Abstract
Recent studies show that exposure to ultraviolet (UV) light suppresses ocular elongation, which causes myopia development. However, the specific mechanisms of this process have not been elucidated. A UV-sensor, Opsin 5 (Opn5) mRNA was shown to be present in extraretinal tissues. To test the possibility that UV-signals mediated by Opn5 would have a direct effect on the outer connective tissues of the eye, we first examined the expression patterns of a mammalian type Opn5 (Opn5m) in the late-embryonic chicken eye. Quantitative PCR showed Opn5m mRNA expression in the cornea and sclera. The anti-Opn5m antibody stained a small subset of cells in the corneal stroma and fibrous sclera. We next assessed the effect of UV-A (375 nm) irradiation on the chicken fibroblast cell line DF-1 overexpressing chicken Opn5m. UV-A irradiation for 30 min significantly increased the expression of Early growth response 1 (Egr1), known as an immediate early responsive gene, and of Matrix metalloproteinase 2 (Mmp2) in the presence of retinal chromophore 11-cis-retinal. In contrast, expression of Transforming growth factor beta 2 and Tissue inhibitor of metalloproteinase 2 was not significantly altered. These results indicate that UV-A absorption by Opn5m can upregulate the expression levels of Egr1 and Mmp2 in non-neuronal, fibroblasts. Taken together with the presence of Opn5m in the cornea and sclera, it is suggested that UV-A signaling mediated by Opn5 in the extraretinal ocular tissues could influence directly the outer connective tissues of the chicken late-embryonic eye.
Keywords: Opsin 5, UV-Absorbing pigment, Fibroblasts, Chicken, Egr1, Mmp2
Abbreviations: cAMP, cyclic adenosine monophosphate; Egr1, Early growth response 1; Gapdh, Glyceraldehyde-3-phosphate dehydrogenase; MAP kinase, mitogen-activated protein kinase; Mmp2, Matrix metalloproteinase 2; Opn5, Opsin 5; Opn5m, mammalian type Opn5; qPCR, quantitative polymerase chain reaction; Tgfb2, Transforming growth factor beta 2; Timp2, Tissue inhibitor of metalloproteinase 2; UV, ultraviolet; UV-A, ultraviolet-A
Highlights
-
•
Opsin 5 (Opn5) is a non-visual ultraviolet-A (UV-A) absorbing photopigment.
-
•
We found an Opn5 (Opn5m) is present in cornea and sclera of late-embryonic chick.
-
•
UV-A absorption by Opn5m upregulated Egr1 and Mmp2 expression in chick fibroblasts.
-
•
UV-A signaling via Opn5m may have a direct effect on the ocular fibroblasts.
1. Introduction
The rhodopsin family, consisting of an opsin protein and its chromophore retinal, are known as photon receptors functioning in the initial stages of vision [1]. There are 9 opsin family genes in the human genome: Rod opsin (RHO), three cone opsin genes (OPN1SW, OPN1MW, OPN1LW), Encephalopsin/Panopsin (OPN3), Melanopin (OPN4), Opsin 5 (OPN5), Retinal G protein coupled receptor (RGR), and Retinal pigment epithelium derived rhodopsin homolog (RRH/peropsin).
Opsin 5 (Opn5; formerly neuropsin), initially identified by genome mining in human and mouse DNA databases [2], has been found in a wide range of genomes from mammals to fish [[3], [4], [5], [6], [7]]. In addition to its presence in the genome, Opn5 was found to be an ultraviolet (UV) (λmax = 380 nm) sensor, which activates Gi-type G-protein and induces further signal transduction pathways via cAMP, Ca2+, and phosphorylation of MAP kinases [5,8,9]. Opn5 is present in the eyes and brains of birds, fish, rodents, and primates [[2], [3], [4], [5], [6], [7], [8],10]. Its functions in the eye were partly elucidated recently: Opn5 in the mouse retina and cornea has roles in the entrainment of their local photoperiodicity and Opn5-null mice exhibit impaired circadian photoentrainment [11,12]. Nevertheless, the functions of Opn5 have not been studied from the viewpoint of ocular development.
The exponential increase in the number of people suffering from myopia globally, and especially in eastern Asia, is a matter of public concern [13]. In myopia, objects viewed at a distance are blurred due to an abnormal elongation of the eyeball. Recent studies have shown that exposure to sunlight or more specifically violet light (360–400 nm), suppresses myopia progression [14,15]. However, the specific mechanisms of this process have not been elucidated.
We hypothesized that UV-A or violet light reception by Opn5 might have a direct inhibitory effect on the elongation of the eyeball, whose wall consists of sclera and cornea, resulting in the suppression of myopia development. To test this hypothesis, we examined the expression levels and distribution of the mammalian type Opn5 (Opn5m) in the sclera and cornea of chicken late-stage embryos. We also assessed the effect of UV-A (375 nm) irradiation on the chicken fibroblast cell line DF-1 overexpressing chicken Opn5m.
2. Materials and methods
2.1. Preparation of chicken late-embryonic eyes, RNA isolation, and synthesis of cDNA
Fertilized chicken eggs (Gallus gallus) were purchased from a commercial farm (Goto-furanjyo, Japan; http://www.gotonohiyoko.co.jp/) and incubated at 37.5 °C in a humidified incubator. According to the American Veterinary Medical Association Guidelines (https://www.avma.org/KB/Policies/Pages/Euthanasia-Guidelines.aspx), we euthanized embryonic day 17 (E17) chicken embryos. Twenty eyes from 10 chicken embryos at E17 were removed and the cornea, fibrous sclera, cartilaginous sclera, chorioretinal tissues, and a portion of liver were isolated in ice-cold phosphate-buffered saline (PBS). They were treated with ice-cold RNAlater stabilization solution (Thermo Fisher Scientific, Waltham, MA, USA), snap-frozen in liquid nitrogen, and stored at −80°C until further use. Total RNA was extracted from the tissues using an RNAqueous-Micro Total RNA Isolation Kit (Thermo). After treatment of DNase I, cDNA was synthesized using random hexamers and reverse transcriptase (SuperScript III First-Strand Synthesis System; Thermo) with completion by RNase H treatment. Polymerase chain reaction (PCR) was performed using Taq polymerase (Ex Taq; Takara, Kusatsu, Japan) and the primers shown in Table 1. The PCR products (459 bp for Opn5m and 551 bp for Gapdh) were sequenced and confirmed to be partial cDNAs for chicken Opn5m and Gapdh.
Table 1.
Primer sequences for PCR used in this study.
| Gene | Accession# (ref#) | Sequences (5′-3′) | Amplicon size (bp) |
|---|---|---|---|
| Opn5m | AB368182 | Forward:5′-ACTTAAAAATCTGTCACTTGGCTTATG-3′ | 459 |
| Reverse:5′-TGAATCTCTGTGTGAAGATAAGGTGTA-3′ | |||
| Opn5m |
AB368182 ( [3]) |
Forward:5′-GGGCTGGCTTCTTCTTTGGCTGTGG-3′ | 128 for qPCR |
| Reverse:5′-CAGGCAGATAAAGGCATGGTGT-3′ | |||
| Gapdh | NM_204305.1 | Forward: 5’ -GAGAAATATGACAAGTCCCTGAAAAT-3′ | 551 |
| Reverse: 5’ -CTGTATCCAAACTCATTGTCATACCAG-3′ | |||
| Gapdh |
NM_204305.1 ( [32]) |
Forward: 5’ -AGATGCAGGTGCTGAGTATGTTG-3′ | 71 for qPCR |
| Reverse: 5’ -GATGAGCCCCAGCCTTCTC-3′ | |||
| Egr1 |
AF026082 ( [33]) |
Forward: 5′-ACTAACTCGTCACATTCGCA-3′ | 241/ for qPCR |
| Reverse: 5′-TGCTGAGACCGAAGCTGCCT-3′ | |||
| Tgfb2 |
NM_001031045.2 ( [25]) |
Forward: 5′-GCTGCGTGTCCCAGGATTT-3′ | 60 for qPCR |
| Reverse: 5′-TGGGTGTTTTGCCAATGTAGTAGA-3′ | |||
| Mmp2 |
U07775 ( [26]) |
Forward: 5′-TGGTGTGCTTCTACCAGCAG-3′ | 122 for qPCR |
| Reverse: 5′-GAGTGCTCTAATCCCATCGC-3′ | |||
| Timp2 |
AF004664 ( [26]) |
Forward: 5′-GATGGAGAAGATCGTGGGCGG-3′ | 169 for qPCR |
| Reverse: 5′-TGGGCTTTCCTACTGGCTACTG-3′ | |||
| Actb |
NM_205518.1 ( [34]) |
Forward: 5′-GCGCTCGTTGTTGACAAT -3′ | 151 for qPCR |
| Reverse: 5′-CATCACCAACGTAGCTGTCTTT-3′ |
2.2. Quantitative PCR (qPCR) analysis
qPCR was performed using a LightCycler Nano (Roche Diagnostics, Penzberg, Germany). Primer sequences used are shown in Table 1. qPCR control genes for the quantification of relative gene expression were Gapdh for Tgfb2 and cytoplasmic beta-actin (Actb) for Opn5m, Egr1, Mmp2, and Timp2.
2.3. In situ hybridization (ISH)
Digoxigenin (DIG)-labeled RNA probes were synthesized using chicken Opn5m (cOpn5m) cDNAs of the coding region (1186 bp) and 3’ untranslated region (UTR) (2679 bp) inserted into the pGEM-T Easy and pBluescript II KS (+) plasmids, respectively. E17 chicken embryonic eyes were dissected and fixed overnight in 4% paraformaldehyde (PFA)/PBS at 4°C. The eyes were then immersed overnight in 20% sucrose in PBS for cryo-protection at 4°C, embedded in optimal cutting temperature compound (Sakura Finetek, Tokyo, Japan), frozen, and stored at −80°C. Histology was confirmed by Hematoxylin and Eosin staining (Supplementary Fig. S1). Sixteen-micron thick frozen sections were cut and subjected to ISH as essentially described [7]. Briefly, rehydrated sections were treated for 15 min with a 2 μg mL−1 proteinase K solution at room temperature, and hybridized with 0.34 μg mL−1 of the DIG-labeled antisense or sense probe at 65°C for 2 days. Color development was performed with alkaline phosophatase reaction buffer (100 mM Tris-HCl, 50 mM MgCl2, 100 mM NaCl, 0.1 % (v/v) Tween 20, 5% (w/v) polyvinyl alcohol (363081; Sigma-Aldrich, St Louis. MO. USA), pH 9.5) containing 50 μg ml−1 Nitroblue Tetrazolium and 175 μg ml−1 5-Bromo-4-chloro-3-indolyl-phosphate for three days with buffer renewal after the first 2 days. Finally, the sections were counter-stained with nuclear fast red, dehydrated with methanol and tissue dehydration solution A (Wako Pure Chemical Industries, Ltd., Osaka, Japan), cleared with G-NOX (Genostaff, Tokyo, Japan), and mounted on coverslips using PARAmount-D mounting medium (Falma, Tokyo, Japan). Histological images were captured with a Nikon digital camera system (DS-Fi1, Nikon, Tokyo, Japan) mounted on a Leica light microscope (DM5000; Leica microsystems, Wetzlar, Germany).
2.4. Immunostaining and confocal laser microscopy
Immunostaining was performed on the frozen sections mentioned above using anti-chicken Opn5m as reported [8]. Nuclei were stained with Hoechst 33342. Fluorescence images were captured by a Zeiss confocal laser microscope (Zeiss LSM 780 with LSM Software ZEN 2011; Carl Zeiss, Oberkochen, Germany). The color of the images was properly adjusted with Adobe Photoshop version 12.0 (Adobe systems, San Jose, CA, USA).
2.5. Retrovirus vector construction, cell culture, and UV irradiation experiments
The RCASBP(A) plasmid containing cOpn5m tagged with the epitope sequence (corresponding to amino acids, ETSQVAPA), recognized by an anti-bovine rhodopsin monoclonal antibody Rho1D4 at the C terminus, was constructed as follows. First, the tagged-cOpn5m cDNA was amplified from the insert in the mammalian expression vector pCAGGS [5] by PCR using the primers: 5′-CCGACCACCATGGCCATGAGTGGGATGGCATCGGA-3’ (forward) and 5′-CTTATCGATAAGCTTTCAGGCAGGCGCCACTTGGC-3’ (reverse), containing recognition sites of the restriction enzymes NcoI and HindIII, respectively. After digestion with NcoI and HindIII, the DNA fragments were ligated to the Cla12Nco adapter vector. After transformation of E. coli DH5α competent cells, insertion of cOpn5m was confirmed by colony-direct PCR using the cOpn5m specific primer pair mentioned above. After digestion with ClaI, the tagged-cOpn5m was ligated to phosphatase-treated RCASBP(A). The insertion and orientation of cOpn5m in RCASBP(A) was confirmed by colony-direct PCR using the primers: 5′-ACGCTTTTGTCTGTGTGCTGC-3’ (forward) and 5′-CGCTATGATGTCAGCTTCTC-3’ (reverse), specific to the sequences in RCASBP(A) and cOpn5m, respectively. Finally, the plasmid RCASBP(A) containing the tagged-cOpn5m was confirmed by sequencing with the forward primer specific to RCASBP(A) shown above. A cell line derived from chicken embryonic fibroblasts, DF-1 [16] (CRL-12203; American Tissue Culture Collection) was cultured in Dulbecco's Modified Eagle Medium (049–32645; Wako) supplemented with 10% fetal bovine serum, 100 units of mL−1 penicillin and 100 μg mL−1 streptomycin at 37°C under 5% CO2 as described previously [17]. For transfection, DF-1 cells were seeded in a 3.5 cm dish, grown to 70% confluency, and transfected with 7.5 μL per dish of Lipofectamine 3000 (Thermo) and 2.5 μg per dish of RCASBP(A) containing the tagged-cOpn5m. After two passages, transfected-cells were seeded into 24-well black clear bottom plates (Perkin Elmer, Waltham, MA, USA) at the 80–90 % confluency. Six days after transfection, 11-cis-retinal was supplied to a final concentration of 2 μM under dim red light. The culture plate was installed on a light plate apparatus, which was made with a three-dimensional printer as described [18] and equipped with a UV-light emitting diode (peak wavelength: 375 nm) (S5UV30; Sousin Digital, Tokyo, Japan). The cells were irradiated with 700 μW cm−2 of UV light, as measured by a USB2000 + spectrometer (Ocean optics, Dunedin, Florida, USA), for half an hour (1.26 J cm−2), 1 h (2.52 J cm−2) or 4 h (10.1 J cm−2). UV irradiation was terminated 1 day after the addition of 11-cis-retinal, irrespective of the duration of exposure. After irradiation, the cells were harvested, lysed in Isogen II solution (Nippon Gene, Tokyo, Japan) and total RNA was extracted. cDNA was synthesized and analyzed by qPCR as mentioned. To estimate transfection efficiency, transfected DF-1 cells were fixed in 4% PFA in PBS and analyzed by standard immunofluorescence using anti-bovine rhodopsin monoclonal antibody Rho1D4 (P2194; Thermo). Through the propagation of the retroviral vector RCASBP, 100% of the transfected cells expressed the tagged-cOpn5m (See Supplementary Fig. S2).
2.6. Statistics
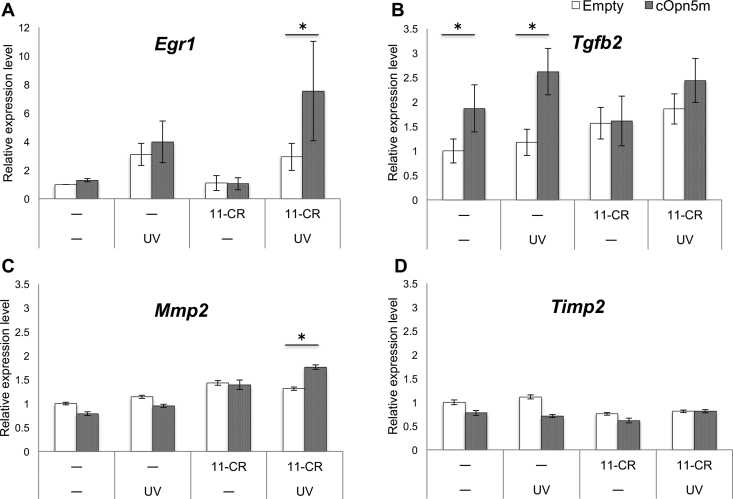
At least three independent replicates of each experiment were performed. The relative expression levels in qPCR were analyzed using Student's t-test comparison between two unpaired groups and differences with P values less than 0.05 were regarded as statistically significant (Fig. 4).
Fig. 4.
Quantitative PCR analysis of myopia-related gene expression in DF-1 cells.
Expression of Egr1 (A), Tgfb2 (B), Mmp2 (C), and Timp2 (D) was examined with or without forced-expression of chicken Opn5m, the addition of 11-cis-retinal (11-CR) to the culture medium, and UV irradiation. White columns show the negative control, in which the empty RCAS vector was transfected; gray columns show cOpn5m expression. Error bars show standard error of the mean. Asterisks indicate a significant difference between the two values (p < 0.05).
3. Results
3.1. PCR analysis of Opn5m expression in chicken embryonic ocular tissues
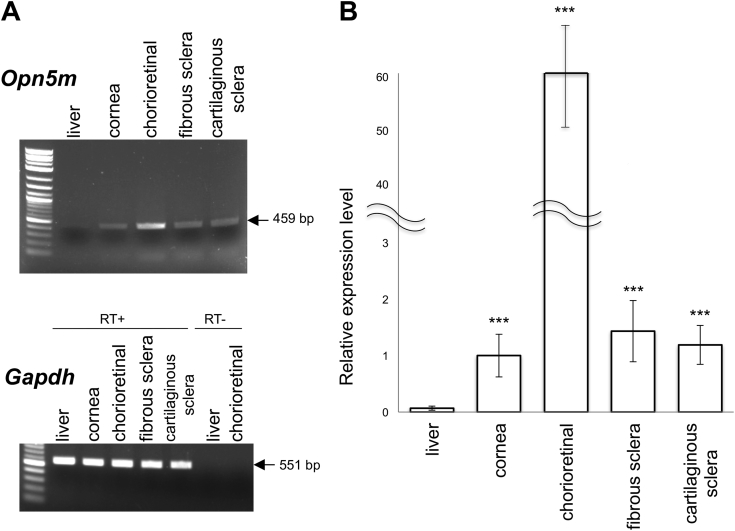
There are three Opn5 subfamily members in Gallus gallus [3,8,19,20], and we focused on Opn5m as it was the most prominent Opn5 receptor in the chicken embryonic retina and is also present in mammals. Reverse transcription PCR analysis showed that Opn5m was expressed in the cornea and sclera of E17 chicken embryos (Fig. 1A). There are two histologically distinct tissues in the chicken sclera; fibrous sclera and cartilaginous sclera and Opn5m was expressed in both. qPCR analysis showed that the expression levels of Opn5m in the cornea and sclera were approximately one sixtieth that of the chorioretinal tissue (Fig. 1B).
Fig. 1.
Expression of Opn5m mRNA in E17 chicken ocular tissues.
A, Electrophoresis of PCR products for Opn5m and Gapdh amplified from cDNA of liver, cornea, chorioretinal tissues, fibrous sclera, and cartilaginous sclera. RT, reverse transcriptase. B, Quantitative PCR showing the relative expression of Opn5m mRNA in each tissue at E17. The level of Opn5m in the E17 cornea was standardized to 1(1.00 ± 0.38). Relative expression levels are 1.43 ± 0.54 in fibrous sclera, 1.19 ± 0.34 in cartilaginous sclera, 60.12 ± 10.01 in chorioretinal tissues, and 0.068 ± 0.036 in liver. Error bars show standard error of the mean. Asterisks indicate a significant difference compared with the level in the liver (p < 0.001).
3.2. Localization of Opn5m mRNA
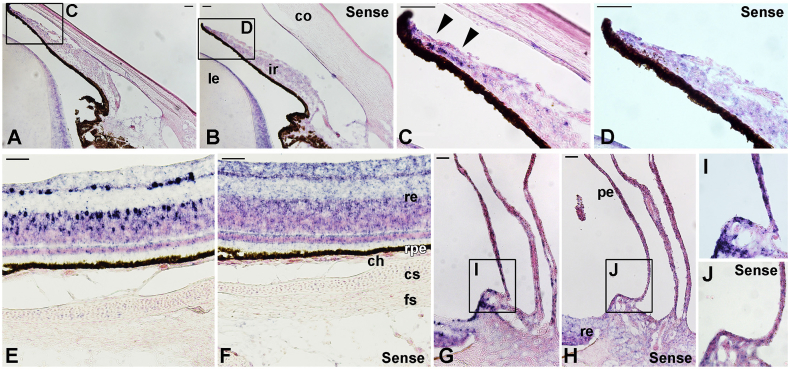
We next sought to identify the Opn5m-expressing cells using ISH on E17 chicken eye sections. In the anterior portion of the eye, however, we could not detect signals for Opn5m mRNA in the cornea (Fig. 2A; Fig. 2B as control), while it was detected in the distal marginal region of the iris (Fig. 2C; Fig. 2D as control). In the posterior portion of the eye, we could not detect obvious signals for Opn5m mRNA in the cartilaginous or fibrous scleral tissue by ISH, either (Fig. 2E; Fig. 2F as control). In addition to distinct signals in the retina, Opn5m mRNA was detected in the pecten, a vascular and pigmented structure unique to the avian species (Fig. 2G, I; Fig. 2H, J as control) [21].
Fig. 2.
In situ hybridization of Opn5m mRNA on E17 chicken ocular tissues.
Signals for Opn5m mRNA are dark blue and the sections are counter-stained with nuclear fast red. A sense probe was used as a negative control (B, D, F, H, J). A, anterior portion of the eye. Enlarged images of the iris (boxed area in A, B) are shown as C and D, respectively. C,Opn5m is expressed in the iris (arrows). E, posterior portion of the eye. There is no obvious staining for Opn5m mRNA in the sclera, while intense signals are observed in a subset of cells of the retina. F, non-specific staining is observed in the retina after a long period of color reaction. G,Opn5m is expressed in the pecten, where strong signals are observed at the base (boxed). Enlarged images of the pecten (boxed area in G, H) are shown as I and J. Abbreviations: ch, choroid; co, cornea (stroma); cs, cartilaginous sclera; fs, fibrous sclera; ir, iris; le, lens; pe, pecten; re, retina, rpe, retinal pigment epithelium. Scale bars, 50 μm.(For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Localization of Opn5m protein
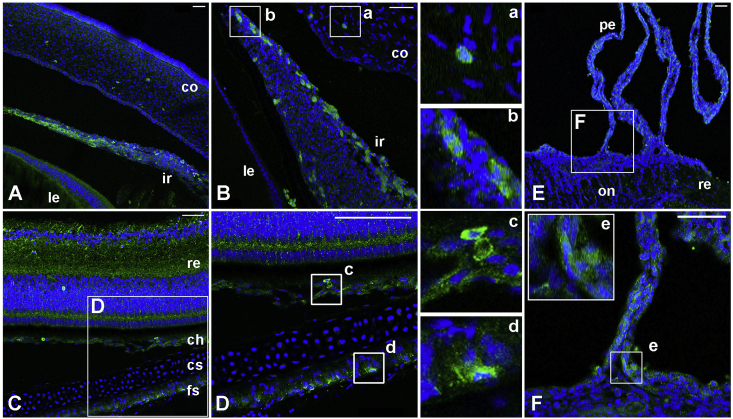
We then determined the localization of Opn5m protein in the ocular tissues by immunostaining. In the anterior portion of the eye, immunoreactivity to chicken Opn5m (green fluorescence) was detected in a subset of the cells of the corneal stroma (Fig. 3A, B, a) as well as in the iris (Fig. 3A, B, b). In the posterior of the eye, low levels of green fluorescence were detected in the choroidal tissue and fibrous sclera (Fig. 3C, D, c, d). We also found signals in the pecten (Fig. 3E, F, e).
Fig. 3.
Immunostaining for Opn5m protein on E17 chicken ocular tissues.
Signals for Opn5m protein are green fluorescence and nuclei are shown in blue. The negative control, without primary antibody against chicken Opn5m, essentially shows no green fluorescence (Supplementary Fig. S3). The boxed area is enlarged and shown in a-e, D, F. A, anterior portion of the eye. Green fluorescence is observed in a subset of cells in the corneal stroma, and iris. Intense signals for Opn5m protein are observed in differentiating sphincter and dilator muscles of the iris. B, in this section, Opn5m protein in the cells just underneath the anterior lining cells and posterior pigmented epithelium. C, posterior portion of the eye. D, chorioretinal tissue and sclera. There is a low level of green fluorescence in the choroid and fibrous sclera. E, F, Opn5m protein is localized to the pecten. a, corneal stroma cells; b, iris stroma cells; c, choroidal stroma cells; d, fibrous scleral cells; e, basal part of pectin oculi. Abbreviations: ch, choroid; co, cornea (stroma); cs, cartilaginous sclera; fs, fibrous sclera; ir, iris; le, lens; pe, pecten; on, optic nerve disc; re, retina. Scale bars, 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Expression analysis of myopia-related genes in chicken fibroblasts after UV irradiation
Myopia-related genes have been identified by experiments using animal myopia models and epidemiological data from human myopia patients. In this study, we focused on four genes, Egr1 [22,23], Tgfb2 [24,25], Mmp2, and Timp2 [26]. To determine whether the expression of these four genes was altered after UV absorption by the Opn5m receptor, chicken Opn5m was overexpressed in the DF-1 fibroblasts. Initially, the Opn5m-expressing cells were UV-irradiated (375 nm) at an intensity of 700 μW cm−2 for half an hour, 1 h or 4 h. Since we found it difficult to obtain consistent results when the cells were UV-irradiated for longer durations, the irradiation time was fixed at 30 min. As it was reported that UV irradiation alone induces Egr1 expression in mouse embryo fibroblasts [27], we saw a similar result in the DF-1 cells (Fig. 4A). Of note, when the Opn5m gene was overexpressed in DF-1 cells and 11-cis-retinal, a chromophore of Opn5m, was added to the culture medium, the expression of Egr1 was significantly increased after UV irradiation (P = 0.01034) (Fig. 4A). We also examined the relative expression levels of Tgfb2, Mmp2, and Timp2 in each experimental condition. Expression of Tgfb2 was significantly elevated after the forced expression of Opn5m without retinal, irrespective of UV irradiation (P = 0.04060 and P = 0.03460, respectively) (Fig. 4B). With the addition of retinal, Tgfb2 expression tended to be elevated with irradiation, although the result was not statistically significant (Fig. 4B). Mmp2 expression was significantly increased after UV irradiation in the presence of retinal (P = 0.01765) (Fig. 4C), and it was not elevated by UV irradiation alone. None of the conditions influenced the expression of Timp2 (Fig. 4D).
4. Discussion
The Opn5m-immunoreactivity was detected in a subset of cells in the cornea and fibrous sclera, but ISH could not detect distinct mRNA signals in these tissues. We think that the levels of Opn5m mRNA in these tissues were too low to be detected by ISH but that the transmembrane receptor protein of Opn5m could be detected by immunostaining, as the structural membrane protein may not require high-abundance mRNA expression. Combined with the results from qPCR and immunostaining, we concluded that Opn5m is present in a subset of cells in the late-embryonic stage chicken corneal stroma and fibrous sclera. Since the expression profile of Opn5m might differ between late-embryonic and young chick, we also examined it in post-hatch day 1 chick (Supplementary Figs. S4–7). The relative expression level of Opn5 was higher in the P1 cornea and sclera compared with E17 (Fig. S4). Immunostaining of the P1 eye tissues showed a similar localization pattern of Opn5m protein (Fig. S6) compared to that of E17. How the Opn5m expression around pre- and early post-hatch days might modulate ocular growth would be the next issue to be examined.
To elucidate the pathophysiology of myopia, the chicken eye provides a good model system [28]. The chicken sclera consists of two layers, the fibrous and cartilaginous layers. Collagen degradation in the chicken sclera is confined to the fibrous layer during scleral remodeling in myopia development [29]. Furthermore, during eyeball growth in chickens and humans, the diameter of collagen fibrils decreases and the structure of the fibrils is altered [24,30]. Thus, it is conceivable that alterations to the expression of genes involved in collagen remodeling such as Mmp2 in the sclera have crucial roles in myopia progression.
We used a chicken fibroblast cell line to examine whether the expression levels of these four genes were altered after UV irradiation combined with the forced expression of Opn5m. We found that the expression of Egr1 and Mmp2 after UV-A exposure was significantly elevated in the Opn5m-overexpressing fibroblasts with 11-cis-retinal. This suggests that UV-A may alter the activity of fibroblasts as revealed by upregulation of Egr1 and Mmp2 via Opn5m. We attempted similar experiments in a primary culture of chicken scleral fibroblasts, but unfortunately the cells deteriorated after transfection with retroviral vectors.
Egr1, also called ZENK or zif268, encodes a zinc finger transcription factor, which regulates transcription of genes involved in tissue remodeling and fibrosis. Its target genes include those of Tgf betas, collagens, Timps, and other growth factors and matrix proteins. Egr1 is one of the major mediators in fibroblasts or myofibroblasts not only in physiological tissue remodeling but also in pathological fibrosis. Egr1 was the first gene to be identified whose synthesis is enhanced when ocular elongation is suppressed [22], and Egr1-knockout mice exhibit axial eye growth [23]. However, all these alterations of Egr1 expression have been observed in the retina. Our study shows that UV-A reception through Opn5m in the presence of 11-cis-retinal significantly upregulates the expression of Egr1 in fibroblasts, which supports the possibility of a direct effect on the sclera or cornea by UV reception via Opn5m.
Mmp2 encodes a protease that initiates degradation of collagens and the extracellular matrix (ECM) complex. In the remodeling of scleral tissue during ocular growth, continuous synthesis and degradation of ECM take place [26]. Mmp2 is expressed in the scleral fibroblasts and the expression of Mmp2 is altered, increased or decreased after imposed myopic defocus or its recovery depending upon the experimental time course [26,31]. Our in vitro study shows that the expression of Mmp2 in chicken fibroblasts is increased by short-time UV-A exposure in the presence of forced-expressed Opn5m and the retinal chromophore 11-cis-retinal. This suggests that UV-A absorption via Opn5m would have a direct effect on the fibroblasts of the sclera and cornea, the outer wall of the eye, for tissue remodeling involving Mmp2 as well as Egr1. Whether biological responses induced by the UV-A-Opm5m phototransduction lead to changes in ECM of the sclera and cornea should be clarified in the next study.
COI
None.
Acknowledgments
We thank Dr. Takahiro Yamashita for his generous gift of 11-cis-retinal. We also thank Central Research Laboratory, Okayama University Medical School for allowing us to conduct the analysis of immunofluorescence using Zeiss LSM 780. This study was partly supported by an academic grant from Pfizer Japan Inc., and grants-in-aid from MEXT (24500382 to HO, 17K15159 to KS), Japan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100665.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shichida Y., Matsuyama T. Evolution of opsins and phototransduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarttelin E.E., Bellingham J., Hankins M.W., Foster R.G., Lucas R.J. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2003;554:410–416. doi: 10.1016/S0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 3.Tomonari S., Migita K., Takagi A., Noji S., Ohuchi H. Expression patterns of the opsin 5-related genes in the developing chicken retina. Dev. Dynam. 2008;237:1910–1922. doi: 10.1002/dvdy.21611. [DOI] [PubMed] [Google Scholar]
- 4.Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., Ebihara S., Kubo Y., Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kojima D., Mori S., Torii M., Wada A., Morishita R., Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieto P.S., Valdez D.J., Acosta-Rodríguez V.A., Guido M.E. Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC-5. Presence of OPN5 in the rat retina. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K., Yamashita T., Haruki Y., Ohuchi H., Kinoshita M., Shichida Y. Two UV-sensitive photoreceptor proteins, Opn5m and Opn5m2 in ray-finned fish with distinct molecular properties and broad distribution in the retina and brain. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita T., Ohuchi H., Tomonari S., Ikeda K., Sakai K., Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22084–22089. doi: 10.1073/pnas.1012498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T., Suzuki H., Takahashi T. Light-induced rapid Ca2+ response and MAPK phosphorylation in the cells heterologously expressing human OPN5. Sci. Rep. 2014;4:5352. doi: 10.1038/srep05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita T., Ono K., Ohuchi H., Yumoto A., Gotoh H., Tomonari S., Sakai K., Fujita H., Imamoto Y., Noji S., Nakamura K., Shichida Y. Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J. Biol. Chem. 2014;289:3991–4000. doi: 10.1074/jbc.M113.514075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhr E.D., Yue W.W.S., Ren X., Jiang Z., Liao H.W.R., Mei X., Vemaraju S., Nguyen M.-T., Reed R.R., Lang R.A., Yau K.-W., Van Gelder R.N. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. U.S.A. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ota W., Nakane Y., Hattar S., Yoshimura T. Impaired circadian photoentrainment in opn5-null mice. IScience. 2018;6:299–305. doi: 10.1016/j.isci.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 14.Sherwin J.C., Reacher M.H., Keogh R.H., Khawaja A.P., Mackey D.A., Foster P.J. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119:2141–2151. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Torii H., Kurihara T., Seko Y., Negishi K., Ohnuma K., Inaba T., Kawashima M., Jiang X., Kondo S., Miyauchi M., Miwa Y., Katada Y., Mori K., Kato K., Tsubota K., Goto H., Oda M., Hatori M., Tsubota K. Violet light exposure can Be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himly M., Foster D.N., Bottoli I., Iacovoni J.S., Vogt P.K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 17.Park T.S., Lee H.J., Kim K.H., Kim J.-S., Han J.Y. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12716–12721. doi: 10.1073/pnas.1410555111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhardt K.P., Olson E.J., Castillo-Hair S.M., Hartsough L.A., Landry B.P., Ekness F., Yokoo R., Gomez E.J., Ramakrishnan P., Suh J., Savage D.F., Tabor J.J. An open-hardware platform for optogenetics and photobiology. Sci. Rep. 2016;6:35363. doi: 10.1038/srep35363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato K., Yamashita T., Ohuchi H., Takeuchi A., Gotoh H., Ono K., Mizuno M., Mizutani Y., Tomonari S., Sakai K., Imamoto Y., Wada A., Shichida Y. Opn5L1 is a retinal receptor that behaves as a reverse and self-regenerating photoreceptor. Nat. Commun. 2018;9:1255. doi: 10.1038/s41467-018-03603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohuchi H., Yamashita T., Tomonari S., Fujita-Yanagibayashi S., Sakai K., Noji S., Shichida Y. A non-mammalian type opsin 5 functions dually in the photoreceptive and non-photoreceptive organs of birds. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischlschweiger W., O'Rahilly R. The ultrastructure of the pecten oculi in the chick. II. Observations on the bridge and its relation to the vitreous body. Z. Zellforsch. Mikrosk. Anat. 1968;92:313–324. doi: 10.1007/BF00455589. [DOI] [PubMed] [Google Scholar]
- 22.Fischer A.J., McGuire J.J., Schaeffel F., Stell W.K. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 1999;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- 23.Schippert R., Burkhardt E., Feldkaemper M., Schaeffel F. Relative axial myopia in Egr-1 (ZENK) knockout mice. Investig. Ophthalmol. Vis. Sci. 2007;48:11–17. doi: 10.1167/iovs.06-0851. [DOI] [PubMed] [Google Scholar]
- 24.Jobling A.I., Nguyen M., Gentle A., McBrien N.A. Isoform-specific changes in scleral transforming growth factor-beta expression and the regulation of collagen synthesis during myopia progression. J. Biol. Chem. 2004;279:18121–18126. doi: 10.1074/jbc.M400381200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Raychaudhuri S., Wildsoet C.F. Imposed optical defocus induces isoform-specific up-regulation of TGFβ gene expression in chick retinal pigment epithelium and choroid but not neural retina. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schippert R., Brand C., Schaeffel F., Feldkaemper M.P. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp. Eye Res. 2006;82:710–719. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Huang R.P., Fan Y., Boynton A.L. UV irradiation upregulates Egr-1 expression at transcription level. J. Cell. Biochem. 1999;73:227–236. doi: 10.1002/(sici)1097-4644(19990501)73:2<227::aid-jcb9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Carr B.J., Stell W.K. The science behind myopia. In: Kolb H., Fernandez E., Nelson R., editors. Webvision: the Organization of the Retina and Visual System. University of Utah Health Sciences Center; Salt Lake City (UT): 1995. http://www.ncbi.nlm.nih.gov/books/NBK470669/ accessed. [PubMed] [Google Scholar]
- 29.Liu H.-H., Gentle A., Jobling A.I., McBrien N.A. Inhibition of matrix metalloproteinase activity in the chick sclera and its effect on myopia development. Investig. Ophthalmol. Vis. Sci. 2010;51:2865–2871. doi: 10.1167/iovs.09-4322. [DOI] [PubMed] [Google Scholar]
- 30.McBrien N.A., Lawlor P., Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Investig. Ophthalmol. Vis. Sci. 2000;41:3713–3719. [PubMed] [Google Scholar]
- 31.Siegwart J.T., Norton T.T. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Investig. Ophthalmol. Vis. Sci. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Liu Y., Wildsoet C.F. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2012;53:6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashby R., Kozulin P., Megaw P.L., Morgan I.G. Alterations in ZENK and glucagon RNA transcript expression during increased ocular growth in chickens. Mol. Vis. 2010;16:639–649. [PMC free article] [PubMed] [Google Scholar]
- 34.Tomonari S., Takagi A., Akamatsu S., Noji S., Ohuchi H. A non-canonical photopigment, melanopsin, is expressed in the differentiating ganglion, horizontal, and bipolar cells of the chicken retina. Dev. Dynam. 2005;234:783–790. doi: 10.1002/dvdy.20600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.