Abstract
Background
Data about the epidemiology of valvular heart disease (VHD) in the elderly is scarce. Hand-held ultrasound devices (HUDs) enable point-of-care ultrasound scanning (POCUS) but their use in an elderly population has not been reported for VHD screening in primary practice.
Methods
One hundred consecutive subjects aged >70 years without a VHD diagnosis had 2D and colour Doppler POCUS by an accredited sonographer, using a contemporary HUD (Vscan), in a primary practice setting. Patients with left-sided valve pathology identified by Vscan were referred for formal echo in the local tertiary cardiac centre.
Results
Mean age (s.d.) was 79.08 (3.74) years (72–92 years); 61 female. By Vscan, we found five patients with ≥moderate aortic stenosis (AS), eight with ≥moderate mitral regurgitation (MR) and none with ≥moderate aortic regurgitation. In the AS and MR groups each, one patient had valve intervention following from the initial diagnosis by Vscan, two and one respectively are under follow-up in the valve clinic, while two and four respectively refused TTE or follow-up. Two patients with moderate MR by Vscan had mild and mild/moderate MR respectively by TTE and were discharged. Total cost for scanning 100 patients was $18,201 – i.e. $182/patient.
Conclusions
Screening with a hand-held scanner (Vscan), we identified 5/100 elderly subjects who needed valve replacement or follow-up in valve clinic, at a cost of $182/patient. These findings have potential significance for the allocation of resources in the context of an ageing population.
Keywords: heart valve disease, hand-held ultrasound, screening, costs
Introduction
Heart valve disease is ‘the next cardiac epidemic’ (1) and imposes an increasing drain on health care resources (2). There are little data about the prevalence of heart valve disease in the old; in major published surveys (3, 4), elderly patients are a minority. An exception is the Ox-Valve study (5) which included only participants over the age of 65 years.
There has been an increase in life expectancy (6) at the same time with the advent of trans-catheter valve interventions. Previously inoperable patients have become candidates for interventional treatment (7, 8), and trans-catheter therapies may replace conventional valve surgery (9, 10). Understanding the burden of VHD in the old becomes essential for planning appropriate allocation of resources.
Echocardiography has been ‘democratised’ by the advent of pocket scanners (11). Their accuracy for the detection of common pathologies is well validated against high-end scanners (12, 13); they allow ultrasound examinations effectively anywhere, revolutionising screening for heart disease (14, 15).
We assessed the prevalence of left-sided heart valve disease in a cohort of adults aged 70 years and above, without a previous formal diagnosis of heart valve disease, by performing hand-held echocardiograms in primary practice.
Methods
Inclusion criteria and patient recruitment
Consecutive patients aged ≥70 years attending a primary health care provider, Swansea Bay Health, in Swansea, Wales, UK were eligible if they gave informed consent and if they had not received a formal diagnosis of heart valve disease or of heart failure before inclusion. Swansea Bay Health covers a population of about 75,000 in and around Swansea.
We recruited using posters and leaflets, available in the waiting area and in the consultation rooms, and we encouraged GPs to mention the study to eligible patients.
Patients in whom the Vscan found significant pathology were encouraged to attend the formal TTE in our tertiary centre (Morriston Cardiac Centre, Swansea, UK).
South East Coast – Surrey Research Ethics Committee had approved the study protocol.
Echocardiography and risk factors
Patients filled in a questionnaire about their cardiac risk factors, and then had a cardiac ultrasound examination. Parasternal long-, short-axis and apical four-chamber, two, three and five-chamber views were obtained in left lateral decubitus. We used the ASE recommendations (16) for POCUS. All scans were performed by one of the authors (CW), an experienced, BSE-accredited, hospital-based cardiac physiologist, who visited the surgery 1 day/week over 6 months and scanned all patients who had given informed consent for the study.
The Vscan (GE Vingmed Ultrasound, Horten, Norway) uses a phased-array probe with a frequency range of 1.7–3.8 MHz; it weighs 390 g and fits inside a pocket. It displays grey-scale images with a fixed sector angle of 75°; the depth can be adjusted up to 25 cm; colour flow has a fixed box size and a fixed pulse repetition frequency. There are no spectral Doppler capabilities. Examinations were saved as one-beat loops on the micro-SD card of the device and were then downloaded on a desktop PC using proprietary software (Vscan Gateway, GE Vingmed Ultrasound). Formal TTE studies were performed on Philips iE33 or GE Vivid 7 machines in the BSE-accredited echocardiographic department of the Morriston Cardiac Centre, according to the BSE dataset.
Image analysis
Video loops of all the examinations were sent for analysis to an ESC-Accredited Laboratory (Prof B Popescu, ‘C Iliescu’ Cardiology Institute, Bucharest, Romania) and studies were reported by an experienced echo doctor (AM) blinded to clinical details of the patients. The following were assessed:
Quality of images in the parasternal and apical views, and of the whole study, for each patient; graded as good, moderate, poor or uninterpretable;
-
Aortic valve (AV):
Aortic stenosis:
First, the core lab provided a qualitative classification of the appearance of the AV: normal, mild, moderate or severe AS;
Then, AV calcification was assessed according to the scheme by Rosenhek et al. (17): 1, no calcification; 2, mildly calcified (small isolated spots); 3, moderately calcified (multiple larger spots) and 4, heavily calcified (extensive thickening and calcification of all cusps). AV sclerosis was defined as thickening of the leaflets without restriction in their movement;
Finally, AV cusp restriction was assessed using the method described by Abe et al. for Vscan (18): we scored each cusp either as having normal mobility (score 0), restricted mobility (score 1) or severely restricted mobility (score 2) and calculated a restriction score for each valve by adding the individual scores of each leaflet;
AV regurgitation:
Its presence and severity was evaluated qualitatively by integrating longitudinal and transverse extension of the jet into the LVOT.
-
Mitral valve (MV):
MV regurgitation: Its presence and severity was assessed qualitatively by assessment of the extent of the colour flow map of the regurgitant jet in the LA;
MV stenosis: Defined qualitatively, by identifying restriction of cusp motion and/or paradoxical posterior leaflet movement in diastole;
LVEF was evaluated by ‘eye-balling’ (normal, reduced, hyperdynamic), and wall-motion abnormalities were identified and described.
Statistics
We analysed the data using SPSS v.22 (SPSS), with chi-square test for comparing proportions and an independent samples t-test for continuous variables; significance level was set at <0.05.
Follow-up
We invited patients with ≥moderate severity of left-sided valve disease by Vscan screening to have formal TTE in the hospital. We followed that with an appointment in the cardiology clinic, with appropriate referral (to the valve clinic for follow-up or to a cardiac surgeon for valve intervention).
Costs
We documented all the costs incurred during the study in UK pounds sterling (GBP, £), using prices and fees supplied by our finance department and rounded them to the nearest decimal point. We then converted the costs to euro (EUR, €) and to US dollars (USD, $), using Google’s online currency convertor (spot exchange rates as of 15 April 2018: 1 GBP = 1.153 EUR; 1 GBP = 1.424 USD).
Results
We recruited 100 consecutive patients (61 female) between June and September 2015, mean age (s.d.) 79.08 (3.74) years, range 72–92 years. Table 1 shows risk factors and clinical characteristics. This represents approximately 1.4% of the total population aged 70 years and above in our catchment area (19).
Table 1.
Clinical characteristics of the patients (n = 100).
| Clinical feature | Status | Frequency/% |
|---|---|---|
| Angina | N | 95 |
| Y | 5 | |
| Hypertension | N | 58 |
| Y | 40 | |
| Unsure | 2 | |
| Hypercholesterolaemia | N | 59 |
| Y | 41 | |
| Current smoking | N | 99 |
| Y | 1 | |
| Diabetes mellitus | N | 90 |
| Y | 10 | |
| Heart murmur | N | 97 |
| y | 2 | |
| Unsure | 1 | |
| Atrial fibrillation | N | 94 |
| Y | 6 | |
| Warfarin | N | 96 |
| Y | 4 | |
| Bronchodilators | N | 84 |
| Y | 16 |
N, no; Y, yes.
Quality of Vscan studies
Poor quality studies were infrequent (7%), and overall the quality of the images was better from the parasternal than from the apical ‘window’.
Left-sided valve disease
By Vscan screening, we found five patients with at least moderate AS, eight with at least moderate MR and none with more than mild AR (Table 2). Restriction and calcification scores for the AV separated normal valves from visually stenosed valves (Table 3). There was one case of mild rheumatic mitral stenosis. In the AS and MR groups each, one patient had valve intervention, two and one respectively are now under follow-up in the valve clinic, while two and four respectively refused formal echo or follow-up in the valve clinic. Two patients in the MR group had mild MR by formal TTE and were discharged. Outcomes and concordance between Vscan and formal TTE are summarised in Tables 4 and 5. Figures 1, 2 and 3 illustrate typical findings in patients with AS and MR.
Table 2.
Echocardiographic findings: valve pathology.
| Frequency/% | |
|---|---|
| Aortic stenosis | |
| No | 48 |
| Mild | 19 |
| Moderate | 4 |
| Severe | 1 |
| Aortic sclerosis | 28 |
| Aortic regurgitation | |
| No | 76 |
| Mild | 24 |
| Mitral regurgitation | |
| No | 9 |
| Mild | 83 |
| Moderate | 6 |
| Severe | 2 |
| Total | 100 |
Table 3.
Aortic valve leaflet restriction and calcification scores, means (s.d.).
| AV | Restriction score | Calcification score |
|---|---|---|
| Normal | 1.82 (1.13) | 2.47 (0.5) |
| Aortic sclerosis | 2.15 (0.98) | 2.67 (0.48) |
| Mild AS | 2.98 (1.20) | 2.75 (0.52) |
| Moderate AS | 5.53 (0.57) | 3.67 (0.57) |
| Severe AS | 6 | 4 |
There was one case of severe AS. P < 0.05 for all degrees of AS vs normal.
Table 4.
Aortic valve pathology: outcomes and concordance of Vscan finding with formal TTE.
| S/N | Gender | Age | AS – Vscan | TTE | AS – TTE |
|---|---|---|---|---|---|
| 29 | M | 78 | Moderate | N | N/A |
| 34 | M | 77 | Moderate | Y | Moderate; f/u in VC |
| 40 | M | 76 | Moderate | N | N/A |
| 46 | F | 81 | Moderate | Y | Moderate; f/u in VC |
| 89 | F | 76 | Severe | Y | Severe; AVR |
AS, aortic stenosis; AVR, aortic valve replacement; f/u, follow-up; N, no; N/A, not available (refused); S/N, study number; VC, valve clinic; Y, yes.
Table 5.
Mitral valve pathology: outcomes and concordance of Vscan findings with formal TTE.
| S/N | Gender | Age | MR – Vscan | TTE | MR – TTE |
|---|---|---|---|---|---|
| 11 | F | 77 | Moderate | Y | Mild/moderate; discharged |
| 17 | F | 80 | Severe | Y | Severe; refused f/u |
| 22 | M | 79 | Moderate | Y | Mild; died – non-cardiac cause |
| 61 | F | 75 | Moderate | Y | Moderate; f/u in VC |
| 64 | M | 77 | Moderate | N | N/A |
| 69 | M | 75 | Moderate | N | N/A |
| 70 | M | 77 | Moderate | N | N/A |
| 74 | F | 76 | Severe | Y | Severe; MV repair |
MR, mitral regurgitation; MV, mitral valve; VC, valve clinic.
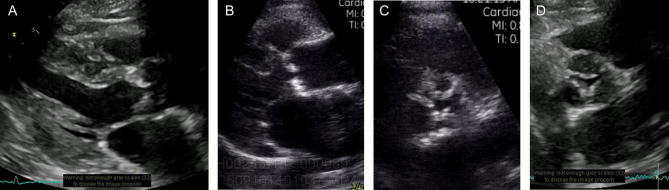
Figure 1.
Parasternal long-axis Vscan image of severe AS, obtained on (A) a high-end scanner and (B) on Vscan. Parasternal short-axis image of severe AS obtained on (C) Vscan and (D) on a high-end scanner.
Figure 2.
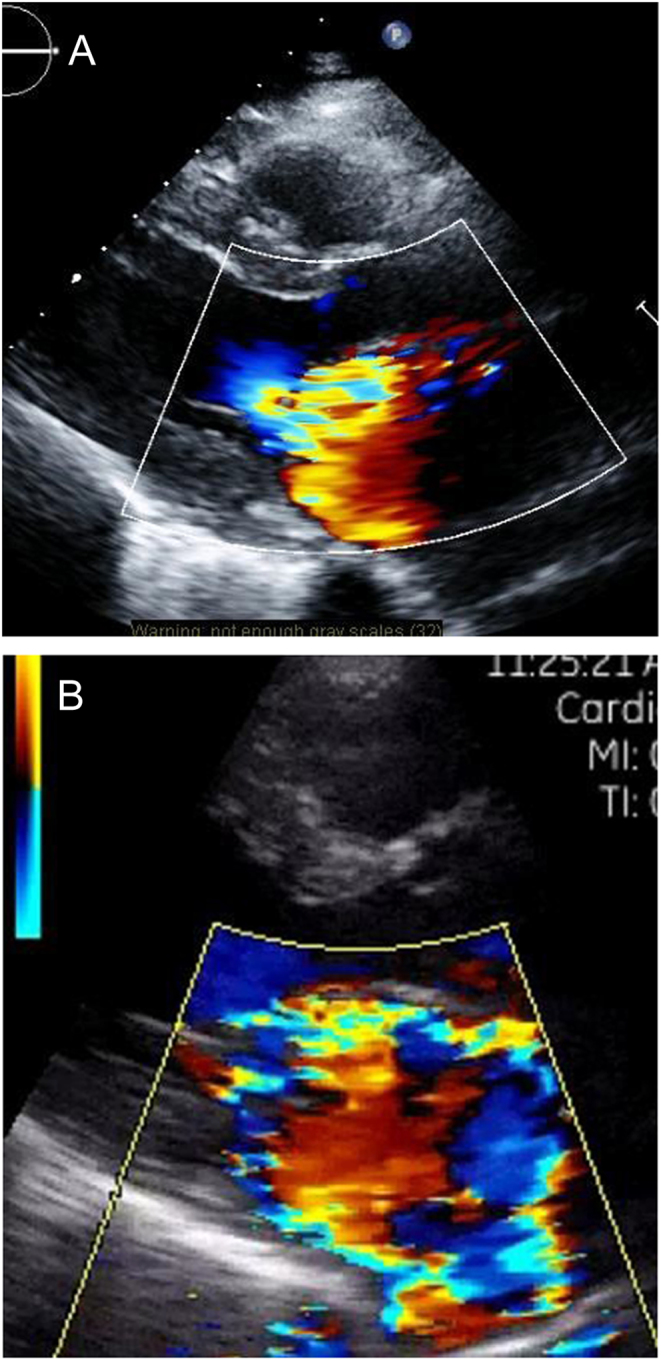
Parasternal image of severe MR obtained on (A) a high-end scanner and (B) on Vscan.
Figure 3.
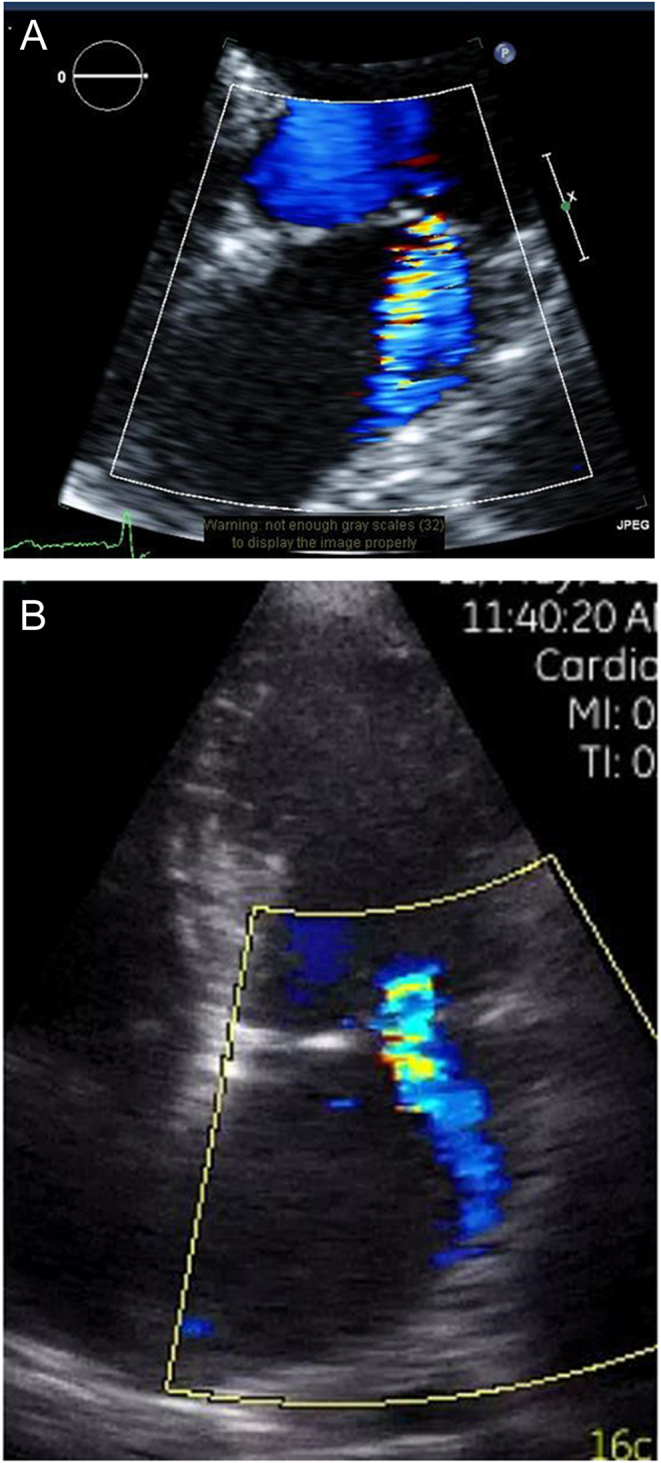
Apical four-chamber image of moderate MR obtained on (A) a high-end scanner and (B) on Vscan.
Association with risk factors
Degenerative AS was more prevalent in patients with a history of angina (P < 0.01).
There were no other associations between left-sided heart valve lesions and clinical characteristics.
Costs
Hand-held ultrasound scans
Performing the 100 Vscan studies incurred a total cost of £5000 (€5765; $7121) in sonographer fees (50 × 100), £1000 (€1153; $1424) in transport costs for the sonographer (all patients attended the surgery by their own means of transportation), £800 (€922; $1139) reporting fee to the core lab, and £5500 (€6341; $7833) in capital costs, that is the cost of buying a Vscan, amounting to a total of £12,300 (€14,181; $17,517).
Formal, hospital-based scans
The sonographer fee for a formal echocardiogram in our centre is £60 (€69; $85), and the hospital charges £265/scan (€306; $377). The cost for scanning eight patients who needed formal TTEs thus amounted to £480 (€553; $684) in fees only (8 × 60), or £2600 (€2998; $3703) including hospital charges (480 + 265 × 8 = 480 + 2120 = 2600).
Total cost
The cost of this study was therefore £12,300 + £480 = £12,780 (€14,734; $18,201 (i.e. £128/€148/$182 per patient)) if we consider only the physiologist fees for the extra formal TTEs in hospital or £12,300 + £2600 = £14,900 (€17,186; $21,221 (i.e. £149/€172/$212 per patient) if we include the total charge levied by the hospital for the extra TTEs.
Discussion
In a study of 100 consecutive elderly subjects attending a primary care centre without a formal, pre-existing diagnosis of valve disease, a detailed grey-scale and colour flow mapping examination performed by an accredited sonographer using a Vscan hand-held ultrasound scanner identified left-sided valve pathology of at least moderate severity in 13%. Two patients had valve intervention based on the findings in the screening study, and three are now under follow-up with the valve clinic in the tertiary centre; a significant proportion refused formal TTE in the hospital.
Comparison with other VHD screening studies
There is a significant literature concerning the use of POCUS and/or of limited scanning protocols for limiting the use of formal outpatient TTE, for elucidating heart murmurs or for screening for valve disease (20, 21, 22, 23, 24). According to Fabich et al. (23): “Structural heart disease may be missed using clinical examination alone and limited echocardiograms or ‘quick-scans’ may be a way to improve rates of detection”. In a population >75 years of age “there were 163 ‘quick scans’ indicated, which were normal in 80 (49%), mildly abnormal in 67 (41%) and significantly abnormal in 16 (10%)”, leading to the conclusion that ‘quick scans’ can detect clinically unexpected pathology. These results are consistent with a global move to use the hand-held ultrasound machine as an extension of the clinical examination (23).
Lindekleiv et al. (24) report that echocardiographic screening of middle-aged participants in the longitudinal Tromso heart study did pick up unexpected pathologies but had no impact on mortality during medium-term follow-up. Potential reasons for this finding may include better health care in a rich, small nation (patients with valve disease are identified effectively by standard clinical practice, without the need for additional screening programmes) as well as the younger age of participants, with screening intercepting valve disease at an earlier stage in its natural progression.
By facilitating timely intervention, screening may have a potential for significant savings and improved outcomes: Approximately 45% of valve interventions are performed on unstable patients (25, 26), who necessitate longer, costlier stays in hospital (25) and who have increased mortality. Reducing the number of urgent and emergency operations would lead to cost savings and better survival.
Another feature of our study is the participation of an experienced, BSE-accredited sonographer performing as complete a scan as possible using the Vscan in a primary care setting. Many previous reports on Vscan have used ‘echo-naïve’ operators, such as medical students, nurses or cardiologists in training, performing focused scans after minimal training and have demonstrated good sensitivity and specificity for the Vscan for limited, specific diagnoses (including screening for AS (27)), when compared to high-end scanners operated by experts. Our aim was different and is part of our current attempts at exploring and developing new models of cardiac care in the community.
Nkomo et al. (4) pooled population-based studies to obtain data for 11,911 subjects aged at least 18 years. They identified moderate or severe valve disease in 615, with an increase in the frequency with age, from 0.7% (95% CI 0.5–1.0) in 18–44 year-olds to 13.3% (11.7–15.0) in the 75 years and older group (P < 0.0001).
Marciniak et al. (28) studied 79,043 patients who were referred to a community open-access echocardiography service over 10 years for suspected heart failure. More than a third of the subjects – 29,682 patients (37.5%) – had mild, 8983 (11.3%) had moderate and 2134 (2.7%) had severe valve disease (total prevalence of clinically significant VHD 14%). The prevalence of AS, regurgitation, mitral stenosis and regurgitation was 10, 8.4, 1 and 12.5% respectively.
Eveborn et al. (29) performed three repeated comprehensive echocardiographic examinations (1994, 2001 and 2008) of a random sample of 3273 participants in the Tromso heart study and found 164 subjects with AS. Prevalence consistently increased with age, from being 0.2% in the 50- to 59-year cohort to 9.8% in the 80- to 89-year cohort, giving an incidence rate of 4.9‰/year.
Lindroos et al. (30) performed TTE screening in 501 elderly participants (75–76, 80–81 and 85–86 years of age) in the Helsinki Heart study and in 55 subjects recruited for the echocardiographic sub-study and found critical native valve stenosis (calculated aortic valve area ≤0.8 cm2 and velocity ratio ≤0.35) in 12 subjects (2.2%), of whom six were symptomatic and potentially eligible for AVR. All subjects with AV stenosis were in the three oldest age groups. The prevalence of critical AV stenosis was 2.9% (95% confidence interval 1.4–5.1%) in the group 75–86 years of age.
We think that our findings are hypothesis-generating and may initiate a rethink of the models of high-quality care for heart valve patients in the community.
We found a prevalence of 8% of ≥moderate MR, 5% of ≥moderate AS (total prevalence 13%). The Ox-Valve study (5), which also recruited in a primary care setting, reported newly diagnosed ‘clinically significant (moderate or severe) VHD’ in 6.4% of participants, with 4.9% of subjects having pre-existing VHD, giving a total population prevalence of moderate or severe VHD of 11.3%. We found a higher prevalence of VHD than in the Ox-Valve study, probably due to a combination of factors. Our study group was older (mean age 79.8 years, 73 years in Ox-Valve), which may increase the prevalence of VHD; it is also possible that Vscan mis-labelled cases that would not have been validated by a formal TTE in the tertiary centre, mainly for regurgitant lesions. In the Ox-Valve, patients only had hospital screening with high-end scanners.
Comparison of standard and HUD echocardiography
The accuracy of HUD for detecting cardiac pathology is good, when compared to standard TTE (31, 32, 33, 34, 35). In our small sample of patients with clinically relevant VHD by Vscan, formal TTE in the hospital confirmed the HUD finding in those with AS but in two patients with MR formal TTE downgraded the HHU findings. This may be an intrinsic limitation of HUD, as previous studies also reported a tendency to over-estimate regurgitant jet area (32).
Cost and implications for screening
Our unsophisticated, preliminary analysis suggests that costs of screening would not be prohibitive. It may be possible to reduce the costs even further, for instance by targeting the scans to groups at high risk for a specific pathology, using an abbreviated protocol for scanning or by teaching enthusiastic GPs to use the Vscan to screen for valve disease as part of the clinical exam of their patients. Importantly, it may be possible to offset some of the costs against savings associated with the positive impact of early detection and timely intervention for VHD.
Our study expands the boundaries of HUD usage beyond those delineated by the relevant EACVI guidelines (36), which recommend FoCUS TTE examinations by HUD as part of the clinical examination and which states they should not be reimbursed.
Detailed TTE using HUD
In the hands of an expert operator, the accuracy of contemporary HUD is comparable to that of high-end stationary scanners, with agreement coefficients ranging between 0.55 and 0.66 (33, 34, 35), albeit a large and systematic comparison of the diagnostic accuracy of comprehensive TTE by HUD vs high-end platforms (33) concluded that “HUD should not be used as a surrogate” for TTE with a high-end machine. Of note, magnifying the image to ‘fill-in’ a 21-inch computer screen improved the diagnostic accuracy of HUD in the only study that reported the comparison with reporting on the Vscan screen (35).
Rationale for reimbursement
The recommendation from EACVI not to reimburse HUD FoCUS exams does not cover our setting. We did not include HUD as part of a physical examination by a clinician, and we used a comprehensive TTE protocol as opposed to a focused or limited one; the only way to have an expert operator perform the scan is to pay them for it. It is likely that as technological progress expands the capabilities of HUD, other legitimate modes of its usage will become established, and our work is a step in that direction.
Limitations of the study
We cannot extrapolate our findings regarding the prevalence of valve disease to a wider population, but our results are in the same range as those of the much larger Ox-Valve study, which reinforces their validity, at least for an UK-based elderly population. We cannot guarantee that patients who took part in the study are representative of the population from which this cohort was sampled, but there is no reason to believe that patients attending the surgery on the days when scanning was performed were substantially different from those attending on other days. Ours was a pragmatic setup, and this was the most accessible sample in a non-academic context. We could have selected patients based on the presence of high risk for finding abnormalities on echo, but this had been done before, and did not address our aim, which was to get an idea on the prevalence of valve disease in the elderly in our catchment area.
This study was designed, performed and written up in a single tertiary centre. In order to avoid any possibility of bias in the interpretation of the POCUS data, our studies were assessed in a core lab, which would not be available for routine clinical work. However, all the cases of significant valve regurgitation were clear-cut and obvious so we would expect similar accuracy for interpretation by an experienced sonographer, once studies with suboptimal quality are excluded from the analysis.
We cannot know how accurate our Vscan diagnoses were in those patients who refused to attend for formal TTEs in the tertiary centre but, at least for AS, the findings by Vscan were concordant with those by formal TTE. We did not set out to assess the right ventricle by POCUS because of its complex shape and limited visibility. We cannot rule out the possibility that patients who put themselves forward for screening may have been worried about symptoms of heart disease, which they had not yet reported. The costs are those provided by our hospital finance department, and may not be representative for other settings. We did not calculate costs for seeing the patients that were referred to the cardiology outpatient clinic because the clinics are reimbursed on a sessional rather than ‘per-patient seen’ basis. Our analysis of costs is basic and unsophisticated, but is hypothesis generating.
Conclusion
Using a pocket-sized scanner (Vscan) in a primary care setting identified at least five elderly patients who needed valve replacement or follow-up in a valve clinic, out of a cohort of 100 elderly subjects presenting without a prior diagnosis of heart valve disease, at a relatively low cost. A model of health care that includes echocardiographic screening for valve disease performed by accredited sonographers in patients above the age of 70 years and using hand-held ultrasound scanners in primary practice may be viable and has a potential to reduce health care-associated costs which needs to be further investigated.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was financed by the Research and Development Department of the Morriston Hospital, ABMU LHB, Swansea, which received an unrestricted grant from Edwards Life Sciences (grant # I 14-312).
Acknowledgements
The authors gratefully thank Prof. Nuno Cardim MD, Hospital da Luz, Lisbon, Portugal, for his valuable editorial comments.
References
- 1.D’Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. Valvular heart disease: the next cardiac epidemic. Heart 2011. 97 . ( 10.1136/hrt.2010.205096) [DOI] [PubMed] [Google Scholar]
- 2.Moore M, Chen J, Mallow PJ, Rizzo JA. The direct health-care burden of valvular heart disease: evidence from US national survey data. ClinicoEconomics and Outcomes Research 2016. 8 . ( 10.2147/CEOR.S112691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, et al A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. European Heart Journal 2003. 24 . ( 10.1016/s0195-668x(03)00201-x) [DOI] [PubMed] [Google Scholar]
- 4.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006. 368 . ( 10.1016/S0140-6736(06)69208-8) [DOI] [PubMed] [Google Scholar]
- 5.d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, et al Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. European Heart Journal 2016. 37 . ( 10.1093/eurheartj/ehw229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office for National Statistics. National life tables, UK: 2013 to 2015. Newport, UK: ONS, 2016. (available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/20132015) [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al Transcatheter versus surgical aortic-valve replacement in high-risk patients. New England Journal of Medicine 2011. 364 . ( 10.1056/NEJMoa1103510) [DOI] [PubMed] [Google Scholar]
- 8.Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, et al Percutaneous repair or surgery for mitral regurgitation. New England Journal of Medicine 2011. 364 . ( 10.1056/NEJMoa1009355) [DOI] [PubMed] [Google Scholar]
- 9.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. New England Journal of Medicine 2017. 376 . ( 10.1056/NEJMoa1700456) [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, et al Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. New England Journal of Medicine 2016. 374 . ( 10.1056/NEJMoa1514616) [DOI] [PubMed] [Google Scholar]
- 11.Mirabel M, Celermajer D, Beraude AS, Jouvena X, Marijona E, Hagège AA. Pocket-sized focused cardiac ultrasound: strengths and limitations. Archives of Cardiovascular Diseases 2015. 108 . ( 10.1016/j.acvd.2015.01.002) [DOI] [PubMed] [Google Scholar]
- 12.Liebo MJ, Israel RL, Lillie EO, Smith MR, Rubenson DS, Topol EJ. Is pocket mobile echocardiography the next-generation stethoscope? A cross-sectional comparison of rapidly acquired images with standard transthoracic echocardiography. Annals of Internal Medicine 2011. 155 . ( 10.7326/0003-4819-155-1-201107050-00005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen GN, Haugen BO, Graven T, Salvesen O, Mjølstad OC, Dalen H. Feasibility and reliability of point-of-care pocket-sized echocardiography. European Journal of Echocardiography 2011. 12 . ( 10.1093/ejechocard/jer108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu JC, Sable C, Ensing GJ, Webb C, Scheel J, Aliku T, Lwabi P, Godown J, Beaton A. Beaton simplified rheumatic heart disease screening criteria for handheld echo-cardiography. Journal of the American Society of Echocardiography 2015. 28 . ( 10.1016/j.echo.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 15.Vanhecke TE, Weber JE, Ebinger M, Bonzheim K, Tilli F, Rao S, Osman A, Silver M, Fliegner K, Almany S, et al Implementation of ultraportable echocardiography in an adolescent sudden cardiac arrest screening program. Clinical Medicine Insights: Cardiology 2014. 8 . ( 10.4137/CMC.S15779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography 2013. 26 . doi:10.1016/j.echo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. New England Journal of Medicine 2000. 343 . ( 10.1056/NEJM200008313430903) [DOI] [PubMed] [Google Scholar]
- 18.Abe Y, Ito M, Tanaka C, Ito K, Naruko T, Itoh A, Haze K, Muro T, Yoshiyama M, Yoshikawa J. A novel and simple method using pocket-sized echocardiography to screen for aortic stenosis. Journal of the American Society of Echocardiography 2013. 26 . ( 10.1016/j.echo.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 19.Swansea Council. Swansea’s Assessment of Local Well-being 2017, Annex 1: A demographic profile of Swansea. Swansea, UK: Swansea Council, 2017. (available at: https://www.swansea.gov.uk/media/19870/Annex-1-A-demographic-profile-of-Swansea/pdf/Annex_1_A_demographic_profile_of_Swansea.pdf) [Google Scholar]
- 20.Smith J, Subbiah S, Hayes A, Campbell B, Chambers JB. Feasibility of an outpatient point-of-care echocardiography service. Journal of the American Society of Echocardiography 2019. 32 . ( 10.1016/j.echo.2019.02.006) [DOI] [PubMed] [Google Scholar]
- 21.Draper J, Subbiah S, Bailey R, Chambers JB. Murmur clinic: validation of a new model for detecting heart valve disease. Heart 2019. 105 . ( 10.1136/heartjnl-2018-313393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arden C, Chambers JB, Sandoe J, Ray S, Prendergast B, Taggart D, Westaby S, Grothier L, Wilson J, Campbell B, et al Can we improve the detection of heart valve disease? Heart 2014. 100 . ( 10.1136/heartjnl-2013-304223) [DOI] [PubMed] [Google Scholar]
- 23.Fabich NC, Harrar H, Chambers JB. ‘Quick-scan’ cardiac ultrasound in a high-risk general practice population. British Journal of Cardiology 2016. 23 . [Google Scholar]
- 24.Lindekleiv H, Løchen ML, Mathiesen EB, Njølstad I, Wilsgaard T, Schirmer H. Echocardiographic screening of the general population and long-term survival: a randomized clinical study. JAMA Internal Medicine 2013. 173 . ( 10.1001/jamainternmed.2013.8412) [DOI] [PubMed] [Google Scholar]
- 25.Widyastutia Y, Stenseth R, Wahba A, Pleym H, Videm V. Length of intensive care unit stay following cardiac surgery: is it impossible to find a universal prediction model? Interactive Cardiovascular and Thoracic Surgery 2012. 15 . ( 10.1093/icvts/ivs302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards FH, Peterson ED, Coombs LP, DeLong ER, Jamieson WR, Shroyer ALW, Grover FL. Prediction of operative mortality after valve replacement surgery. Journal of the American College of Cardiology 2001. 37 . ( 10.1016/s0735-1097(00)01202-x) [DOI] [PubMed] [Google Scholar]
- 27.Gulic TG, Jana Makuc J, Prosen G, Dinevski D. Pocket-size imaging device as a screening tool for aortic stenosis. Wiener Klinische Wochenschrift 2016. 128 . ( 10.1007/s00508-015-0904-6) [DOI] [PubMed] [Google Scholar]
- 28.Marciniak A, Glover K, Sharma R. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ Open 2017. 7 e012240 ( 10.1136/bmjopen-2016-012240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis: the Tromsø study. Heart 2013. 99 . ( 10.1136/heartjnl-2012-302265) [DOI] [PubMed] [Google Scholar]
- 30.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. Journal of the American College of Cardiology 1993. 21 . ( 10.1016/0735-1097(93)90249-z) [DOI] [PubMed] [Google Scholar]
- 31.Prinz C, Voigt JU. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. Journal of the American Society of Echocardiography 2011. 24 . ( 10.1016/j.echo.2010.10.017) [DOI] [PubMed] [Google Scholar]
- 32.Kono Y, Fukuda S, Shimada K, Oe H, Maeda K, Kawasaki T, Fujimoto H, Otsuka K, Kubo T, Jissho S, et al Pocket-sized echo for evaluation of mitral and tricuspid regurgitation. JACC: Cardiovascular Imaging 2011. 4 921 ( 10.1016/j.jcmg.2011.02.022) [DOI] [PubMed] [Google Scholar]
- 33.Mirabel M, Celermajerd D, Beraudef AS, Jouven X, Marijon E, Hagège AA. Pocket-sized focused cardiac ultrasound: strengths and limitations. Archives of Cardiovascular Diseases 2015. 108 . ( 10.1016/j.acvd.2015.01.002) [DOI] [PubMed] [Google Scholar]
- 34.Cullen MW, Blauwet LA, Vatury OM, Mulvagh SL, Behrenbeck TR, Scott CG, Pellikka PA. Diagnostic capability of comprehensive handheld vs. transthoracic echocardiography. Mayo Clinic Proceedings 2014. 89 . ( 10.1016/j.mayocp.2013.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Testuz A, Mueller H, Keller PF, Meyer P, Stampfli T, Sekoranja L, Vuille C, Burri H. Diagnostic accuracy of pocket-size handheld echocardiographs used by cardiologists in the acute care setting. European Heart Journal Cardiovascular Imaging 2013. 14 . ( 10.1093/ehjci/jes085) [DOI] [PubMed] [Google Scholar]
- 36.Cardim N, Dalen H, Voigt JU, Ionescu A, Price S, Neskovic AN, Edvardsen T, Galderisi M, Sicari R, Donal E, et al The use of handheld ultrasound devices: a position statement of the European Association of Cardiovascular Imaging (2018 update). European Heart Journal Cardiovascular Imaging 2019. 20 . ( 10.1093/ehjci/jey145) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a