Due to intrinsic multidrug resistance, pulmonary infections with Mycobacterium abscessus are extremely difficult to treat. Previously, we demonstrated that bedaquiline is highly effective against Mycobacterium abscessus both in vitro and in vivo. Here, we report that verapamil improves the efficacy of bedaquiline activity against M. abscessus clinical isolates and low-level resistant strains, both in vitro and in macrophages.
KEYWORDS: ATP synthase, MmpL, Mycobacterium abscessus, bedaquiline, drug resistance, efflux pump, verapamil
ABSTRACT
Due to intrinsic multidrug resistance, pulmonary infections with Mycobacterium abscessus are extremely difficult to treat. Previously, we demonstrated that bedaquiline is highly effective against Mycobacterium abscessus both in vitro and in vivo. Here, we report that verapamil improves the efficacy of bedaquiline activity against M. abscessus clinical isolates and low-level resistant strains, both in vitro and in macrophages. Verapamil may have clinical potential as adjunctive therapy provided that sufficiently high doses can be safely achieved.
INTRODUCTION
The Mycobacterium abscessus complex, comprising three subspecies, M. abscessus sensu stricto, M. bolletii, and M. massiliense (1, 2), represents the most important cause of pulmonary infections by rapidly growing nontuberculous mycobacteria (NTM) in patients with chronic lung diseases, such as bronchiectasis and cystic fibrosis (CF) (3, 4). Chronic M. abscessus infection in these patients correlates with greater rates of pulmonary function decline compared to patients without NTM infections (5, 6). Moreover, pulmonary infections with M. abscessus remain extremely difficult to treat, with a cure rate of only 25 to 58% (7, 8). In addition, prolonged treatment regimes not only induce severe side effects in patients but also cause a high economic burden to society (9).
Clofazimine (CFZ) and bedaquiline (BDQ) are currently being evaluated in clinical trials against M. abscessus pulmonary infections as repositioned drugs with gaining interest. BDQ is a diarylquinoline approved by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of multidrug-resistant tuberculosis (10). We and others have recently shown that BDQ exhibits very low MIC values against NTM, including clinical M. abscessus strains from CF and non-CF patients (11–13). To determine the cooperative potential of BDQ with companion drugs for new treatment regimens against M. abscessus, evaluation of combinations of BDQ and other antimicrobials in synergism is necessary. In this context, we tested whether verapamil (VER), a cationic amphiphilic membrane stress inducer, previously shown to potentiate the effect of BDQ in M. tuberculosis (14, 15), increases the efficacy of BDQ in M. abscessus.
On Middlebrook 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase (OADC) enrichment (7H10OADC), VER alone at 50 μg/ml did not impact on M. abscessus growth but clearly augmented growth inhibition by BDQ, although it did not for CFZ (Fig. 1A). A similar augmentative effect was not observed with the efflux inhibitor, reserpine (Fig. 1A). These results were reproduced in cation-adjusted Mueller-Hinton broth, the CLSI-recommended medium for antimicrobial testing against NTM (16) (Fig. 1B). Moreover, varying the concentration of BDQ over that of VER showed a hyperbolic relationship between the MIC of BDQ and the concentration of VER that was suggestive of a strong synergistic effect between the two compounds (Fig. 1B, upper panel). Importantly, high concentrations of up to 250 μg/ml VER did not affect M. abscessus growth (data not shown), while concentrations as low as 2 to 4 μg/ml resulted in an ∼2-fold decrease in the BDQ MIC (Fig. 1B, upper panel). In the context of well documented toxicity of VER (17, 18), the maximal range of systemic verapamil concentrations that have been achieved in the clinic by rapid continuous intravenous infusion without major toxicity is in the order of 2 μg/ml (19). To investigate whether VER and BDQ in combination exert a bacteriostatic or bactericidal activity against M. abscessus, we determined the growth kinetics of the bacteria in the presence of BDQ at 1× MIC (0.125 μg/ml) in the presence of VER. As shown in Fig. 1C, VER at 50 μg/ml failed to exert an additive bactericidal effect along with BDQ against M. abscessus (Fig. 1C).
FIG 1.
VER potentiates the in vitro efficacy of BDQ against M. abscessus. (A) Assessment of BDQ and clofazimine (CFZ) MICs against M. abscessus in the presence of verapamil (VER) or reserpine (RES) in 7H10OADC agar medium. An exponential-phase culture of M. abscessus CIP104536T (smooth variant) was serially 10-fold diluted, and 5-μl volumes of each dilution were spotted onto the agar plates, which were then incubated at 37°C for 4 days before pictures were taken. (B) Line graph showing a hyperbolic decrease in BDQ MIC as the VER concentration is increased (top panel). No decrease in CFZ MIC against M. abscessus is observed by addition of increasing concentrations of VER (bottom panel). These MIC determinations were done in cation-adjusted Mueller-Hinton broth at 30°C. Symbols and error bars represent means and standard errors of the mean calculated from at least two independent experiments. (C) Growth kinetics of M. abscessus in cation-adjusted Mueller-Hinton broth containing (or not) 0.125 μg/ml BDQ, as well as 0, 2, or 50 μg/ml VER. (D) Assessment in the presence of VER of BDQ MICs against M. abscessus mutants that overexpress (ΔMAB_2299c) or lack the MAB_2300-MAB_2301 BDQ-efflux pump (ΔMAB_2299c ΔMAB_2300-2301). An exponential-phase culture of each strain was serially 10-fold diluted, and 5-μl volumes of each dilution were spotted onto the agar plates, which were then incubated at 37°C for 4 days before pictures were taken.
Next, we tested the potency of the VER/BDQ combination against a wide range of M. abscessus clinical isolates, including isolates from all three M. abscessus subspecies, and obtained from both non-CF and CF patients. As shown in Table 1, VER drastically improved the efficacy of BDQ against all isolates tested, with an improvement of the BDQ MIC in the presence of VER ranging between 4- and 8-fold. These results are in line with previous studies demonstrating that VER decreases the MIC of BDQ against M. tuberculosis in vitro (14, 15, 20). However, the addition of VER to the medium failed to change the MIC of the clinically used drugs amikacin, imipenem, or cefoxitin (see Table S1 in the supplemental material). This highlights the specificity of VER in potentiating the effect of BDQ and indicates that VER has no beneficial value when combined with other M. abscessus drugs.
TABLE 1.
MICs of BDQ in cation-adjusted Mueller-Hinton broth at 30°C without verapamil (0 VER) or with 50 μg/ml verapamil (50 VER) for 17 clinical isolates from CF and non-CF patients belonging to the M. abscessus complexa
| Isolate | Morphotype | Source | BDQ MIC (ng/ml) |
Fold change | |||
|---|---|---|---|---|---|---|---|
| Replicate 1 |
Replicate 2 |
||||||
| 0 VER | 50 VER | 0 VER | 50 VER | ||||
| M. abscessus | |||||||
| CIP104536T | S | Non-CF | 64 | 16 | 64 | 8 | 4–8 |
| 1298 | S | CF | 64 | 8 | 32 | 8 | 4–8 |
| 2069 | S | Non-CF | 64 | 8 | 64 | 8 | 8 |
| 3321 | S | Non-CF | 32 | 8 | 32 | 8 | 4 |
| 2587 | S | CF | 32 | 8 | 32 | 4 | 4–8 |
| 2648 | R | CF | 64 | 8 | 32 | 4 | 8 |
| 2524 | R | CF | 64 | 8 | 128 | 16 | 8 |
| 3022 | R | Non-CF | 128 | 64 | 64 | 16 | 2–4 |
| M. massiliense | |||||||
| 210 | R | CF | 64 | 16 | 64 | 16 | 4 |
| 179 | R | CF | 64 | 8 | 64 | 8 | 8 |
| 140 | S | CF | 64 | 8 | 64 | 8 | 8 |
| 185 | S | CF | 128 | 16 | 128 | 16 | 8 |
| M. bolletii | |||||||
| 114 | S | CF | 128 | 16 | 128 | 16 | 8 |
| 116 | S | CF | 32 | 4 | 32 | 4 | 8 |
| 17 | S | Non-CF | 32 | 4 | 32 | 4 | 8 |
| 112 | R | CF | 64 | 4 | 64 | 4 | 16 |
| 19 | R | Non-CF | 32 | 4 | 64 | 16 | 4–8 |
| 108 | R | CF | 32 | 8 | 32 | 8 | 4 |
The fold change in BDQ MIC in the presence of verapamil compared to in its absence is shown. The experiment was completed in duplicate, with technical replicates included in each experiment. Morphotype: R, resistant; S, susceptible.
We recently identified a TetR repressor, MAB_2299c, responsible for low-level resistance to BDQ and CFZ in M. abscessus (21). Loss-of-function mutations or targeted deletion of MAB_2299c led to overexpression of the MAB_2300-MAB_2301-encoded MmpS-MmpL efflux pump system. This resulted in the exclusion of BDQ and CFZ and, hence, a low level of resistance toward these antibiotics. On the other hand, targeted deletion of MAB_2300-MAB_2301 led to a 4-fold decrease in the already low MIC of BDQ against M. abscessus, implicating the MAB_2300-MAB_2301 efflux pump in intrinsic resistance to BDQ. We exploited these strains (Table S2) to address whether VER’s potentiating action in BDQ sensitivity is dependent upon the BDQ efflux mechanism encoded by MAB_2300-MAB_2301. As shown in Fig. 1D and Table 2, VER still exerted a potentiating effect on BDQ sensitivity in strains that normally exhibit low-level resistance to BDQ due to MAB_2300-MAB_2301 overexpression (ΔMAB_2299c and CFZ-R6), as well as in a BDQ-hypersensitive strain lacking both MAB_2299c and MAB_2300-MAB_2301 (ΔMAB_2299c ΔMAB_2300-2301). These results indicate that the VER/BDQ combination may be successful in mitigating low-level BDQ resistance. Moreover, the latter strain became extremely susceptible to BDQ/VER exposure (BDQ MIC of 4 to 8 ng/ml), apparently excluding this efflux pump as a target of VER. That VER augments the activity of BDQ in M. tuberculosis through its deleterious effect on membrane energetics, rather than by directly inhibiting efflux, was recently reported (22). However, VER at 50 μg/ml did not increase the sensitivity toward BDQ of M. abscessus AtpE target mutants (atpE with a mutation at D29V [atpED29V] and atpEA64P), expressing high-level resistance to BDQ (13) (Fig. S1), consistent with previous work in M. tuberculosis (23).
TABLE 2.
MICs of BDQ in 7H10OADC agar at 37°C without verapamil (0 VER) or with 50 μg/ml (50 VER) or 250 μg/ml (250 VER) verapamil against genetically modified M. abscessus strains either overexpressing the MAB_2300-2301-encoded MmpS-MmpL efflux pump (ΔMAB_2299c deletion mutant or CFZ-R6 carrying a point mutation in MAB_2299c) or in which the gene set has been deleted (ΔMAB_2299c ΔMAB_2300-2301)a
| Strain | BDQ MIC (ng/ml) |
||||
|---|---|---|---|---|---|
| 0 VER | 50 VER | Fold change (50 VER) | 250 VER | Fold change (250 VER) | |
| Wild type | 256 | 64 | 4 | 16 | 16 |
| ΔMAB_2299c | 512 | 256 | 2 | 64 | 8 |
| ΔMAB_2299c Compl | 256 | 64 | 4 | 32 | 8 |
| ΔMAB_2299c ΔMAB_2300-2301 | 32 | 8 | 4 | 4 | 8 |
| CFZ-R6 | 512 | 256 | 2 | 64 | 8 |
The fold change values shown represent the BDQ MIC in the presence of verapamil (for 50 VER and 250 VER each) compared to in its absence. Data shown are representative of three independent experiments. 7H10OADC, Middlebrook 7H10 supplemented with OADC.
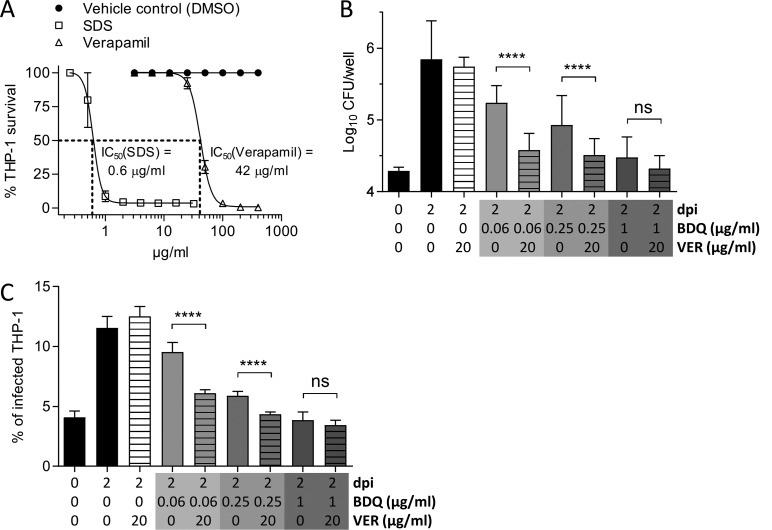
To further demonstrate the potential of VER to improve BDQ treatment outcomes during infection, we assayed the VER/BDQ combination in a THP-1 macrophage infection model. The cytotoxicity of VER was first examined using a procedure described elsewhere (24). As can be seen in Fig. 2A, VER exhibited a relatively low level of toxicity against differentiated THP-1 macrophages, with a 50% inhibitory concentration (IC50) of 42 μg/ml. We thus decided to fix the concentration of VER to one-half the IC50 in BDQ/VER cotreatment experiments against M. abscessus-infected macrophages. Cells were infected with M. abscessus (multiplicity of infection [MOI] of 2:1) at 37°C in 5% CO2 for 2 h, followed by three gentle washes with phosphate-buffered saline (PBS) and an incubation with fetal bovine serum-supplemented RPMI (Gibco) medium (RPMIFBS) containing 250 μg/ml amikacin for 2 h to kill remaining extracellular bacteria, prior to the addition of RPMIFBS alone (negative control) or containing increasing concentrations of BDQ alone or in combination with 20 μg/ml VER. At 2 days postinfection, macrophages were extensively washed with PBS and lysed with 1% (vol/vol) Triton X-100, and serial dilutions were plated to monitor the intracellular viable bacterial units. As shown in Fig. 2B, after 2 days of treatment, VER significantly restricted intracellular growth of M. abscessus at 0.06 and 0.25 μg/ml BDQ. Furthermore, to determine the percentage of infected macrophages, cells were infected with tdTomato-expressing M. abscessus (25) (MOI of 2:1) prior to treatment with medium containing increasing concentrations of BDQ alone or in combination with 20 μg/ml VER. Fixation was performed using 4% paraformaldehyde in PBS for 20 min, and samples were examined using a confocal microscope (63× objective; Zeiss, LSM880). For each condition, hundreds of macrophages were counted to determine the percentage of infected cells. A pronounced reduction in the number of infected THP-1 cells treated with increasing concentrations of BDQ at 2 days postinfection compared to untreated controls was observed, and this effect was further exacerbated by the addition of 20 μg/ml VER, particularly in the presence of low BDQ doses (0.06 and 0.25 μg/ml) (Fig. 2C). These results are in agreement with previous reports on potentiation of BDQ activity against M. tuberculosis by VER in macrophages and in the mouse model of infection (15, 20, 26).
FIG 2.
Augmentation by VER of BDQ activity against M. abscessus in macrophages. (A) Cytotoxicity assay of VER toward THP-1 macrophages. SDS was included as a control compound that is known to be toxic to the cells at low concentrations. (B) M. abscessus-infected THP-1 macrophages were treated with different concentrations of BDQ without or with 20 μg/ml VER and incubated for 2 days before they were lysed, the lysates were plated, and CFU enumerations were performed. Histograms and error bars represent medians and 95% confidence intervals calculated from three independent experiments done in triplicate. Differences between treatments were assessed by a two-tailed Mann Whitney test. ****, P < 0.0001; ns, nonsignificant. (C) THP-1 macrophages were infected with M. abscessus expressing tdTomato, treated with different concentrations of BDQ without or with VER, and incubated for 2 days before they were observed under a confocal microscope and the percentage of infected macrophages determined. Histograms and error bars represent medians and 95% confidence intervals calculated from three independent experiments completed in triplicate. Differences between treatments were assessed by a two-tailed Mann-Whitney test. ****, P < 0.0001; ns, nonsignificant.
In summary, we illustrated, for the first time, the potential of a membrane stress inducer, VER, to increase BDQ sensitivity in M. abscessus, and we also confirmed that VER has adjunctive activity in macrophages. Due to limitations on the verapamil plasma concentrations that are obtained by oral verapamil dosing (27), its potential might be limited in clinical settings to improve outcomes of BDQ treatment in M. abscessus infections. However, this study, along with previous reports that VER augments BDQ activity against M. tuberculosis (14, 15), proves that pharmacological potential exists to improve the activity of BDQ against tuberculous and nontuberculous mycobacteria. Subsequent investigations should be initiated to discover analogous compounds to VER that have reduced toxicity and that act in a similar manner to increase the sensitivity of mycobacteria to BDQ.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Fondation pour la Recherche Médicale (grant DEQ20150331719 to L.K.) and the Association Gregory Lemarchal and Vaincre la Mucoviscidose (grant RIF20180502320 to C.R.). M.D.J. received a postdoctoral fellowship granted by Labex EpiGenMed, an Investissements d’Avenir program (ANR-10-LABX-12-01). The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication.
The authors have no conflict of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00705-19.
REFERENCES
- 1.Choo SW, Wee WY, Ngeow YF, Mitchell W, Tan JL, Wong GJ, Zhao Y, Xiao J. 2014. Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential. Sci Rep 4:4061. doi: 10.1038/srep04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan JL, Ngeow YF, Choo SW. 2015. Support from phylogenomic networks and subspecies signatures for separation of Mycobacterium massiliense from Mycobacterium bolletii. J Clin Microbiol 53:3042–3046. doi: 10.1128/JCM.00541-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 4.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. U.S. Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esther CR, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catherinot E, Roux A-L, Macheras E, Hubert D, Matmar M, Dannhoffer L, Chinet T, Morand P, Poyart C, Heym B, Rottman M, Gaillard J-L, Herrmann J-L. 2009. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J Clin Microbiol 47:271–274. doi: 10.1128/JCM.01478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon K, Kwon OJ, Lee NY, Kim B-J, Kook Y-H, Lee S-H, Park YK, Kim CK, Koh W-J. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 8.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 9.Strollo SE, Adjemian J, Adjemian MK, Prevots DR. 2015. The burden of pulmonary nontuberculous mycobacterial disease in the United States. Ann Am Thorac Soc 12:1458–1464. doi: 10.1513/AnnalsATS.201503-173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matteelli A, Carvalho AC, Dooley KE, Kritski A. 2010. TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol 5:849–858. doi: 10.2217/fmb.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang Y, Zheng H, Tan Y, Song Y, Zhao Y. 2017. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother 61:e02627-16. doi: 10.1128/AAC.02627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesenbeckh S, Schönfeld N, Roth A, Bettermann G, Krieger D, Bauer TT, Rüssmann H, Mauch H. 2017. Bedaquiline as a potential agent in the treatment of Mycobacterium abscessus infections. Eur Respir J 49:1700083. doi: 10.1183/13993003.00083-2017. [DOI] [PubMed] [Google Scholar]
- 13.Dupont C, Viljoen A, Thomas S, Roquet-Banères F, Herrmann J-L, Pethe K, Kremer L. 2017. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob Agents Chemother 61:e01225-17. doi: 10.1128/AAC.01225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Tyagi S, Bishai WR. 2015. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob Agents Chemother 59:673–676. doi: 10.1128/AAC.04019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace RJ. 2011. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes: approved standard, 2nd ed M24-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 17.St-Onge M, Dubé P-A, Gosselin S, Guimont C, Godwin J, Archambault PM, Chauny J-M, Frenette AJ, Darveau M, Le Sage N, Poitras J, Provencher J, Juurlink DN, Blais R. 2014. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol 52:926–944. doi: 10.3109/15563650.2014.965827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batalis NI, Harley RA, Schandl CA. 2007. Verapamil toxicity: an unusual case report and review of the literature. Am J Forensic Med Pathol 28:137–140. doi: 10.1097/01.paf.0000257399.58935.28. [DOI] [PubMed] [Google Scholar]
- 19.Toffoli G, Robieux I, Fantin D, Gigante M, Frustaci S, Nicolosi GL, De Cicco M, Boiocchi M. 1997. Non-linear pharmacokinetics of high-dose intravenous verapamil. Br J Clin Pharmacol 44:255–260. doi: 10.1046/j.1365-2125.1997.t01-1-00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Tasneen R, Peloquin CA, Almeida DV, Li S-Y, Barnes-Boyle K, Lu Y, Nuermberger E. 2018. Verapamil increases the bioavailability and efficacy of bedaquiline but not clofazimine in a murine model of tuberculosis. Antimicrob Agents Chemother 62:e01692-17. doi: 10.1128/AAC.01692-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richard M, Gutiérrez AV, Viljoen A, Rodriguez-Rincon D, Roquet-Baneres F, Blaise M, Everall I, Parkhill J, Floto RA, Kremer L. 2018. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother 63:e01316-18. doi: 10.1128/AAC.01316-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Gardete S, Jansen RS, Shetty A, Dick T, Rhee KY, Dartois V. 2018. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob Agents Chemother 62:e02107-17. doi: 10.1128/AAC.02107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefebvre A-L, Le Moigne V, Bernut A, Veckerlé C, Compain F, Herrmann J-L, Kremer L, Arthur M, Mainardi J-L. 2017. Inhibition of the β-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 61:e02440-16. doi: 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernut A, Herrmann J-L, Kissa K, Dubremetz J-F, Gaillard J-L, Lutfalla G, Kremer L. 2014. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc Natl Acad Sci U S A 111:E943–E952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams KN, Szumowski JD, Ramakrishnan L. 2014. Verapamil, and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210:456–466. doi: 10.1093/infdis/jiu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John DN, Fort S, Lewis MJ, Luscombe DK. 1992. Pharmacokinetics and pharmacodynamics of verapamil following sublingual and oral administration to healthy volunteers. Br J Clin Pharmacol 33:623–627. doi: 10.1111/j.1365-2125.1992.tb04091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.