Abstract
A new delivery microdevice, based on hydrophobic oleic acid‐capped mesoporous silica particles and able to payload release in the presence of surfactants, has been developed. The oleic acid functionalization confers to the system a high hydrophobic character, which avoids cargo release unless surfactant molecules are present. The performance of this oleic‐acid capped microdevice in the presence of different surfactants is presented and its zero‐release operation in the absence of surfactants is demonstrated.
Keywords: mesoporous materials, oleic acid, surfactants, controlled delivery, molecular gate
Controlled delivery has become an essential field of research for the design of systems able to liberate a cargo upon the application of specific stimuli, as stimuli‐responsive systems allow to minimize side effects caused by non‐controlled administration.1 Traditionally, the delivery systems were based on polymers that released the cargo by diffusion‐controlled processes or through degradation of the polymer container.2, 3 In the last years, porous hybrid organic‐inorganic materials have merged as a noteworthy and large class of materials.4, 5, 6, 7 These materials show high surface area, tunable pore size and versatility in their framework composition, which confer them an outstanding performance in many fields such as drug delivery, species adsorption and sensing applications among others.8, 9, 10, 11, 12 In particular, a promising alternative for controlled delivery systems are mesoporous silica particles (MSPs) due to their versatile properties such as low toxicity, high internal volume and easy chemical functionalization.13 The opportunity of functionalizing the external surface of the MSPs with capping agents and uncap them with predefined stimuli makes these supports ideal candidates for controlled delivery applications. A number of examples able to payload deliver in the presence of physical, chemical and biochemical stimuli have been widely described.14, 15, 16, 17, 18
Despite these reported examples, the design of MSPs gated materials able to control the delivery through the action of surfactants is still emerging. To the best of our knowledge, only one example involving surfactant‐responsive mesoporous silica particles has been recently described.19 In that work, mesoporous materials loaded with a dye and capped with a lipid bilayer was prepared. The solid was opened by the rupture of the lipid bilayer induced by the presence of the surfactant DTAB (dodecyltrimethylammonium bromide). However, and despite the appeal of this attractive stimuli‐responsible strategy on MSPs, no more examples of surfactant triggers have been reported.
Surfactant nature of bile salts from the duodenum has been thoroughly reported in literature. In addition to play an important role in cholesterol elimination route, in hormone secretion or in the stimulation of intestinal immune system, bile contains surfactant molecules that emulsify ingested fats facilitating their intestinal absorption.17, 18 However, this character of bile salts has not yet been explored as a possible trigger for capped MSPs to deliver certain molecules in the small intestine. The small intestine is a relevant place where controlled release is needed, being the site where the bioactive molecules absorption takes place.20 Traditionally, the particles developed to deliver active molecules in the small intestine use pH changes and the presence of enzymes as external stimuli, despite the inconvenience that these stimulus can be also present in other parts of the digestive tract.21, 22
In this scenario, with the aim of exploring the surfactant properties of bile salts, we present here MSPs capped with strong hydrophobic lipid molecules (oleic acid) and loaded with a reporter, rhodamine B (RhB). Our original approach relies on proving that using lipid‐capped silica mesoporous particles a remarkable delivery control can be obtained as only surfactants should trigger the release of the cargo, whereas in their absence, zero release should be maintained.
Lipid molecules have been widely used as carriers of bioactive molecules, but their use as capping molecules is scarce in the literature.23, 24 The attachment of lipid molecules on the MSPs surface originates a hydrophobic layer around the particles that avoids the release of the loaded cargo. In our case, oleic acid was selected as capping agent, which confers the aforementioned hydrophobic character and increases the bioapplicability of the particles.25 The presence of oleic acid in capped‐mesoporous silica particles has been described but, as far as we know, its use as capping system for surfactant‐responsive controlled release has not been reported yet. The presence of surfactant molecules (as bile salts) was expected to allow the release of the cargo entrapped into the MSPs, via the emulsifier action of surfactants on the hydrophobic layer (oleic acid). The novelty of this work relies on the remarkable effect of the surfactant nature of bile salts in the controlled release of the cargo loaded inside the pores of the oleic acid‐capped mesoporous silica particles. As far as we know, this is the first example of bile salts surfactant‐responsive‐induced delivery using lipid‐capped silica mesoporous particles.
SBA‐15 microparticles were chosen as support in the envisioned functional MSPs system. SBA‐15 was prepared using Pluronic 123 as micellar template and tetraethylortosilicate (TEOS) as inorganic precursor. The as‐made solid was calcined at 550 °C for 5 h in order to remove the organic template, and the resulted porous solid was loaded with RhB (solid S0). Solid S0 was functionalized firstly with (3‐aminopropyl)triethoxysilane (APTES) to produce the coating of the surface with amino groups (solid S1) and then with oleic acid by means of amidation reaction, giving the final solid S2, see Scheme 1. All the solids were then characterized using standard techniques (see Supporting information for details).26 Powder X‐ray diffraction (PXRD), transmission electron microscopy (TEM) and N2 adsorption‐desorption isotherms carried out on the initial SBA‐15 mesoporous particles, clearly showed a mesoporous structure that remained in solid S2 regardless of the loading process and further functionalization with APTES and oleic acid. DLS measurements pointed out an average particle size of 1 μm for the prepared solids. FTIR spectra was used to follow the functionalization process through the anchoring of the APTES moieties in S1 and the formation of the amide bond between the anchored amines and the oleic acid to form the gating system in solid S2. Measurements of ζ potential of the different materials were in agreement with the functionalization process reaching a value of 44.43 mV in S2, indicative of a stable final material. Thermogravimetric analysis (TGA) and elemental analysis (EA) showed an organic matter content of 43.1 mg of rhodamine B per gram of SiO2 and 84.7 mg of molecular gate per gram of SiO2 in S2.
Scheme 1.
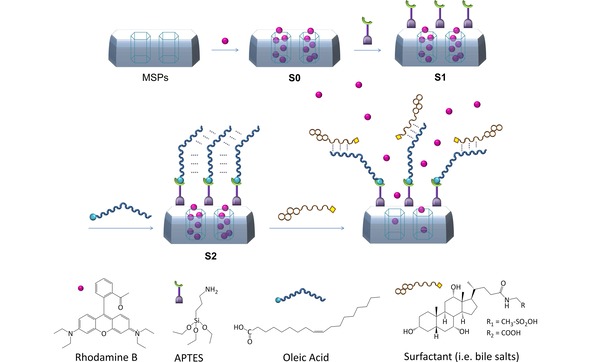
Representation of mesoporous silica material encapsulated with a cargo molecule (RhB) and capped with APTES and the oleic acid layer attached to the free amine of the APTES. Addition of surfactant (i. e. bile salts) to S2 would uncap the mesopores via emulsifier action.
In addition to the characterization process applied to all solids,26 the release profile of RhB from solid S2 was studied in the presence and absence of bile salts at 37 °C. In a typical experiment, 5 mg of S2 were suspended in 10 mL of PBS or in 10 mL of a bile extract solution. At different times an aliquot was taken, filtered, and the fluorescence signal of RhB measured (λex=555 nm, λem=572 nm). As depicted in Figure 1 no cargo release was observed when the solid was suspended in PBS. In contrast, a remarkable delivery was achieved when bile salts were present. After 4 hours of the experiment, almost 80 % of the total cargo release was reached.
Figure 1.
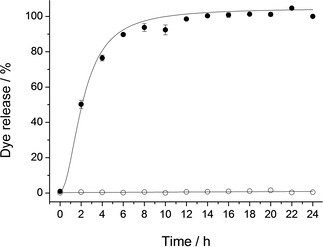
Kinetic release profile of RhB dye from S2 in a PBS suspension without bile salts (○) and when bile salts are present (•).
In order to study the surfactant‐responsive controlled‐release protocol in detail, several experiments of RhB release from S2 in the presence of bile salts, different gastrointestinal enzymes (pepsin, pancreatin, pronase, and esterase) and different surfactants (CTAB, SDS, Triton™ X‐100) were carried out. For this purpose, 5 mg of S2 were suspended in 10 mL of the corresponding solution, and the suspension was stirred for 10 h to allow maximum cargo delivery. Figure 2 shows no RhB release when S2 was suspended in water, PBS or solutions containing enzymes. However, a high cargo release was induced when surfactants were present. Comparing the effect of different surfactants, the release achieved with CTAB, SDS or Triton™ X‐100 was higher than that found with bile salts. It is tentatively attributed to the fact that the surfactant concentration on bile extract used is between 3 and 8×10−3 M, lower than the one used for CTAB, SDS or Triton™ X‐100. These experiments demonstrated that only surfactant molecules were able to open the S2 system, while the solid remains capped when surfactants are not present.
Figure 2.
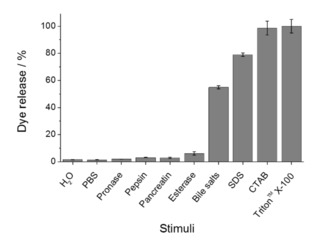
Percentages of dye released from S2 with different stimuli. Enzymes solutions were prepared in PBS at their optimal pH (7.5 for pancreatin, pronase and esterase; and 2 for pepsin), in the same concentration as the one of bile extract (10 mg/mL). Surfactants concentration was 10−2 M.
Having this in mind and taking into account that active molecules are absorbed in the small intestine,20 S2 was tested in an in vitro digestion. The assay was carried out simulating the digestive steps and conditions of mouth, stomach and small intestine (see Figure 3). In this experiment, the corresponding fluids were added after certain times to 5 mg of S2 (3, 6 and 9 mL of simulated saliva, gastric and intestinal fluids, respectively). Aliquots were taken at different times to measure the fluorescence signal of the RhB release over 5 hours.26 Figure 3 shows no cargo release from S2 during the mouth and stomach digestion (saliva and gastric fluid addition), while in the small intestine, when bile salts were added, the signal of the cargo increased. This difference is in agreement with the selective opening of S2 in the presence of the bile salts acting as surfactant.
Figure 3.
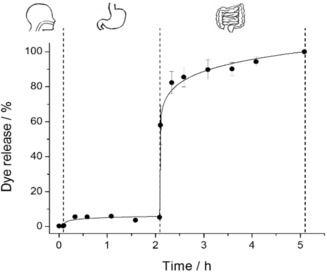
In vitro digestion of S2, in saliva, gastric fluid and intestinal fluid for 5 hours.
Finally, in order to validate the system for biological or nutritional application, the bioactive molecule riboflavin‐5P, one of the vitamers of vitamin B2, was loaded into the pores of MSPs. Riboflavin plays important roles in energy metabolism and is required in the metabolism of amino acids, fatty acids and carbohydrates. Moreover, it is well established that water‐soluble vitamins, as riboflavin, are absorbed in the intestine via specific carrier‐mediated processes.27 Interference with absorption, which occurs in a variety of conditions (e. g. congenital defects in the digestive or absorptive system, intestinal disease/resection, drug interaction and chronic alcohol use), leads to the development of deficiency (and sub‐optimal status) and results in clinical abnormalities.27 Riboflavin deficit is related with different diseases as migraine and hypertension,28 anemia,29 brain injury,30 intestinal disorders31, 32 and also cell carcinomas.33 A dietary supplementation with riboflavin could be an effective therapy for these diseases, or serve as palliative treatment to decrease the symptoms.28
Based on the above‐mentioned facts, encapsulation and controlled release of riboflavin appears to be an important approach. Then, SBA‐15 was loaded with riboflavin‐5P, and afterwards functionalized in two steps with APTES and oleic acid to obtain the final solid S3 (ζ potential 39.71 mV, 50.0 mg of Riboflavin‐5P per gram of SiO2 and 167.2 mg of molecular gate per gram of SiO2). Solid S3 release was tested in similar conditions than solid S2, using water as blank and a surfactant solution as stimulus, and an analogous behavior was observed. The riboflavin‐5P release in the blank solution was negligible compared with the one achieved in the presence of surfactant.26
In summary, although several controlled release systems based on mesoporous silica particles have been described, none of them uses bile salts or other surfactant as opening stimulus for gastrointestinal delivery. Lipid systems have been used as carriers of bioactive molecules, but its performance as release controllers in the presence of specific stimuli has not been reported yet. The microdevice presented here has many advantages such as the high loading capacity typical of mesoporous silica materials, an expected good biocompatibility due to the external lipid coating, and specific controlled release in the presence of surfactants. In conclusion, the synthesis of new oleic acid‐capped mesoporous silica particles as surfactant‐responsive delivery systems for on‐command delivery applications has been described. The zero‐release system's behavior in the absence of surfactant molecules could make it a useful protective support for sensitive molecules, and its selective response to surfactant molecules can be used as specific trigger for both controlled delivery and sensory applications. In fact, very good performance of bile salts, similar to that of commercial surfactants, in the controlled release of the cargo loaded inside the pores of the oleic acid‐capped mesoporous silica particles was demonstrated. As far as we know, this is the first example of surfactant‐responsive‐induced delivery using lipid‐capped silica mesoporous particles. We believe that our microdevice presented here, possesses high potential for finding application in the food and pharmaceutical industry.
Experimental Section
Solids Synthesis
In a typical synthesis, 300 mg of SBA‐15 were suspended in 20 mL of an aqueous solution of RhB (115 mg, 0.24 mol) and stirred during 24 h to obtain the loaded solid S0. After that, an excess of APTES (1.75 mL, 7.48 mmol) was added to the previous mixture, and the mixture was stirred during 5.5 h. Solid S1 was isolated by centrifugation and was dried at 37 °C overnight.
Then, 300 mg of S1 were added to a mixture of 50 mg of EDC and 5 mL of oleic acid in 18 mL of ethanol in order to obtain the final loaded and functionalised solid S2. This solid was washed twice with an ethanol: water (2 : 1) mixture and dried at 37 °C overnight.
In order to obtain the riboflavin loaded and functionalized solid (S3), 150 mg of SBA‐15 were suspended in a riboflavin solution [75 mg in 6 mL of a water:methanol (2 : 1) mixture] and stirred during 24 h. After that, the solid was centrifuged and promptly re‐suspended in 5 mL of acetone. Then an excess of APTES (0.876 mL, 3.74 mmol) was added. The reaction was stirred during 5.5 h, centrifuged and dried at 37 °C overnight. 50 mg of the dried solid were added to a mixture of oleic acid (1 mL) and DCC (17 mg) in acetone (10 mL), and the reaction was stirred overnight. The final solid S3 was centrifuged, washed twice with ethanol: water (2 : 1) and dried at 37 °C overnight.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The authors want to thank the Spanish Government (projects MAT2015‐64139‐C4‐1‐R, AGL2015‐70235‐C2‐1‐R and AGL2015‐70235‐C2‐2‐R (MINECO/FEDER)) and RTI2018‐100910‐B‐C41, RTI2018‐101599‐B‐C22 and RTI2018‐101599‐B‐C21 (MCUI/AEI/FEDER, UE)) and Generalitat Valenciana (project PROMETEO/2018/024) for support. E.P.‐R. thanks the Generalitat Valenciana for her predoctoral fellowship. A.B. wants to acknowledge the Spanish Government for the financial support Juan de la Cierva Incorporación IJCI‐2014‐21534. The authors also thank the Electron Microscopy Service at the UPV for support.
E. Poyatos-Racionero, É. Pérez-Esteve, M. Dolores Marcos, J. M. Barat, R. Martínez-Máñez, E. Aznar, A. Bernardos, ChemistryOpen 2019, 8, 1052.
Contributor Information
Prof. Dr. M. Dolores Marcos, Email: mmarcos@qim.upv.es
Prof. Dr. Ramón Martínez‐Máñez, Email: rmaez@qim.upv.es
References
- 1. Harrison K., Biomed. Polym. (Ed.: M. Jenkins), Cambridge: Woodhead Publishing, UK, 2007, pp. 33–56. [Google Scholar]
- 2. Shankar G., Agrawal Y. K., Int. J. Pharm. Sci. Res. 2017, 8, 4983–4991. [Google Scholar]
- 3. Bourganis V., Karamanidou T., Kammona O., Kiparissides C., Eur. J. Pharm. Biopharm. 2017, 111, 44–60. [DOI] [PubMed] [Google Scholar]
- 4. El-Safty S. A., Shenashen M. A., Ismail A. A., Chem. Commun. 2012, 48, 9652–9654. [DOI] [PubMed] [Google Scholar]
- 5. El-Safty S. A., Khairy M., Shenashen M. A., Elshehy E., Warkocki W., Sakai M., Nano Res. 2015, 8, 3150–3163. [Google Scholar]
- 6. El-Safty S. A., Shenashen M. A., Ismael M., Khairy M., Adv. Funct. Mater. 2012, 22, 3013–3021. [Google Scholar]
- 7. Shenashen M. A., Shahat A., El-Safty S. A., J. Hazard. Mater. 2013, 244–245, 726–735. [DOI] [PubMed] [Google Scholar]
- 8. El-Safty S. A., Shenashen M. A., Ismael M., Khairy M., Chem. Commun. 2012, 48, 6708–6710. [DOI] [PubMed] [Google Scholar]
- 9. El-Safty S. A., Shenashen M. A., Shahat A., Small 2013, 9, 2288–2296. [DOI] [PubMed] [Google Scholar]
- 10. El-Safty S. A., Shenashen M. A., Sens. Actuators B 2013, 183, 58–70. [Google Scholar]
- 11. El-Sewify I. M., Shenashen M. A., Shahat A., Yamaguchi H., Selim M. M., Khalil M. M. H., El-Safty S. A., J. Lumin. 2018, 198, 438–448. [Google Scholar]
- 12. Emran M. Y., El-Safty S. A., Shenashen M. A., Minowa T., Sens. Actuators B 2019, 284, 456–467. [Google Scholar]
- 13. Kickelbick G., Angew. Chem. Int. Ed. 2004, 43, 3102–3104; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 3164–3166. [Google Scholar]
- 14. Aznar E., Oroval M., Pascual L., Murguía J. R., Martínez-Máñez R., Sancenón F., Chem. Rev. 2016, 116, 561–718. [DOI] [PubMed] [Google Scholar]
- 15. Candel I., Aznar E., Mondragón L., De La Torre C., Martínez-Máñez R., Sancenón F., Marcos M. D., Amorós P., Guillem C., Pérez-Payá E., Nanoscale 2012, 4, 7237–7245. [DOI] [PubMed] [Google Scholar]
- 16. Díez P., Sánchez A., Gamella M., Martínez-Ruíz P., Aznar E., De La Torre C., Murguía J. R., Martínez-Máñez R., Villalonga R., Pingarrón J. M., J. Am. Chem. Soc. 2014, 136, 9116–9123. [DOI] [PubMed] [Google Scholar]
- 17. De La Torre C., Agostini A., Mondragón L., Orzáez M., Sancenón F., Martínez-Máñez R., Marcos M. D., Amorós P., Pérez-Payá E., Chem. Commun. 2014, 50, 3184–3186. [DOI] [PubMed] [Google Scholar]
- 18. Pérez-Esteve É., Fuentes A., Coll C., Acosta C., Bernardos A., Amorós P., Marcos M. D., Sancenón F., Martínez-Máñez R., Barat J. M., Microporous Mesoporous Mater. 2015, 202, 124–132. [Google Scholar]
- 19. González-Alvarez M., Coll C., Gonzalez-Alvarez I., Giménez C., Aznar E., Martínez-Bisbal M. C., Lozoya-Agulló I., Bermejo M., Martínez-Máñez R., Sancenón F., Mol. Pharm. 2017, 14, 4442–4453. [DOI] [PubMed] [Google Scholar]
- 20. Shimizu M., Biosci. Biotechnol. Biochem. 2010, 74, 232–241. [DOI] [PubMed] [Google Scholar]
- 21. Bernardos A., Aznar E., Coll C., Martínez-Mañez R., Barat J. M., Marcos M. D., Sancenón F., Benito A., Soto J., J. Controlled Release 2008, 131, 181–189. [DOI] [PubMed] [Google Scholar]
- 22. Bernardos A., Aznar E., Marcos M. D., Martínez-Máñez R., Sancenón F., Soto J., Barat J. M., Amorós P., Angew. Chem. Int. Ed. 2009, 48, 5884–5887; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 5998–6001. [Google Scholar]
- 23. Han N., Wang Y. Y., Bai J., Liu J., Wang Y. Y., Gao Y., Jiang T., Kang W., Wang S., Microporous Mesoporous Mater. 2016, 219, 209–217. [Google Scholar]
- 24. Zhang J., Desai D., Rosenholm J. M., Adv. Funct. Mater. 2014, 24, 2352–2360. [Google Scholar]
- 25. Van Schooneveld M. M., Vucic E., Koole R., Zhou Y., Stocks J., Cormode D. P., Tang C. Y., Gordon R. E., Nicolay K., Meijerink A., Nano Lett. 2008, 8, 2517–2525. [DOI] [PubMed] [Google Scholar]
- 26.See Supporting information for details.
- 27. Said H. M., Biochem. J. 2011, 437, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thakur K., Tomar S. K., Singh A. K., Mandal S., Arora S., Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [DOI] [PubMed] [Google Scholar]
- 29. Lane M., Alfrey C. P. Jr, Blood J. 1965, 25, 432–442. [PubMed] [Google Scholar]
- 30. Bosch A. M., Abeling N. G. G. M., Ijlst L., Knoester H., Van Der Pol W. L., Stroomer A. E. M., Wanders R. J., Visser G., Wijburg F. A., Duran M., J. Inherited Metab. Dis. 2011, 34, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Said H. M., Ortiz A., Moyer M. P., Yanagawa N., Am. J. Physiol. Cell Physiol. 2000, 278, C270–C276. [DOI] [PubMed] [Google Scholar]
- 32. Nakano E., Mushtaq S., Heath P. R., Lee E. S., Bury J. P., Riley S. A., Powers H. J., Corfe B. M., Dig. Dis. Sci. 2011, 56, 1007–1019. [DOI] [PubMed] [Google Scholar]
- 33. Li S.-S., Xu Y.-W., Wu J.-Y., Tan H.-Z., Wu Z.-Y., Xue Y.-J., Zhang J.-J., Li E.-M., Xu L.-Y., Nutr. Cancer 2017, 69, 21–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


