age children, adolescents and adults (6).
It is not very clear whether a strict diet is essential in GLUT1 deficiency, but it is certain that a more permissive diet is better tolerated in the long term. Current research, with the development of animal models with GLUT 1 deficiency syndrome, is promising in understanding the mechanism of action of the diet and developing alternative therapies in this syndrome (7).
In 2007, in a retrospective review, Klepper highlighted the lack of reported patients in Eastern and Southern European countries as well as the need for more communication and awareness for this disorder (1). Things have changed since then, with more recognised and reported cases from this part of the world (8, 9). Even though access to genetic testing is not readily available in some countries, awareness for the disease has been increasing lately in the clinical setting.
CONCLUSION
Ketogenic diet is the treatment of choice for GLUT1 deficiency syndrome. Recognition of the disorder is the key for appropriate management among clinicians. Diet compliance is an important issue in school age children.
Conflicts of interest: none declared.
Financial support: none declared.
TABLE 1.
Clinical and laboratory features but also response to Ketogenic diet in patients with GLUT1 deficiency syndrome Abbreviations: KD =ketogenic diet; MAD=modified Atkins diet; M=male; No.=number; NA=not applicable; yrs=years; mo=months, IQ=Intelligence Quotient , CSF=cerebrospinal fluid.
Contributor Information
Carmen SANDU, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Pediatric Neurology Clinic, “Prof. Dr. Alexandru Obregia” Clinical Hospital, Bucharest, Romania.
Carmen Magdalena BURLOIU, Pediatric Neurology Clinic, “Prof. Dr. Alexandru Obregia” Clinical Hospital, Bucharest, Romania.
Diana Gabriela BARCA, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Pediatric Neurology Clinic, “Prof. Dr. Alexandru Obregia” Clinical Hospital, Bucharest, Romania.
Sanda Adriana MAGUREANU, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Dana Cristina CRAIU, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Pediatric Neurology Clinic, “Prof. Dr. Alexandru Obregia” Clinical Hospital, Bucharest, Romania.
References
- 1.Klepper J, Leiendecker B. GLUT1 deficiency syndrome–2007 update. Dev Med Child Neurol. 2007;49:707–716. doi: 10.1111/j.1469-8749.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 2.De Vivo DC, Trifiletti RR, Jacobson RI, et al. Defective glucose transport across the blood–brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. The New England Journal of Medicine. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. [DOI] [PubMed] [Google Scholar]
- 3.Mueckler M, Caruso C, Baldwin SA, et al. Sequence and structure of a human glucose transporter. Science. 1985;229:291–295. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 4.Klepper J, Sheffer H, Elsaid MF, et al. Autosomal recessive inheritance of GLUT1 deficiency syndrome. Neuropediatrics. 2009;40:207–210. doi: 10.1055/s-0030-1248264. [DOI] [PubMed] [Google Scholar]
- 5.Kossoff EH, Hartman AL. Ketogenic diets: new advances for metabolism-based therapies. Current Opinion in Neurology. 2012;25:73–78. doi: 10.1097/WCO.0b013e3283515e4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentina De Giorgis, Pierangelo Veggiotti. GLUT1 deficiency syndrome 2013: Current state of the art. Seizure. 2013;22:803–811. doi: 10.1016/j.seizure.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Klepper J. Glucose transporter deficiency syndrome (GLUT1DS) and the ketogenic diet. Epilepsia. 2008;49:46–49. doi: 10.1111/j.1528-1167.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 8.Szczepanik E, Terczyńska I, Kruk M, et al. Glucose transporter type 1 deficiency due to SLC2A1 gene mutations-a rare but treatable cause of metabolic epilepsy and extrapyramidal movement disorder; own experience and literature review. Dev Period Med. 2015;19:454–463. [PubMed] [Google Scholar]
- 9.Ivanova N, Peycheva V, Kamenarova K, et al. Three novel SLC2A1 mutations in Bulgarian patients with different forms of genetic generalized epilepsy reflecting the clinical and genetic diversity of GLUT1-deficiency syndrome. Seizure. 2018;54:41–44. doi: 10.1016/j.seizure.2017.11.014. [DOI] [PubMed] [Google Scholar]