Abstract
Purpose
This study evaluated the association between night-shift work (NSW) and breast cancer risk as well as subtypes of breast cancer in Korean women.
Patients and methods
The study population included 1721 female breast cancer cases and 1721 female controls matched by age. The subtypes of breast cancer were determined based on estrogen, progesterone, and human epidermal growth factor receptor 2 statuses by reviewing pathology reports. Odds ratios (ORs) for NSW experience, age at commencement of NSW, frequency, and duration were estimated using conditional logistic regression and were adjusted for confounders such as parity and socioeconomic status–related factors.
Results
Among 1721 pairs, 10.58% of cases and 9.59% of controls had ever engaged in NSW. NSW was not associated with breast cancer risk in terms of ever having night-shift exposure (adjusted OR was 1.11, 95% confidence interval [CI] =0.89–1.40), duration, frequency, or cumulative working time. The OR for >10 years of lifetime duration of NSW was 1.55 (95% CI 0.89–2.69, P=0.124). In addition, the OR for >35,000 hrs for cumulative frequency of night work was OR=1.42 (95% CI=0.73–2.74, P=0.304). There was no heterogeneity in ORs of ever having NSW and cumulative duration of NSW between four subtypes of breast cancer.
Conclusion
NSW including long-term and heavy working exposure was not associated with breast cancer risk.
Keywords: night-shift work, breast cancer, hormone receptor, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2
Introduction
Breast cancer is the most common type of cancer in women worldwide which accounts for 30% of all new cancer diagnoses in women.1 The incidence rates vary worldwide with a higher incidence in Western Europe and lower incidence in Africa and East Asia. Higher risk of breast cancer in developed countries and rapid risk increment in developing countries2 have suggested that industrialization of societies is an important determinant of breast cancer risk. In Korea where rapid industrialization has been achieved, female breast cancer has increased continuously since 1999 and is the most common cancer in women.3 In the industrialized world, people often undergo some levels of circadian disruption through exposure to electric light at night or their working condition.4
Night-shift work (NSW) is common in industrialized societies with a prevalence of about 14% in women in Western countries.5,6 In Korea, 10.2–14.5% of the total workers are estimated to engage in NSW. The International Agency for Research on Cancer (IARC) evaluated the carcinogenicity of NSW and classified it as a probable carcinogen to humans in 2007 based on the results from epidemiological studies and biological plausibility including suppression of melatonin, modification of estrogen, or change in the expression of clock genes.7 Following the IARC report, several epidemiological studies have been implemented to evaluate the association between NSW and breast cancer. However, studies have shown varied results.6,8–22 Findings from some studies showed an increased association between working night shifts and risk of breast cancer in ever night workers,8 in women who worked for 20 or more years,9,10 in postmenopausal female workers,9 or in “graveyard shift” workers,12 and a dose–response relationship was found in a meta-analysis.6 However, results from other analyses showed weak evidence or no association15–18 and even protective relationship in overnight shift workers compared to day workers.19 In addition, the evidence from a meta-analysis of 10 large prospective studies with a total of 1.4 million women suggested a null association between NSW and breast cancer risk.20 The most recent studies denoted no overall short-term effect of night-shift work on risk of breast cancer,21 but the effect was found in long-term rotating night-shift workers who performed shift work during young adulthood.22
However, most of the epidemiological studies regarding NSW and risk of breast cancer were conducted in Western countries and there have been only two studies that investigated this association in the Asian population thus far.15,16 Both the studies were conducted in Chinese women and yielded null associations. Thus, in the Asian population, the remaining aspects regarding the association between NSW and breast cancer risk might include the inconsistency among published studies and limited amount of evidence. In addition, with reference to the different association of NSW according to hormonal receptor status of breast tissues,23,24 the differential effect of NSW according to hormone receptors needs to be explored in Asia as well. Therefore, we evaluated the association between NSW and breast cancer as well as stratified the risk by shift intensity and breast cancer subtypes based on hormone receptor status in the Korean female population where rapid Westernization has occurred during the past few decades.
Materials and methods
Study population
Women aged 20 years old or more who visited the Breast Cancer Center or Health Examination Center at the National Cancer Center in Korea from 2012 February to 2018 January were enrolled. Eligible cases of incident breast cancer were histologically confirmed by pathologists and breast cancer surgeons. Controls were selected from among women who visited the centers for health check-ups between 2012 and 2016. All eligible cases and controls were asked to give written informed consent. After obtaining informed consent by trained interviewers, information on night-work experience, demographic characteristics, reproductive factors, and lifestyle behaviors were collected using a structured questionnaire through face-to-face interviews. Controls were confirmed as being free-of-cancer based on medical record review, results of health checkup, and answers of questionnaires regarding past medical history. If they had any type of cancers, they were excluded from the analysis. The study protocol was reviewed and approved by the National Cancer Center Institutional Review Board (IRB number: NCCNCS13717). All procedures were performed in accordance with the Declaration of Helsinki 7th version.
During the enrollment period, a total of 2058 female patients with breast cancer and 1938 female controls with NSW information were recruited. The cases and controls were matched by 10-year age groups. For breast cancer patients, pathology reports were reviewed to access information about the hormone receptor status of breast cancer tissue including estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2). After checking medical records of enrolled patients, we classified breast cancer into four subtypes based on the receptor status: luminal A (ER and/or PR positive and HER2 negative), luminal B (ER and/or PR positive and HER2 positive), HER2-enriched (ER and PR negative and HER2 positive), and triple-negative (all receptors negative).25
Exposure measurement
NSW was defined as ever having worked in night shifts regularly between 9:00 pm and 8:00 am for at least 2 months in their lifetime. In addition, age at starting and ending NSW, clock-time of starting and finishing night shifts, the number of working days per week, the average length of shift cycles, and years on night schedule were obtained using a standardized questionnaire.
NSW exposure was classified as having ever or never engaged in night work, in addition to the length of exposure and the shift schedule. Shift schedule was detailed for age at starting to work night shifts, number of days per week for NSW exposure, clock time at starting night shifts, and lifetime duration of night work as recommended by the IARC working group.26 The duration of NSW involvement was classified into three levels: 10 years and under, longer than 10 years. To integrate the shift duration and intensity, the lifetime cumulative hours of night work were computed by multiplying the number of years with the number of days per week with NSW, and hours of working per NSW.
Statistical analysis
The distribution of demographic characteristics and other covariates in the study population was described by frequencies and percentages. The associations between all selected risk factors and breast cancer as well as with NSW were determined by univariate logistic regression models.
The association between NSW and breast cancer risk was presented as odds ratios (ORs) with 95% confidence intervals (CIs) and adjusted ORs. Confounding factors to be adjusted for were assessed via two steps: literature review and confirmation in our dataset. Ijaz et al14 had systematically assessed confounding for this association using directed acyclic graph and selected age, ethnicity, parity, and socioeconomic status as confounders that may truly affect the association between NSW and breast cancer. Thus, we applied the selected confounders from Ijaz et al to our analysis, except ethnicity because our all study population was Korean. For age, we frequently matched cases and controls by age group and adjusted for age as continuous variable to avoid residual confounding effect. As a proxy for socioeconomic status, educational level (less than university, university or higher) was considered. For parity-related factors, we considered the number of pregnancies and age at birth of the first child.
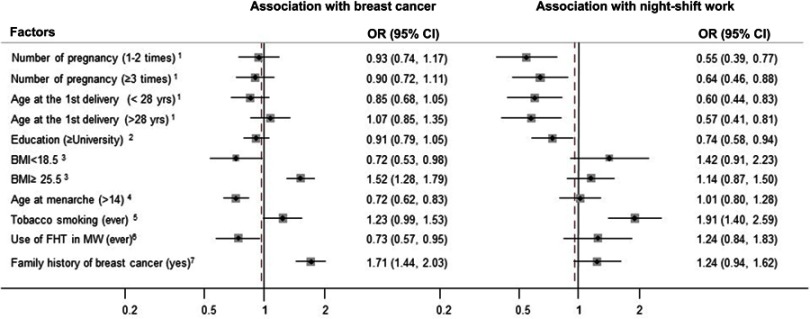
We checked whether these factors were confounders in our data by using univariate logistic regression and assessing whether they were related to both NSW and breast cancer (Figure 1). We additionally considered whether the established risk factors for breast cancer such as body mass index (BMI), age at menarche, tobacco smoking, female hormone treatment therapy, and family history of breast cancer were associated with both NSW and breast cancer.27
Figure 1.
The odds ratios (ORs) showed the association between all the selected factors and night-shift work as well as breast cancer. 1Never pregnancy as reference, 2high school and under as the reference, 318.5≤ BMI<25 as reference, 4aged 14 and under as reference, 5never smoking tobacco as reference, 6never use of female hormone treatment in menopausal women as reference, 7not having family history of breast cancer in first-degree relatives as reference.
Abbreviations: yrs, years; BMI, body mass index (kg/m2); FHT, female hormone treatment; MW, menopausal women.
This included age at diagnosis time for cases and age at interviewing time for controls, the number of pregnancies, age at birth of first child, and educational levels to yield a minimally adjusted result. The fully adjusted ORs incorporated all established risk factors including age, number of pregnancies, age at birth of first child, educational levels, BMI, age at menarche, tobacco smoking, use of female hormone treatment, and family history of breast cancer to yield a fully adjusted result. This was performed by multivariate conditional logistic regression using never worked in night shift as the reference. The missing information on detailed NSW information and covariates was treated as a dummy variable and included in the analysis.
To investigate whether the association between NSW and breast cancer would be altered by the hormone receptor status, we also performed multiple logistic regression models according to luminal A, luminal B, HER2-enriched, and triple-negative subtypes, and the results were presented as ORs and 95%CIs. The heterogeneity among the results by breast cancer subtypes was tested by using the likelihood ratio test. All analyses were performed using SAS software version 9.4.
Results
A total of 1721 cases and 1721 10-year age-group matched controls were included in the analysis. The distribution of baseline characteristics in our matched study population is presented in Table 1. We confirmed that the age as a matching variable was not different between cases and controls. The distribution of known risk factors for breast cancer, including pregnancy, number of pregnancies, age at birth of first child, BMI, age at menarche, tobacco smoking, and family history of breast cancer, were significantly different between the two groups.
Table 1.
Distribution of baseline characteristics in age-matched breast cancer patients and controls
| Characteristics | Controls (N=1721) | Cases (N=1721) | ||
|---|---|---|---|---|
| N | N | % | N | % |
| Age group (years) | ||||
| <30 | 38 | 2.3 | 38 | 2.3 |
| 30–39 | 205 | 11.9 | 205 | 11.9 |
| 40–49 | 752 | 43.7 | 752 | 43.7 |
| 50–59 | 530 | 30.8 | 530 | 30.8 |
| 60–69 | 174 | 10.1 | 174 | 10.1 |
| ≥70 | 22 | 1.3 | 22 | 1.3 |
| Number of pregnanciesa | ||||
| Nullipara | 208 | 12.2 | 187 | 11.2 |
| 1–2 | 586 | 34.4 | 564 | 33.9 |
| ≥3 | 909 | 53.4 | 912 | 54.8 |
| Age at birth of first child (years)b | ||||
| Nullipara | 208 | 12.5 | 187 | 11.7 |
| <28 | 867 | 52.2 | 919 | 57.5 |
| ≥28 | 586 | 35.3 | 492 | 30.8 |
| Educationc | ||||
| <High school | 722 | 48.9 | 704 | 46.4 |
| ≥University | 756 | 51.2 | 813 | 53.6 |
| BMI (kg/m2)d | ||||
| <18.5 | 1181 | 70.3 | 1279 | 75.5 |
| ≥18.5–24.9 | 73 | 4.3 | 110 | 6.5 |
| ≥25 | 426 | 25.4 | 304 | 18.0 |
| Age at menarche (years)e | ||||
| ≤14 | 1213 | 71.1 | 1090 | 63.9 |
| >14 | 493 | 28.9 | 616 | 36.1 |
| Tobacco smokingf | ||||
| Never | 1521 | 88.6 | 1544 | 90.6 |
| Ever | 195 | 11.4 | 161 | 9.4 |
| Use of female hormone treatment in menopausal womeng | ||||
| Never | 455 | 73.4 | 530 | 78.9 |
| Ever | 165 | 26.6 | 142 | 21.1 |
| Family history of breast cancer in first-degree relativesh | ||||
| No | 1434 | 84.7 | 1290 | 76.3 |
| Yes | 260 | 15.3 | 400 | 23.7 |
Notes: a18 cases and 58 controls with missing information; b60 cases and 123 controls with missing information; c204 cases and 243 controls with missing information; d41 cases and 28 controls with missing information; e15 cases and 15 controls with missing information; f5 cases and 16 controls with missing information; gestimated in 721 postmenopausal cases and 645 postmenopausal controls, 49 cases and 25 controls with missing information; h31 cases and 27 controls with missing information.
Abbreviations: N, number; BMI, body mass index (kg/m2).
The associations between all considered covariates and breast cancer as well as night shifts are presented in Figure 1. The number of pregnancies, age at birth of first child, educational level, and tobacco smoking were significantly associated with NSW. However, the association between these variables and breast cancer risk was not observed. Furthermore, BMI, age at menarche, and family history of breast cancer were significantly associated with breast cancer risk. Thus, we adjusted for age at birth of first child, number of pregnancies, and educational levels, in addition to all considered factors in our analysis.
The frequencies of having ever engaged in NSW were 10.6% and 9.6% in breast cancer patients and controls, respectively. In our study population, there was no significant association between ever engaging in NSW and breast cancer indicated by the minimally adjusted OR of 1.11 (95%CI 0.89–1.40, Table 2). Furthermore, age at starting their first NSW, frequency of NSW (number of NSWs per week), and NSW starting time did not show association with breast cancer risk. The minimally adjusted ORs and fully adjusted ORs were similar. Women with more than 10 years of cumulative NSW exposure were not associated with risk of breast cancer compared with women who had never engaged in NSW (minimally adjusted OR=1.55, 95% CI=0.89–2.69). A similar effect was found with respect to cumulative NSW exposure expressed in hours. No significant association between more than 35,000 hrs of night-shift exposure and risk of breast cancer was observed (minimally adjusted OR=1.42, 95% CI=0.73–2.74). Increase in lifetime cumulative frequency of night-shift work did not correspond to an increased risk of breast cancer (P-trend=0.168).
Table 2.
Distribution of night-shift work characteristics and associations with breast cancer in age-matched breast cancer patients and controls
| Characteristics | Controls | Cases | aAdjusted OR (95%CI) | bAdjusted OR (95%CI) | ||
|---|---|---|---|---|---|---|
| (N=1721) | (N=1721) | |||||
| N | % | N | % | |||
| Night-shift work status | ||||||
| Had job but no NSW | 1556 | 90.4 | 1539 | 89.4 | ref | ref |
| Ever | 165 | 9.6 | 182 | 10.6 | 1.11 (0.89–1.40) | 1.10 (0.88–1.39) |
| Age at starting working night shifts (years)c | ||||||
| ≤30 | 86 | 5.0 | 96 | 5.6 | 1.11 (0.82–1.51) | 1.08 (0.8–1.49) |
| >30 | 72 | 4.2 | 82 | 4.8 | 1.16 (0.84–1.62) | 1.16 (0.83–1.63) |
| Number of days per weekd | ||||||
| 1–5 days | 77 | 4.5 | 88 | 5.2 | 1.17 (0.85–1.60) | 1.13 (0.82–1.57) |
| >5 days | 66 | 3.9 | 78 | 4.6 | 1.17 (0.84–1.65) | 1.16 (0.83–1.66) |
| Time starting NSW (clock time)e | ||||||
| Before midnight | 137 | 8.0 | 163 | 9.5 | 1.20 (0.94–1.52) | 1.17 (0.93–1.51) |
| Midnight and after | 7 | 0.4 | 7 | 0.4 | 0.98 (0.34–2.80) | 0.85 (0.30–2.49) |
| Lifetime duration of night work (years)f | ||||||
| ≤10 | 139 | 8.1 | 145 | 8.4 | 1.07 (0.83–1.36) | 1.05 (0.83–1.36) |
| >10 | 21 | 1.2 | 35 | 2.0 | 1.55 (0.89–2.69) | 1.44 (0.82–2.55) |
| p-trend | ||||||
| Lifetime cumulative frequency of night work (hrs)f | ||||||
| ≤10,000 | 86 | 5.0 | 94 | 5.5 | 1.11 (0.82–1.50) | 1.08 (0.80–1.49) |
| >10,000 – ≤35,000 | 59 | 3.7 | 63 | 3.9 | 1.09 (0.76–1.57) | 1.09 (0.76–1.59) |
| >35,000 | 15 | 0.6 | 23 | 1.1 | 1.42 (0.73–2.74) | 1.30 (0.66–2.58) |
Notes: 95% CI estimated using conditional logistic regression models; aadjusted for age, educational level, number of pregnancies, and age at birth of first child; badjusted for age, educational level, number of pregnancies, age at birth of first child, body mass index, age at menarche, alcohol consumption, smoking, use of female hormone treatment, and family history of breast cancer in first degree relatives; c4 cases and 7 controls with missing information; d16 cases and 22 controls with missing information; e12 cases and 21 controls with missing information; f2 cases and 5 controls with missing information.
Abbreviation: N, number.
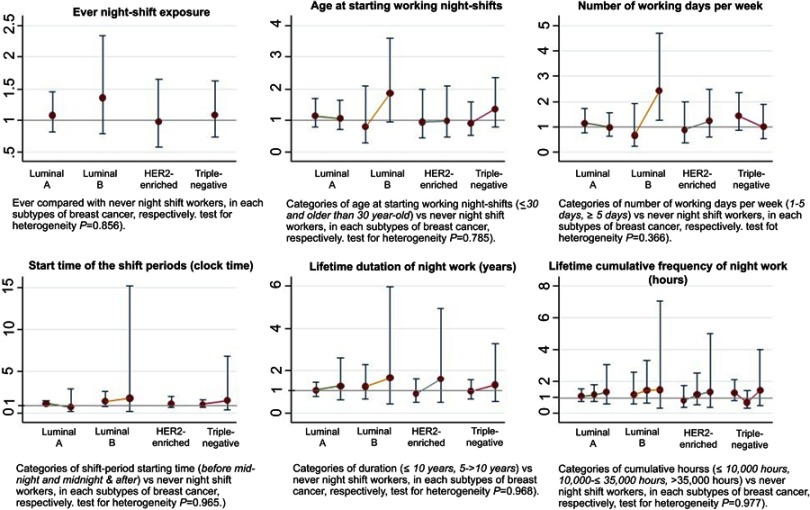
Among 1721 cases, 1529 had hormonal receptor status in ER and PR and HER2. The numbers of luminal A, luminal B, HER2-enriched, and triple-negative breast cancer were 860, 134, 187, and 348, respectively. When the association between NSW and subtypes of breast cancer was investigated (Figure 2 and detailed in Supplementary 1), overall, ever having night-shift exposure did not significantly increase breast cancer risk on every subtype of breast cancer. Although the point estimate for the risk of HER2-enriched breast cancer was an OR of 0.98 [95%CI=0.58–1.66], which was lower compared to luminal A (OR=1.09, 95%CI=0.82–1.45), luminal B (OR =1.36, 95%CI=0.79–2.33), and triple-negative (OR=1.10, 95%CI=0.74–1.62) subtypes, the heterogeneity among the ORs was not confirmed (p=0.856). Analyses for NSW characteristics showed null findings except for luminal B. Women who worked night shifts more than 5 days per week had a significantly higher risk of luminal B breast cancer than never workers at 2.42 times (95%CI=1.25–4.69). There were no heterogeneous associations between NSW and breast cancer among the four subtypes of breast cancer with respect to age at starting their first NSW, frequency of NSW, NSW starting time, and cumulative exposure to NSW (Figure 2 and Supplementary 1).
Figure 2.
Adjusted OR (minimal adjustment) and 95% CI in 4 molecular subtypes of breast cancer (x axis) for odds ratios for breast cancer (y axis). The rounded points and CIs represent the estimated risks from each NSW exposure level in the study. While luminal A (ER- and/or PR-positive and HER2-negative), luminal B (ER- and/or PR-positive and HER2-positive), HER2-enriched (ER- and PR-negative and HER2-positive), and Triple-negative (all receptors negative), and OR and 95%CI from the multivariate logistic regression models.
In terms of the association between NSW and breast cancer risk stratified by menopausal status, no significant association or difference was found in the risk of breast cancer regarding high intensity and heavy exposure with NSW between premenopausal and postmenopausal women (Supplementary 2).
Discussion
In this case–control study among Korean women, NSW was not associated with the risk of breast cancer. The null findings were observed regardless of any shift work intensity and breast cancer subtypes.
Our findings were consistent with recent studies that showed no association between NSW and breast cancer15–17,20,22,28–30 even with long-term exposure to NSW15,16,20,28 or any hormone receptor status of breast cancer.24 Although several previous studies9,12,31–34 and meta-analysis11 have shown an increased risk among women who had engaged in NSW, recent meta-analyses have shown inconsistent results.13,14,20 The increased risk of NSW on breast cancer in previous studies could be possibly attributed to the characteristics of the study populations. Previous studies included women engaged in particular occupations with high intensity of NSW such as nurses, flight attendants, or militaries,9,32,35 or in women with “graveyard” shift work.12 All of the workers had heavy exposure levels of night shift compared to the general workers.
In terms of the general population, some studies found an increased risk of breast cancer in long-term workers with working periods longer than 15–20 years or heavy work frequencies;12,23,33,34 however, these were not observed in other prospective studies.15-17,20 It has been suggested that the report from the IARC regarding shiftwork in 20077 was primarily based on experimental evidence and considered epidemiological evidence was limited, followed by overestimation of the effect.20,36 A recent meta-analysis20 using 10 large prospective cohort studies provided evidence for no association between 20 or more years of NSW and breast cancer risk (RR=1.01; 95% CI=0.93–1.10), suggesting lack of an association even with long duration of NSW. In our study, even the duration of cumulative NSW of more than 10 years or 35,000 hrs did not show any significant differences despite increased OR point estimates of 1.44 or 1.30. Considering a small number of subjects included in these categories (1–2% of cases or controls), the insignificant results would be caused by no real association as previous studies15,16,20,28 or false-negative results due to low power. Although the definitions of NSW varied, the International Labor Organization defined night shift as full-time working period between 24.00 and 05.00.37 The 24.00–05.00 includes the time for physiological peak of melatonin biosynthesis which is around 01.00–02.00 AM. We categorized the time of NSW initiation before and after midnight and compared them with those who had never engaged in NSW. However, we could not accord significance to the results with similar OR irrespective of the time of NSW initiation. Further, it has been suggested that age at starting NSW in relation to the status of the breast tissue would be critical, and women exposed to NSW at a young age were more vulnerable to breast cancer.22 However, our results did not show an association between age at starting NSW and breast cancer. Inconsistent results of the previous studies may be due to, in part, varied definitions of NSW and its duration, potential recall bias, and limited adjustment for confounders. In addition, we evaluated the association between NSW and breast cancer risk stratified by menopausal status considering its effect on circulating estradiol levels;38 however, no differences were observed.
Two studies regarding the association between NSW and breast cancer conducted in China showed no association.15,16 In these studies, even longer durations and higher frequencies of exposure were not associated with the risk of breast cancer. Biological differences between Asian women and women in Western countries in terms of human endocrinology, particularly melatonin or difference in the circadian genes, were suggested as factors accounting for the differences. The decrease of the circulating melatonin concentrations could result in cancer development,39 and after night work, the amount of reduction in urinary 6-sulfatoxymelatonin levels was lower in Asian people, suggesting that Asian night workers were better at maintaining their melatonin production.40 Asian populations have lower frequencies of rs23051560 Thr/Thr, one of the neuronal PAS domain protein 2 genes associated with breast cancer risk, compared to European populations, suppressing the effect of shift work.41
When the association between NSW and breast cancer subtypes based on hormone receptor status was observed, we did not find any heterogeneity among ORs. This may be due in part to the small number of participants in each category. Although several previous studies showed stronger associations in hormone receptor–positive groups,22–24,42,43 other studies showed higher association in ER-negative breast cancer29 or did not present heterogeneous results according to the hormone receptor status.42,43
To the best of our knowledge, this is the first study evaluating the association between NSW and breast cancer in Korean women and the third study conducted in the Asian population. Our findings will provide additional evidence about the association between NSW and breast cancer risk in the Asian population. One of the strengths of our study included detailed night-shift characteristics (shift systems, rotation cycle, years on night-shift schedule, recurred the second exposure) in general population. This was a well-designed case–control study, where possible confounding factors were considered. Adequate pathology information about the hormone receptors located in the breast cells (ER±, PR±, and HER2±) was obtained to classify breast cancers into detailed breast cancer subtypes.
There are several limitations in this study. This was a hospital-based case–control study and our findings may not be generalizable. We compared the prevalence of NSW with the result from the sixth (2013–2015) Korean National Health and Nutrition Examination Survey (KNHANES), which provides nationally representative results in Korea in the same period as our study.44 The KNHANES evaluated the night-shift work status of participants by the question “Do you usually work during the day (between 6 am and 6 pm), or do you work at a different time in last one year?”. Because of the questionnaire approaches were different, we did not have enough data to compare the prevalence of night-shift work of general and our study population. The results for last-one-year exposure of this study population were lower than KNHANES result (3.0% in KNHANES vs 1.1% in the control group). Furthermore, around 1.27 million workers involved in night-shift work accounting for 11.2% of total workers in the Survey Report on Labor Conditions by Employment Type.45 Thus, our study population was deemed to have a similar prevalence of night-shift work (10.1%) compared to general Korean population. To increase comparability between cases and controls to minimize the association being likely to be attributable to differences in basic characteristics, we matched age groups of 10-year interval, but this might cause a selection bias. Another limitation is recall bias. Participants in the case group are usually better in recalling the previous exposures to risk factors compared with those in the control group. However, our data showed similar night-shift exposure in the case and control group, which can possibly explain our null findings. Insignificant associations possibly caused by low power due to the small number of subjects with experience of high intensity of NSW would be also suspected. Although we included subjects with NSW information (ever or never), detailed information on NSW such as age at initiation, number of days, start time, or duration and covariates was not obtained from a few participants. They were all included in the analysis by treating missing variables as dummy, but it might cause information bias. The period of recruitment of breast cancer cases and controls was different, and time bias might affect the result. Regarding adjusted variables for the association between NSW and breast cancer, among the covariates from Ijaz et al,14 the number of pregnancies, age at birth of first child, and educational level were significantly associated with NSW but were not associated with breast cancer. Thus, as the definition of a confounder,27 parity-related factors may not confound the association between NSW and breast cancer. Thus, adjusting for both parity-related factors (age at birth of first child and number of pregnancies) and educational levels, which represent socioeconomic status, in addition to all considered factors in our analysis, might be an over-adjustment, followed by less significant result. In summary, ever engaged in NSW, long-term or heavy NSW exposure was not associated with breast cancer risk. It is untimely to conclude night-shift work as a probable cause of cancer. Our results, in combination with other Asian studies, provide evidence that may explain the effects of NSW on Asian and Caucasian populations.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1C1B1013621 and NRF-2019R1H1A1079862). The funders had no role in study design, data collection, and analysis, decision to present, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2018, Ministry of Health and Welfare; 2018. Available from: http://ncc.re.kr/main.ncc?uri=english/sub04_Statistics. Accessed July29, 2017.
- 4.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014;64(3):207–218. doi: 10.3322/caac.21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parent-Thirion A, Vermeylen G, Van Houten G, et al. Fifth European Working Conditions Survey. Luxembourg: Publications Office of the European Union; 2012. [Google Scholar]
- 6.Wang F, Yeung KL, Chan WC, et al. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol. 2013;24(11):2724–2732. doi: 10.1093/annonc/mdt283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IARC Monographs on the Evaluation of Cardinogenic Risks to HumansPainting, Firefighting, and Shiftwork. Vol. 98 Lyon: IARC Press, International Agency for Research on Cancer; 2010. [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. [DOI] [PubMed] [Google Scholar]
- 9.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563 [DOI] [PubMed] [Google Scholar]
- 10.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–111. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Lu Y, Wu K, et al. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37(3):197–206. doi: 10.1016/j.canep.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557 [DOI] [PubMed] [Google Scholar]
- 13.Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138(1):291–301. doi: 10.1007/s10549-013-2433-1 [DOI] [PubMed] [Google Scholar]
- 14.Ijaz S, Verbeek J, Seidler A, et al. Night-shift work and breast cancer–a systematic review and meta-analysis. Scand J Work Environ Health. 2013;39(5):431–447. doi: 10.5271/sjweh.3371 [DOI] [PubMed] [Google Scholar]
- 15.Li W, Ray RM, Thomas DB, et al. Shift work and breast cancer among women textile workers in Shanghai, China. Cancer Causes Control. 2015;26(1):143–150. doi: 10.1007/s10552-014-0493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pronk A, Ji BT, Shu XO, et al. Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol. 2010;171(9):953–959. doi: 10.1093/aje/kwq029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesch B, Harth V, Rabstein S, et al. Night work and breast cancer - results from the German GENICA study. Scand J Work Environ Health. 2010;36(2):134–141. doi: 10.5271/sjweh.2890 [DOI] [PubMed] [Google Scholar]
- 18.Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007;336–343. doi: 10.5271/sjweh.1150 [DOI] [PubMed] [Google Scholar]
- 19.O’leary ES, Schoenfeld ER, Stevens RG, et al. Shift work, light at night, and breast cancer on long Island, New York. Am J Epidemiol. 2006;164(4):358–366. doi: 10.1093/aje/kwj211 [DOI] [PubMed] [Google Scholar]
- 20.Travis RC, Balkwill A, Fensom GK, et al. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J Natl Cancer Inst. 2016;108(12):djw169. doi: 10.1093/jnci/djw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vistisen HT, Garde AH, Frydenberg M, et al. Short-term effects of night shift work on breast cancer risk: a cohort study of payroll data. Scand J Work Environ Health. 2017;43(1):59–67. doi: 10.5271/sjweh.3603 [DOI] [PubMed] [Google Scholar]
- 22.Wegrzyn LR, Tamimi RM, Rosner BA, et al. Rotating night shift work and risk of breast cancer in the nurses’health studies. Am J Epidemiol. 2017;186(5):532–540. doi: 10.1093/aje/kwx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy A, Richardson H, Burstyn I, et al. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013;70(12):831–838. doi: 10.1136/oemed-2013-101482 [DOI] [PubMed] [Google Scholar]
- 24.Cordina-Duverger E, Koudou Y, Truong T, et al. Night work and breast cancer risk defined by human epidermal growth factor receptor-2 (HER2) and hormone receptor status: a population-based case-control study in France. Chronobiol Int. 2016;33(6):783–787. doi: 10.3109/07420528.2016.1167709 [DOI] [PubMed] [Google Scholar]
- 25.Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens RG, Hansen J, Costa G, et al. Considerations of circadian impact for defining ‘shift work’in cancer studies: IARC working group report. Occup Environ Med. 2011;68(2):154–162. doi: 10.1136/oem.2009.053512 [DOI] [PubMed] [Google Scholar]
- 27.Gordis L. Epidemiology. 5th ed. Vol. 15 Philadelphia, PA: Elsevier Saunders; ; 2014. [Google Scholar]
- 28.Koppes LL, Geuskens GA, Pronk A, Vermeulen RC, De Vroome EM. Night work and breast cancer risk in a general population prospective cohort study in the Netherlands. Eur J Epidemiol. 2014;29(8):577–584. doi: 10.1007/s10654-014-9938-8 [DOI] [PubMed] [Google Scholar]
- 29.Rabstein S, Harth V, Pesch B, et al. Night work and breast cancer estrogen receptor status–results from the German GENICA study. Scand J Work Environ Health. 2013;39(5):448–455. doi: 10.5271/sjweh.3360 [DOI] [PubMed] [Google Scholar]
- 30.Menegaux F, Truong T, Anger A, et al. Night work and breast cancer: a population‐based case–control study in France (the CECILE study). Int J Cancer. 2013;132(4):924–931. doi: 10.1002/ijc.27669 [DOI] [PubMed] [Google Scholar]
- 31.Knutsson A, Alfredsson L, Karlsson B. et al. Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scand J Work Environ Health;2013. 170–177. doi: 10.5271/sjweh.3323 [DOI] [PubMed] [Google Scholar]
- 32.Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur J Cancer. 2012;48(11):1722–1729. doi: 10.1016/j.ejca.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Lie J-AS, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control. 2006;17(1):39–44. doi: 10.1007/s10552-005-3639-2 [DOI] [PubMed] [Google Scholar]
- 34.Akerstedt T, Knutsson A, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and breast cancer in women: a Swedish cohort study. BMJ Open. 2015;5(4):e008127. doi: 10.1136/bmjopen-2015-008127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69(8):551–556. doi: 10.1136/oemed-2011-100240 [DOI] [PubMed] [Google Scholar]
- 36.Noone P. Nightshift breast cancer, flour dust and blue-light risk. Occup Med (Chic Ill). 2010;60(6):499. doi: 10.1093/occmed/kqq096 [DOI] [PubMed] [Google Scholar]
- 37.C171 - Night Work Convention; 1990. Accessed August25, 2017.
- 38.Bracci M, Copertaro A, Manzella N, et al. Influence of night-shift and napping at work on urinary melatonin, 17-beta-estradiol and clock gene expression in pre-menopausal nurses. J Biol Regul Homeost Agents. 2013;27(1):267–274. [PubMed] [Google Scholar]
- 39.Mirick DK, Davis S. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol Prev Biomarker. 2008;17(12):3306–3313. doi: 10.1158/1055-9965.EPI-08-0605 [DOI] [PubMed] [Google Scholar]
- 40.Bhatti P, Mirick DK, Davis S. Racial differences in the association between night shift work and melatonin levels among women. Am J Epidemiol. 2013;177(5):388–393. doi: 10.1093/aje/kws278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monsees GM, Kraft P, Hankinson SE, Hunter DJ, Schernhammer ES. Circadian genes and breast cancer susceptibility in rotating shift workers. Int J Cancer. 2012;131(11):2547–2552. doi: 10.1002/ijc.27564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papantoniou K, Castaño-Vinyals G, Espinosa A, et al. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur J Epidemiol. 2016;31(9):867–878. doi: 10.1007/s10654-015-0073-y [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Ren FM, Lin Y, et al. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16(4):462–468. doi: 10.1016/j.sleep.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 44.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention, Korea Health Statistics Korea National Health and Nutrition Examination Survey (KNHANES); 2007–2015. Available from: https://knhanes.cdc.go.kr/knhanes/eng/index.do. Accessed July29, 2017.
- 45.Lee H-E, Lee J, Jang T-W, Kim I-A, Park J, Song J. The relationship between night work and breast cancer. Ann Occup Environ Med. 2018;30(1):11. doi: 10.1186/s40557-018-0273-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2018, Ministry of Health and Welfare; 2018. Available from: http://ncc.re.kr/main.ncc?uri=english/sub04_Statistics. Accessed July29, 2017.