Abstract
Radiomics is a relatively new concept that consists of extracting data from images and applies advanced characterization algorithms to generate imaging features. These features are biomarkers with prognostic and predictive value, which provide a characterization of tumor phenotypes in a non-invasive manner. The clinical application of radiomics is hampered by challenges such as lack of image acquisition and analysis standardization. Textural features extracted from computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) images of patients diagnosed with head and neck cancers can be used in the pre-therapeutic evaluation of the response to multimodal chemo-radiotherapy. For patients with positive HPV-oropharyngeal cancers, the correlation of the radiomic textural features from the tumor with p16 values from the pathological sample can identify tumor specific signatures in CT imaging, an entity with favorable prognosis and a better response to chemo-radiotherapy. Pretreatment contrast CT-scans were extracted and radiomics analysis of gross tumor volume were performed using MaZda package apart from MaZda software containing B11 program for texture analysis and visualization. Data set was randomly divided into a training dataset and a test dataset and machine learning algorithms were applied to identify a textural radiomic signature. Radiomic texture analysis and machine learning algorithms demonstrate a predictive potential related to the capability of stratification for subclasses of platinum-chemotherapy resistance and radioresistant head and neck cancers requiring an intensification of multimodal treatment.
Keywords:radiomics, head and neck oncology, machine learning, texture, features.
INTRODUCTION
In recent years, oropharyngeal squamous cell carcinoma has shown an increase in incidence over other types of head and neck cancers, being related to Human Papilloma Virus (HPV) infections, which are biologically and clinically different from HPV-negative oropharyngeal cancer, often associated with smoking and alcohol consumption. Head and neck cancer associated with HPV virus infection has been shown to have a superior response to radio-chemotherapy, with higher rates of local control (over 80%) and overall survival at five years. Less than 50% of patients with HPV negative oropharyngeal cancers survive at five years although they are being treated with the correct multimodal treatment protocol. Young age and favorable prognosis make it necessary to evaluate the possibilities for reducing late toxicities and to implement some clinical trials for treatment de-escalation.
Immunohistochemistry for investigation of p16 expression level strongly correlated with HPV infection but also in situ hybridization for viral DNA, HPV DNA or PCR RNAs are standard methods for identifying the viral etiology of this type of cancer (1).
Radiomics has shown the potential to predict HPV status in head and neck cancer. Indeed, studies have reported radiological differences between HPV positive and negative, demonstrating that heterogeneity of image-based density is potentially associated with HPV infection with oropharynx cancers (2). In oncology, the identification of biomarkers that describe the characteristics of malignancy from different points of view (clinical, histological, molecular) is an essential objective, but some biomarkers can also predict the patient’s response to the administered treatment, a gradually translation from fundamental research to clinical practice being necessary. Screening tools wearing the “omics” suffix offer incredible analytical opportunities in the context of access to a large number of medical information stored in databases. “Radiomics” is the newcomer in the “omics” family and is based on radiological medical images, from which multiple features can be extracted and correlated with clinical, biological, genomic data in order to obtain predictive models to personalize the treatment (3).
The use of medical imaging like computer tomography (CT), magnetic resonance imaging (IRM) and, more recently, positron emission tomography (PET-CT) is a standard for diagnosis, staging of cancers and evaluating the therapeutic response, but with the introduction of image-guided radiotherapy (IGRT) is a essential step in planning of modern radiation therapy. By extracting quantitative information from clinical imaging, radiomics has proven possible ways to maximize radiotherapy efficiency by identifying the radio-resistance areas in the tumor and to modulate treatment in such a way as to obtain the best tumor control with a lower toxicity rate. The concept of “big data” in oncology, particularly in head and neck cancers, and computational data processing promises to bring new light to the molecular mechanisms that determine the pathogenesis of these malignancies by identifying new prognostic and predictive factors (4).
Tumors include clonal populations of cells form dynamic complex systems that show a rapid evolution as a result of their interaction with the tumor microenvironment, and this interaction is modulated by the treatment. Different properties of the microenvironment depend on factors such as increase of metastatic capacity and immunological characteristics being highlighted by differences in metabolic activity, oxygenation levels, cell proliferation rates, pH, hypoxia, and vascularization of intratumoral necroses. Such intratumoral differences are related to the concept of tumor heterogeneity, a feature that can be observed with significantly different characteristics even in tumors of the same histopathological type. Thus, tumors present an increased heterogeneity even if they are identical histopathological forms, intratumoral heterogeneity being associated with resistance to treatment, and to the risk of progression, relapse and metastasis (5, 6).
MATHERIALS AND METHODS
Computed tomography images for three patients with locally advanced non-metastatic head and neck cancers who were proposed for curative multimodal treatment (induction chemotherapy 3-4 cycles) and radiotherapy. All patients benefited from a CT imaging assessment for staging and evaluating of the tumor size before performing the first cycle of chemotherapy and after performing at least three cycles of chemotherapy. Only patients who received TPF (taxanes, platinum, fluorouracil) chemotherapy were included. Cases were selected from each category of response after induction chemotherapy: progressive disease, stationary disease and partial response according to RECIST criteria. Delta radiomic features (skewness and kurtosis) were calculated for each case.
RESULTS
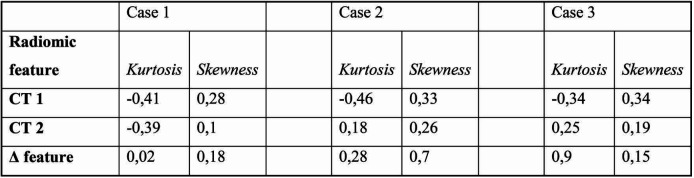
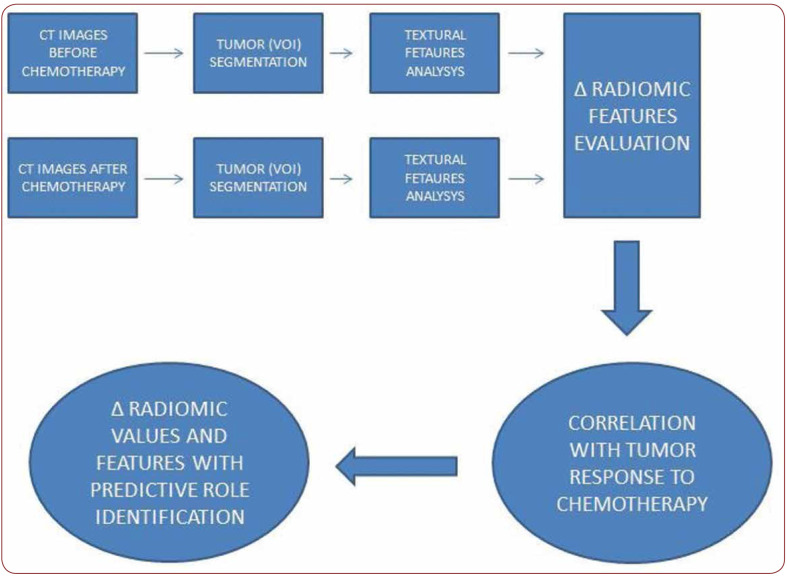
In all three cases, there was a variation of radiomic parameters evaluated for the volume of the primary tumor extracted using MazDa, a free software for radiomic feature extraction and analysis. Delta kurtosis is between 0.02 and 0.9 (minimum and maximum) and the skewness delta is between 0.15 and 0.17 (minimum and maximum) (Table 1). The algorithm designed to introduce the radiomic prediction of the response to induction chemotherapy is shown in Figure 1.
DISCUSSION
The results of the Phase II OPTIMA trial indicate that HPV-associated head and neck cancer patients, even those with advanced nodal involvement, may receive lower radiation doses without compromising local control if they respond initially to induction chemotherapy. In this case local control rates exceed 92% two years after the treatment a reduction in the incidence of side effects compared to those who received standard therapy. Compared to HPV negative head and neck cancer, which is usually caused by smoking and alcohol consumption, positive HPV disease is more receptive to radiation therapy and chemotherapy and is associated with significantly higher rates of healing. The non-invasive identification of these “good responders” may have particular implications for reducing adverse effects. Although reducing the intensity of cancer treatment offers to patients a better quality of life but this approach should only be used with caution for patients with a favorable biology to de-escalation of therapy (7). The use of the radiomic features of the different regions of the tumor volumes delineated on imaging can highlight tumor heterogeneity, Haussdorf and fractal dimension and shape analysis are associated with the expression of growth factors and modifying angiogenic tumors and tumor proliferation with different rates in different regions of the tumor volume having finally a prognostic value for resistance to chemotherapy and radiotherapy (8).
The emergence of the radiomic profile concept might be possible to predict prognosis or response to induction chemotherapy guiding the therapeutic protocol to intensification or de-escalation of multimodal treatment. The success and enthusiasm of radiomics can reach the highest level using these applications for modulation and refinement of treatment in oncology, because the impact it may have in the modern context of precision medicine is extraordinary. However, we have insufficient data standardization protocols and insufficient evidence in radiomics, problem that leads to a variability with significant differences in terms of clinical trials methodology (9).
Although initial results using structural imaging, in particular CT, medical imaging are promising, several studies have explored PET-radiomics used to delineate the target volumes for radiotherapy planning, assessing the prognostic value of radiomics to identify tumor failure response to treatment, the high recurrence risk, and even the ability to predict the risk of cancer related high mortality rate. As in other radiomic studies, the methodological diversity for extracting and analyzing the selected features remains one of the unsolved problems of PET radiomics. The use of radiomics in PET-CT imaging analysis shows their potential to non-invasively enhanced by deep learning algorithms for tumor characterization, and could allow empowering of the feature selection of and the creation of powerful prognostic models. Deep learning methods are very effective when the number of samples available is high during a training phase, therefore one of the main challenges in implementing deep learning results from the limited number of training samples available to build models without suffering overload (10, 11).
Radiomic studies extract a large number of imaging features to increase the discriminating power of features, and selection of size reduction techniques are typically used to reduce the difficulties in sample size selection and improve predictive performance. Most feature selection techniques are designed in a supervised method to identify discriminatory features by optimizing the performance of prediction models based on validation data sets. Extraction of dates from small samples can cause errors in estimating the predictive value of the selected features (12). Methods for texture analysis in head and neck cancers include first- and second-order texture features based on the intensity values within a region of interest (ROI). Others features can also be extracted from histograms of intensity values or can be extracted from the shape of the ROI. Texture features for head and neck cancers are based on the same parent matrices that are used in other radiomics studies for various malignancies. The most used features based on gray levels extracted from images are gray-level cooccurrence matrix (GLCM) (13).
Multiple open-source and commercial software solutions that facilitate are used to develop radiomic research in head and neck cancer. Imaging Biomarker Explorer (IBEX) by Zhang et al. compatible with CT, PET, and MR modalities. The authors described it an “open infrastructure software platform that flexibly supports common radiomics workflow tasks such as multimodality image data import and review, development of feature extraction algorithms, validation model, and consistent data sharing among multiple institutions” (14).
Mazda is an open-source software for texture analysis designed at the base for magnetic resonance imaging (MRI) texture analysis which supports various feature selection algorithms for model generation. MazDa is available at http:// www.eletel.p.lodz.pl/programy/mazda/index. php?action=mazda (15). Specific applications for texture and radiomics analysis in head and neck tumors have already demonstrated the ability to obtain results from these techniques in the following areas of head and neck cancer: tumor segmentation and pathological classification, risk stratification, prognostic and predictive biomarker, monitoring the change in normal tissue as a late toxicity of the radiotherapy (16). The concept of variation in radiomic feature values from baseline to the next imaging investigation was promoted by Nasief et al. and Cherezov and colleagues in pancreatic and lung studies, respectively, proving the utility in screening and the possibility of predicting the early response chemotherapy (17, 18).
CONCLUSION
Delta radiomic features demonstrates value in predicting the response to induction chemotherapy in pancreatic and lung cancer studies. Based on this premise, the analysis of the delta values of some radiomic parameters obtained from CT imaging performed prior to the beginning of chemotherapy and after the first induction chemotherapy cycle in head and neck cancers could be a biomarker of response to chemotherapy. However, the data should be carefully assessed in view of the difficulties in standardizing the acquisition and the subjectivity in selecting volumes of interest, the use of large standardized data bases being a solution to overcome these limits.
Conflicts of interest: none declared.
Financial support: none declared.
TABLE 1.
Radiomic fetaures values extracted from CT images (before and after induction chemotherapy)
FIGURE 1.
Algorithm designed to introduce radiomic prediction of response to chemotherapy induction
Contributor Information
Camil Ciprian MIRESTEAN, Regional Institute of Oncology, Iasi, Romania; University of Medicine and Pharmacy Craiova, Dolj, Romania.
Ovidiu PAGUTE, Regional Institute of Oncology, Iasi, Romania.
Calin BUZEA, Regional Institute of Oncology, Iasi, Romania.
Roxana Irina IANCU, “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania; “Sf. Spiridon” Universitary Hospital, Iasi, Romania dUniversity of Medicine and Pharmacy Craiova, Dolj, Romania.
Dragos Teodor IANCU, Regional Institute of Oncology, Iasi, Romania; “Gr. T. Popa” University of Medicine and Pharmacy, Iasi, Romania.
References
- 1.Leijenaar RT, Bogowicz M, Jochems A. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol. 2018;1086:20170498. doi: 10.1259/bjr.20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leijenaar RT, Bogowicz M, Jochems A. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol. 2018;1086:20170498. doi: 10.1259/bjr.20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheckenbach K1, Colter L, Wagenmann M. Radiomics in Head and Neck Cancer: Extracting Valuable Information from Data beyond Recognition. ORL J Otorhinolaryngol Relat Spec. 2017;1-2:65–71. doi: 10.1159/000455704. [DOI] [PubMed] [Google Scholar]
- 4.Resteghini C, Trama A, Borgonovi E. Big Data in Head and Neck Cancer. Curr Treat Options Oncol. 2018;2:62. doi: 10.1007/s11864-018-0585-2. [DOI] [PubMed] [Google Scholar]
- 5.Rybinski B, Yun K. Addressing intra-tumoral heterogeneity and therapy resistance. Oncotarget. 2016;44:72322–72342. doi: 10.18632/oncotarget.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderwood SK. Tumor Heterogeneity, Clonal Evolution, and Therapy Resistance: An Opportunity for Multitargeting Therapy. Discov Med. 2013;82:188–194. [PMC free article] [PubMed] [Google Scholar]
- 7.Yu K, Zhang Y, Yu Y, et al. Radiomic analysis in prediction of Human Papilloma Virus status. Clin Transl Radiat Oncol. 2017;7:49–54. doi: 10.1016/j.ctro.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann B, Frenzel T, Schmitz R, et al. Modeling Growth of Tumors and Their Spreading Behavior Using Mathematical Functions. Methods Mol Biol. 2019;1878:263–277. doi: 10.1007/978-1-4939-8868-6_16. [DOI] [PubMed] [Google Scholar]
- 9.Vallières M, Kay-Rivest E, Perrin LJ, et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci Rep. 2017;1:10117. doi: 10.1038/s41598-017-10371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen D, Wu G, Suk H-I. Deep Learning in Medical Image Analysis. Annu Rev Biomed Eng. 2017;19:221–248. doi: 10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, Fan Y. Direct sparsity optimizationbased feature selection for multiclass classification. In: Proceedings of the twenty-fifth international joint conference on artificial intelligence. (IJCAI-16) 2016. pp. 1918–1924.
- 12.Galperin-Aizenberg M, Li H, Pryma D. Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiother Oncol. 2018;2:218–226. doi: 10.1016/j.radonc.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam SW. Texture feature extraction using gray level gradient based co-occurrence matrices. Systems, Man, and Cybernetics. IEEE International Conference. 1996;1:267–271. [Google Scholar]
- 14.Zhang L, Fried DV, Fave XJ, et al. IBEX: an open infrastructure software platform to facilitate collaborative work in radiomics. Med Phys. 2015;42:1341–153. doi: 10.1118/1.4908210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown AM, Nagala S, McLean MA, et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn Reson Med. 2016;75:1708–1716. doi: 10.1002/mrm.25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AJ, Kanwar A, Mohamed AS. Radiomics in head and neck cancer: from exploration to application. Transl Cancer Res. 2016;4:371–382. doi: 10.21037/tcr.2016.07.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherezov D, Hawkins SH, Goldgof DB, et al. Delta radiomic features improve prediction for lung cancer incidence: A nested case–control analysis of the National Lung Screening Trial Cancer Medicine. Magn Reson Med. 2018;12:6340–6356. doi: 10.1002/cam4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasief HG, Hall WA, Schott D, et al. Delta-Radiomics of Daily CTs Acquired During Chemo-Radiation Therapy of Pancreatic Cancer. Conference paper. July 2018. 2018.