A total of 598 Neisseria gonorrhoeae isolates obtained from patients in Taiwan from 2001 to 2018 were evaluated. The MICs of ceftriaxone (CRO) and azithromycin (AZM) against the isolates were determined by the agar dilution method. N. gonorrhoeae isolates with AZM MICs of ≥1 μg/ml were identified and characterized by the presence of AZM resistance determinants.
KEYWORDS: Neisseria gonorrhoeae, Taiwan, azithromycin resistance, ceftriaxone
ABSTRACT
A total of 598 Neisseria gonorrhoeae isolates obtained from patients in Taiwan from 2001 to 2018 were evaluated. The MICs of ceftriaxone (CRO) and azithromycin (AZM) against the isolates were determined by the agar dilution method. N. gonorrhoeae isolates with AZM MICs of ≥1 μg/ml were identified and characterized by the presence of AZM resistance determinants. For high-level AZM-resistant (AZM-HLR) isolates (MIC ≥ 256 μg/ml), genotyping was performed using multilocus sequence typing (MLST) and N. gonorrhoeae multiantigen sequence typing (NG-MAST). Among the N. gonorrhoeae isolates studied, 8.7% (52/598) exhibited AZM MICs of ≥1 μg/ml. Thirteen of the 52 isolates contained A2059G (23S rRNA NG-STAR type 1) or C2611T (23S rRNA NG-STAR type 2) mutations. The prevalence of the A2059G mutation was higher in AZM-HLR isolates (P < 0.001). The −35A deletion in the promoter region of the mtrR gene did not differ between AZM-HLR isolates (100%, 10/10) and the isolates with AZM MICs of 1 μg/ml to 64 μg/ml (95.2%, 40/42) (P = 1.000). The presence of mutations in the mtrR coding region was significantly different between these two groups at 90% (9/10) and 26.2% (11/42), respectively (P < 0.001). The AZM-HLR isolates, all carrying four mutated A2059G alleles, a −35A deletion, and G45D, were classified as MLST 12039/10899 and NG-MAST 1866/16497. In conclusion, Taiwan is among the countries reporting gonococci with high-level resistance to AZM so that a single dose of 1 g ceftriaxone intramuscularly as the first choice for management of N. gonorrhoeae infection should be evaluated.
INTRODUCTION
Sexually transmitted disease caused by Neisseria gonorrhoeae is a global problem, and in 2012, the World Health Organization (WHO) estimated that the number of new cases of gonococcal infection worldwide was 78.3 million (1). In Taiwan, the incidence of N. gonorrhoeae infection has increased continuously, from 6.7 cases per 100,000 people in 2005 to 19.5 cases per 100,000 people in 2017 (2).
A wide spectrum of clinical manifestations and complications of N. gonorrhoeae infection has been observed, and no vaccine against N. gonorrhoeae is available at present; therefore, infection control predominantly relies on timely diagnostics with appropriate antibiotic therapy (1, 3, 4). Over time, the rapid development of N. gonorrhoeae resistance to a broad range of antimicrobial agents worldwide has threatened the management of N. gonorrhoeae infection (1). As the waning susceptibility of N. gonorrhoeae and the emergence of treatment failure with cephalosporins have been documented in several countries in the 2000s, dual therapy with ceftriaxone (CRO) (250 mg or 500 mg administered intramuscularly) plus azithromycin (AZM) (1 g or 2 g given orally) has been widely accepted as the first choice for the treatment of gonorrhea to ensure effective treatment and limit the development of antimicrobial resistance (3).
AZM exercises its capability to inhibit protein synthesis of N. gonorrhoeae by binding to the 23S rRNA component of the 50S ribosomal subunit. Specific mutations of 23S rRNA, i.e., A2059G and C2611T (Escherichia coli numbering), have been demonstrated to be associated with AZM resistance in N. gonorrhoeae (3). Mutations either in the coding region of the mtrR gene or in the mtrR promoter, leading to overexpression of the MtrCDE efflux pump, also affect AZM susceptibility (3). Other unusual mutations in N. gonorrhoeae, such as mutations in the genes erm, mef, rplD, and rplV, also conferred AZM resistance (3, 5).
In Taiwan, the rate of AZM resistance in N. gonorrhoeae isolates collected in northern Taiwan from 2001 to 2013 was demonstrated to be 1.3% based on the current MIC interpretive criteria recommended by the Clinical and Laboratory Standards Institute (CLSI), while the rate of non-wild type (NWT) was demonstrated to be 14.6% using the current MIC interpretive criteria recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (6). Because of the emergence of N. gonorrhoeae isolates with resistance to both CRO and AZM globally (7, 8), the continuous monitoring of resistance to AZM and the comprehension of relevant molecular mechanisms at a national level is crucial, particularly for regions with the presence of isolates exhibiting high-level resistance to AZM (AZM-HLR) (MICs of ≥256 μg/ml).
In the present study, we investigated nationwide trends of resistance to AZM and the related molecular mechanisms of N. gonorrhoeae isolates in Taiwan from 2001 to 2018.
RESULTS
Antimicrobial susceptibilities of the isolates.
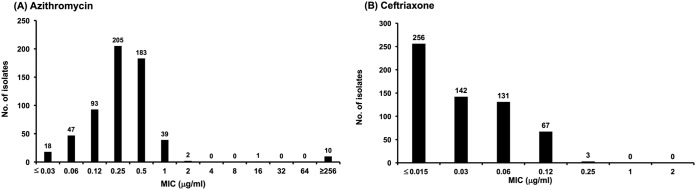
The MIC distributions of CRO and AZM for the 598 N. gonorrhoeae isolates are demonstrated in Fig. 1. The MICs of CRO ranged from 0.015 μg/ml to 0.25 μg/ml, with an MIC50 of 0.03 μg/ml and an MIC90 of 0.12 μg/ml. Only three isolates (0.5%) exhibited resistance to CRO (MIC > 0.125 μg/ml). The MICs of AZM ranged from 0.03 μg/ml to ≥256 μg/ml, with an MIC50 and MIC90 of 0.25 μg/ml and 0.5 μg/ml, respectively. Fifty-two isolates (8.7%) exhibited AZM MICs of ≥1 μg/ml. Among the 598 isolates, 13 (2.2%) were AZM NWT isolates (MICs of ≥2 μg/ml). Of the 13 AZM NWT isolates, two (0.3%) exhibited MICs of 2 μg/ml, one (0.17%) had an MIC of 16 μg/ml, and 10 (1.7%) had MICs of ≥256 μg/ml (AZM-HLR). No isolates exhibiting resistance to CRO and NWT to AZM were noted.
FIG 1.
Distribution of the MICs for azithromycin (A) and ceftriaxone (B) against 598 isolates of Neisseria gonorrhoeae from January 2001 to September 2018.
Azithromycin resistance determinants and penA allele.
Among the 52 isolates with AZM MICs of ≥1 μg/ml, 13 (25%) contained A2059G (23S rRNA NG-STAR type 1) or C2611T (23S rRNA NG-STAR type 2) mutated alleles in domain V of 23S rRNA (Table 1). Ten of those isolates possessed the A2059G substitution in all four alleles of 23S rRNA, demonstrating high-level resistance to AZM with MICs of ≥256 μg/ml, and one isolate had a single mutated A2059G allele with an MIC of 1 μg/ml. The prevalence of the A2059G mutation was higher in AZM-HLR isolates (P < 0.001). In the other two isolates, a C2611T substitution in 1 or 4 alleles of 23S rRNA were detected with an MIC of 1 μg/ml and 2 μg/ml, respectively.
TABLE 1.
Comparison of antimicrobial resistance determinants in isolates with azithromycin MICs of 1 μg/ml to 64 μg/ml versus high-level azithromycin-resistant (MIC ≥ 256 μg/ml) Neisseria gonorrhoeae isolates
| Resistance determinants | No. (%) of N. gonorrhoeae isolates with indicated MICs (n = 52) |
P value | |
|---|---|---|---|
| MIC = 1–64 μg/ml (n = 42) | MIC ≥ 256 μg/ml (n = 10) | ||
| 23S rRNA | |||
| A2059G | |||
| Wild type | 41 (97.6) | 0 (0.00) | <0.001 |
| A2059G in 1–2 alleles | 1 (2.40) | 0 (0.00) | <0.001 |
| A2059G in 3–4 alleles | 0 (0.00) | 10 (100) | <0.001 |
| C2611T | |||
| Wild type | 40 (95.2) | 10 (100) | 1.000 |
| C2611T in 1–2 alleles | 1 (2.44) | 0 (0.00) | 1.000 |
| C2611T in 3–4 alleles | 1 (2.44) | 0 (0.00) | 1.000 |
| mtrR promoter | |||
| Wild type | 2 (4.88) | 0 (0.00) | 1.000 |
| −35A deletion | 40 (95.2) | 10 (100) | 1.000 |
| mtrR coding region | |||
| Wild type | 31 (73.8) | 1 (10.0) | <0.001 |
| A39T | 8 (19.0) | 0 (0.00) | <0.001 |
| G45D | 3 (7.14) | 9 (90.0) | <0.001 |
Fifty (96.1%, 50/52) isolates bore a −35A deletion in the 13-bp inverted repeat sequence. Of the 42 isolates with AZM MICs of 1 μg/ml to 64 μg/ml, the majority (73.8%, 31/42) were wild type (WT) for the mtrR coding region, 19.0% (8/42) had A39T, and 7.14% (3/42) had G45D. Of the 10 AZM-HLR isolates, no A39T was identified, while 10% (1/10) were wild type for the mtrR coding region and 90.0% (9/10) had G45D. Analysis of the mtrR promoter region revealed that the prevalence of the −35A deletion was no different in AZM-HLR isolates (100%, 10/10) from that in the isolates with AZM MICs of 1 μg/ml to 64 μg/ml (95.2%, 40/42) (P = 1.000) (Table 1). In contrast, the presence of mutation in the mtrR coding region was significantly different between these two groups (P < 0.001) (Table 1).
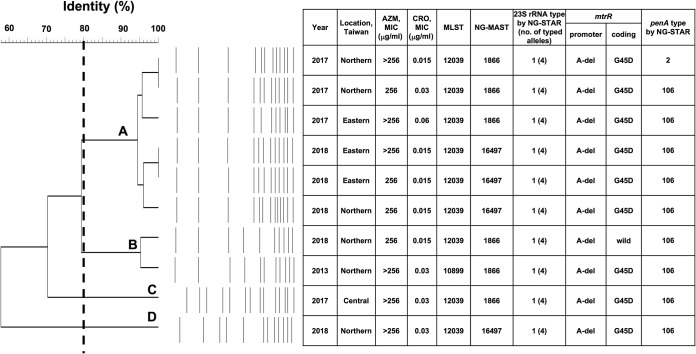
All of the 10 AZM-HLR isolates carried the nonmosaic penA allele, including nine isolates of NG-STAR type 106 and one of NG-STAR type 2 (Fig. 2).
FIG 2.
Pulsed-field gel electrophoresis profiles using the restriction enzyme NheI and a dendrogram of the 10 high-level azithromycin-resistant isolates. With a cutoff at 80% of similarity, four major clusters (A, B, C, and D) were identified. The year and region of collection and MICs of azithromycin and ceftriaxone are shown. Genetic relatedness determined by MLST and NG-MAST and resistance determinants, including the A2059G mutation, promoter region, and coding region of the mtrR gene and penA, are indicated. AZM, azithromycin; CRO, ceftriaxone; MLST, multilocus sequence typing; NG-MAST, N. gonorrhoeae multiantigen sequence typing; A-del, −35A deletion.
Genetic relatedness of N. gonorrhoeae isolates with AZM MICs of ≥1 μg/ml.
The 52 N. gonorrhoeae isolates with AZM MICs of ≥1 μg/ml comprised 24 different multilocus sequence types (MLSTs). Thirteen (54.2%, 13/24) sequence types (STs) were represented by only a single isolate, and the remaining STs were represented by two or more isolates. The most prevalent STs among the 42 isolates with AZM MICs of 1 μg/ml to 64 μg/ml were ST 1901 (21.4%; n = 9), ST 1583 (7.1%; n = 3), ST 7371 (7.1%; n = 3), and ST 11196 (7.1%; n = 3). Among the 10 AZM-HLR isolates, nine isolates (90%) were identified as MLST 12039 and one (10%) belonged to MLST 10899. N. gonorrhoeae multiantigen sequence typing (NG-MAST) analysis of the 10 AZM-HLR isolates revealed the following two types: NG-MAST 1866 (60%; n = 6) and NG-MAST 16497 (40%; n = 4) (Fig. 2).
PFGE and phylogenetic analysis of AZM-HLR isolates.
Pulsed-field gel electrophoresis (PFGE) analysis of the 10 AZM-HLR isolates revealed 12 ± 2 bands (Fig. 2). Cluster analysis of the resulting dendrograms defined four clusters (clusters A to D) at the level of ≥80% similarity. Cluster A encompassed six isolates, collected from northern and eastern Taiwan, and all were determined to be MLST 12039 but belonged to two different NG-MASTs (1866 and 16497). In contrast, the two isolates in cluster B belonged to the same NG-MAST 1866 but were defined as MLST 12039 and 10899, respectively. Cluster C consisted of only one isolate, typed as MLST 12039 and NG-MAST 1866. Lastly, cluster D contained one isolate designated as MLST 12039 and NG-MAST 16497 (Fig. 2).
Clinical characteristics of the 10 patients infected with AZM-HLR isolates.
The 10 patients (9 males and 1 female) with a median age of 37 years (range, 21 to 83 years) had gonococcal urethritis caused by AZM-HLR isolates. Nine of them were diagnosed during the 2017 to 2018 period, and one was infected in 2013 (Fig. 2). Geographical spread was throughout Taiwan as follows: six from northern Taiwan, three from eastern Taiwan, and one from central Taiwan. No sexual contact between each of them was reported. All of them were negative for HIV and syphilis infection. None of them had macrolide exposure within 60 days of diagnosis of N. gonorrhoeae infection or recent international travel history, except one patient who lived in Shenzhen, China.
DISCUSSION
In 2019, EUCAST recommended epidemiologic cutoff values (ECOFF) of 1 μg/ml for AZM among N. gonorrhoeae isolates to segregate bacterial populations into those belonging to the WT and those with acquired and/or mutational resistance mechanisms (NWT) so that N. gonorrhoeae isolates with AZM MICs of ≤1 μg/ml were classified as WT (9). In 2019, CLSI recommended clinical breakpoints of AZM and defined isolates with AZM MICs of ≤1 μg/ml as susceptible (10). However, many studies, including our present study, showed N. gonorrhoeae strains with an AZM MIC of 1 μg/ml carried the C2611T mutated 23S rRNA allele or mtrR gene (5, 11–13). It is noteworthy that AZM resistance determinants were also recognized in N. gonorrhoeae isolates with AZM MICs of <1 μg/ml (11, 12, 14). Close monitoring and further research is needed to monitor the evolution of wild-type N. gonorrhoeae.
Emergence of AZM-HLR N. gonorrhoeae has been described in many countries, including Argentina in 2009 (15), the United States in 2012 (16), Australia in 2015 (17), Canada in 2016 (11), China in 2016 (18), and the United Kingdom in 2018 (12). In this study, we first report the emergence and clustering of AZM-HLR N. gonorrhoeae in Taiwan. During the study period, 10 AZM-HLR isolates were gathered from different patients as follows: one in 2013, four in 2017, and five in 2018. None of these patients had been treated with a macrolide within 60 days of diagnosis of N. gonorrhoeae infection, which is compatible with the earlier study showing no association between previous azithromycin exposure and subsequent azithromycin-resistant N. gonorrhoeae isolates (19). Although this finding cannot imply development of azithromycin resistance, either bystander or direct selection (20), analysis of molecular typing of these AZM-HLR isolates is necessary. These isolates were sequenced as MLST 12039/10899 and NG-MAST 1866/16497. All of them had nonmosaic penA alleles and the same AZM resistance determinants, including 4 mutated 23S rRNA copies with the A2059G mutation, −35A deletion in the promoter region of the mtrR gene, and a G45D mutation in the mtrR coding region. The results of genotyping and PFGE suggest the clonal spread of AZM-HLR isolates in Taiwan and the circulation of a certain strain through sexual networks instead of de novo development. Furthermore, through the implementation of MLST and NG-MAST genotyping, it could be inferred that the AZM-HLR isolates circulating in Taiwan may originate from an internationally successful clone that evolved with spontaneous genetic events to the current variation observed in our study. China reported that AZM-HLR N. gonorrhoeae isolates belonging to NG-MAST 1866 in Hangzhou city in 2011 and 2012 carried identical AZM resistance determinants to our isolates, including the A2059G mutation, −35A deletion, and G45D mutation (18). Furthermore, in early 2018, the United Kingdom and Australia reported CRO- and AZM-resistant isolates G97687 and A2735, respectively, and they were typed as the same genotype, MLST 12039 and NG-MAST 16848 (7, 8). Compared to G97687 and A2735, our AZM-HLR isolates share the same MLST, 12039, and almost identical NG-MAST profile, NG-MAST 1866 and 16497, with a difference of 2 to 3 single nucleotide polymorphisms (SNPs), implying that our AZM-HLR isolates and G97687/A2735 might be clonally related. The discrepancy between the typing results of the MLST and NG-MAST may arise from different targeted DNA sequences. MLST identifies seven relatively conserved housekeeping genes (abcZ, adk, aroE, fumC, gdh, pdhC, and pgm) and is more suitable to deal with macroepidemiology, whereas NG-MAST associates with two variable genes, porB and tbpB, which explains its potential for microepidemiological investigations and its greater discriminatory ability than MLST. However, due to horizontal transfer of porB alleles among N. gonorrhoeae isolates, it is difficult to precisely describe the phylogenetic relationship between N. gonorrhoeae isolates solely based on the method of NG-MAST (21, 22). Therefore, whole-genome sequencing (WGS) of globally reported AZM-HLR isolates to provide a higher level of discrimination is needed to confirm international transmission.
An earlier study found one A2059G mutated 23S rRNA allele in AZM-susceptible isolates (MIC of 0.25 μg/ml) and also demonstrated that AZM-HLR isolates were descendants of the low-level resistant isolates (MIC of 1 μg/ml) and the susceptible isolates, supporting the theory that once a mutated allele exists within the organism, the further acquisition of multiple mutated alleles occurs without high barriers (12, 23). Owing to one isolate harboring an A2059G mutation in a single allele of 23S rRNA with an AZM MIC of 1 μg/ml in our study, it is of concern that our isolates with AZM MICs of <1 μg/ml may carry the A2059G mutation, and endogenous homologous recombination can be anticipated under the selection pressure, leading to a yield of AZM-HLR isolates (23). Moreover, as the frequency of chromosomal DNA transformation in gonococci can be very high (10−2/μg DNA/108 CFU) (3), we should be concerned that our isolates were only one step away from horizontal transfer of the mosaic penA allele to display resistance to both CRO and AZM. To keep the efficacy of CRO and AZM for the treatment of gonorrhea, substantially comprehensive surveillance of antimicrobial resistance and adequate prescription of AZM is necessary.
The contributions of mtrR mutations to AZM resistance have been controversial. Formerly, the hierarchy of the mtrR locus mutation regarding the MtrCDE efflux pump system had been established; that is, the promoter mutation expressed high levels of resistance (by ≥10-fold) while the missense mutations in the mtrR coding sequence typically resulted in low- to midlevel resistance (by 2- to 4-fold) (24). However, our results showed that only the mutation in the mtrR coding region, not the presence of the −35A deletion, has an impact on AZM MICs, a similar finding to the study of Wan et al. (25). In contrast, WGS analysis of AZM-resistant (MIC > 2 μg/ml) N. gonorrhoeae isolates (n = 75) in Europe from 2009 to 2014 revealed that neither mutations in mtrR nor its promoter significantly affect the MIC of AZM (26). Grad et al. further revealed that mtrR mutations were not associated with AZM resistance, and 81% of the collected isolates in the United States from 2000 to 2013 were resistant to AZM with unexplained mechanism (27). The complex may be partially explained by epistatic interactions at the mtrR gene obtained from multiple commensal Neisseria spp., such as Neisseria meningitidis and Neisseria lactamica, through DNA transformation (28).
This study had several potential limitations. First, not all of the collected isolates were tested to determine the presence of antimicrobial resistance determinants causing the underestimation of the clinical impact of these determinants. Second, information on antibiotic exposure history was lacking, so selection pressure on the dynamics of N. gonorrhoeae strains cannot be clearly elucidated. Third, with the lack of comparison of WGS data, it is hard to sharply draw the links between our isolates and internationally reported AZM-resistant strains to take timely action to preserve the efficacy of AZM for N. gonorrhoeae infection.
In conclusion, we presented the clonal spread of AZM-HLR isolates in Taiwan through sexual networks, and these isolates may originate from internationally successful isolates. A2059G mutated 23S rRNA was encountered in our study, and therefore we should pay close attention to the possible emergence of N. gonorrhoeae isolates with resistance to both CRO and AZM through spontaneous mutation and DNA transformation. Taiwan is now on the list of countries reporting gonococci with high-level resistance to AZM, compromising the efficacy of dual therapy with CRO plus AZM, so that we may follow the recommendation of the British Association for Sexual Health and HIV and take a single dose of ceftriaxone 1 g intramuscularly as the first choice for management of N. gonorrhoeae infection (29). Comprehensive efforts to avoid untreatable gonorrhea and reduce the burden of disease also should be taken across regional and national boundaries.
MATERIALS AND METHODS
Bacterial isolates.
From January 2001 to September 2018, 598 N. gonorrhoeae isolates were recovered from various sources, including urethral discharge, vaginal secretion, skin pus, eye discharge, blood, surgical wound, gastric juice, synovial fluid, and Bartholin abscess, of 598 patients, mainly from a medical center in northern Taiwan (62.2%, 372/598). The remaining isolates were obtained from another eight medical centers/regional hospitals in different geographical areas of Taiwan as follows: northern Taiwan, 17.6% (n = 105); central Taiwan, 13.0% (n = 78); southern Taiwan, 4.5% (n = 27); and eastern Taiwan, 2.7% (n = 16). These isolates were identified as oxidase-positive, Gram-negative, and kidney-shaped diplococci with slightly concave adjacent surfaces in smears and were confirmed by the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). All isolates were stored in Trypticase soy broth with 20% glycerol at −70°C. For further testing, these isolates were retrieved from storage, inoculated on chocolate agar, and incubated at 37°C in a 5% CO2-enriched atmosphere (6).
Collection of patient data.
Patients with N. gonorrhoeae infection were identified through review of microbiology laboratory records at the participating hospitals in Taiwan. The medical records of patients with AZM-HLR N. gonorrhoeae infection were retrospectively reviewed; this included history of human immunodeficiency virus (HIV) infection, syphilis, macrolide exposure within 60 days when N. gonorrhoeae infection was diagnosed, and travel history. The Institutional Review Board (IRB) of the National Taiwan University Hospital (201609066RINB) approved this study. Informed consent was waived by the IRB due to the retrospective nature of the project, and the study was performed in accordance with the Declaration of Helsinki.
Antimicrobial susceptibility testing.
MICs of the isolates resistant to CRO and AZM were determined by the agar dilution method using GC agar (Difco GC medium base; BBL Microbiology Systems, Cockeysville, MD) with a 1% defined growth supplement (IsoVitaleX; BBL Microbiology Systems, Becton and Dickinson, Sparks, MD, USA), as described previously (6). CRO and AZM were purchased from Sigma (Taipei, Taiwan). N. gonorrhoeae ATCC 49226 was used as the control strain.
N. gonorrhoeae isolates with AZM MICs of ≤1 μg/ml were defined as susceptible to AZM by the guidelines recommended by the CLSI (10). However, the EUCAST recommended an epidemiologic cutoff value (ECOFF) of 1 μg/ml AZM for defining WT (MICs of ≤1 μg/ml) and NWT (MICs of >1 μg/ml) isolates (9). In this study, N. gonorrhoeae isolates with AZM MICs of ≥256 μg/ml were defined as AZM-HLR. The MIC breakpoints used to determine resistance to CRO (MIC > 0.125 μg/ml) were in accordance with the 2019 EUCAST guideline (9).
Antimicrobial resistance determinants.
To identify the presence of mutations, gene sequences of domain V of the 23S rRNA, the mtrR, and the penA genes were amplified by PCR (30, 31). The DNA sequencing data was uploaded to a publicly accessible database on the NG-STAR website (https://ngstar.canada.ca), hosted by the Public Health Agency of Canada, National Microbiology Laboratory, to determine the antimicrobial resistance markers, including numbers of alleles with mutated A2059G or C2611T, −35A deletion of mrtR promoter region, mtrR coding gene, and penA type.
Multilocus sequence typing and multiantigen sequence typing for isolates with AZM MICs of ≥1 μg/ml.
Genetic relatedness of N. gonorrhoeae isolates with AZM MICs of ≥1 μg/ml was determined by using the MLST and NG-MAST methods as previously described (32, 33). In the N. gonorrhoeae MLST scheme, seven housekeeping genes, namely abcZ, adk, aroE, fumC, gdh, pdhC, and pgm, were amplified and sequenced. The sequences were submitted to the MLST website (https://pubmlst.org/neisseria/) to assign sequence types (STs). AZM-HLR N. gonorrhoeae isolates were additionally typed by the NG-MAST method. NG-MAST allele numbers of porB and tbpB and STs were obtained through the NG-MAST website (http://www.ng-mast.net) (33).
Pulsed-field gel electrophoresis for AZM-HLR isolates.
PFGE analysis was performed for AZM-HLR isolates only. DNA of the isolates was digested by the restriction enzyme NheI, and the restriction fragments were separated in a CHEF-DR III unit (Bio-Rad Laboratories, Hercules, CA, USA) at 200 V for 27 h. The pulsotypes were analyzed using the Bio-Rad CHEF Mapper apparatus (Bio-Rad Laboratories, Hercules, CA). Cluster analysis was performed using BioNumerics version 5.0 (Applied Maths, Sint-Martens-Latem, Belgium) and the unweighted pair-group method with arithmetic averages (UPGMA). The Dice correlation coefficient was used with a tolerance of 1% in order to analyze any similarities between banding patterns. Isolates showing identical PFGE patterns were considered to be the same strain (same pulsotypes), and isolates exhibiting PFGE patterns with a similarity of >80% were considered to represent closely related strains.
Statistical analysis.
Statistical significance was assessed using SPSS version 13.0 (SPSS Inc., Chicago, IL). Fisher’s exact test was used for statistical analyses. A P value of <0.001 was considered to be significant.
REFERENCES
- 1.Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK. 2017. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 17:e235–e279. doi: 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 2.Taiwan Centers for Disease Control. Taiwan CDC disease surveillance express. Taiwan Centers for Disease Control, Taipei, Taiwan: http://www.cdc.gov.tw/professional/report.aspx?v=D3C5BBCF8E60CF3D&treeid=3f2310b85436188d&nowtreeid=F78C19E5D2014555. [Google Scholar]
- 3.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao HY, Wang CH. 2017. Preseptal cellulitis caused by Neisseria gonorrhoeae: a rare disease need to be vigilant. J Microbiol Immunol Infect 50:397–398. doi: 10.1016/j.jmii.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Belkacem A, Jacquier H, Goubard A, Mougari F, La Ruche G, Patey O, Micaelo M, Semaille C, Cambau E, Bercot B. 2016. Molecular epidemiology and mechanisms of resistance of azithromycin-resistant Neisseria gonorrhoeae isolated in France during 2013–14. J Antimicrob Chemother 71:2471–2478. doi: 10.1093/jac/dkw182. [DOI] [PubMed] [Google Scholar]
- 6.Liu YH, Huang YT, Liao CH, Hsueh PR. 2018. Antimicrobial susceptibilities and molecular typing of Neisseria gonorrhoeae isolates at a medical centre in Taiwan, 2001–2013 with an emphasis on high rate of azithromycin resistance among the isolates. Int J Antimicrob Agents 51:768–774. doi: 10.1016/j.ijantimicag.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Whiley DM, Jennison A, Pearson J, Lahra MM. 2018. Genetic characterisation of Neisseria gonorrhoeae resistant to both ceftriaxone and azithromycin. Lancet Infect Dis 18:717–718. doi: 10.1016/S1473-3099(18)30340-2. [DOI] [PubMed] [Google Scholar]
- 8.Eyre DW, Sanderson ND, Lord E, Regisford-Reimmer N, Chau K, Barker L, Morgan M, Newnham R, Golparian D, Unemo M, Crook DW, Peto TE, Hughes G, Cole MJ, Fifer H, Edwards A, Andersson MI. 2018. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Euro Surveill 23:1800323. doi: 10.2807/1560-7917.ES.2018.23.27.1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0, 2019. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf.
- 10.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing. CLSI M100-ED29. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Demczuk W, Martin I, Peterson S, Bharat A, Van Domselaar G, Graham M, Lefebvre B, Allen V, Hoang L, Tyrrell G, Horsman G, Wylie J, Haldane D, Archibald C, Wong T, Unemo M, Mulvey MR. 2016. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 54:1304–1313. doi: 10.1128/JCM.03195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fifer H, Cole M, Hughes G, Padfield S, Smolarchuk C, Woodford N, Wensley A, Mustafa N, Schaefer U, Myers R, Templeton K, Shepherd J, Underwood A. 2018. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. Lancet Infect Dis 18:573–581. doi: 10.1016/S1473-3099(18)30122-1. [DOI] [PubMed] [Google Scholar]
- 13.Whiley DM, Kundu RL, Jennison AV, Buckley C, Limnios A, Hogan T, Enriquez R, El Nasser J, George CR, Lahra MM. 2018. Azithromycin-resistant Neisseria gonorrhoeae spreading amongst men who have sex with men (MSM) and heterosexuals in New South Wales, Australia, 2017. J Antimicrob Chemother 73:1242–1246. doi: 10.1093/jac/dky017. [DOI] [PubMed] [Google Scholar]
- 14.Wind CM, Bruisten SM, Schim van der Loeff MF, Dierdorp M, de Vries HJC, van Dam AP. 2017. A case-control study of molecular epidemiology in relation to azithromycin resistance in Neisseria gonorrhoeae isolates collected in Amsterdam, the Netherlands, between 2008 and 2015. Antimicrob Agents Chemother 61:e02374-16. doi: 10.1128/AAC.02374-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galarza PG, Alcala B, Salcedo C, Canigia LF, Buscemi L, Pagano I, Oviedo C, Vazquez JA. 2009. Emergence of high level azithromycin-resistant Neisseria gonorrhoeae strain isolated in Argentina. Sex Transm Dis 36:787–788. doi: 10.1097/OLQ.0b013e3181b61bb1. [DOI] [PubMed] [Google Scholar]
- 16.Katz AR, Komeya AY, Soge OO, Kiaha MI, Lee MV, Wasserman GM, Maningas EV, Whelen AC, Kirkcaldy RD, Shapiro SJ, Bolan GA, Holmes KK. 2012. Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin Infect Dis 54:841–843. doi: 10.1093/cid/cir929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens K, Zaia A, Tawil S, Bates J, Hicks V, Whiley D, Limnios A, Lahra MM, Howden BP. 2015. Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Australia. J Antimicrob Chemother 70:1267–1268. doi: 10.1093/jac/dku490. [DOI] [PubMed] [Google Scholar]
- 18.Ni C, Xue J, Zhang C, Zhou H, van der Veen S. 2016. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J Antimicrob Chemother 71:2355–2357. doi: 10.1093/jac/dkw131. [DOI] [PubMed] [Google Scholar]
- 19.Clifton S, Town K, Furegato M, Cole M, Mohammed H, Woodhall SC, Dunbar JK, Fifer H, Hughes G. 2018. Is previous azithromycin treatment associated with azithromycin resistance in Neisseria gonorrhoeae? A cross-sectional study using national surveillance data in England. Sex Transm Infect 94:421–426. doi: 10.1136/sextrans-2017-053461. [DOI] [PubMed] [Google Scholar]
- 20.Olesen SW, Grad YH. 8 April 2019. Deciphering the impact of bystander selection for antibiotic resistance in Neisseria gonorrhoeae. J Infect Dis. doi: 10.1093/infdis/jiz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goire N, Lahra MM, Chen M, Donovan B, Fairley CK, Guy R, Kaldor J, Regan D, Ward J, Nissen MD, Sloots TP, Whiley DM. 2014. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol 12:223–229. doi: 10.1038/nrmicro3217. [DOI] [PubMed] [Google Scholar]
- 22.Unemo M, Dillon JA. 2011. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev 24:447–458. doi: 10.1128/CMR.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 54:3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner DM, Shafer WM, Jerse AE. 2008. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol 70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan C, Li Y, Le WJ, Liu YR, Li S, Wang BX, Rice PA, Su XH. 2018. Increasing resistance to azithromycin in Neisseria gonorrhoeae in eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsson S, Golparian D, Cole M, Spiteri G, Martin I, Bergheim T, Borrego MJ, Crowley B, Crucitti T, Van Dam AP, Hoffmann S, Jeverica S, Kohl P, Mlynarczyk-Bonikowska B, Pakarna G, Stary A, Stefanelli P, Pavlik P, Tzelepi E, Abad R, Harris SR, Unemo M. 2016. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother 71:3109–3116. doi: 10.1093/jac/dkw279. [DOI] [PubMed] [Google Scholar]
- 27.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadsworth CB, Arnold BJ, Sater MRA, Grad YH. 2018. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 9:e01419-18. doi: 10.1128/mBio.01419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bignell C, Fitzgerald M, Guideline Development Group, British Association for Sexual Health and HIV UK. 2011. UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 22:541–547. doi: 10.1258/ijsa.2011.011267. [DOI] [PubMed] [Google Scholar]
- 30.Ng LK, Martin I, Liu G, Bryden L. 2002. Mutation in 23S rRNA associated with macrolide resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:3020–3025. doi: 10.1128/AAC.46.9.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao M, Gu WM, Yang Y, Dillon JA. 2011. Analysis of mutations in multiple loci of Neisseria gonorrhoeae isolates reveals effects of PIB, PBP2 and MtrR on reduced susceptibility to ceftriaxone. J Antimicrob Chemother 66:1016–1023. doi: 10.1093/jac/dkr021. [DOI] [PubMed] [Google Scholar]
- 32.Jolley KA. 2001. Multi-locus sequence typing. Methods Mol Med 67:173–186. doi: 10.1385/1-59259-149-3:173. [DOI] [PubMed] [Google Scholar]
- 33.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. 2004. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis 189:1497–1505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]