Abstract
The study re-visited malaria burden and pre-hospital medication among malarious subjects in Maiduguri, Northeast Nigeria. A total of 1,657 febrile subjects were screened for malaria by microscopy at two health institutions. Giemsa-stained blood smears were examined for parasitaemia and gametocytaemia; and parasite density (PD), gametocyte density (GD) and gametocyte sex ratio (GSR) were determined. The mean age of the 1,657 subjects was 27.5 ± 12.2 years and 7.8% (130/1,657) of the subjects aged <5 years. Sex distribution showed 47.0% (778/1,657) males and 53.0% (879/1657) females. Parasitaemia was recorded in 22.6% (375/1,657) with geometric mean PD of 8,925 (320–275,000) parasites/μl blood. The prevalence of parasitaemia was highest among subjects <5 years (χ2 = 401.1; df = 5; p < 0.0001) and in August and September (χ2 = 406.9; df = 11; p < 0.0001). Prevalence of gametocytaemia was 12.8% (48/375) with geometric mean GD of 109 (8–464) gametocytes/μl blood. The prevalence was higher in dry (16.5%, 29/176) than wet (9.5%, 19/199) months (χ2 = 4.0; df = 1; p = 0.045). The weighted mean GSR was 0.4 ± 0.1 with highest value in March (0.7 ± 0.2). Pre-hospital medication was recorded in 74.1% (278/375) of the subjects with parasitaemia. Analgesics (51.7%; 194/375) accounted for the highest proportion of drug consumed while 9.3% (35/375) of the subjects took antimalarial drugs. Malaria persisted in Maiduguri especially among subjects <5 years during wet months and pre-hospital medication is a common practice. These findings could serve as guide for policy decision that could contribute to effective treatment and control of malaria in the region.
Keywords: Infectious disease, Public health, Malaria, Burden, Pre-hospital medication, Nigeria, Maiduguri
1. Introduction
Malaria persistently affects public health and health policies especially in sub-Saharan Africa and Southeast Asia that are most hit by the burden. In 2016, 216 million malaria cases were reported with 445,000 deaths as against 217 million cases and 487,000 deaths reported in 2012. About 90% of the morbidity and 91% of the mortality were from Africa (WHO, 2018) where malaria is largely caused by Plasmodium falciparum (WHO, 2017). Meanwhile, few cases of other species (P. ovale, P. vivax and P. malariae) have been reported (Poirier et al., 2016; Ruas et al., 2017). Malaria disproportionately affects individuals of all ages, though children under five years bear the worst burden due to their relatively minimal previous malaria experience (WHO, 2005; Todd et al., 2007).
The interventional strategies deployed to mitigate the burden of malaria include prophylactic [e.g. intermittent preventive therapies (IPTs) and seasonal malaria chemoprevention (SMC)] (WHO, 2013); therapeutic [e.g. artemisinin combination therapies (ACTs)] (WHO, 2015) and control (insecticide-treated nets, indoor and outdoor spraying) measures (WHO, 2006; Roberts and Mayon-White, 2015). In 2016 alone, the cumulative efforts to curb the persisted burden of malaria gulped an estimated sum of USD 1.7 trillion globally (WHO, 2017). The cornerstone of effective treatment and control of malaria is ACTs that provide therapeutic options such as artemether-lumefantrine (AL), artesunate-amodiaquine (AA) and dihydroartemisinin-piperaquine among others (WHO, 2015). ACTs have significantly contributed to the fight against malaria since it was introduced in 2001 (WHO, 2001) and adopted by many countries including Nigeria in 2005 (FMH, 2005).
Malaria transmission occurs all year round in Nigeria with variation in transmission intensity across the regions due to climate diversity (NPC/NMCP/ICF, 2012). In 2016, Nigeria accounted for 27% and 24% of malaria morbidity and mortality, respectively (WHO, 2017) making her the leading contributor to global malaria burden. AL and AA were adopted as first- and second-line drugs for malaria treatment in Nigeria (FMH, 2005) and they are readily available and widely used across the country. Maiduguri is the largest and most populous city in Northeast Nigeria with population of 736,696 people as at 2006 (NPC, 2006) and seasonal malaria transmission. However, the population has increased exponentially due to influx of people displaced by persisted armed conflict in the region. This could have worsened the burden of malaria and other communicable diseases in the region. The available epidemiological data on malaria burden in Maiduguri (Salako et al., 1990) predated ACTs. Most of the relatively recent studies focused on specific cohorts (e.g. pregnant women and children) and were conducted over short period (Bako et al., 2009; Balogun et al., 2010, 2016; Kokori et al., 2013). Thus, there is dearth of robust epidemiological data on malaria burden in Maiduguri. In addition, pre-hospital medication is a common phenomenon among febrile patients (Fehintola and Balogun, 2010; Balogun et al., 2010). ACTs are readily available in Maiduguri partly due to free provision by governmental and non-governmental organizations and this could predispose the drugs to irrational consumption.
To this background, the present study re-visited malaria burden in Maiduguri in all ages and assessed pre-hospital medication among malarious subjects. This study is the first of its kind in recent time and provided scientific evidence that could guide polices on malaria in the region.
2. Materials and methods
2.1. Study area and population
The study was conducted at University of Maiduguri Teaching Hospital (UMTH) and University of Maiduguri Medical Centre (UMMC), Maiduguri, Borno State, Nigeria with subjects drawn from Maiduguri. Maiduguri, the capital of Borno State, is the largest city in Northeast Nigeria located on latitude 11o40′N – 11o44′N and longitude 13o05′E – 13o14′E with land area of 300 km2 and estimated population of over one million people due to influx of people displaced by persisted armed conflict (WPR, 2018). The inhabitants are predominantly Kanuri; other ethnic groups are Babur, Bura, Marghi, Shuwa, Hausa, Fulani, Yoruba and Igbo among others. The major economic activities of the people include farming, livestock keeping, trading, fishing, artisanship and civil service. The climate is characterized by a harmattan or cool-dry season (October–February), hot season (March–May/June) and a rainy season (June or July–September) [Waziri, 2009]. Sanitation provision in Maiduguri is grossly deficient with poor drainage system, inadequate portable water, unhealthy waste disposal system and presence of stagnant water around households (Jimme et al., 2016; Mukhtar and Akpan, 2018). The healthcare facilities in Maiduguri comprise primary (e.g. UMMC), secondary and tertiary health institutions (e.g. UMTH). The average annual rainfall is 562 mm and the average annual climatic temperature is 26.9 °C (average high is 34.7 °C and average low is 19.1 °C) [WCH, 2018]. Relative humidity varies throughout the year, ranging from 15% in March to 72% in August (Weather2, 2018). Malaria is mesoendemic in Maiduguri with seasonal transmission of 4–6 months during the raining season (NPC/NMCP/ICF, 2012) and a reported prevalence of 35.6% (Ayanugwo and Kalu, 1997).
2.2. Study design and subjects enrolment
The study was a cross-sectional prospective study aimed at evaluating the burden of malaria among the general population and pre-hospital medication among malarious subjects in Maiduguri. Febrile subjects and subjects with history of fever, who were presented at UMTH and UMMC between May, 2011 and November, 2012 were enrolled following informed consent obtained from the subjects or the caregivers where applicable. Concise demographic and medical records of the subjects were obtained using standard case record form and they were subjected to comprehensive physical examination by the clinicians. The data obtained included age, sex, duration of illness, axillary temperature and pre-hospital medication. The detail of the study was clearly explained to the subjects before enrolment and they were assured of the confidentiality of the information provided. Subjects with obvious danger signs were excluded from the study. The ethical approval was obtained from Research Ethics Committee, UMTH.
2.3. Laboratory investigations
Giemsa-stained thick and thin blood smears were prepared from finger-pricked blood samples of all enrolled subjects as described by WHO (2003). The thin smears were used for identification of Plasmodium species while the thick smears were used for qualitative and quantitative assessment of malaria asexual parasitaemia and gametocytaemia. Parasite (asexual) and gametocyte densities were estimated according to WHO protocol (WHO, 2005). Smears were declared negative when no parasite or gametocyte was found after viewing 100hpf by two independent assessors (STB and JJ).
Malaria parasitaemia, defined as presence of asexual or/and sexual stage of malaria parasite in peripheral blood, was estimated by counting asexual stage parasites relative to 200 leukocytes. Gametocytaemia, defined as presence of sexual (gametocyte) stage of malaria parasite in peripheral blood, was estimated by counting sexual stage parasite against 1000 leukocytes. The parasite and gametocyte densities were calculated assuming a leukocyte count of 8000 cells/μl blood using the formulae below (WHO, 2003):
Gametocyte sex ratio (GSR), defined as the proportion of gametocytes in peripheral blood that were male (Pickering et al., 2000; West et al., 2001), was determined if the gametocyte density was at least 24 gametocytes/μl blood. The gametocytes were sexed into microgametocyte (male) and macrogametocyte (female) using the features described by Robert et al. (1996) and the GSR determined using the formula below:
In addition, haematocrit value was established for all subjects using microhaematocrit method as described by Cheesbrough (2000) and the proportion of the subjects with anaemia (haematocrit <30%) was determined.
2.4. Treatment of the subjects
Subjects with malaria parasitaemia were re-evaluated and those who met the inclusion criteria were enrolled into an antimalarial efficacy study (results are presented elsewhere) while others were treated with AL as prescribed by WHO (2015). Non-parasitaemic subjects were referred for further laboratory and clinical examinations and they were treated accordingly.
2.5. Data analyses
Data generated from the studies were analyzed using SPSS version 16.0 (SPSS Inc., USA). Proportions were compared by Chi Square with Yates’ or Fisher exact tests, correlation was assessed by Pearson test while means were compared by analysis of variance (ANOVA). Significance was inferred if p < 0.05.
3. Results
3.1. Demographic and clinical characteristics of the subjects
The demographic characteristics of the 1,657 subjects screened during the study are presented in Table 1. The mean age ±standard deviation (range) of the subjects was 27.5 ± 12.2 (4.0–72.0) years with subjects aged <5 years accounted for 7.8% (130/1,657). Symptomatic subjects with malaria parasitaemia were significantly younger (p < 0.0001) and had lower haematocrit level (p = 0.023) than symptomatic non-parasitaemic subjects. In addition, 18.5% (307/1,657) of the subjects were anaemic at enrolment and this cohort was higher among parasitaemic than non-parasitaemic subjects (p = 0.004).
Table 1.
Demographic and clinical characteristics of the subjects screened.
| Variable | Parasitaemic Subjects | Non-Parasitaemic Subjects | All Subjects | ap-value |
|---|---|---|---|---|
| Number (%) | 375 (22.6) | 1,282 (77.4) | 1,657 (100.0) | < 0.0001 |
| Age (years) | ||||
| Mean ± SD | 20.1 ± 14.5 | 34.5 ± 19.7 | 27.5 ± 12.2 | < 0.0001 |
| Range | 4.0–62.0 | 4.0–72.0 | 4.0–72.0 | - |
| Number <5 (%) | 37 (9.9) | 142 (11.1) | 130 (10.8) | 0.571 |
| DOI (Days) | ||||
| Mean ± SD | 2.3 ± 1.2 | 2.7 ± 1.3 | 2.8 ± 1.3 | 0.374 |
| Range | 1–4 | 1–6 | 1–6 | - |
| Number >2 (%) | 106 (28.3) | 476 (37.1) | 582 (35.1) | 0.002 |
| Haematocrit (%) | ||||
| Mean ± SD | 30.6 ± 8.1 | 32.7 ± 9.2 | 32.6 ± 9.2 | 0.023 |
| Range | 27.0–46.0 | 27.0–48.0 | 27.0–48.0 | - |
| Number with <30 (%) | 89 (23.7) | 218 (17.0) | 307 (18.5) | 0.004 |
| Sex | ||||
| Female (%) | 206 (12.4) | 673 (40.6) | 879 (53.0) | 0.411 |
| Male (%) | 169 (10.2) | 609 (36.8) | 778 (47.0) | - |
| Temperature (°C) | ||||
| Mean ± SD | 38.4 ± 3.2 | 38.1 ± 3.1 | 38.5 ± 3.2 | 0.524 |
| Range | 36.7–41.3 | 36.5–40.4 | 36.5–41.3 | - |
DOI Duration of illness.
SD Standard deviation.
Comparison between parasitaemic and non-parasitaemic subjects.
3.2. Burden of parasitaemia in Maiduguri
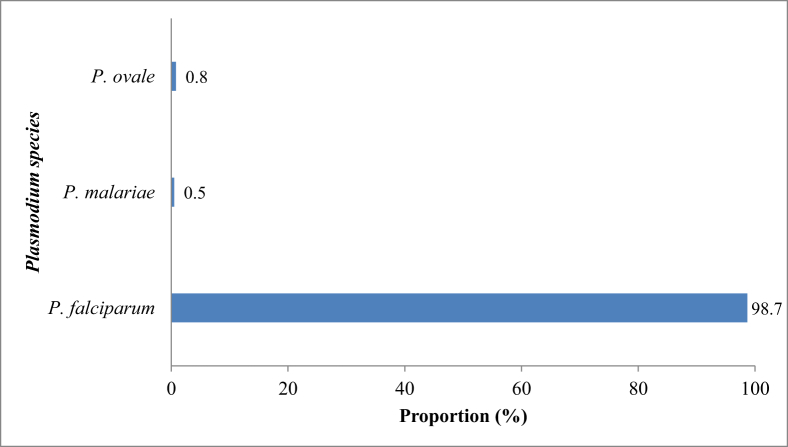
The prevalence of malaria parasitaemia recorded among the subjects was 22.6%. This was significantly highest in August (74.0%) and September (84.2%) [χ2 = 406.9; df = 11; p < 0.0001] (Table 2) and among subjects aged <5 years (χ2 = 401.1; df = 5; p < 0.0001) [Table 3]. P. falciparum accounted for significantly highest proportion (98.7%; 370/375) of the infections in Maiduguri (χ2 = 457.9; df = 3; p < 0.0001) as shown in Fig. 1. The geometric mean parasite density, GMPD (range) of the parasitaemic subjects was 8,925 (320–275,000) asexual parasites/μl blood. The GMPD was significantly highest in subjects screened in August and September with 22,900 and 27,100 asexual parasites/μl blood, respectively (χ2 = 401.1; df = 5; p < 0.0001) [Table 2] and subjects aged <5 years with 11,500 asexual parasites/μl blood (χ2 = 401.1; df = 5; p < 0.0001) [Table 3]. Severe malaria was recorded in 5 of the 375 parasitaemic subjects giving a prevalence of 1.3%. All the cases of severe malaria were due to P. falciparum, occurred in subject <5 years and had GMPD above 200,000 asexual parasites/μl blood.
Table 2.
Monthly burden of malaria in Maiduguri.
| Month | Number Screened | Parasitaemia |
Gametocytaemia |
||||
|---|---|---|---|---|---|---|---|
| Number | aPOP (%) | bGMPD | Number | cPOG (%) | dGMGD | ||
| January | 217 | 22 | 10.0 | 14,422 | 2 | 9.1 | 124 |
| February | 236 | 20 | 8.5 | 22,604 | 4 | 20.0 | 112 |
| March | 213 | 23 | 10.8 | 4,517 | 2 | 8.7 | 106 |
| April | 254 | 19 | 7.5 | 42,543 | 5 | 26.3 | 122 |
| May | 186 | 25 | 13.4 | 117,520 | 8 | 32.0 | 106 |
| June | 74 | 32 | 43.2 | 54,909 | 2 | 6.3 | 139 |
| July | 69 | 38 | 55.1 | 30,000 | 2 | 5.3 | 142 |
| August | 73 | 54 | 74.0 | 12,752 | 8 | 14.8 | 120 |
| September | 57 | 48 | 84.2 | 5,924 | 5 | 10.4 | 84 |
| October | 76 | 33 | 43.4 | 7,120 | 2 | 6.1 | 68 |
| November | 97 | 32 | 33.0 | 7,665 | 5 | 15.6 | 122 |
| December | 105 | 29 | 27.6 | 11,538 | 3 | 10.3 | 89 |
| Total | 1,657 | 375 | 22.6 | 8,925 | 48 | 12.8 | 109 |
(p < 0.0001a, < 0.0001b, 037c, and 0.10d).
GMGD Geometric mean gametocyte density (gametocytes/μl blood).
GMPD Geometric mean parasite density (asexual parasites/μl blood).
POG Prevalence of gametocytaemia (expressed as percentage of subjects with parasitaemia).
POP Prevalence of parasitaemia (expressed as percentage of subjects screened).
Table 3.
Age distribution of malaria burden in Maiduguri.
| Variables | Age Groups (years) |
Total | ap-value | |||||
|---|---|---|---|---|---|---|---|---|
| <5 | 5–9 | 10–19 | 20–29 | 30–39 | ≥40 | |||
| Number Screened | 130 | 276 | 484 | 330 | 255 | 182 | 1,657 | - |
| Parasitaemia | ||||||||
| Number | 102 | 133 | 59 | 31 | 28 | 22 | 375 | - |
| Prevalence (%) | 78.5 | 48.2 | 12.2 | 9.4 | 11.0 | 12.1 | 22.6 | < 0.0001 |
| GMPD (/μl blood) | 11,500 | 8,702 | 8,193 | 7,262 | 8,549 | 5,894 | 8,925 | 0.037 |
| Minimum PD | 2,100 | 900 | 388 | 1,100 | 820 | 340 | 340 | - |
| Maximum PD | 275,000 | 150,000 | 67,000 | 100,000 | 120,000 | 97,000 | 275,000 | - |
| Gametocytaemia | ||||||||
| Number | 9 | 14 | 10 | 6 | 5 | 4 | 48 | - |
| bPrevalence (%) | 8.8 | 10.5 | 16.9 | 19.4 | 17.9 | 18.2 | 12.8 | 0.370 |
| GMGD (/μl blood) | 235 | 98 | 104 | 76 | 77 | 89 | 109 | 0.516 |
| Minimum GD | 80 | 8 | 8 | 8 | 8 | 24 | 8 | - |
| Maximum GD | 416 | 464 | 400 | 368 | 376 | 392 | 464 | - |
GD Gametocyte density Gametocyte density.
GMGD Geometric Mean.
GMPD Geometric Mean Parasite density.
PD Parasite density.
Comparison between age groups using χ2 test (prevalence) and student-t test (GMPD and GMGD).
Expressed as percentage of subjects with parasitaemia.
Fig. 1.
Proportion of Plasmodium species found in Maiduguri, Nigeria.
3.3. Burden of gametocytaemia in Maiduguri
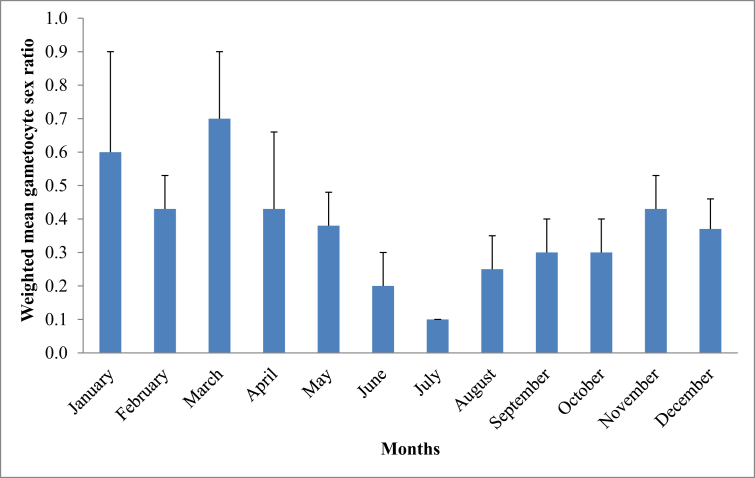
The prevalence of gametocytaemia among the 375 subjects with malaria was 12.8% (48/375). Altogether, subjects in the dry months of January, February, March, April, May, November and December had significantly higher gametocyte carriage (16.5 %, 29/176) than subjects in the wet months of June, July, August, September and October (9.5 %, 19/199) [χ2 = 4.0; df = 1; p = 0.045] (Table 2). The geometric mean gametocyte density, GMGD (range) of the 48 subjects was 109 (8–464) gametocytes/μl blood. The GMGD was similar across the months (p = 0.10) [Table 2] and among various age groups examined (p = 0.516) [Table 3]. Of the 48 subjects with gametocytaemia, 11 subjects had pure gametocytaemia and 37 subjects had both parasitaemia and gametocytaemia. The subjects with pure gametocytaemia were older than those with both parasitaemia and gametocytaemia (p = 0.035). The GSR was determined in subjects with at least gametocyte density of 24 gametocytes/μl blood and presented in Fig. 2. Overall, 91.2 % (8,435/9,249 gametocytes) of the total gametocytes counted were sexed in 39 subjects with density ≥24 gametocytes/μl blood. The weighted mean GSR in these subjects was 0.4 ± 0.1, this was highest and least in March and July, respectively (Fig. 2). The proportion of subjects with GSR ≥0.5 was 30.8 % (12/39) and the distribution was similar in the dry and wet months (χ2 = 2.28; df = 1; p = 0.131).
Fig. 2.
Gametocyte sex ratios of the 39 subjects with gametocyte density ≥24 gametocytes/μl.
3.4. Presenting symptoms
The symptoms of malaria parasitaemia were recorded in the 375 subjects and were compared with that of the 1,282 symptomatic non-parasitaemic subjects. Overall, fever and headache accounted for the highest proportion of 75.2% (1,246/1,657) and 59.3% (983/1,657), respectively (χ2 = 133.3; df = 8; p < 0.05). The symptoms observed were similar in the two groups except for body ache (41.1 %; 154/375; p = 0.0001) and nausea/vomiting (47.7 %; 179/375; p = 0.0001) which were higher in parasitaemic subjects (Table 4).
Table 4.
Presenting symptoms in subjects with and without malaria parasitaemia.
| Symptoms | Parasitaemic Subjects (N = 375) |
Non-parasitaemic Subjects (N = 1,282) |
Total (N = 1,657) |
bp-value |
|---|---|---|---|---|
| Frequency (%) | Frequency (%) | Frequency (%) | ||
| Abdominal pain | 162 (43.2) | 542 (42.3) | 704 (42.5) | 0.796 |
| Body ache | 154 (41.1) | 388 (30.3) | 542 (32.7) | 0.0001 |
| Diarrhoea | 37 (9.8) | 141 (11.0) | 178 (10.7) | 0.571 |
| Fever | 290 (77.3) | 956 (74.6) | 1246 (75.2) | 0.307 |
| Headache | 227 (60.5) | 756 (59.0) | 983 (59.3) | 0.632 |
| Nausea/vomiting | 179 (47.7) | 398 (31.0) | 577 (34.8) | 0.0001 |
| Poor appetite | 133 (35.5) | 519 (40.5) | 652 (39.3) | 0.082 |
| Weakness | 198 (52.8) | 647 (50.5) | 845 (51.0) | 0.445 |
| Othersa | 54 (14.4) | 213 (16.7) | 267 (16.1) | 0.338 |
N Number.
Constipation, prostration, fainting, catarrh, herpes labialis and inability to swallow.
Comparison between parasitaemic and non-parasitaemic subjects.
3.5. Pre-hospital medication among the subjects with malaria parasitaemia
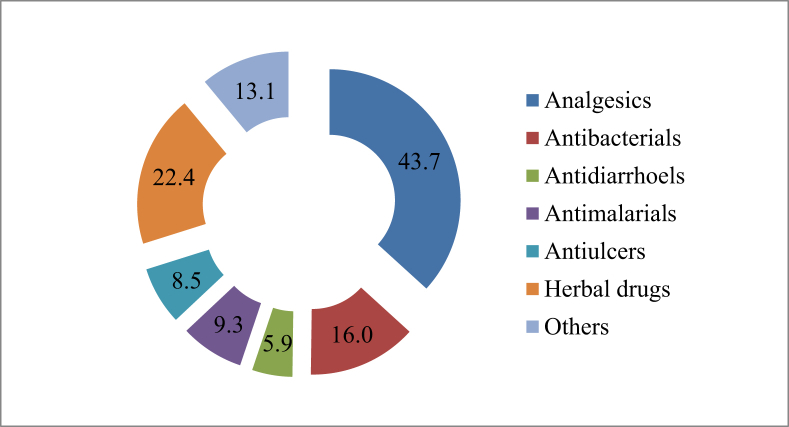
Fig. 3 presents the pre-hospital medication among the subjects with malaria parasitaemia. In all, 74.1% (278/375) of the subjects consumed at least a medication prior to hospital presentation; 25.5% (71/278) of this cohort concurrently consumed at least two drugs. In addition, the pre-hospital medication intake is common among all age groups studied in the present study (χ2 = 3.72; df = 5; p < 0.073). Analgesics accounted for the highest proportion (51.7%; 194/375) of drugs consumed (p < 0.001). In addition, only 9.3% (35/375) of the subjects took required antimalarial medication while 16.0% (60/375) wrongly consumed antibacterial drugs before hospital presentation. It is noteworthy to state that significant proportion (22.4%; 84/375) of the subjects consumed herbal preparations.
Fig. 3.
Pre-hospital medication among the subjects with malaria parasitaemia.
4. Discussion
Antimalarial chemotherapy plays significant role in the treatment and control of malaria with ACTs as the frontline drugs (WHO, 2015). Reduction in global malaria burden has been reported following adoption of ACTs as first line treatment of uncomplicated malaria. Despite, many regions especially in sub-Saharan Africa experience persisted burden of malaria (WHO, 2018). In the present study, the burden of malaria in Maiduguri, Northeast Nigeria and pre-hospital medication among malarious subjects were evaluated years after adoption of ACTs in Nigeria.
In the present study, the prevalence of malaria in Maiduguri remains relatively high and the transmission disproportionately occurs throughout the year. However, the present average prevalence of 22.6% is about one-third lower (over seven years) than 35.2% and 35.6% reported during pre-ACTs era (Molta et al.,1993; Ayanugwo and Kalu, 1997). The pre-ACTs studies were conducted in particular season of the year while the present study spanned through the year, thus, the low transmission during the dry months could be partly responsible for the lower average prevalence recorded in the present study. In addition, ACTs affect gametocyte generation and could in turn influence malaria transmission (Bousema et al., 2006; Sowunmi et al., 2007) especially in areas of low transmission intensity (Nosten et al., 2000). Thus, this reduction could also be attributed to the impact of ACTs on malaria transmission in the region as previously modeled by Okell et al. (2008). The fact that the reduction recorded in our study is lower than the expected based on impact model (Okell et al., 2008) could be due to concurrent use of sulphadoxine-pyrimethamine (SP) by the subjects and incomplete coverage of ACTs deployment in the region. SP favours malaria transmission by gametocyte generation (Sowunmi et al., 2009; Fehintola et al., 2012) and the impact model is based on the assumption of 100% ACTs coverage (Okell et al., 2008). The highest prevalence recorded in the months of August and September coincided with the wet months when mosquitos’ breeding is optimum. This finding is in agreement with previous study that reported seasonal malaria transmission in Maiduguri (Samdi et al., 2005). The high proportion of falciparum malaria recorded in the present study is similar to that reported by Molta et al. (1993), thus, it appears the proportion remains constant in two decades. Despite high proportion of P. falciparum in the region, severe malaria was low (1.3%) and this could be partly attributed to the relatively low parasite density recorded in majority of the subjects who were mostly adult. The mean duration of illness recorded (2.3 days) is an indication of timely presentation at the hospital, hence, the low parasite density.
Gametocyte carriage and gametocyte sex ratio (GSR) are some of the parasite factors that promote malaria transmission (Price et al., 1999). There is dearth of scientific data on pre-ACTs gametocyte carriage and GSR reported from Maiduguri, thus, the present study will complement the existing data. The present carriage is similar to previously reported values from the study area (Watila et al., 2006) and other parts of Nigeria (Sowunmi et al., 2007; Fehintola et al., 2012). However, it is significantly lower than 26.3% reported from Damboa, a town less than 100km from the study area (Samdi, 2012). We do not have immediate explanation for this wide variation within same region, but factors such as age, transmission intensity, parasite density, haematological factors and environmental conditions could have contributed (Drakeley et al., 1999; Price et al., 1999). The higher gametocyte carriage observed during the dry months is similar to previous report (Boudin et al., 1991) and could be survival means by the parasites to circumvent the unsuitable condition. The pure gametocytaemia observed in some older subjects with minimal symptoms could be a subtle reservoir of infection. This could contribute to persisted malaria transmission in the region despite the control measures. Thus, routine mass screening for malaria and effective treatment targeting all ages could minimized the transmission impact of this cohort.
The presenting symptoms by the subjects at enrolment in the present study are consistent with known non-specific nature of malaria symptoms which may be easily confused with other febrile illnesses. The WHO recommended serological and parasitological diagnosis of malaria (WHO, 2015) may not be readily available in regions with inadequate technical and financial resources and for children requiring urgent treatment. Thus, clinical diagnosis based on presenting symptoms becomes the only option. In such situation, presentation of body ache and nausea/vomiting by the subjects may be better indicator of malaria as observed in the present study. However, this should be done with caution especially in children since the present study did not evaluate the relationship between presenting symptoms and age of the subjects.
The present study also examined pre-hospital medication among subjects with malaria parasitaemia. The high proportion of the subjects who consumed at least a medication before visiting hospital is an indication of irrational drug use across all ages in the population. This practice could result into unnecessary expenditure on drugs, endanger the health of the consumers and predispose the drugs to resistance. Analgesics largely consumed by these subjects have been implicated in drug-induced nephrotoxicity (Vadivel et al., 2007; Sampathkumar et al., 2016), thus, the increasing renal disorders seen at UMTH (unpublished data) may be partly due to analgesics consumption. The fact that significant proportion of the subjects consumed herbal preparations is in accordance with health service seeking pattern across Africa (WHO, 1993). Medicinal plants and herbal preparations with antimalarial activities have been well documented (Ene et al., 2010; Adebayo and Krettli, 2011; Thiengsusuk et al., 2013), thus, the herbal preparations consumed by these subjects could have halted the disease progression. However, gametocyte carriage recorded in numbers of these subjects raised research concern about the effect of the herbs on gametocyte generation. Thus, urgent studies are required to investigate the impact of medicinal plants on gametocyte generation. Meanwhile, pre-hospital consumption of drugs cannot be completely discouraged but must be done with caution.
In conclusion, the present study reported persisted malaria burden in Maiduguri, Northeast Nigeria years after adoption of ACTs, gametocyte carriage and pre-hospital medication practice among the population. These findings provide scientific evidence that could serve as guide for policy decision on malaria treatment and control in the region and Nigeria at large.
Declarations
Author contribution statement
Sulayman T. Balogun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Umar K. Sandabe, Fatai A. Fehintola: Performed the experiments.
Kenneth O. Okon: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ayodele O. Akanmu: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by part financial support received from University of Maiduguri Study Fellowship awarded to STB.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors acknowledged the technical supports provided by Mr. Justus Jibrin (Department of Clinical Pharmacology and Therapeutics, College of Medical Sciences, University of Maiduguri, Nigeria), Mr. Isyaka Tom (Department of Medical Microbiology, College of Medical Sciences, University of Maiduguri, Nigeria) and Mr. Mohammed (University of Maiduguri Medical Centre, Maiduguri, Nigeria.
References
- Adebayo J.O., Krettli A.U. Potential antimalarials from Nigerian plants: a review. J. Ethnopharmacol. 2011;133(2):289–302. doi: 10.1016/j.jep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Ayanugwo M.C., Kalu A.U. Variations in the prevalence of malaria in northern east Nigeria: studies on Maiduguri metropolis. Ann. Borno. 1997;13/14 320-320. [Google Scholar]
- Bako B., Geidam A.D., Maigari A.G., Malah B.M., Ngadda H.A., Musa A.B., Sadauki H.M. The effect of intermittent preventive therapy for malaria on pregnancy outcome at UMTH, Maiduguri. Kanem J. Med. Sci. 2009;3(1):23–28. [Google Scholar]
- Balogun S.T., Sandabe U.K., Bdliya D.N., Adedeji W.A., Okon K.O., Fehintola F.A. Asymptomatic falciparum malaria and genetic polymorphisms of Pfcrt K76T and Pfmdr1 N86Y among almajirai in Northeast Nigeria. J. Infect. Dev. Countries. 2016;10(3):290–297. doi: 10.3855/jidc.6853. [DOI] [PubMed] [Google Scholar]
- Balogun S.T., Mishara R., Okon K.O., Adesina O.O., Kutdang E.T., Fehintola F.A. Prevalence of malaria in febrile patients with symptoms clinically compatible with malaria in Maiduguri. J. Life Environ. Sci. 2010;11:641–648. [Google Scholar]
- Boudin C., Lyannaz J., Bosseno M.F., Carnevale P., Ambroise-Thomas P. Epidemiology of Plasmodium falciparum in a rice field and a savannah area in Burkina Faso: seasonal fluctuations of gametocytaemia and malarial infectivity. Ann. Trop. Med. Parasitol. 1991;85:377–385. doi: 10.1080/00034983.1991.11812580. [DOI] [PubMed] [Google Scholar]
- Bousema J.T., Schneider P., Gouagna L.C., Drakeley C.J., Tostmann A., Houben R., Githure J.I., Ord R., Sutherland C.J., Omar S.A., Sauerwein R.W. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. JID (J. Infect. Dis.) 2006;193(8):1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- Cheesbrough M. University Press; Cambridge, UK: 2000. District Laboratory Practice in Tropical Countries (Part I) [Google Scholar]
- Drakeley C.J., Secka I., Correa S., Greenwood B.M., Targett G.A. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae s.s. mosquitoes. Trop. Med. Int. Health. 1999;4:131–138. doi: 10.1046/j.1365-3156.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Ene A.C., Atawodi S.E., Ameh D.A., Kwanashie H.O., Agomo P.U. Locally used plants for malaria therapy amongst the Hausa, Yoruba and Ibo communities in Maiduguri, Northeastern Nigeria. Indian J. Tradit. Knowl. 2010;9(3):486–490. [Google Scholar]
- Fehintola F.A., Balogun S.T. Malaria: Passive case detection and healthcare providers’ choices of chemotherapy. Afr. J. Med. Sci. 2010;39:60–65. [PubMed] [Google Scholar]
- Fehintola F.A., Balogun S.T., Adeoye S.B. Intermittent preventive treatment during pregnancy with sulphadoxine-pyrimethamine may promote Plasmodium falciparum gametocytogenesis. Med. Princ. Pract. 2012;21(1):63–67. doi: 10.1159/000332405. [DOI] [PubMed] [Google Scholar]
- FMH . Federal Ministry of Health; Abuja, Nigeria: 2005. National Antimalarial Treatment Guidelines. [Google Scholar]
- Jimme M.A., Bukar W.M., Monguno A.K. Contamination levels of domestic water sources in Maiduguri metropolis, Borno state, Northeast Nigeria. Ethiop. J. Environ. Stud. Manag. 2016;9(6):760–768. [Google Scholar]
- Kokori M.M., Turaki Z.S.G., Garba A.M. Influence of parasiatemia on anaemia in Pf treated children at lake-alau, Borno state. Int. J. Sci. Eng. Res. 2013;1(1) 2347-2389-93. [Google Scholar]
- Molta N.B., Daniel H.I., Oguche S.O., Watila I.M. Malaria treatment failures in Northeastern Nigeria: an update. Niger. J. Parasitol. 1993;14:21–26. [Google Scholar]
- Mukhtar A., Akpan J.C. Assessment of challenges facing solid waste management in Maisandari neighbourhood of Maiduguri Metropolis, Borno State, Nigeria. Int. J. Res. Innov. Soc. Sci. 2018;2(7):170–177. [Google Scholar]
- Nosten F., van Vugt M., Price R., Luxemburger C., Thway K.L., Brockman A., McGready R., ter Kuile F., Looareesuwan S., White N.J. Effects of artesunate-mefloquine combination on incidence of P. falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- NPC . National Population Commission; Abuja, Nigeria: 2006. Nigeria Population Census 2006. [Google Scholar]
- NPC/NMCP/ICF . National Population Commission, National Malaria Control Programme, and ICF International; Abuja, Nigeria: 2012. Nigeria Malaria Indicator Survey 2010. [Google Scholar]
- Okell L.C., Drakeley C.J., Bousema T., Whitty C.J.M., Ghani A.C. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008;5(11):e226. doi: 10.1371/journal.pmed.0050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering J., Read A.F., Guerrero S., West S.A. Sex ratio and virulence in two species of lizard malaria parasites. Evol. Ecol. Res. 2000;2:171–184. [Google Scholar]
- Poirier P., Doderer-Lang C., Atchade P.S., Lemoine J., Coquelin de l’Isle M., Abou-bacar A., Pfaff A.W., Julie Brunet J., Arnoux L., Haar E., Filisetti D., Perrotey S., Chabi N.W., Akpovi C.D., Anani L., Bigot A., Sanni A., Candolfi E. The hide and seek of Plasmodium vivax in West Africa: report from a large-scale study in Beninese asymptomatic subjects. Malar. J. 2016;15:570. doi: 10.1186/s12936-016-1620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R., Nosten F., Simpson J.A., Luxemburger C., Phaipun L., Kuile F., van Vugt M., Chongsuphajaisiddhi T., White N.J. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 1999;60:1019–1023. doi: 10.4269/ajtmh.1999.60.1019. [DOI] [PubMed] [Google Scholar]
- Robert V., Read A.F., Essong J., Tchuinkam T., Mulder B., Verhave J.P., Carnevale P. Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 1996;90:621–624. doi: 10.1016/s0035-9203(96)90408-3. [DOI] [PubMed] [Google Scholar]
- Roberts N., Mayon-White D. 2015. A Leading Practice in Malaria Control. A Study Conducted by Sentinel Consulting, Commissioned by Rio Tinto and Facilitated by GBCHealth. [Google Scholar]
- Ruas R., Pinto A., Nuak J., Sarmento A., Abreu C. Non-falciparum malaria imported mainly from Africa: a review from a Portuguese hospital. Malar. J. 2017;16:298. doi: 10.1186/s12936-017-1952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salako L.A., Ajayi F.O., Sowunmi A., Walker O. Malaria in Nigeria: a revisit. Ann. Trop. Med. Parasitol. 1990;84:435–445. doi: 10.1080/00034983.1990.11812493. [DOI] [PubMed] [Google Scholar]
- Samdi L.M. University of Jos; Nigeria: 2012. A Study on the Malaria Vector (Anopheles Spp) in a Sudano-Sahelian Savannah Area of Borno State North Eastern Nigeria and the Insect Growth Regulator Pyriproxyfen (S-31183) A PhD Thesis submitted to the School of Postgraduate Studies. [Google Scholar]
- Samdi L.M., Oguche S., Molta W.M.B., Kalu M.K., Watila I.M., Anyanwu G.I., Agomo P.U. A comparative longitudinal Study of Seasonal variation of malaria parasite and vector densities in the sahel, Northeastern Nigeria. Niger. J. Exp. Appl. Biol. 2005;6(1):77–85. [Google Scholar]
- Sampathkumar K., Rajiv A., Sampathkumar D. Analgesic nephropathy - a painful progression. Clin. Med. Insights Urol. 2016;9:7–10. [Google Scholar]
- Sowunmi A., Balogun S.T., Gbotosho G.O., Happi C.T. Plasmodium falciparum gametocyte sex ratios in symptomatic children treated with antimalarial drugs. Acta Trop. 2009;109(2):108–117. doi: 10.1016/j.actatropica.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Sowunmi A., Balogun T., Gbotosho G.O., Happi C.T., Adedeji A.A., Fateye B.A., Fehintola F.A. Activities of amodiaquine, artesunate, and artesunate-amodiaquine against asexual- and sexual-stage parasites in falciparum malaria in children. Antimicrob. Agents Chemother. 2007;51:1694–1699. doi: 10.1128/AAC.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiengsusuk A., Chaijaroenkul W., Na-Bangchang K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol. Res. 2013;112(4):1475–1481. doi: 10.1007/s00436-013-3294-6. [DOI] [PubMed] [Google Scholar]
- Todd C.W., Udhayakumar V., Escalante A.A., Lal A.A. Malaria vaccines. In: Tibayrenc M., editor. Encyclopedia of Infectious Diseases. John Wiley and Sons; New Jersey, US: 2007. [Google Scholar]
- Vadivel N., Trikudanathan S., Singh A.K. Analgesic nephropathy. Kidney Int. 2007;72:517–520. doi: 10.1038/sj.ki.5002251. [DOI] [PubMed] [Google Scholar]
- Watila I.M., Oguche S., Shaa K.K., Samdi L.M., Molta N.B. A study of efficacy, safety and tolerability of prepackage Amodiaquine/artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in children age 6 months to 6 years, Northeastern Nigeria. Hosp. Digest. 2006;212:13–16. [Google Scholar]
- Waziri M. Adamu Joji Publishers; Kano city, Nigeria: 2009. Spatial Pattern of Maiduguri City. Researchers Guide. [Google Scholar]
- WCH . 2018. Climate, Global Warming and Daylight Charts and Data: Maiduguri, Nigeria. World Climate Home.http://www.climate-charts.com/Locations/n/NI65082.php Available at. [Google Scholar]
- Weather2 . 2018. Local Weather: Maiduguri. My Weather.www.myweather2.com/city-Town/Nigeria/Maiduguri/climate-profile.aspx?month=6 Available at. [Google Scholar]
- West S.A., Reece S.E., Read A.F. Evolution of gametocyte sex ratios in malaria and related apicomplexan (protozoan) parasites. Trends Parasitol. 2001;17:525–531. doi: 10.1016/s1471-4922(01)02058-x. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization, Regional Office; Manila, Philippines: 1993. Guidelines for Evaluation of Herbal Medicines, Report No 611. [Google Scholar]
- WHO . Report of a WHO Technical Consultation. World Health Organization; Geneva, Switzerland: 2001. Antimalarial drug combination therapy. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2003. World Health Organization. Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2005. Susceptibility of Plasmodium Falciparum to Antimalarial Drugs: Report on Global Monitoring: 1996-2004. [Google Scholar]
- WHO . third ed. World Health Organization; Geneva, Switzerland: 2015. Guidelines for the Treatment of Malaria. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2006. Malaria Vector Control and Personal protection. WHO Technical Report Series, 936. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2017. World Malaria Report 2017. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2018. Ready to Beat Malaria. World Malaria Day 2018.www.who.int WHO/CDS/GMP/2018.06. Available at. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2013. Seasonal Malaria Chemoprevention with Sulfadoxine-Pyrimethamine Plus Amodiaquine in Children: a Field Guide. [Google Scholar]
- WPR . World Population Review; 2018. Population of Cities in Nigeria 2018.www.worldpopulationreview.comcom/countries/nigeria-population/cities/ Available at. [Google Scholar]