High dosages of ceftriaxone are used to treat central nervous system (CNS) infections. Dosage adaptation according to the glomerular filtration rate is currently not recommended. Ceftriaxone pharmacokinetics (PK) was investigated by a population approach in patients enrolled in a French multicenter prospective cohort study who received high-dose ceftriaxone for CNS infection as recommended by French guidelines (75 to 100 mg/kg of body weight/day without an upper limit).
KEYWORDS: modeling, simulation, pharmacokinetics, antibiotics, nomogram
ABSTRACT
High dosages of ceftriaxone are used to treat central nervous system (CNS) infections. Dosage adaptation according to the glomerular filtration rate is currently not recommended. Ceftriaxone pharmacokinetics (PK) was investigated by a population approach in patients enrolled in a French multicenter prospective cohort study who received high-dose ceftriaxone for CNS infection as recommended by French guidelines (75 to 100 mg/kg of body weight/day without an upper limit). Only those with suspected bacterial meningitis were included in the PK analysis. A population model was developed using Pmetrics. Based on this model, a dosing nomogram was developed, using the estimated glomerular filtration rate (eGFR) and total body weight as covariates to determine the optimal dosage allowing achievement of targeted plasma trough concentrations. Efficacy and toxicity endpoints were based on previous reports, as follows: total plasma ceftriaxone concentrations of ≥20 mg/liter in >90% of patients for efficacy and ≤100 mg/liter in >90% of patients for toxicity. Based on 153 included patients, a two-compartment model including eGFR and total body weight as covariates was developed. The median value of the unbound fraction was 7.57%, and the median value of the cerebral spinal fluid (CSF)/plasma ratio was 14.39%. A nomogram was developed according to a twice-daily regimen. High-dose ceftriaxone administration schemes, used to treat meningitis, should be adapted to the eGFR and weight, especially to avoid underdosing using current guidelines. (This study has been registered at ClinicalTrials.gov under identifier NCT01745679.)
TEXT
Ceftriaxone is a broad-spectrum cephalosporin recommended for empirical treatment of bacterial meningitis (1–3). This antibiotic displays original pharmacokinetics (PK) with dual biliary and renal clearance and an extended half-life allowing a twice-daily or even a once-daily administration scheme. Recommended ceftriaxone dosages for treating meningitis are different from one learned society to another. The IDSA (Infectious Diseases Society of America) and the ESCMID (European Society for Clinical Microbiology and Infectious Diseases) recommended 4 g per day in adults regardless of patient weight (2, 3), while French guidelines promote a dose-weighted adjustment in a range of 75 mg/kg to 100 mg/kg of body weight per day without an upper limit dosage (1). None of them recommends adaptation according to the glomerular filtration rate (GFR), except for French guidelines in the case of a GFR of <10 ml/min.
Dosages used in central nervous system (CNS) infections are widely higher than those usually prescribed. Hence, previous studies investigated ceftriaxone population PK after administration of a dosage generally equal to or lower than 2 g per day (4–8), and no specific study was performed in populations receiving more than 4 g per day. High GFRs are frequently observed in critically ill patients with community-acquired acute infectious meningitis and are likely to affect ceftriaxone clearance (9, 10) These changes, frequently observed during meningitis, justify investigating specifically ceftriaxone PK in this population. The aim of this work was to describe the PK of ceftriaxone in a large cohort of patients treated for suspected bacterial meningitis with a high daily dosage (≥75 mg/kg or 4 g per day). Based on the finally developed model, Monte Carlo simulations were performed to propose an optimal ceftriaxone scheme of administration in patients treated for suspected bacterial meningitis according to renal function and weight.
(Preliminary results of this study were presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases, 22 to 25 April 2017, Vienna, Austria.)
RESULTS
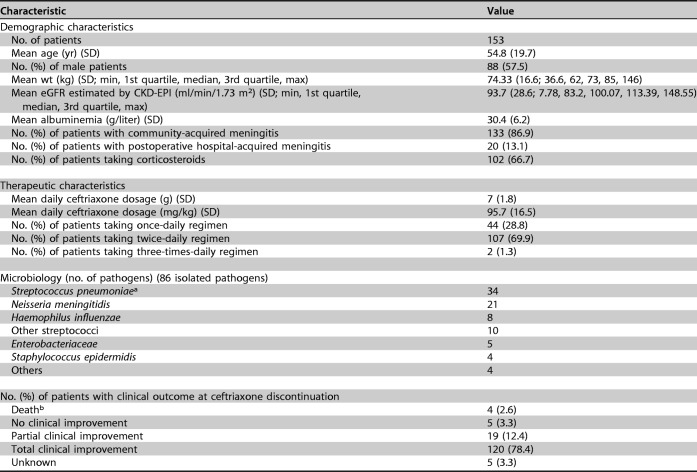
One hundred fifty-three patients were included, 133 with suspected or proven community-acquired meningitis and 20 with suspected or proven hospital-acquired meningitis.
A description of study patients and their clinical, microbiological, and therapeutic characteristics are reported in Table 1.
TABLE 1.
Baseline demographic and therapeutic characteristics, microbiology, and clinical outcomes of studied patients
aMIC values were determined for Streptococcus pneumoniae and ranged from <0.016 to 0.5 mg/liter.
bDeaths were caused by septic shock (n = 2), stroke (n = 1), and cerebral venous thrombosis (n = 1).
For each patient, 1 to 4 blood samples were collected at least 23 h after the beginning of therapy (mean delay, 92 h [range, 23 to 240 h]). Three hundred one total concentrations, 214 unbound concentrations, and 11 cerebral spinal fluid (CSF) concentrations were sampled. The elapsed time from the last dose to plasma measurement ranged from 5 min to 24 h.
A two-compartment model was chosen as the basic PK model. Ke (elimination rate constant from the central compartment) was modeled as Ke1 + (eGFR/94 × Ke2), where 94 is the median value for the population, and V (volume of the central compartment) was modeled as V1 × (WT/74)V2, where WT is weight and 74 is the median value for the population.
A gamma error model with a starting value of 2 was chosen, and values for C0, C1, C2, and C3 were 0.5, 0.05, 0, and 0 for the standard deviation (SD) polynomial, respectively. The final cycle value of gamma was 2.45. This indicates an acceptable process noise.
The estimates of the population PK parameters are presented in Table 2. The median value of the unbound fraction was calculated from 214 individual observed concomitant data and was 7.57% (from 1.61 to 49.30%). Protein binding was saturable, especially when total concentrations exceeded 100 mg/liter. The relationship between linked and total concentrations was best described by a polynomial model where the percentage of protein binding was modeled as −5E−09x3 + 6E−07x2 − 0.0004x + 0.9393, where x is the total concentration of ceftriaxone (R2, 0.3224). The median value of the CSF/plasma ratio was calculated from 8 individual observed concomitant data and was 14.39% (from 5.86 to 65.94%), with a median concentration in CSF of 14.95 mg/liter (from 1.65 to 27.1 mg/liter), a median total plasma concentration of 63.55 mg/liter (from 21.6 to 201.3 mg/liter), and a median time after infusion of 12 h (from 3.67 to 48 h).
TABLE 2.
Population parameter estimates and pharmacokinetics dataa
| Parameter | Value |
||
|---|---|---|---|
| Median (95% CI) | MAWD (95% CI) | Range | |
| Ke1 (h−1) | 0.158 (0.104–0.272) | 0.119 (0.0.065–0.186) | 0.001–1 |
| Ke2 (h−1) | 0.465 (0.0.367–0.546) | 0.138 (0.066–0.190) | 0.001–1 |
| KCP (h−1) | 72.082 (59.067–79.618) | 13.154 (8.285–21.189) | 25–100 |
| KPC (h−1) | 11.127 (7.490–12.904) | 3.262 (1.279–4.830) | 5–20 |
| V1 (liters) | 3.712 (2.874–5.169) | 1.595 (0.948–2.934) | 1–20 |
| V2 (liters) | 0.602 (0.204–0.993) | 0.336 (0.006–0.483) | 0.001–1 |
CI, confidence interval of the estimates; MAWD, median absolute weighted deviation, used as an estimate of the variance for a nonparametric distribution; range, interval of values set before the run. In the model, Ke = Ke1 + (eGFR/94 × Ke2), where Ke is the elimination rate constant from the central compartment (per hour) and eGFR/94 is the estimated glomerular filtration rate (milliliters per minute) normalized to the population mean. KCP is the constant of transfer from the central compartment to the peripheral compartment (per hour). KPC is the constant of transfer from the peripheral compartment to the central compartment (per hour). V = V1 × (WT/74)V2, where V is the volume of the central compartment (liters) and WT/74 is the weight (kilograms) normalized to the population mean. The median (minimum to maximum) value of the unbound fraction from 214 individual observed data was 7.57% (1.61 to 49.30%). The median (minimum to maximum) value of CSF/plasma diffusion from 8 individual observed data was 14.39% (5.86 to 65.94%).
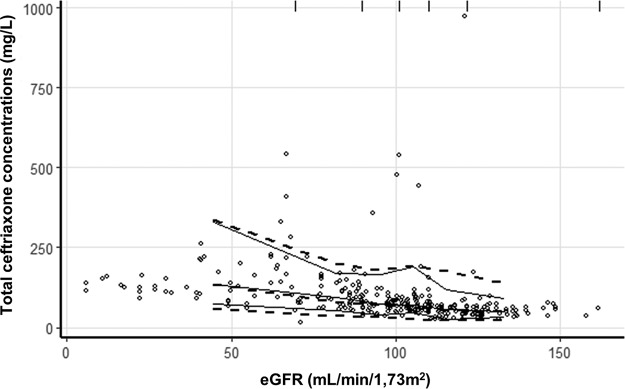
Diagnostic plots are shown in Fig. 1. Equations established by linear regression of observed concentrations versus individual predicted concentrations are close to the identity line, analysis of residuals appears to be satisfactory, and a visual predictive check (VPC) plot shows that the model correctly described the observed data (Fig. 2).
FIG 1.
Graphical representations allowing the validation of the population pharmacokinetic model. (A and B) Observed concentrations plotted against population (A) or individual (B) predicted concentrations (milligrams per liter) (R2 = 0.684 and 0.947, respectively). (C) Weighted residuals plotted against individual predicted concentrations (milligrams per liter). (D) Weighted residuals plotted against time postadministration (hours).
FIG 2.
Visual predictive checks of ceftriaxone concentrations against estimated glomerular filtration rate (eGFR) values by the CKD-EPI. Open circles represent all observed ceftriaxone concentrations included in modelization. Solid lines represent the 5th, 50th, and 95th percentiles for observed concentrations. Dashed lines represent the 5th, 50th, and 95th percentiles for simulated concentrations. Vertical lines at the top of the plots are bin separators.
From the validated model, the dosing nomogram was constructed. Figure 3 shows the nomogram according to a twice-daily regimen. The eGFR window range is from 15 ml/min/1.73 m2 to 160 ml/min/1.73 m2 according to our population data.
FIG 3.
Nomogram of the daily dose of ceftriaxone per kilogram of total weight to be administered to achieve a trough concentration target of 20 mg/liter (full line) and to not exceed 100 mg/liter (broken line) with a probability of 0.9, accounting for renal function estimated by the CKD-EPI formula (eGFR) using a twice-daily regimen. Dotted lines represent the 95% confidence interval.
DISCUSSION
This first population PK study in adult patients treated with high doses of ceftriaxone (above 4 g or 75 mg/kg per day) for suspected bacterial meningitis suggests that the ceftriaxone administration scheme and posology should be adapted to the eGFR and weight.
However, few obese patients were included in our work, and the proposed nomogram was not adapted for these patients. It should therefore be used with caution in this population, knowing that, for most beta-lactams, adjustment in proportion to excess weight is not required.
Only 86 pathogens were isolated in proven single-strain infections. For the other 67 patients, microbiological research was unable to isolate the pathogen, or the infection was not proven. If ceftriaxone was given in the case of a strong suspicion of bacterial meningitis, the absence of infection in some patients could not be excluded.
The population modeling results are consistent with previous studies and confirm the influence of the eGFR on ceftriaxone clearance (7, 11, 12). According to Schleibinger et al., ceftriaxone concentrations seem to depend only on the clearance of unbound ceftriaxone, which depends on renal function but not on the unbound fraction (13). Here, the influence of renal function was observed whatever the degree of renal insufficiency but not in a previous study with a lower-dose ceftriaxone regimen, but owing to higher concentrations, the percentage of binding is very variable (1.6 to 43%) and probably may result in a greater role of the kidney in total elimination (7). According to Heinemeyer et al., this result suggests that biliary ceftriaxone clearance does not compensate for the decrease in renal elimination in patients with renal failure (14).
In this work, the volume of distribution was correlated with the total body weight and demonstrates the interest in adapting the dosage to the total body weight as suggested by French recommendations (1). A previous study showed similar results, where total body weight was integrated into population modeling (15). Other recent population studies did not integrate body weight into modeling but integrated other covariates, such as serum albumin (12).
The unbound fraction in our study was heterogeneous compared to healthy volunteers (1.6 to 43% versus 5 to 15%) and seems lower than those in other previously reported studies of critically ill patients (14 to 43%; 33%) (13, 16, 17). This could be due to the characteristics of our group, which was constituted partly of patients with viral meningitis with less systemic inflammation and subsequent hypoalbuminemia.
The median value of the CSF/plasma ratio of total ceftriaxone was calculated for 8 observed concomitant data and was 14.39% (from 5.86 to 65.94%). These values were higher than those observed in a previous study (from 0.6 to 1.8% for those without meningeal inflammation to 2 to 7% in cases of meningeal inflammation) (18–20).
Finally, it would have been preferable to evaluate the probability of target attainment (PTA) for efficacy in CSF, but the number of concentration data for the CSF was insufficient. Nevertheless, the CSF/plasma ratio of 14.39% reported here suggests that when trough plasma concentrations are above 20 mg/liter, concentrations in CSF should be higher than the upper bound of ceftriaxone-intermediate strains of Streptococcus pneumoniae (0.5 < MIC ≤ 2 mg/liter) (21). A recent study showed that during community-acquired pneumococcal meningitis, in which the level of meningeal inflammation is the highest, ceftriaxone diffusion in CSF could be very high (20).
The first infectious agents found in our population were Streptococcus pneumoniae (34/86 isolated pathogens), followed by Neisseria meningitidis and Haemophilus influenzae, which is consistent with previous data (22). MIC values were determined for Streptococcus pneumoniae and ranged from <0.016 to 0.5 mg/liter, confirming EUCAST epidemiological cutoff values used for determination of the PK-pharmacodynamic (PD) target of 20 mg/liter (21).
The DALI study suggests that the PK-PD target is 100% fT>MIC (dosing interval during which the free plasma concentration of ceftriaxone is above the MIC of the causative bacteria) in critically ill patients (23). Given the severity of illness in these patients, a fixed target for the unbound trough plasma concentration higher than 4 times the MIC of the targeted bacteria appears to be reasonable (24). This margin of safety is justified notably by the imprecision in the determination of the MIC (25). Moreover, the variability of the diffusion of ceftriaxone in the CNS could justify this margin, and an elevated target appears to be more accurate to prevent an insufficient level of diffusion and to have a maximum bactericidal rate (24, 26, 33). Therefore, a target of 20 mg/liter was chosen based on protein binding of around 90% and a maximum MIC value of 0.5 mg/liter, as confirmed by our results. This target is currently recommended by French guidelines (27).
The target for toxicity was fixed at 100 mg/liter according to recently reported data (28). This plasma concentration threshold could be associated with a higher risk of having a ceftriaxone-related adverse drug reaction, and even if it is uncertain, it justifies clinical monitoring of the patient, especially at the neurological level. In addition, it is unlikely that exceeding these concentrations will provide a benefit in terms of effectiveness. This target has been confirmed by recent guidelines of the French Society of Anesthesia and Intensive Care/French Society of Pharmacology (27).
A nomogram was developed to propose a twice-daily regimen knowing that more than one-quarter of our group received ceftriaxone by a once-daily regimen and more than two-thirds received it by a twice-daily regimen. A once-daily regimen nomogram was not presented here because it failed to allow achieving sufficient concentrations while being safe in terms of toxicity. Moreover, a once-daily regimen necessitates higher daily dosages than a twice-daily regimen.
For example, a patient with an eGFR of 90 ml/min/1.73 m2 and weighing 75 kg (approximatively the mean patient eGFR and weight in this study) will require a daily dosage of 47 to 71 mg/kg (3.5 to 5.3 g) with a twice-daily regimen based on the nomogram. In this case, the proposed posology is consistent with European and American guidelines. In the case of a higher eGFR (>140 ml/min/m2), a probably frequent and underestimated situation in patients with bacterial meningitis, the same patient weighing 75 kg would require at least 78 mg/kg (5.8 g) daily without exceeding 108 mg/kg (8 g) to obtain the same PTA with a twice-daily regimen. This patient would probably be underdosed according to European and American guidelines (and potentially with a once-daily regimen). According to nomogram, European and American guidelines could increase the risk of subtherapeutic concentrations of ceftriaxone, especially in cases of patients with an increased GFR and obese patients, even in the case of infection by susceptible strains of S. pneumoniae with an MIC of 0.5 mg/liter. On the contrary, French guidelines probably overestimate posology in cases of renal impairment. Unfortunately, the 3 sets of guidelines neglect the importance of kidney function in dosage adjustment.
This work suffers from several limitations. First, the population model did not integrate unbound plasma and CSF concentrations because of missing data for many patients. Integration of unbound concentrations has been tested but was not satisfactory, and it was chosen to develop the model based only on total plasma concentrations to obtain a reliable model for Monte Carlo simulations. Moreover, targeted concentrations have been defined by French guidelines as total concentrations, and modeling on total concentrations allowed being coherent with those.
The second limitation of this work is the absence of correction of the eGFR by the body surface area because there are missing data for many patients. Despite this limitation, the model appears to be robust, and eGFR not corrected by the body surface area is probably more easily reachable for posology adaptation.
Third, the proposed schemes do not take into account the potential excessive peaks that may also lead to toxicity (neurological or renal), and dosage proposals higher than those recommendations are to be used with caution. In this context of a high daily dose, the shortening of the dosing interval could be discussed.
Finally, two sites were used for quantification of ceftriaxone total plasma concentrations, which potentially increased analytical variability. Moreover, for technical reasons, all unbound plasma (and CSF) concentrations were determined in a single site. Knowing that analytical practices, clinical practices, and types of patients included were the same in both centers, we considered that the impact of this point was negligible.
Conclusion.
High-dose ceftriaxone administration schemes, used to treat meningitis, should be adapted to both eGFR and weight, but weight adjustment should be discussed for obese patients.
MATERIALS AND METHODS
Ethics and patients.
This work is an ancillary population PK study from an open-label, prospective, multicenter study, entitled High-Dose Ceftriaxone in Central Nervous System Infections, aiming to determine PK and tolerance of high-dose ceftriaxone in patients with CNS infections (28). Written informed consent was obtained from all patients enrolled in the study. The study design and consent form were approved by regulatory authorities and research clinical practices, and the study was conducted according to the principles of the Declaration of Helsinki (22 October 2008 version) and to French law. A license was issued by the French national drug regulatory authority. This study has been registered at ClinicalTrials.gov under identifier NCT01745679.
Patients suffering from suspected or proven CNS infections treated with a ceftriaxone daily dosage equal or higher than 4 g or 75 mg/kg were enrolled in 6 French centers in infectious diseases departments or intensive care units from the west of France (Nantes University Hospital, Angers University Hospital, Saint-Nazaire Hospital, Tours University Hospital, La Roche-sur-Yon Hospital, and Rennes University Hospital) between December 2012 and July 2015, and only those patients suspected of having community- or hospital-acquired meningitis were included in the PK analysis.
Collection of clinical and therapeutic data.
Clinical evolution during ceftriaxone treatment was monitored by the physician in charge of the patient, and clinical and therapeutic data were listed in a case report form.
Quantification of ceftriaxone concentrations.
Plasma samples were obtained by direct venipuncture or through a catheter, and CSF samples were collected by lumbar puncture.
Ceftriaxone plasma concentrations were determined by validated high-performance liquid chromatography methods with UV detection in the pharmacology departments of Nantes and Rennes University Hospitals. Concentrations for patients included from Rennes University Hospital were measured using a previously validated assay (29).
Total ceftriaxone plasma concentrations for other patients were centralized in the pharmacology department of Nantes University Hospital, and the consistency of results between the two departments was checked using a similar quality control program (Asqualab, Paris, France). A liquid-liquid extraction procedure was used in Nantes by mixing a 1-ml aliquot of plasma with 1 ml acetonitrile. The mixture was centrifuged at 1,800 × g for 5 min at +4°C. The supernatant layer (1.6 ml) was added to dichloromethane (8 ml). Tubes were horizontally shaken for 10 min and centrifuged at 1,800 × g for 5 min at +4°C, and 50 μl of the upper aqueous layer was injected into the system. The mobile phase (0.03 M Na2HPO4 [pH 1.9]–acetonitrile [85/15, vol/vol]) was delivered at 1.3 ml/min, and separation was performed on a Waters Symmetry column (5-μm C18 column [250- by 4.6-mm internal diameter {ID}]; Waters, Milford, MA, USA). The ceftriaxone plasma concentration was detected by the UV absorbance at 260 nm.
Chromatographic conditions defined in this assay were also applied to plasma ultrafiltrates obtained according to a previously validated method using an Amicon Ultra 0.5-ml 30,000-molecular-weight-cutoff centrifugal filter device (Millipore, Cork, Ireland) to measure the ceftriaxone unbound plasma concentration (30).
These chromatographic conditions were also applied to measure ceftriaxone concentrations in the CSF.
All unbound plasma and CSF concentrations were determined at the pharmacology department of Nantes University Hospital. The limits of quantitation were 1 mg/liter in plasma and in CSF. The methods were accurate (interday and intraday inaccuracy of <15%) and showed good precision (interday and intraday imprecision of <15%).
Total ceftriaxone concentrations were the only data used for model building. Unbound and CSF concentrations were used to assay binding and diffusion ratios in our population and to compare these data to those reported previously and were used to build current guidelines.
Population pharmacokinetics analysis.
Ceftriaxone PK analysis was performed using a nonparametric method implemented in Pmetrics (Laboratory of Applied Pharmacokinetics, University of Southern California, CA) (31). Pmetrics is a library package for R using Fortran.
Only total plasma concentrations were used to build the model. An initial analysis was conducted to estimate the parameters of the structural model without covariates. Three kinds of structural models were tested: a one-compartment model, a two-compartment model, and a three-compartment model.
Additive and multiplicative error models were tested, where observations were weighted by (SD2 + λ2)0.5 and SD × γ, respectively. Lambda/gamma represented process noise such as sampling time uncertainty and model misspecification. SD was the standard deviation of each observation, modeled by a polynomial equation, C0 + C1 × [obs] + C2 × [obs]2 + C3 × [obs]3, where [obs] is the observation.
The influence of covariates on parameters was assessed using a backward stepwise process and visual examination of the parameter-versus-covariate plot. Linear, exponential, power associations and allometric scaling were assessed. The following covariates were tested: age, sex, total body weight, creatinine serum concentration measured by an enzymatic assay (Roche, Basel, Switzerland), Modification of Diet in Renal Disease estimated glomerular filtration rate (eGFR), Chronic Kidney Disease - Epidemiology Collaboration (CKD-EPI) eGFR, serum albumin concentration, corticoid use, mechanical ventilation, and type of meningitis (community or hospital acquired).
The selection of the best model was based on the Akaike information criterion (AIC) value and the Bayesian information criterion (BIC) value. The model displaying the lower AIC and BIC values was chosen. Models were also assessed by visual examination of the diagnostic plots (observed concentration versus predicted concentrations, weighted residuals versus time or individual predicted concentrations, and visual predictive checks [VPCs]). Bias (mean weighted error of predictions minus observations) and imprecision (bias-adjusted mean weighted squared error of predictions minus observations) were also factored into the model selection.
VPCs were performed using Monte Carlo simulations (n = 1,000) from each of the patients to take into account each dose regimen and each time postdose (32). Medians and 5th and 95th percentiles for observed and simulated concentrations were then compared visually. Plotting was done using the “vpc” package for R.
Monte Carlo simulations of dosage regimens and nomogram.
Based on the parameters of the structural model, Monte Carlo simulations were generated (n = 1,000) from patient profiles with various eGFRs and weights reflective of the population observed. For each of these profiles, exposure to ceftriaxone was assessed for doses ranging from 500 mg/day to 25,000 mg/day according to a twice-daily regimen.
Targeted plasma trough concentrations for total ceftriaxone were defined from 20 mg/liter to 100 mg/liter. During bacterial meningitis, no targeted plasma concentration has ever been studied specifically, but in severe infections, the unbound trough concentrations should be at least 4 times higher than the MIC (100% fT>4× MIC) to achieve efficacy (23, 27). The most resistant bacterium found during community-acquired bacterial meningitis is Streptococcus pneumoniae with reduced susceptibility to penicillin. Therefore, the EUCAST epidemiological cutoff MIC for S. pneumoniae (0.5 mg/liter) was used to set the target of efficacy (21). Knowing that a 100% fT>4× MIC was targeted, it corresponded to an unbound trough concentration of ceftriaxone in plasma of 2 mg/liter. Accounting for the high protein binding of ceftriaxone (around 85 to 95%), the target of 20 mg/liter for the total ceftriaxone trough concentration was determined. This cutoff was consistent with recent guidelines of the French Society of Anesthesia and Intensive Care/French Society of Pharmacology (27).
The 100-mg/liter target was defined as an upper limit beyond which the toxicity risk could be largely increased. This target was recently reported in the study part investigating the tolerability of high-dose ceftriaxone in the same cohort (28).
To conceive the dosing nomogram, the lowest dose required to achieve a probability of target attainment (PTA) of at least 90% was reported on a graph in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) for 20 mg/liter as the trough targeted concentration, while the highest dose allowed to not reach a PTA of more than 10% was reported for 100 mg/liter as the trough targeted concentration. Confidence intervals (95%) were calculated for each prediction and reported on the graph.
ACKNOWLEDGMENTS
This work was supported by a grant from the French Ministry of Health (Interregional French Clinical Hospital Research Program grant PHRCi 2012-API12N037).
We declare that we have no competing interest regarding this work.
We thank all members of the High-Dose CRO CNS Infections Study Group: trial steering committee members Pierre Abgueguen, Natahalie Asseray (principal investigator), Louis Bernard, David Boutoille, Cédric Bretonnière, Jocelyne Caillon, Anne Chiffoleau, Eric Dailly, Martin Dary, Denis Garot, Thomas Guimard, Jérôme Hoff, Monique Marguerite, Dominique Navas, Maja Ogielska, François Raffi (chair), Véronique Sébille, Pierre Tattevin, and Yves-Marie Vandamme; expert committee members David Boutoille, Anne Chiffoleau, Martin Dary, and Dominique Navas; Angers investigators Pierre Abgueguen and Nicolas Crochette; La Roche sur Yon investigators Jean Baptiste Lascarrou, Christine Lebert, Eve Trebouet, Isabelle Vinatier, Maud Fiancette, Aihem Yehia, Jean Reignier, Jea-Claude Lacherade, Laurent Martin-Lefevre, Matthieu Henry-Lagarrigue, Elsa Bieber, Bertrand Weys, Gwenaël Colin, Aurélie Joret, and Kostas Bakoumas; Nantes investigators Marie Dalichampt, Guillaume Deslandes, Mathieu Grégoire, Monique Marguerite, Marion Rigot, Cédric Bretonnière, Jocelyne Caillon, Laurent Brisard, Syvie Raoul, Anne-Catherine Di Prizio, Charlotte Biron, Maeva Lefebvre, Magali Brière, Samuel Pineau, Jérémie Orain, Line Happi Djeukou, Laurene Leclair, Arnaud Peyre, Armelle Magot, and Guillemette Favet; Rennes investigators Solène Patrat-Delon, Paul Sauleau, Mathieu Revest, Cédric Arvieux, Caroline Piau-Couapel, Enora Ouamara-Digue, Maja Ratajczak, and Adèle Lacroix; Saint Nazaire investigators Céline Chevalier, Patricia Courouble, and Alix Phelizot; and Tours investigators Frédéric Bastides, Guillaume Gras, Maja Ogielska, Rodolphe Buzele, Emmanuelle Mercier, Pierre-François Dequin, Annick Legras, Antoine Guillon, Youenn Jouan, Stephan Ehrmann, Laeticia Bodet-Contentin, Emmanuelle Rouve, and Karine Fevre.
V.S., D.N., E.D., and N.A. drafted the protocol. Funding research was done by V.S., D.N., E.D., and N.A. M.G., E.D., D.G., T.G., L.B., P.T., Y.-M.V., J.H., F.L., M.-C.V., G.D., A.C., D.B., and N.A. performed data collection. M.G., E.D., F.L., and M.-C.V. performed concentration measurements. M.G., E.D., and R.B. performed population pharmacokinetic analysis. M.G., E.D., P.L.T., D.N., and N.A. drafted the article. M.G., E.D., P.L.T., and N.A. wrote the article.
REFERENCES
- 1.Société de Pathologie Infectieuse de Langue Française. 2009. 17th Consensus Conference. Consensus conference on bacterial meningitis. Short text. Med Mal Infect 39:175–186. (In French.) doi: 10.1016/j.medmal.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 2.van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard C, Pagliano P, Pfister HW, Read RC, Sipahi OR, Brouwer MC, ESCMID Study Group for Infections of the Brain. 2016. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22(Suppl 3):S37–S62. doi: 10.1016/j.cmi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ. 2004. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 4.Simon N, Dussol B, Sampol E, Purgus R, Brunet P, Lacarelle B, Berland Y, Bruguerolle B, Urien S. 2006. Population pharmacokinetics of ceftriaxone and pharmacodynamic considerations in haemodialysed patients. Clin Pharmacokinet 45:493–501. doi: 10.2165/00003088-200645050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Iida S, Kawanishi T, Hayashi M. 2011. Indications for a ceftriaxone dosing regimen in Japanese paediatric patients using population pharmacokinetic/pharmacodynamic analysis and simulation. J Pharm Pharmacol 63:65–72. doi: 10.1111/j.2042-7158.2010.01179.x. [DOI] [PubMed] [Google Scholar]
- 6.Iida S, Kinoshita H, Kawanishi T, Hayashi M. 2009. The pharmacokinetics of ceftriaxone based on population pharmacokinetics and the prediction of efficacy in Japanese adults. Eur J Drug Metab Pharmacokinet 34:107–115. doi: 10.1007/BF03191159. [DOI] [PubMed] [Google Scholar]
- 7.Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequi PF, Le Guellec C. 2011. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 72:758–767. doi: 10.1111/j.1365-2125.2011.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodise TP, Nau R, Kinzig M, Jones RN, Drusano GL, Sörgel F. 2007. Comparison of the probability of target attainment between ceftriaxone and cefepime in the cerebrospinal fluid and serum against Streptococcus pneumoniae. Diagn Microbiol Infect Dis 58:445–452. doi: 10.1016/j.diagmicrobio.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Lautrette A, Phan T-N, Ouchchane L, Aithssain A, Tixier V, Heng A-E, Souweine B. 2012. High creatinine clearance in critically ill patients with community-acquired acute infectious meningitis. BMC Nephrol 13:124. doi: 10.1186/1471-2369-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitby M, Finch R. 1986. Bacterial meningitis. Rational selection and use of antibacterial drugs. Drugs 31:266–278. doi: 10.2165/00003495-198631030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Ziegler WH. 1981. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther 29:650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- 12.Bos JC, Prins JM, Mistício MC, Nunguiane G, Lang CN, Beirão JC, Mathôt RAA, van Hest RM. 2018. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: a population pharmacokinetic modelling study. J Antimicrob Chemother 73:1620–1629. doi: 10.1093/jac/dky071. [DOI] [PubMed] [Google Scholar]
- 13.Schleibinger M, Steinbach CL, Töpper C, Kratzer A, Liebchen U, Kees F, Salzberger B, Kees MG. 2015. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 80:525–533. doi: 10.1111/bcp.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinemeyer G, Link J, Weber W, Meschede V, Roots I. 1990. Clearance of ceftriaxone in critical care patients with acute renal failure. Intensive Care Med 16:448–453. doi: 10.1007/BF01711224. [DOI] [PubMed] [Google Scholar]
- 15.Sharma VD, Singla A, Chaudhary M, Taneja M. 2016. Population pharmacokinetics of fixed dose combination of ceftriaxone and sulbactam in healthy and infected subjects. AAPS PharmSciTech 17:1192–1203. doi: 10.1208/s12249-015-0454-2. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Pea F, Lipman J. 2013. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet 52:1–8. doi: 10.1007/s40262-012-0018-5. [DOI] [PubMed] [Google Scholar]
- 17.Tsai D, Stewart P, Goud R, Gourley S, Hewagama S, Krishnaswamy S, Wallis SC, Lipman J, Roberts JA. 2016. Total and unbound ceftriaxone pharmacokinetics in critically ill Australian Indigenous patients with severe sepsis. Int J Antimicrob Agents 48:748–752. doi: 10.1016/j.ijantimicag.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Latif R, Dajani AS. 1983. Ceftriaxone diffusion into cerebrospinal fluid of children with meningitis. Antimicrob Agents Chemother 23:46–48. doi: 10.1128/aac.23.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrasekar PH, Rolston KV, Smith BR, LeFrock JL. 1984. Diffusion of ceftriaxone into the cerebrospinal fluid of adults. J Antimicrob Chemother 14:427–430. doi: 10.1093/jac/14.4.427. [DOI] [PubMed] [Google Scholar]
- 20.Le Turnier P, Grégoire M, Garot D, Guimard T, Duval X, Bernard L, Boutoille D, Dailly E, Navas D, Asseray D. 2019. CSF concentration of ceftriaxone following high-dose administration: pharmacological data from two French cohorts. J Antimicrob Chemother 74:1753–1755. doi: 10.1093/jac/dkz047. [DOI] [PubMed] [Google Scholar]
- 21.EUCAST. 2019. MIC and zone distributions and ECOFFs. http://www.eucast.org/mic_distributions_and_ecoffs/.
- 22.Swartz MN. 2004. Bacterial meningitis—a view of the past 90 years. N Engl J Med 351:1826–1928. doi: 10.1056/NEJMp048246. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients. Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 24.Mouton JW, Punt N, Vinks AA. 2007. Concentration-effect relationship of ceftazidime explains why the time above the MIC is 40 percent for a static effect in vivo. Antimicrob Agents Chemother 51:3449–3451. doi: 10.1128/AAC.01586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J. 2018. MIC-based dose adjustment: facts and fables. J Antimicrob Chemother 73:564–568. doi: 10.1093/jac/dkx427. [DOI] [PubMed] [Google Scholar]
- 26.Goessens WHF, Mouton JW, ten Kate MT, Bijl AJ, Ott A, Bakker-Woudenberg IA. 2007. Role of ceftazidime dose regimen on the selection of resistant Enterobacter cloacae in the intestinal flora of rats treated for an experimental pulmonary infection. J Antimicrob Chemother 59:507–516. doi: 10.1093/jac/dkl529. [DOI] [PubMed] [Google Scholar]
- 27.Société Française d’Anesthésie et de Réanimation. 2018. Optimisation du traitement par bêta-lactamines. Société Française d’Anesthésie et de Réanimation, Paris, France: https://sfar.org/optimisation-du-traitement-par-beta-lactamines-chez-le-patient-de-soins-critiques/. [Google Scholar]
- 28.Le Turnier P, Navas D, Garot D, Guimard T, Bernard L, Tattevin P, Vandamme YM, Hoff J, Chiffoleau A, Dary M, Leclair-Visonneau L, Grégoire M, Pere M, Boutoille D, Sébille V, Dailly E, Asseray N. 2019. Tolerability of high-dose ceftriaxone in CNS infections: a prospective multicentre cohort study. J Antimicrob Chemother 74:1078–1085. doi: 10.1093/jac/dky553. [DOI] [PubMed] [Google Scholar]
- 29.Verdier M-C, Tribut O, Tattevin P, Le Tulzo Y, Michelet C, Bentué-Ferrer D. 2011. Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother 55:4873–4879. doi: 10.1128/AAC.00533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briscoe SE, McWhinney BC, Lipman J, Roberts JA, Ungerer JPJ. 2012. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 907:178–184. doi: 10.1016/j.jchromb.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, Sörgel F. 1993. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother 37:1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]