Abstract
Welfare states increasingly rely on aging in place policies and have cut back on institutional long-term care (LTC) provision. Simultaneously, the major determinants of LTC use, that is, dementia and living to very old age, are increasing. We investigated how increasing longevity and concomitant dementia were associated with changes in round-the-clock LTC use in the last 5 years of life between 1996 and 2013. Retrospective data drawn from national registers included all those who died aged 70+ in 2007 and 2013, plus a 40% random sample from 2001 (N = 86,554). A generalized estimating equations (GEE) were used to estimate the association of dementia and age with LTC use during three study periods 1996-2001, 2002-2007, and 2008-2013. Between the study periods, the total number of days spent in LTC increased by around 2 months. Higher ages at death and the increased number of persons with dementia contributed to this increase. The group of the most frequent LTC users, that is, people aged 90+ with or without dementia, grew the most in size, yet their LTC use decreased. The implications of very old age and concomitant dementia for care needs must be acknowledged to guarantee an adequate quantity and quality of care.
Keywords: longevity, dementia, long-term care, age at death
Introduction
Population aging and increasing longevity are driving up the number of people with old-age chronic conditions such as memory disorders and all-cause dementia (Alzheimer Europe, 2013; Public Health Agency of Canada, 2014; Taylor, Greenlund, McGuire, Lu, & Croft, 2017; World Health Organization [WHO], 2017). This increase in longevity and dementia are therefore changing care needs especially during the last years of life when care needs are usually at their highest. Dementia, old age, and closeness of death are important drivers of long-term care (LTC) use (Taylor et al., 2017). Of these three mutually dependent determinants of LTC use, previous research suggests that dementia is the most important (Aguero-Torres, von Strauss, Viitanen, Winblad, & Fratiglioni, 2001; Gianino et al., 2017; Martikainen, Murphy, Metsa-Simola, Hakkinen, & Moustgaard, 2012).
The growing number of people with dementia in the older population has led to predictions of an increased need for care in the future, especially LTC (Gianino et al., 2017; Martikainen et al., 2012). Despite these predictions, financial pressures partly caused by population aging have seen Finland and many other welfare states in Europe and North America deciding to cut back on the supply of round-the-clock LTC, and to keep older people living in their homes instead (Anttonen & Häikiö, 2011; Da Roit, 2012; Gianino et al., 2017; Menec, Means, Keating, Parkhurst, & Eales, 2011; Ministry of Social Welfare and Health, 2012; Rosenwohl-Mack, Schumacher, Fang, & Fukuoka, 2018; Schön, Lagergren, & Kåreholt, 2015). We lack research on the impact of these two opposite developments—the increase in dementia and longevity and the simultaneous cutbacks in care provision—on care utilization at the national level.
In Finland, the provision of LTC is the responsibility of local municipalities. They can provide the services themselves and purchase them from nongovernmental organizations (NGOs), the for-profit private sector, or other municipalities. Institutional care is offered in two round-the-clock care sites: in nursing homes and in primary care hospital inpatient wards. The third and increasingly common LTC site is service housing with 24-hr assistance (also known as sheltered housing with 24-hr assistance; Aaltonen et al., 2014). Since around 2000, LTC in nursing homes and primary care hospital inpatient wards has decreased. Simultaneously, LTC in service housing with 24-hr assistance has increased—although the latter has not offset the former. Furthermore, even though aging in place has become a policy priority in Finland, community-based LTC, that is, home care services, has not expanded to support this agenda. In fact, the coverage of home care has declined (Official Statistics of Finland [OSF], 2018).
Despite the cutbacks in LTC overall, a previous study showed that the use of round-the-clock LTC in the last 2 years of life has increased among older adults in Finland (Forma et al., 2017). This is partly explained by the postponement of death to an increasingly advanced age, when LTC use is at its highest. However, we lack information about the joint impact of dementia and longevity on these changes in LTC use; it is possible that it is not only very old age but very old age and dementia together that adds to LTC use at the population level. This study explores the impact of dementia alone and in combination with age at death on round-the-clock LTC use in the last 5 years of life. This is studied during the period from 1996 to 2013, when structural changes were made to old-age services in Finland. This information is crucial especially in rapidly aging Finland (United Nations, 2015), where the number of deaths with dementia as underlying cause of death has multiplied by 40 since the 1980s (OSF, 2017) and proportion of people with dementia in whole population is higher than the European Union (EU) average (Alzheimer Europe, 2013). The results will provide information about (a) the potential impact of increasing dementia and longevity on LTC needs and (b) how the LTC system has responded to these changes in older population.
Method
This was a retrospective study using register-based data of all persons who died at the age of 70 or older in Finland in 2007 or 2013 (N = 72,837) and a 40% random sample of those who died in 2001 (N = 13,717). The data were drawn from comprehensive national registers: the Care Register for Health Care and the Care Register for Social Welfare (National Institute for Health and Welfare) and the Causes of Death Register (Statistics Finland). Data sets were linked using personal identity codes (PICs) that remain unchanged throughout the person’s lifetime.
To identify the impact of approaching death on LTC use, we followed the number of days spent in round-the-clock LTC for the last 5 years of life (1,825 days) for each individual from time of death in 2001, 2007, or 2013 (N = 86,554). Thus, care use was studied for three 5-year periods: 1996-2001, 2002-2007, and 2008-2013.
Permission to access the registers was obtained from the register maintainers (National Institute for Health and Welfare and Statistics Finland). Researchers had no access to PICs of the participants. The research plan was approved by the Pirkanmaa Hospital District Ethics Committee (Decision R08192). Written informed consent for individual information from the participants was not possible to obtain due to the fact that all information was collected from already deceased individuals.
Outcome Variable
In this study, use of LTC refers to the number of days spent in
nursing homes and service housing units with 24-hr assistance and
primary care hospitals’ (also known as health care centers) inpatient wards when the length of stay was ≥90 days.
Explanatory Variables
The dementia group included all those for whom any cause of death (immediate, underlying, intermediate, or contributing) was dementia (Causes of Death Register), or for whom dementia was recorded in the care registers (Care Register for Health Care, Care Register for Social Welfare), using codes from the International Classification of Diseases, 10th Revision (ICD10): F00 (dementia in Alzheimer’s disease), F01 (vascular dementia), F02 (dementia in other diseases), F03 (unspecified dementia), or G30 (Alzheimer’s disease). Age at death was categorized as 70 to 79, 80 to 89, and 90+ years. In addition, we constructed variable that combined age at death and dementia to demonstrate the joint impact of age and dementia on LTC use: (a) no dementia, age 70 to 79 years; (b) no dementia, age 80 to 89 years; (c) no dementia, age 90+ years; (d) dementia, age 70 to 79 years; (e) dementia, age 80 to 89 years; and (f) dementia, age 90+ years. D+ indicates the group suffering from dementia and D– indicates the group without a dementia diagnosis. Year of death refers to 2001, 2007, and 2013. Other diagnoses besides dementia were used as covariates to adjust the models for comorbidities. Comorbidities were combined into 10 groups: cancer (C00-C97), diabetes (E10-E14), psychosis, depressive symptoms or other mental health disorders excluding dementia (F04-F99), Parkinson’s disease or other neurological diseases (G00-G99 excluding G30), chronic asthma and chronic obstructive pulmonary disease (COPD) or other respiratory diseases (J00-J99), hip fracture (S72), stroke (I60-I69), ischemic and other heart diseases excluding rheumatic and alcoholic heart diseases (I20-I25, I30-I425, I427-I52), and other diseases of the circulatory system (I00-I15, I26-I28, I70-I99), arthritis or osteoarthritis (M05-M06, M15-M19).
Statistical Analysis
Generalized estimating equations (GEE) were used to estimate the impact of dementia and age on LTC use in the last 5 years of life, and to explore the changes in LTC use between the three study periods 1996-2001, 2002-2007, and 2008-2013. GEE method can take into account the within-subjects correlation, that is, the correlation between repeated observations of days in care in each year (Galenkamp, Braam, Huisman, & Deeg, 2013; Kleinbaum & Klein, 2010). Five observations describing LTC use of each individual were constructed retrospectively for everyone from time of death to 5 years before death. The year of death was included to analysis as one of the explanatory variables. Hence, this study takes advantage on both, longitudinal and cross-sectional study compositions. Days in care was a highly skewed count variable with observed variance larger than the mean. Such data would be overdispersed and therefore negative binomial regression was applied instead of Poisson regression to estimate the incidence rate ratios (IRR; Hilbe, 2008).
In statistical analyses after the univariate models (Model 1), dementia and other diagnoses, age, gender, and year of death were entered in Model 2. In Model 3, to investigate the joint association of gradually increasing age and concomitant dementia with LTC use, we ran GEE models for the association of the combined dementia and age variable with days in LTC. In Model 4, we constructed the interaction term Dementia × Age to show the interaction between dementia and the three age groups. In Model 5, interaction between dementia and year of death and, in Model 6, interaction between age and year of death were analyzed to show possible changes in the impact of dementia and age on LTC use over time. Diagnoses other than dementia were entered in the models as binary variables.
Standardization
In the final phase of our analysis, we wanted to establish to what extent the increase in total LTC use was explained by the increase in dementia and age at time of death. Different populations may have the similar age-specific care utilization, but different overall rates of care use due to differences in their age distributions. Standardization can be used to provide numbers and comparisons that show the influence of age and other factors—in this case age and dementia (Schoenbach, 1999). Therefore, we standardized age and prevalence of dementia in 2008-2013 to the level in 1996-2001, using those who died in 2001 as the standard population.
Results
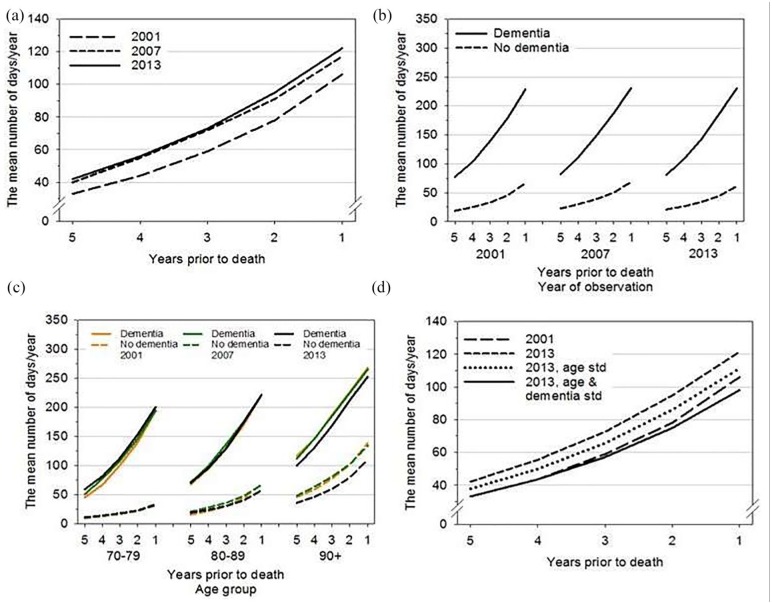
Both the proportion of people with dementia during the last 5 years of life and age at time of death increased over the three study periods (Table 1). Of all dementia cases identified in the register data, nearly 83% were dementia cases as cause of death in 2013. In the whole group who died at age 70+, the proportion of those who died at age 80+ with dementia increased from 19.3% in 2001 to 31.0% in 2013 (age groups 80+ and 90+ with dementia combined). From 1996 to 2001 onward, the proportion of LTC users and the average number of days in LTC increased in the 70+ years study population (Table 1, Figure 1a).
Table 1.
Description of the Study Population.
| Year of death | 2001 | 2007 | 2013 |
|---|---|---|---|
| N | 13,717 | 34,750 | 38,087 |
| Dementia (%) | 24.5 | 30.2 | 35.6 |
| % of dementia cases to whom dementia was a cause of death | 72.5 | 73.8 | 82.7 |
| Age, average (in years) | 82.8 | 83.6 | 84.3 |
| No dementia (D–) | 82.0 | 82.5 | 82.9 |
| Dementia (D+) | 85.3 | 86.0 | 86.9 |
| Age groups (%) | |||
| 70-79 years | 35.8 | 30.8 | 27.2 |
| 80-89 years | 44.8 | 47.0 | 46.7 |
| 90+ years | 19.3 | 22.2 | 26.0 |
| Average number of diagnoses (range 0-9)a | 3.53 | 3.72 | 3.74 |
| Age and dementia distribution among 70+ deaths (%) | |||
| D− 70-79 | 30.6 | 25.9 | 22.5 |
| D− 80-89 | 32.4 | 31.1 | 28.6 |
| D− 90+ | 12.5 | 12.9 | 13.3 |
| D+ 70-79 | 5.2 | 4.9 | 4.7 |
| D+ 80-89 | 12.4 | 15.9 | 18.2 |
| D+ 90+ | 6.9 | 9.3 | 12.8 |
| Women (%) | 58.8 | 57.2 | 56.5 |
| LTC days, averageb | 320 | 375 | 387 |
| % who used LTCc | 44.2 | 48.1 | 50.1 |
Note. LTC = long-term care.
Dementia (F00-F03, G30), cancer (C00-C97), diabetes (E10-E14), psychosis, depressive symptoms or other mental health disorders excluding dementia (F04-F99), Parkinson’s disease or other neurological diseases (G00-G99 excluding G30), chronic asthma and chronic obstructive pulmonary disease or other respiratory diseases (J00-J99), hip fracture (S72), stroke (I60-I69), ischemic and other heart diseases excluding rheumatic and alcoholic heart diseases (I20-I25, I30-I425, I427-I52), and other diseases of the circulatory system (I00-I15, I26-I28, I70-I99), arthritis or osteoarthritis (M05-M06, M15-M19).
Average number of days in last 5 years.
Proportion of those ≥1 day in last 5 years.
Figure 1.
Mean number of days in long-term care in the last 5 years of life.
Note. Number 1 is the last year of life. (a) Total observed use of LTC in the whole study population in 2001, 2007, and 2013. (b) Difference in LTC use between people with and without dementia in the last 5 years of life. (c) Joint impact of age and dementia on LTC. Numbers of days in care are age-standardized means within each age group, calculated using marginal means from negative binomial regression analysis to remove the impact of within age group variation in the mean age at death. (d) Age and frequency of dementia in 2008-2013 standardized to their level among those who died in 2001. Those who died in 2001 are used as the standard population. LTC = long-term care.
Association of Dementia and Age With LTC Use
Both dementia and age were strongly associated with number of days in LTC. This was true for all study years (Figure 1b and 1c; Table 2, Models 1 and 2). Number of days in LTC increased as death approached in both the D+ and D– groups, irrespective of age (Figure 1b and 1c).
Table 2.
Days in Long-Term Care in the Last 5 Years of Life Among Those Who Died in 2001, 2007, and 2013: IRRs From Negative Binomial Regression Models Using Generalized Estimating Equations.
| Univariate models | Model 2 | Model 3a,b | Model 4a,b interaction | Model 5a,b interaction | Model 6a,b interaction | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 70-79 (ref.) | ||||||
| 80-89 | 2.07** | 1.85** | 2.03** | 1.85** | 1.87** | |
| 90+ | 3.51** | 3.41** | 4.64** | 3.41** | 3.82** | |
| Gender | ||||||
| Men (ref.) | ||||||
| Women | 2.02** | 1.58** | ||||
| Dementia | ||||||
| No (ref.) | ||||||
| Yes | 3.81** | 3.48** | 5.73** | 3.47** | 3.49** | |
| Other diagnoses (no disease ref.)a | ||||||
| Cancer | 0.35** | 0.52** | ||||
| Diabetes | 0.89** | 1.18** | ||||
| Mental health disorders | 1.30** | 1.89** | ||||
| Neurological diseases | 1.15** | 1.52** | ||||
| Respiratory diseases | 1.19** | 1.34** | ||||
| Hip fracture | 1.50** | 1.32** | ||||
| Stroke | 1.25** | 1.79** | ||||
| Ischemic heart disease | 0.67** | 0.75** | ||||
| Other diseases of the circulatory system | 0.73** | 0.81** | ||||
| Arthritis | 0.82** | 0.93** | ||||
| Year of death | ||||||
| 2001 (ref.) | ||||||
| 2007 | 1.17** | 1.12** | 1.11** | 1.11** | 1.13** | 1.10** |
| 2013 | 1.20** | 1.05** | 1.05** | 1.05** | 1.03** | 1.13** |
| Age and dementia | ||||||
| D− 70-79 years (ref.) | ||||||
| D− 80-89 years | 2.03** | |||||
| D− 90+ years | 4.64** | |||||
| D+ 70-79 years | 5.72** | |||||
| D+ 80-89 years | 7.42** | |||||
| D+ 90+ years | 9.80** | |||||
| Interaction terms | ||||||
| Age × Dementia | ||||||
| D− all, D+ 70-79 (ref.) | 0.64** | |||||
| D+ 80-89 | 0.37** | |||||
| D+ 90+ | ||||||
| Year of Death × Dementia | ||||||
| 2007 | 0.95* | |||||
| 2013 | 1.04* | |||||
| Year of Death × Age | ||||||
| 2001 all age groups (ref.) | ||||||
| 2007 80-89 years | 1.02 | |||||
| 2007 90+ years | 0.98 | |||||
| 2013 80-89 years | 0.95* | |||||
| 2013 90+ years | 0.79** | |||||
Note. IRR = incidence rate ratio.
Analyses are adjusted for morbidity including cancer (C00-C97), diabetes (E10-E14), psychosis, depressive symptoms or other mental health disorders excluding dementia (F04-F99), Parkinson’s disease or other neurological diseases (G00-G99 excluding G30), chronic asthma and chronic obstructive pulmonary disease or other respiratory diseases (J00-J99), hip fracture (S72), stroke (I60-I69), ischemic and other heart diseases excluding rheumatic and alcoholic heart diseases (I20-I25, I30-I425, I427-I52), and other diseases of the circulatory system (I00-I15, I26-I28, I70-I99), arthritis or osteoarthritis (M05-M06, M15-M19).
Analyses are adjusted for gender and other diagnoses. IRRs are the same than in Model 2.
p < .05. **p < .001.
The six-category combined variable of age and dementia (Model 3) and the interaction term Age × Dementia (Model 4) show the joint association of age and dementia with LTC use. LTC use increased progressively from the youngest to the oldest age group (Model 3). In every age group, dementia diagnosis greatly increased the number of days in LTC. In the age group 70 to 79 years, the number of days in LTC was 5.7 times higher in the D+ than in the D– group. The impact of dementia was somewhat weaker in the older age groups: at age 90+, the number of LTC days was twice as high in the D+ group than in the D– group (Table 2, Model 3). The interaction term in Model 4 confirms the stronger association of dementia with LTC use in the younger than the older group.
Changes in LTC Use From 1996 to 2013 by Dementia and Age
LTC use remained relatively stable in the D+ and D– groups across the three study periods (Figure 1b). The interaction between year of death and dementia (Table 2, Model 5) implied a somewhat weaker impact of dementia in 2007 than in 2001, but a slightly stronger impact between 2001 and 2013. In all, the association of dementia with LTC days showed no major changes between the three study periods when other explanatory variables were taken into account.
Changes in LTC use between the study years were different for different age groups, however. In the two younger age groups, LTC use was about the same in different study periods in the D+ and D– groups (Figure 1c). In the oldest group in both people with and without dementia, LTC use was higher in the earlier than in the later study years, suggesting that those who died in 2013 at the age of 90+ used less LTC than those who died in earlier years. The interaction between year of death and age (Table 2, Model 6) confirms that the greatest change occurred between 2001 and 2013 in the oldest age group. LTC use in the oldest group moved slightly closer to that in the youngest group.
We show the age and dementia standardized numbers of LTC days in Figure 1d. If mean age at death for those who died in 2013 had been the same (Figure 1d, 2013 age standardized) as for those who died in 2001 (i.e., lower mean age), the number of days spent in LTC would have been lower than that observed for those who died in 2013 (on average, 4 days lower in 5 years before death and 10 days lower in the last year of life). Still, the number of LTC days would have been higher than that observed for those who died in 2001. Furthermore, if in addition to age at death the frequency of dementia in 2008-2013 had been the same as in 1996-2001 (i.e., lower frequency of dementia, 2013 age and dementia standardized in Figure 1d), the number of days in LTC would have been slightly lower in 2008-2013 than in 1996-2001. After standardized for age at death and the frequency of dementia, the LTC days in 2008-2013 decreased for 24 days in the final year of life.
Discussion
This study shows the combined association of increasing age at death and dementia with changes in LTC use over time and demonstrates that deaths in very old age with concomitant dementia are likely to increase the need for LTC services at the end of life. This development sets new challenges for end-of-life care at the individual and population levels. Our results show that dementia and old age, and especially living to a very old age with concomitant dementia, were associated with an increasing number of days in round-the-clock LTC in the last years of life. Between the study periods, the proportion of people aged 80+ with dementia increased. Even though LTC use decreased over time among the oldest-old, this age group and especially those with dementia remained the most frequent LTC users. Thus, the increase from 1998-2001 to 2008-2013 in LTC use among all who died at the age of 70+ is mainly attributable to increase in this “heavy-user” group(s), that is, in people who lived to a very old age with dementia.
LTC availability and use show declining trends over time in different countries. In most (but not in all) EU countries, the number of LTC beds available and the proportion of older LTC recipients have decreased in recent decades (Gianino et al., 2017). This indicates that round-the-clock LTC is increasingly provided to people in the greatest need of care (de Meijer, Bakx, van Doorslaer, & Koopmanschap, 2015; Gianino et al., 2017). In Finland, access to publicly funded LTC services is based on a professional needs assessment to ensure that the services are targeted to those most in need in terms of health and functional ability. In this light, it might be suggested that in our study, the reduced use of LTC observed among the oldest-old implies a reduced need for LTC among the oldest-old. However, recent Finnish studies (Jylhä, Enroth, & Luukkaala, 2013) suggest there has been no improvement in the health and functional status of the oldest-old during this period. If the general health status has not improved, but the use of LTC has decreased, it is possible that people are now living in private homes with a level of disability that formerly would have kept them in round-the-clock LTC. Although older people’s disability trajectories and needs for care are, at least to some extent, predictable (Gill, Gahbauer, Han, & Allore, 2010), policy decisions and their implementation imply that projected needs for care do not necessarily translate into real use of health and social care services.
In the younger-old population, the adoption of healthier lifestyles can postpone the onset of dementia (Ngandu et al., 2015). However, we still do not know whether dementia can be prevented among the oldest-old. Those who die from dementia are typically older than those with other causes of death (Dufouil, Beiser, Chêne, & Seshadri, 2018; Gill et al., 2010; Lunney, Lynn, & Hogan, 2002; Martikainen et al., 2012), and it is possible that increasing longevity will postpone the onset of dementia to an even older age (Dufouil et al., 2018). This may increase the risk of ending life with long periods of frailty and disability (Robine, 2018).
The prospect of long periods of frailty and disability near the end of life clearly underscores the importance of providing adequate care, in terms of both quality and quantity. The consequences of advanced dementia such as mental confusion, behavioral problems and functional deterioration (Mitchell, Teno, Miller, & Mor, 2005; Van Der Steen, Van Soest-Poortvliet, Achterberg, Ribbe, & De Vet, 2011) present a very specific set of challenges for care offered in the community. Even though many older adults express their desire to live at home as long as possible (Wiles, Leibing, Guberman, Reeve, & Allen, 2012), aging in place becomes more challenging with increasing disability and dementia (Thoma-Lürken, Bleijlevens, Lexis, de Witte, & Hamers, 2018).
Based on our results, we make no assumptions about whether round-the-clock LTC or home- and community-based care leads to better outcomes of care (Wysocki et al., 2012). We do, however, want to raise discussion about this issue, given the growing number of very old people, many with dementia, living in their private homes near the end of life. More in-depth research is needed to assess the ability of home care and other support services to provide adequate care for home-dwelling older people at the end of life. A recent study of home care workers’ reflections on their work (Kröger, Van, Aerschot, & Puthenparambil, 2018) suggest that their increasingly demanding work with clients with multiple care needs has not been taken thoroughly into account in home care planning. It might also be necessary to add to the resources available for round-the-clock LTC to guarantee good-quality end-of-life care for the older population, given that formal home care topped up with informal care is not available to everyone.
Our study was subject to some limitations. The register data provide no information on functional limitations, the availability and use of informal care, and on formal home care, all of which affect the need for and use of round-the-clock LTC (Andersen & Newman, 2005). It is, however, well established that functional ability usually decreases with increasing age and approaching death (e.g., Gill et al., 2010; Guralnik, LaCroix, Branch, Kasl, & Wallace, 1991). Therefore, it is reasonable to assume that the oldest-old in their final years of life in our study were the most likely to have severe functional limitations. It is difficult to estimate the impact of changes in care policy and care allocation on LTC use because no register data are available on these aspects; all we could do was relate the year of observation to the historical period, a method that has been used in previous studies of temporal changes in care use (e.g., Alders, Comjis, & Deeg, 2017; Hoben et al., 2019; Swinkels, Suanet, Deeg, & Broese van Groenou, 2016). Awareness of dementia improved during the period under study. It was likely diagnosed earlier, and treatment was more active than previously. We acknowledge that the increase in dementia that we observed in the register data is partly explained by these changes in diagnostic practice. Since 2005, cause of death statistics in Finland have followed international guidelines which limit the use of pneumonia as an underlying cause of death in connection with several chronic diseases. If a person who has pneumonia is also suffering from advanced dementia, the latter is now identified as the underlying cause of death (OSF, 2016). This change might have contributed to the increasing prevalence of dementia. However, our study was based on data from registers of health and social care as well as cause of death data, irrespective of whether dementia was an immediate, underlying, intermediate, or contributing cause of death. As far as we understand, it is unlikely that the new guidelines for recording cause of death have significantly affected our findings. There is no extensive variation in dementia as cause of death between the study periods: The majority of people with dementia had dementia as a cause of death in all three time points. Because of the inevitable time lag in obtaining register data, the data set for our study covered the period from 1996 to 2013. Further research will need more up-to-date data to be able to explore whether there have been more recent changes in the use and availability of round-the-clock LTC.
Regardless of these shortcomings, a major strength of our study was the comprehensive register data, which provide reliable information on round-the-clock care use among the older population. To our knowledge, this was a unique undertaking, in that we investigated LTC use for almost two decades, essentially covering all older people during their last years of life in one country. This research design takes into account the increasing effect of proximity of death on LTC use, but, on the contrary, makes it difficult to compare the results with other research on changes in LTC use over time (e.g., Hoben et al., 2019; Viana, Bicalho, Moraes, & Romano-Silva, 2015), which are not based on similar study composition. Yet, similar study composition could be constructed from administrative claims data, available, for example, in the United States and in Canada. Our results not only are relevant to Finland but have international value because several countries have seen—or in the future will see—a similar increase in longevity and possibly in dementia, and a growing trend toward deinstitutionalization.
Conclusions and Implications
This study revealed the joint impact of increasing longevity and dementia on LTC use in the last years of life; not only very old age or only dementia but very old age and dementia together increased the use of LTC the most. The joint impact of increasing longevity and dementia on care needs is affecting both individual lives and aging societies as a whole; yet, this has largely been overlooked in elderly care reforms. For the purposes of future LTC service allocation, it is paramount to give careful assessment to the implications of very old age and concomitant dementia to the need for and use of care near the end of life. The consequences of the substantial growth of the oldest-old population and the increasing number of those dying from dementia must be acknowledged in health and social care policy making to guarantee adequate care—in quality and quantity—near the time of death.
Footnotes
Authors’ Note: This work was conducted as part of a research project on “The longevity revolution: Implications for the need and cost of health and social care” (COCTEL) and in collaboration with Social Inequalities in Aging (SIA) project. COCTEL is one of the research projects underway at the Center of Excellence in Research on Ageing and Care.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Permission to access the registers was obtained from the register maintainers (National Institute for Health and Welfare and Statistics Finland). Researchers had no access to personal identity codes (PICs) of the participants. The research plan was approved by the Pirkanmaa Hospital District Ethics Committee (Decision R08192). Written informed consent for individual information was not possible to obtain from the participants due to the fact that all information was collected from already deceased individuals.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant to Mari S. Aaltonen from the Academy of Finland (318985) and grants to Professor Marja K. Jylhä from the Academy of Finland (312311 and 287372). The funding bodies did not influence any aspect of the design of the study, analysis or interpretation of data. The views expressed in the submitted article are the authors’ and not an official position of their respective institutions or funder.
ORCID iD: Mari S. Aaltonen  https://orcid.org/0000-0002-6620-4968
https://orcid.org/0000-0002-6620-4968
References
- Aaltonen M., Raitanen J., Forma L., Pulkki J., Rissanen P., Jylhä M. (2014). Burdensome transitions at the end of life among long-term care residents with dementia. Journal of the American Medical Directors Association, 15, 643-648. [DOI] [PubMed] [Google Scholar]
- Aguero-Torres H., von Strauss E., Viitanen M., Winblad B., Fratiglioni L. (2001). Institutionalization in the elderly: The role of chronic diseases and dementia. Cross-sectional and longitudinal data from a population-based study. Journal of Clinical Epidemiology, 54, 795-801. [DOI] [PubMed] [Google Scholar]
- Alders P., Comjis H. C., Deeg D. J. H. (2017). Changes in admission to long-term care institutions in the Netherlands: Comparing two cohorts over the period 1996-1999 and 2006-2009. European Journal of Ageing, 14, 123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer Europe. (2013). 2013: The prevalence of dementia in Europe. Retrieved from https://www.alzheimer-europe.org/Policy-in-Practice2/Country-comparisons/2013-The-prevalence-of-dementia-in-Europe
- Andersen R., Newman J. F. (2005). Societal and individual determinants of medical care utilization in the United States. The Milbank Quarterly, 83(4), 1-28. [PubMed] [Google Scholar]
- Anttonen A., Häikiö L. (2011). Care “going market”: Finnish elderly-care policies in transition [Special issue]. Nordic Journal of Social Research, 2, 70-90. [Google Scholar]
- Da Roit B. (2012). The Netherlands: The struggle between universalism and cost containment. Health and Social Care in the Community, 20, 228-237. [DOI] [PubMed] [Google Scholar]
- de Meijer C., Bakx P., van Doorslaer E., Koopmanschap M. (2015). Explaining declining rates of institutional LTC use in the Netherlands: A decomposition approach. Health Economics, 24, 18-31. [DOI] [PubMed] [Google Scholar]
- Dufouil C., Beiser A., Chêne G., Seshadri S. (2018). Are trends in dementia incidence associated with compression in morbidity? Evidence from the Framingham heart study. The Journals of Gerontology, Series B: Psychological Sciences & Social Sciences, 73(Suppl. 1), S65-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forma L., Jylhä M., Pulkki J., Aaltonen M., Raitanen J., Rissanen P. (2017). Trends in the use and costs of round-the-clock long-term care in the last two years of life among old people between 2002 and 2013 in Finland. BMC Health Services Research, 17(1), Article 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galenkamp H., Braam A. W., Huisman M., Deeg D. J. (2013). Seventeen-year time trend in poor self-rated health in older adults: Changing contributions of chronic diseases and disability. The European Journal of Public Health, 23, 511-517. [DOI] [PubMed] [Google Scholar]
- Gianino M. M., Lenzi J., Martorana M., Bonaudo M., Fantini M. P., Siliquini R., . . .Damiani G. (2017). Trajectories of long-term care in 28 EU countries: Evidence from a time series analysis. The European Journal of Public Health, 27, 948-954. [DOI] [PubMed] [Google Scholar]
- Gill T. M., Gahbauer E. A., Han L., Allore H. G. (2010). Trajectories of disability in the last year of life. The New England Journal of Medicine, 362, 1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik J. M., LaCroix A. Z., Branch L. G., Kasl S. V., Wallace R. B. (1991). Morbidity and disability in older persons in the years prior to death. American Journal of Public Health, 81, 443-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbe J. M. (2008). Negative binomial regression. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Hoben M., Chamberlain S. A., Gruneir A., Knopp-Sihota J. A., Sutherland J. M., Poss J. W., . . .Estabrooks C. A. (2019). Nursing home length of stay in 3 Canadian health regions: Temporal trends, jurisdictional differences, and associated factors. Journal of the American Medical Directors Association. Advance online publication. doi: 10.1016/j.jamda.2019.01.144 [DOI] [PubMed] [Google Scholar]
- Jylhä M., Enroth L., Luukkaala T. (2013). Trends of functioning and health in nonagenarians: The vitality 90+ study. Annual Review of Gerontology and Geriatrics, 33, 313-332. [Google Scholar]
- Kleinbaum D. G., Klein M. (2010). Logistic regression: A self-learning text (3rd ed.). New York, NY: Springer. [Google Scholar]
- Kröger T., Van Aerschot L., Puthenparambil J. (2018). Hoivatyö muutoksessa: Suomalainen vanhustyö pohjoismaisessa vertailussa [Care work in change: Finnish eldercare in Nordic comparison] (Available only in Finnish). YFI julkaisuja, (6). [Google Scholar]
- Lunney J. R., Lynn J., Hogan C. (2002). Profiles of older Medicare decedents. Journal of the American Geriatrics Society, 50, 1108-1112. [DOI] [PubMed] [Google Scholar]
- Martikainen P., Murphy M., Metsa-Simola N., Hakkinen U., Moustgaard H. (2012). Seven-year hospital and nursing home care use according to age and proximity to death: Variations by cause of death and socio-demographic position. Journal of Epidemiology & Community Health, 66, 1152-1158. [DOI] [PubMed] [Google Scholar]
- Menec V. H., Means R., Keating N., Parkhurst G., Eales J. (2011). Conceptualizing age-friendly communities. Canadian Journal on Aging/La Revue Canadienne Du Vieillissement, 30, 479-493. [DOI] [PubMed] [Google Scholar]
- Ministry of Social Welfare and Health. (2012). The act on supporting the functional capacity of the older population and on social and health services for older persons [in Finnish laki ikääntyneen väestön toimintakyvyn tukemisesta sekä iäkkäiden sosiaali- ja terveyspalveluista]. Retrieved from https://www.finlex.fi/fi/laki/kaannokset/2012/en20120980.pdf
- Mitchell S. L., Teno J. M., Miller S. C., Mor V. (2005). A national study of the location of death for older persons with dementia. Journal of the American Geriatrics Society, 53, 299-305. [DOI] [PubMed] [Google Scholar]
- Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., . . .Kivipelto M. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet, 385, 2255-2263. [DOI] [PubMed] [Google Scholar]
- Official Statistics of Finland. (2016). Causes of death. Deaths from dementia and Alzheimer’s disease are increasing. Retrieved from http://www.stat.fi/til/ksyyt/2015/ksyyt_2015_2016-12-30_kat_003_en.html
- Official Statistics of Finland. (2017). Causes of death. Tables. Retrieved from https://www.stat.fi/til/ksyyt/tau_en.html
- Official Statistics of Finland. (2018). Statistical yearbook on social welfare and health care 2017. Service structure and coverage in care and services for older people, 1990-2016. Helsinki, Finland: National Institute for Health and Welfare, Social Protection. [Google Scholar]
- Public Health Agency of Canada. (2014). Mapping connections: An understanding of neurological conditions in Canada. Ottawa, Ontario: Government of Canada. [Google Scholar]
- Robine J. M. (2018). Age at death, the return of an old metric whose importance is growing. Aging Clinical and Experimental Research, 30, 1147-1149. [DOI] [PubMed] [Google Scholar]
- Rosenwohl-Mack A., Schumacher K., Fang M. L., Fukuoka Y. (2018). Experiences of aging in place in the United States: Protocol for a systematic review and meta-ethnography of qualitative studies. Systematic Reviews, 7(1), Article 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbach V. J. (1999). Standardization. Retrieved from http://www.epidemiolog.net/evolving/Standardization.pdf
- Schön P., Lagergren M., Kåreholt I. (2015). Rapid decrease in length of stay in institutional care for older people in Sweden between 2006 and 2012: Results from a population-based study. Health and Social Care in the Community, 24, 631-638. doi: 10.1111/hsc.12237 [DOI] [PubMed] [Google Scholar]
- Swinkels J. C., Suanet B., Deeg D. J. H., Broese van Groenou M. I. (2016). Trends in the informal and formal home-care use of older adults in the Netherlands between 1992 and 2012. Ageing & Society, 36, 1870-1890. [Google Scholar]
- Taylor C. A., Greenlund S. F., McGuire L. C., Lu H., Croft J. B. (2017). Deaths from Alzheimer’s disease—United States, 1999-2014. Morbidity and Mortality Weekly Report, 66, 521-526. doi: 10.15585/mmwr.mm6620a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma-Lürken T., Bleijlevens M. H. C., Lexis M. A. S., de Witte L. P., Hamers J. P. H. (2018). Facilitating aging in place: A qualitative study of practical problems preventing people with dementia from living at home. Geriatric Nursing, 39, 29-38. doi: 10.1016/j.gerinurse.2017.05.003 [DOI] [PubMed] [Google Scholar]
- United Nations. (2015). World population ageing 2015. New York, NY: Department of Economic and Social Affairs Population Division, United Nations. [Google Scholar]
- Van Der Steen J. T., Van Soest-Poortvliet M. C., Achterberg W. P., Ribbe M. W., De Vet H. C. W. (2011). Family perceptions of wishes of dementia patients regarding end-of-life care. International Journal of Geriatric Psychiatry, 26, 217-220. doi: 10.1002/gps.2577 [DOI] [PubMed] [Google Scholar]
- Viana B. M., Bicalho M. A. C., Moraes E. N., Romano-Silva M. A. (2015). Twenty-four–year demographic trends of a Brazilian long-term care institution for the aged. Journal of the American Medical Directors Association, 16, 174.e1-174.e6. doi: 10.1016/j.jamda.2014.11.012 [DOI] [PubMed] [Google Scholar]
- Wiles J. L., Leibing A., Guberman N., Reeve J., Allen R. E. (2012). The meaning of “aging in place” to older people. Gerontologist, 52, 357-366. doi: 10.1093/geront/gnr098 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2017). Dementia—Fact sheet. Retrieved from www.who.int/mediacentre/factsheets/fs362/en/
- Wysocki A., Butler M., Kane R. L., Kane R. A., Shippee T., Sainfort F. (2012). Long-term care for older adults: A review of home and community-based services versus institutional care (Comparative Effectiveness Review No. 81, Minnesota Evidence-Based Practice Center). Rockville, MD: Agency for Healthcare Research and Quality; Retrieved from www.effectivehealthcare.ahrq.gov/reports/final.cfm [PubMed] [Google Scholar]