Abstract
Background:
Arsenic, cadmium, lead, and mercury are ubiquitous toxicants that may be especially harmful to unborn children. We therefore sought to identify temporal trends and predictors of toxic metal biomarkers among US women of reproductive age, including those who were pregnant and/or breastfeeding.
Methods:
Interviews and examinations were performed among a representative sample of women, aged 20 to 44 years, as part of the 2003-2014 National Health and Nutrition Examination Surveys. A range of sociodemographic, lifestyle, and dietary factors were evaluated as predictors of urinary inorganic arsenic, urinary cadmium, blood mercury, and blood lead concentrations.
Results:
Levels of all four toxic metal biomarkers declined during the study period. Older age, racial/ethnic minorities, and a birthplace outside of the US were independently associated with higher toxic metal concentrations. Associations were similar for women who were pregnant or breastfeeding and those who were not.
Conclusion:
US women of reproductive age were exposed to lower levels of toxic metals in 2013-2014 compared to 2003-2004. However, because the long-term health effects of early life exposures are unclear, public health efforts to address toxic metals should pay particular attention to older, non-white, and foreign-born women if they are pregnant or planning to become pregnant.
Keywords: toxic metals, arsenic, cadmium, mercury, lead, reproductive health, pregnancy
Introduction
Arsenic, cadmium, lead, and mercury are pervasive in the environment. In 2017, the United States Agency for Toxic Substances and Disease Registry ranked these four toxic metals and metalloids within the top ten priority substances according to their frequency, toxicity, and potential for human exposure.(1) Epidemiologic studies have recently begun to suggest toxic metal exposures are associated with adverse reproductive health and birth outcomes. Notably, arsenic has been linked to increased risk of miscarriage, stillbirth, and low birth weight; cadmium with low birth weight; lead with miscarriage; and mercury with reduced fetal growth.(2–5) As metals readily cross the placenta and can be detected in breast milk, it is also essential to consider their potential impacts on offspring.(6, 7) For example, numerous studies have identified associations of prenatal mercury exposure with neurocognitive delays in childhood.(8–10)
Public health concerns regarding exposures to metal mixtures have additionally been increasing in recent years, as most individuals are exposed to multiple toxic metals on a daily basis. Data from Belgium suggest prenatal co-exposures to arsenic and cadmium may reduce birth weights more than either metal alone.(11) Moreover, the findings from a United States (US)-based retrospective cohort study observed children whose mothers lived in areas with high soil concentrations of arsenic and lead while pregnant appeared to be most at risk for developing intellectual disabilities.(12) It has been estimated arsenic, cadmium, lead, and mercury are detectable in nearly one in four US women of reproductive age.(13) Few studies to date, however, have characterized potential sources of toxic metal exposures or identified sub-populations who may be of particularly high risk. To that end, we used data from the 2003-2014 cycles of the National Health and Nutrition Examination Survey (NHANES) to examine temporal trends and determinants of blood lead, blood mercury, urinary inorganic arsenic, and urinary cadmium levels among US women aged 20-44 years.
Subjects and Methods
Study Population
The NHANES is a nationally-representative survey of the non-institutionalized US population. It is conducted annually by the National Center for Health Statistics and employs multi-stage random sampling. Participants undergo in-person interviews and physical examinations, including the collection of blood and urine specimens. We combined NHANES cycles from 2003 to 2014 during which 7,573 women of reproductive age (20-44 years) were evaluated. Of these, we restricted our analyses to participants with complete data on selected potential determinants including sociodemographics, lifestyle characteristics, and dietary factors who had been selected for blood measurements of lead and mercury concentrations (n=4,285) or urinary measurements of arsenic and cadmium concentrations (n=1,562).
Biomarkers of Toxic Metal Exposures
Blood and spot urine samples were collected in a mobile examination center. All study participants aged 1 year and above were eligible for the assessment of blood lead and total mercury levels for NHANES cycles 2003-2004 through 2011-2012; for the 2013-2014 cycle, a random one-half sub-sample of participants was selected. Urinary total arsenic, arsenic species, and cadmium concentrations were measured in a random one-third sub-sample of participants aged 6 years and above for NHANES 2003-2014. Of note, NHANES measured cadmium, lead, and mercury in both urine and blood samples. We evaluated total blood mercury concentrations rather than urinary concentrations because it is more likely to reflect methylmercury exposure amongst non-occupationally exposed individuals.(14) Methylmercury is of significant concern for women of reproductive age since the developing fetus is extremely sensitive to exposure.(15) We selected measures of cadmium in urine and lead in blood because these have longer half-lives than their blood or urine counterparts reflecting longer term exposures.(16, 17)
Biospecimens were shipped on dry ice to the National Center for Environmental Health in Atlanta, GA. There, metal concentrations were measured using inductively coupled plasma dynamic reaction cell-mass spectrometry. Concentrations of the organic arsenic species arsenobetaine was determined by high performance liquid chromatography. Urinary creatinine concentrations (mg/dL) were measured by an enzymatic method and are considered to be a measure of urine dilution. For all toxic metals, any concentration below the respective limit of detection (LOD) for each NHANES cycle was substituted with the LOD/√2. Fill values for samples below the LOD ranged from 0.07 to 0.20 μg/dL for blood lead, 0.10 to 0.23 μg/L for blood mercury, 0.28 to 0.88 μg/L for urinary arsenic, 0.28 to 0.84 μg/L for urinary arsenobetaine, and 0.025 to 0.04 μg/L for urinary cadmium.
Because inorganic arsenic and its metabolites are more toxic than organic arsenic species, we used a validated statistical method to isolate urinary inorganic arsenic species from urinary total arsenic concentrations.(18) First, using linear regression, we regressed log-transformed urinary total arsenic on log-transformed urinary arsenobetaine (an organic arsenical resulting from recent seafood consumption) concentrations, accounting for the complex NHANES survey design. We then summed the model residuals, which represent inorganic arsenic species independent of seafood consumption, with the conditional geometric mean total arsenic concentration from participants with undetectable arsenobetaine concentrations (3.50 μg/L). The resulting values were interpreted as a biomarker of inorganic arsenic exposure. (18)
Sociodemographic and Lifestyle Characteristics
Participants self-reported information regarding age, race/ethnicity, education, employment, family income, health insurance coverage, marital status, birthplace, gravidity (i.e., number of prior pregnancies), and breastfeeding as part of household interviews conducted by trained staff. Participants who reported they were either “looking for work” or “not working at a job or business” during the last week were considered unemployed; those who reported working at least one hour in the prior week were classified as employed. The ratio of self-reported family income to federal poverty guidelines, specific to family size, year, and state, was computed as a marker of socioeconomic status. The average number of alcoholic drinks consumed during two non-consecutive 24-hour recalls was calculated to ascertain alcohol consumption. Serum cotinine, measured by isotope-dilution high-performance liquid chromatography, served as a biomarker of tobacco smoke exposure. Non-exposure was defined as non-detectable cotinine concentrations (<0.015 ng/mL), whereas secondhand exposure was defined as serum cotinine levels < 10 ng/mL and active smoking as ≥10 ng/mL.(19) Pregnant women were identified through urine pregnancy tests administered in the mobile examination center. Breastfeeding status was ascertained via a Reproductive Health questionnaire.
All participants were asked to report the use of any dietary supplements in the previous 30 days and provide the supplement container or label. From these product labels, NHANES staff documented the supplement ingredients and their quantities; if the supplement container was unavailable, staff obtained this information directly from the product manufacturer. To identify prenatal supplement users, we queried the NHANES Product Label Database, which contains detailed information on all dietary supplements reported by survey participants since 1999, for products with names or labels indicating use for pregnant women only. Based on the most common ingredients listed in the reported prenatal formulations, we defined prenatal supplements as multi-vitamins/multi-minerals containing vitamins A, B1, B2, B3, B6, B9 (folic acid), B12, C, D, and E, as well as the minerals calcium, iron, and zinc. We also identified users of other multi-vitamin/multi-mineral supplements (i.e., non-prenatal formulations), which we operationally defined as products containing at least 3 vitamins and at least 1 mineral.(20) Anthropometric data were collected by trained technicians in the mobile examination center. Body weight was measured with a digital scale and standing height with a stadiometer. Body mass index (BMI) was subsequently calculated as weight in kilograms divided by height in meters squared and rounded to one decimal place.
Dietary Factors
Participants were asked to report all foods and beverages consumed in the prior 24-hour period during the health examination component. A second 24-hour dietary recall was performed via telephone approximately 3-10 days following the in-person recalls. Trained interviewers administered the recalls using an automated multiple-pass approach in order to elicit as many details as possible.
All foods and beverages were coded according to the United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies. Containing over 7,000 items, the database provides nutrient values and weights for typical portion sizes. The amount of kilocalories in each item reported as consumed was summed to obtain the daily total; we calculated the average across both recall days. The USDA Food Patterns Equivalents Database was used to convert each reported item into the following components: alcoholic beverages, drinking water, seafood, meat, poultry, vegetables, legumes, nuts and seeds, fruit, eggs, dairy, whole grains, and refined grains.(21) Amounts were quantified as the number of alcoholic drinks; grams of drinking water; ounce equivalents for grains and protein foods; and cup equivalents for fruit, vegetables, and dairy. For the 88.3% of participants who completed both recalls, we calculated the average amount reported; for the remaining 11.7%, we used the amount reported on whichever recall was available.
Within the United States, public drinking water supplies are routinely monitored for contaminants, including toxic metals, by federal law. However, populations relying on unregulated, private drinking water systems may be at higher risk of ingesting toxic metals via drinking water.(22) Participants were asked to report the source of their tap water (community supply, well or rain cistern, spring, or don’t drink tap water) during the dietary recall components. We used the reported amount of drinking water consumed in the entire 48-hour dietary assessment period and water source to derive the average amount of public (community supply) and private (well, rain water cistern, or spring) water consumed in grams per day.
Metal Mixtures
To evaluate metal mixtures, we derived a multiple metal index that reflected having high levels of all four biomarkers (defined as concentrations exceeding the geometric means) for the sub-sample of participants with complete urine and blood metal biomarkers available. Participants from the 2013-2014 cycle were excluded from this portion of the analysis because of the limited overlap between the random one-half blood metal sub-sample and the random one-third urinary metal sub-sample. Logistic regression was used to estimate odds ratios and 95% confidence intervals associated with high exposure to inorganic arsenic, cadmium, lead, and mercury.
Statistical Analyses
Toxic metal concentrations were log-transformed to approach more normal distributions. Multivariable linear regression models were fit to calculate β-coefficients for independent associations between predictors with each toxic metal. Survey cycle was included as a covariate in all models to account for changes over time. Models of urinary metal concentrations additionally included urinary creatinine as a covariate to control for inter-individual differences in urine dilution.(23) Because continuous variables were measured on various scales, we converted each to z-scores. Model coefficients were back-transformed by exponentiating β-values and are presented as percent differences for interpretability.
Each regression model was fit to the overall sample of women of reproductive age, as well as stratified by reproductive status (i.e., pregnant and/or breastfeeding), with the exception of the logistic regression model evaluating metal mixtures due to data sparseness. The complex sampling design of NHANES was accounted for by incorporating metal sub-sample weights (blood or urine, as appropriate) and survey design variables. Standard errors were estimated with Taylor series linearization. Statistical analyses were performed with Stata/SE version 15 (College Station, TX). To correct for multiple hypothesis testing of the numerous predictor variables, we calculated false discovery rates and considered q-values < 0.05 to be statistically significant.(24)
Sensitivity Analysis
We conducted a sensitivity analysis to examine the impact of urine dilution on the derivation of our multiple metal index. First, we divided urinary inorganic arsenic and cadmium concentrations (μg/L) by creatinine (mg/dL) and multiplied by 100 so the resulting metal biomarker values were expressed as μg/g creatinine. Then, we used creatinine-corrected urinary inorganic arsenic and cadmium concentrations to rederive the multiple metal index. We re-ran our logistic regression model substituting the rederived index as the dependent variable and dropping urinary creatinine from the list of covariates.
Results
Biomarker Concentrations and Temporal Trends
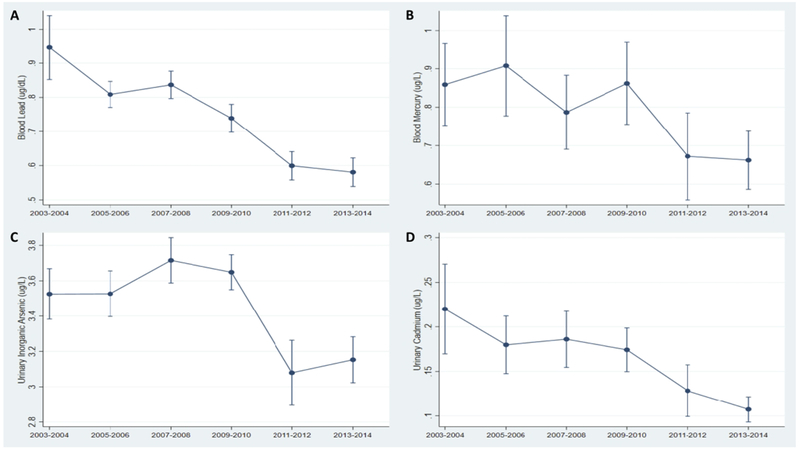
Metals had high detection rates among women of reproductive age. Lead and mercury were detected in 99.0% and 86.9% of blood samples, whereas total arsenic, arsenobetaine, and cadmium were detected in 97.8%, 56.2%, 87.9% of urine samples. Table 1 provides ranges and geometric means with standard errors of each metal biomarker for all women of reproductive age, and by reproductive status. In general, pregnant/breastfeeding women tended to have lower levels of toxic metals than their non-pregnant/non-lactating counterparts. Overall, metal biomarker concentrations tended to decrease throughout the study period (Figure 1).
Table 1.
Ranges and geometric means of toxic metal biomarkers overall and by reproductive status
| Overall (N=4,285) |
Not Pregnant/Breastfeeding (N=3,639) |
Pregnant/Breastfeeding (N=646) |
||||
|---|---|---|---|---|---|---|
| Blood Metals | Range | Geometric Mean ± SE | Range | Geometric Mean ± SE | Range | Geometric Mean ± SE |
| Lead (μg/dL) | 0.11-15.01 | 0.73 ± 0.01 | 0.15-15.01 | 0.74 ± 0.01 | 0.11-12.00 | 0.62 ± 0.02 |
| Mercury (μg/L) | 0.10-30.70 | 0.78 ± 0.02 | 0.10-19.30 | 0.79 ± 0.02 | 0.10-30.70 | 0.71 ± 0.04 |
| Overall (N=1,562) |
Not Pregnant/Breastfeeding (N=1,334) |
Pregnant/Breastfeeding (N=228) |
||||
| Urinary Metals | Range | Geometric Mean ± SE | Range | Geometric Mean ± SE | Range | Geometric Mean ± SE |
| Inorganic arsenic1 (μg/L) | 0.86-6.70 | 3.43 ± 0.03 | 0.86-5.97 | 3.42 ± 0.03 | 1.77-6.70 | 3.54 ± 0.07 |
| Cadmium (μg/L) | 0.03-2.30 | 0.16 ± 0.01 | 0.03-2.30 | 0.16 ± 0.01 | 0.03-1.52 | 0.13 ± 0.01 |
Estimated by removing the contribution of arsenobetaine (an organic seafood-derived arsenical) from total urinary arsenic via a residual-based method
Figure 1. Temporal trends in metal biomarkers among women of reproductive age, NHANES 2003-2014.
A. Geometric mean concentrations of blood lead (μg/dL); B. Geometric mean concentrations of blood mercury (μg/L); C. Geometric mean concentrations of urinary inorganic arsenic (μg/L); D. Geometric mean concentrations of urinary cadmium (μg/L).
Characteristics of US Women of Reproductive Age
Participant characteristics by sub-sample and reproductive status are displayed in Table 2. Women who were currently pregnant and/or breastfeeding tended to be younger and were more likely to be born within the US, unemployed, insured, and married or living with a partner. They reported consuming fewer alcoholic drinks on average, had lower serum cotinine concentrations, were more likely to use prenatal supplements, and had previously experienced a higher number of pregnancies than their non-pregnant and non-breastfeeding counterparts. Average BMIs did not appear to differ by reproductive status. Levels of urinary creatinine were similar between those who were currently pregnant/breastfeeding (data not shown). In terms of dietary intakes, pregnant/breastfeeding women reported consuming greater daily amounts of calories, vegetables, legumes, fruits, eggs, dairy, and grains.
Table 2.
Characteristics of reproductive-age women by reproductive status and metal biomarker sub-sample, NHANES 2003—2014.
| Blood Metal Sub-Sample | Urine Metal Sub-Sample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Overall (N=4,285) |
Not Pregnant/Breastfeeding (N=3,639) |
Pregnant/Breastfeeding (N=646) |
Overall (N=1,562) |
Not Pregnant/Breastfeeding (N=1,334) |
Pregnant/Breastfeeding (N=228) |
| Sociodemographics | ||||||
| Age (years), Mean ± SE | 32.2 ± 0.2 | 32.5 ± 0.2 | 29.0 ± 0.4 | 32.1 ± 0.2 | 32.4 ± 0.3 | 28.6 ± 0.5 |
| Race/Ethnicity, % | ||||||
| Non-Hispanic white | 64.4 | 64.8 | 60.6 | 64.6 | 65.2 | 58.6 |
| Non-Hispanic black | 12.6 | 12.7 | 12.4 | 12.2 | 12.3 | 11.6 |
| Hispanic | 15.7 | 15.5 | 18.0 | 15.9 | 15.6 | 19.7 |
| Other | 7.3 | 7.0 | 9.0 | 7.2 | 6.9 | 10.1 |
| Educational attainment, % | ||||||
| Less than high school diploma | 13.5 | 13.3 | 15.5 | 13.6 | 13.6 | 14.0 |
| High school diploma/GED | 18.6 | 18.9 | 15.7 | 18.9 | 19.2 | 15.7 |
| At least some college | 36.8 | 37.2 | 32.3 | 37.7 | 37.8 | 36.0 |
| College graduate or above | 31.1 | 30.6 | 36.5 | 29.9 | 29.4 | 34.3 |
| Employment status, % | ||||||
| Unemployed | 30.4 | 28.9 | 46.7 | 30.0 | 28.6 | 44.8 |
| Employed | 69.6 | 71.1 | 53.3 | 70.0 | 71.4 | 55.2 |
| Family income: poverty line, Mean ± SE | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 3.0 ± 0.2 |
| Health insurance status, % | ||||||
| No current coverage | 21.4 | 22.1 | 13.4 | 20.3 | 20.9 | 13.8 |
| Current private or public coverage | 78.6 | 77.9 | 86.6 | 79.7 | 79.1 | 86.2 |
| Marital status, % | ||||||
| Single | 38.0 | 40.2 | 14.7 | 38.0 | 40.1 | 16.1 |
| Married or living with partner | 62.0 | 59.8 | 85.3 | 62.0 | 59.9 | 83.9 |
| Nativity, % | ||||||
| Born in the US | 84.0 | 84.5 | 79.1 | 83.6 | 84.3 | 76.1 |
| Born in a US territory or another country | 16.0 | 15.5 | 20.9 | 16.4 | 15.7 | 23.9 |
| Lifestyle Factors | ||||||
| Alcohol (drinks/day), Mean ± SE | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.1 ± 0.03 | 0.5 ± 0.04 | 0.5 ± 0.04 | 0.1 ± 0.04 |
| Cigarette smoke exposure, % | ||||||
| Unexposed (Non-detectable serum cotinine) | 25.5 | 23.9 | 42.4 | 24.3 | 22.8 | 40.0 |
| Secondhand (<10 ng/mL serum cotinine) | 50.0 | 50.1 | 48.0 | 51.8 | 52.1 | 49.2 |
| Active (≥10 ng/mL serum cotinine) | 24.5 | 26.0 | 9.6 | 23.9 | 25.1 | 10.8 |
| Prenatal supplement use, % | ||||||
| No | 93.8 | 97.6 | 54.3 | 93.2 | 97.6 | 47.7 |
| Yes | 6.2 | 2.4 | 45.8 | 6.8 | 2.4 | 52.3 |
| Other multi-vitamin/multi-mineral use, % | ||||||
| No | 71.2 | 70.6 | 77.3 | 72.2 | 71.6 | 77.1 |
| Yes | 28.8 | 29.4 | 22.7 | 27.9 | 28.4 | 22.9 |
| Gravidity, Mean ± SE | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.6 ± 0.1 | 2.0 ± 0.07 | 2.0 ± 0.08 | 2.5 ± 0.15 |
| BMI (kg/m2). Mean ± SE | 28.5 ± 0.2 | 28.5 ± 0.2 | 28.3 ± 0.4 | 28.3 ± 0.2 | 28.4 ± 0.2 | 27.8 ± 0.5 |
| Dietary Factors | ||||||
| Calories (kcal/day), Mean ± SE | 1888 ± 12 | 1858 ± 13 | 2184 ± 39 | 1887 ± 22 | 1855 ± 22 | 2190 ± 69 |
| Seafood (ounces/day), Mean ± SE | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.5 ± 0.06 | 0.5 ± 0.03 | 0.4 ± 0.03 | 0.5 ± 0.09 |
| Meat (ounces/day), Mean ± SE | 1.3 ± 0.03 | 1.3 ± 0.04 | 1.5 ± 0.09 | 1.3 ± 0.05 | 1.3 ± 0.05 | 1.5 ± 0.1 |
| Poultry (ounces/day), Mean ± SE | 1.4 ± 0.04 | 1.4 ± 0.04 | 1.4 ± 0.09 | 1.4 ± 0.05 | 1.4 ± 0.06 | 1.4 ± 0.1 |
| Vegetables (cups/day), Mean ± SE | 1.4 ± 0.02 | 1.4 ± 0.02 | 1.6 ± 0.06 | 1.4 ± 0.03 | 1.4 ± 0.03 | 1.6 ± 0.1 |
| Legumes (ounces/day), Mean ± SE | 0.1 ± 0.04 | 0.1 ± 0.04 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.02 |
| Nuts and seeds (ounces/day), Mean ± SE | 0.5 ± 0.03 | 0.5 ± 0.03 | 0.7 ± 0.2 | 0.5 ± 0.04 | 0.5 ± 0.05 | 0.6 ± 0.09 |
| Fruit (cups/day), Mean ± SE | 0.8 ± 0.02 | 0.8 ± 0.02 | 1.4 ± 0.07 | 0.9 ± 0.04 | 0.8 ± 0.04 | 1.4 ± 0.1 |
| Eggs (ounces/day), Mean ± SE | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.5 ± 0.03 | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.6 ± 0.05 |
| Dairy (cups/day), Mean ± SE | 1.5 ± 0.03 | 1.5 ± 0.03 | 2.0 ± 0.08 | 1.5 ± 0.04 | 1.5 ± 0.04 | 2.1 ± 0.1 |
| Whole grains (ounces/day), Mean ± SE | 0.7 ± 0.02 | 0.7 ± 0.02 | 0.9 ± 0.07 | 0.7 ± 0.03 | 0.7 ± 0.04 | 0.9 ± 0.1 |
| Refined grains (ounces/day), Mean ± SE | 5.3 ± 0.06 | 5.2 ± 0.06 | 6.5 ± 0.2 | 5.3 ± 0.1 | 5.2 ± 0.1 | 6.6 ± 0.3 |
| Tap water source, % | ||||||
| Community supply | 73.7 | 73.7 | 74.5 | 73.7 | 73.6 | 75.0 |
| Well, rain cistern, or spring | 10.1 | 9.9 | 11.6 | 9.5 | 9.4 | 9.7 |
| Don’t drink tap water | 16.2 | 16.4 | 13.9 | 16.8 | 17.0 | 15.3 |
Blood Lead and Mercury Levels
In separate survey-weighted linear regression models, a number of predictors were associated with blood lead and mercury concentrations after correcting for multiple comparisons (Table 3). Specifically, age, non-Hispanic black or other race/ethnicity, being born in a US territory or outside of the country, serum cotinine, and public drinking water intakes were positively associated with blood lead levels among all 4,285 women of reproductive age. In contrast, higher educational attainment, current employment, current health insurance coverage, prenatal supplement use, other multi-vitamin/multi-mineral supplement use, and BMI were inversely related to blood lead levels. Point estimates from models stratified by reproductive status were similar, with the strongest associations observed for women who were born outside of the 50 United States, who were non-Hispanic black, or who were of another race/ethnicity. Our models explained between 32% and 40% of the variation in blood lead levels (Table 3).
Table 3.
Adjusted1 model coefficients for blood lead and mercury concentrations among women of reproductive-age, NHANES 2003—2014.
| Blood Lead | Blood Mercury | |||||
|---|---|---|---|---|---|---|
| Characteristic2 | Overall (N=4,285) |
Not Pregnant/Breastfeeding (N=3,639) |
Pregnant/Breastfeeding (N=646) |
Overall (N=4,285) |
Not Pregnant/Breastfeeding (N=3,639) |
Pregnant/Breastfeeding (N=646) |
| Sociodemographics | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Age z-score | 10.2 (8.2, 12.2)* | 9.5 (7.5, 11.5)* | 16.0 (8.4, 24.2)* | 5.9 (2.8, 9.0)* | 5.2 (2.0, 8.4)* | 14.9 (2.8, 28.3) |
| Race/Ethnicity | ||||||
| Non-Hispanic white | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Non-Hispanic black | 15.5 (9.4, 21.8)* | 15.4 (9.2, 21.8)* | 22.0 (4.3, 42.8) | 35.7 (21.1, 52.0)* | 36.7 (22.1, 53.0)* | 20.8 (−4.6, 52.9) |
| Hispanic | 1.3 (−5.2, 8.2) | −0.5 (−7.1, 6.6) | 16.4 (−2.2, 38.5) | 14.5 (3.7, 26.5)* | 15.7 (3.9, 28.9)* | −10.9 (−31.2, 15.4) |
| Other | 17.4 (9.4, 26.0)* | 16.5 (8.4, 25.2)* | 27.3 (6.0, 52.9) | 71.4 (51.4, 93.9)* | 79.2 (57.1, 104.4)* | 15.9 (−18.6, 65.1) |
| Educational attainment | ||||||
| Less than high school diploma | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| High school diploma/GED | −9.8 (−15.7, −3.5)* | −9.3 (−15.6, −2.5)* | −13.5 (−26.7, 2.1) | −0.4 (−10.0, 10.3) | −1.9 (−12.1, 9.5) | 11.8 (−16.4, 49.6) |
| At least some college | −9.7(−15.3, −3.7)* | −8.8 (−14.8, −2.4)* | −18.1 (−30.6, −3.3) | 10.0 (−0.9, 22.1) | 7.5 (−4.5, 20.9) | 34.0 (4.8, 71.3) |
| College graduate or above | −7.5 (−13.8, −0.9) | −6.8 (−13.9, 0.7) | −17.1 (−31.8, 0.7) | 27.6 (14.9, 41.7)* | 26.8 (12.6, 42.9)* | 20.6 (−9.5, 60.7) |
| Employment status | ||||||
| Unemployed | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Employed | −4.6 (−8.0, −1.1)* | −3.8 (−7.6, 0.3) | −12.2 (−22.3, −0.8) | 1.5 (−5.0, 8.4) | −0.2 (−7.1, 7.3) | 6.4 (−11.5, 28.1) |
| Family income: poverty line | −0.5 (−2.4, 1.5) | −0.2 (−1.9, 1.6) | −1.5 (−8.9, 6.5) | 10.4 (6.1, 14.9)* | 10.0 (5.6, 14.7)* | 10.8 (−0.4, 23.2) |
| Health insurance status | ||||||
| No current coverage | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Current private or public coverage | −4.9 (−8.8, −0.9)* | −4.9 (−9.0, −0.7) | −6.6 (−21.0, 10.3) | 0.2 (−7.7, 8.8) | 0.2 (−7.8, 9.0) | 5.5 (−18.6, 36.8) |
| Marital status | ||||||
| Single | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Married or living with partner | −2.1(−6.3, 2.3) | −1.9 (−6.1, 2.6) | 0.6 (−13.1, 16.5) | −5.3 (−11.6, 1.4) | −4.6 (−11.2, 2.6) | 1.9 (−19.1, 28.3) |
| Nativity | ||||||
| Born in the US | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Born in a US territory or another country | 36.6 (27.0, 47.0)* | 37.7 (27.4, 48.8)* | 28.2 (11.4, 47.7)* | 19.7 (9.2, 31.1)* | 21.3 (9.8, 33.9)* | 6.5 (−13.9, 31.9) |
| Lifestyle Factors | ||||||
| Alcohol z-score | 7.1 (5.2, 9.0) | 6.6 (4.8, 8.5) | 10.1 (5.0, 15.4)* | 11.6 (8.4, 14.9) | 11.2 (7.9, 14.6)* | 7.0 (−1.8, 16.6) |
| Serum cotinine z-score | 10.7 (9.0, 12.4)* | 10.4 (8.7, 12.2)* | 10.1 (3.6, 16.9)* | −2.3 (−5.0, 0.6) | −2.3 (−5.1, 0.6) | −4.2 (−12.6, 5.1) |
| Prenatal supplement use3 | ||||||
| No | 0.0 (ref.) | -- | 0.0 (ref.) | 0.0 (ref.) | -- | 0.0 (ref.) |
| Yes | −12.7 (−19.1, −5.7)* | -- | −6.6 (−17.6, 5.9) | 1.6 (−10.0, 14.7) | -- | 15.5 (−1.8, 35.8) |
| Other multi-vitamin/multi-mineral use3 | ||||||
| No | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Yes | −5.1 (−8.8, −1.2)* | −5.9 (−9.7, −2.0)* | 2.3 (−9.9, 16.1) | 4.3 (−2.9, 11.9) | 3.9 (−3.3, 11.6) | 19.0 (−3.2, 46.2) |
| Gravidity z-score | −1.3 (−3.1, 0.5) | −1.1 (−2.9, 0.9) | −1.9 (−7.8, 4.4) | −2.5 (−5.1, 0.2) | −2.4 (−5.0, 0.2) | −0.6 (−9.4, 9.2) |
| BMI z-score | −4.6 (−5.7, −3.4)* | −4.4 (−5.6, −3.3)* | −5.3 (−10.8, 0.6) | −6.7 (−9.1, −4.2)* | −6.5 (−8.9, −3.9)* | −11.0 (−18.4, −2.9) |
| Dietary Factors | ||||||
| Calories z-score | −0.6 (−3.3, 2.1) | 0.3 (−2.4, 3.1) | −8.2 (−17.7, 2.5) | −8.6 (−12.7, −4.3)* | −7.3 (−11.7, −2.7)* | −18.4 (−28.7, −6.6)* |
| Seafood z-score | −0.1 (−1.3, 1.0) | −0.5 (−1.6, 0.6) | 5.5 (0.5, 10.7) | 15.3 (11.4, 19.4)* | 14.7 (10.7, 18.8)* | 26.1 (18.2, 34.5)* |
| Meat z-score | 1.3 (−0.3, 2.9) | 1.1 (−0.4, 2.6) | 4.0 (−3.0, 11.4) | 4.3 (1.1, 7.6)* | 3.7 (0.4, 7.1) | 11.5 (2.1, 21.8) |
| Poultry z-score | 0.1 (−1.3, 1.5) | −0.2 (−1.5, 1.2) | 3.8 (−2.2, 10.2) | 4.9 (2.3, 7.5)* | 4.7 (2.1, 7.4)* | 10.0 (1.6, 19.0) |
| Vegetables z-score | 1.6 (−0.2, 3.4) | 1.5 (−0.3, 3.4) | −1.6 (−6.8, 4.0) | 3.8 (−0.6, 8.4) | 3.6 (−0.9, 8.4) | −0.3 (−8.8, 9.1) |
| Legumes z-score | 1.5 (0.2, 2.8) | 1.1 (−0.3, 2.5) | 5.5 (0.4, 10.8) | 1.8 (−1.1, 4.8) | 1.6 (−1.5, 4.8) | 6.5 (−2.3, 16.1) |
| Nuts and seeds z-score | 0.0 (−1.2, 1.3) | −0.4 (−1.6, 0.8) | 4.8 (−2.6, 12.8) | 2.9 (0.5, 5.4)* | 3.3 (0.7, 6.0)* | −8.0 (−16.9, 1.7) |
| Fruit z-score | 0.6 (−1.2, 2.5) | 0.4 (−1.5, 2.3) | 3.1 (−1.7, 8.1) | 0.0 (−3.4, 3.5) | −0.3 (−3.8, 3.3) | 5.6 (−3.4, 15.4) |
| Eggs z-score | −0.3 (−1.7, 1.1) | −0.3 (−1.6, 1.1) | −3.5 (−8.7, 1.9) | 5.9 (1.9, 10.1)* | 5.6 (1.5, 9.9)* | 11.8 (4.2, 20.0)* |
| Dairy z-score | −1.3 (−3.0, 0.4) | −1.8 (−3.6, 0.0) | 4.5 (−2.1, 11.4) | 1.2 (−1.7, 4.2) | 1.5 (−1.6, 4.6) | −2.1 (−9.5, 6.0) |
| Whole grains z-score | −1.7 (−3.3, −0.2) | −1.5 (−3.0, 0.0) | −3.0 (−10.7, 5.5) | 3.2 (−0.3, 6.8) | 3.6 (0.0, 7.4) | −2.1 (−11.0, 7.8) |
| Refined grains z-score | −1.6 (−3.4, 0.2) | −2.1 (−4.0, −0.2) | 5.6 (−2.6, 14.4) | 0.2 (−3.3, 3.9) | −0.8 (−4.4, 2.9) | 16.8 (3.5, 31.9) |
| Public water z-score | 3.4 (1.8, 5.1)* | 3.6 (1.9, 5.3)* | 1.0 (−5.2, 7.5) | 2.1 (−0.8, 5.1) | 2.3 (−0.8, 5.4) | 0.7 (−8.7, 11.1) |
| Private water z-score | 1.3 (−0.5, 3.1) | 1.6 (−0.1, 3.3) | −1.2 (−9.3, 7.6) | 2.2 (−0.7, 5.1) | 2.0 (−0.8, 4.9) | 4.5 (−1.6, 11.1) |
| Model R2 | 0.32 | 0.32 | 0.40 | 0.23 | 0.23 | 0.30 |
Adjusted for all variables listed in the Table in addition to survey cycle (2003-2004, 2005-2006, 2007-2008, 2009-2010, 2011-2012, or 2013-2014)
For all continuous variables, the adjusted percent difference in biomarker concentration corresponds to a 1-standard deviation increase
Because women who were not pregnant or breastfeeding rarely used prenatal supplements (2.4%), we combined prenatal with other multi-vitamin/multi-mineral supplement use for this sub-group
Q-value < 0.05
For blood total mercury, older age, minority race/ethnicity, a college degree or above, higher family incomes, a birthplace in a US territory or another country, seafood, meat, poultry, nut and seed, and egg intakes were predictive of higher concentrations, whereas higher BMIs and caloric intakes were predictive of lower concentrations (Table 3). Overall, the strongest associations were observed for women of other racial/ethnic groups (e.g., Asian Americans) who had blood mercury levels 71.4% (95% CI: 51.4-93.9%) greater than non-Hispanic white women, and for non-Hispanic black women who had levels 35.7% (95% CI: 21.1-52.0%) greater than white women. Stratified analyses revealed the inverse association observed for daily caloric intakes and the positive associations observed for seafood and egg intakes with blood mercury levels were more pronounced among women who were pregnant or breastfeeding. Collectively, the selected variables in the regression models explained less than one-third of the variation in blood mercury concentrations (Table 3).
Urinary Inorganic Arsenic and Cadmium Levels
Hispanic women of reproductive age and women of other racial/ethnic groups tended to have the highest urinary inorganic arsenic concentrations (Table 4). A 1-standard deviation increase in daily seafood and fruit intakes were associated with 1.4% (95% CI: 0.7-2.1%) and 1.1% (95% CI: 0.3-1.9%) higher urinary arsenic levels, respectively. Results were similar by reproductive status, although we did observe a positive association between refined grain intakes and arsenic concentrations only among women not currently pregnant or breastfeeding. Altogether, sociodemographic, lifestyle, and dietary factors explained 45-60% of the variation in urinary inorganic arsenic levels (Table 4).
Table 4.
Adjusted1 model coefficients for urinary inorganic arsenic and cadmium concentrations among women of reproductive-age, NHANES 2003—2014.
| Urinary Inorganic Arsenic | Urinary Cadmium | |||||
|---|---|---|---|---|---|---|
| Characteristic2 | Overall (N=1,562) |
Not Pregnant/Breastfeeding (N=1,334) |
Pregnant/Breastfeeding (N=228) |
Overall (N=1,562) |
Not Pregnant/Breastfeeding (N=1,334) |
Pregnant/Breastfeeding (N=228) |
| Sociodemographics | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) |
| Age z-score | −0.8 (−1.9, 0.3) | −0.5 (−1.6, 0.7) | −2.0 (−4.8, 0.9) | 29.7 (24.2, 35.3)* | 29.7 (24.1, 35.5) | 30.6 (16.6, 46.2)* |
| Race/Ethnicity | ||||||
| Non-Hispanic white | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Non-Hispanic black | −0.8 (−3.7, 2.2) | −0.8 (−3.9, 2.4) | −2.8 (−9.6, 4.6) | 13.2 (−0.9, 29.1) | 13.8 (−0.6, 30.2) | 24.0 (−5.6, 62.7) |
| Hispanic | 4.3 (1.8, 6.9)* | 4.1 (1.6, 6.7)* | 3.7 (−3.8, 11.8) | 26.4 (14.3, 39.7)* | 29.6 (15.8, 45.1)* | −3.4 (−29.7, 32.7) |
| Other | 5.8 (1.9, 9.9)* | 6.2 (1.9, 10.6)* | 6.1 (−4.7, 18.2) | 36.4 (16.7, 59.5)* | 31.9 (11.5, 56.1)* | 57.3 (15.4, 114.4)* |
| Educational attainment | ||||||
| Less than high school diploma | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| High school diploma/GED | −0.9 (−3.9, 2.2) | −0.9 (−4.1, 2.5) | 1.2 (−5.1, 7.9) | −12.8 (−24.6, 0.7) | −12.6 (−25.1, 2) | −11.2 (−38.0, 27.2) |
| At least some college | −3.4 (−6.1, −0.7) | −3.4 (−6.3, −0.4) | −1.7 (−7.0, 3.9) | −9.7 (−21.2, 3.4) | −12.1 (−23.7, 1.4) | 11.9 (−18.1, 52.9) |
| College graduate or above | −3.2 (−6.4, 0.1) | −2.6 (−5.9, 0.8) | −7.9 (−15.7, 0.6) | −12.2 (25.4, 3.4) | −13.0 (−26.7, 3.3) | 4.7 (−27.3, 50.6) |
| Employment status | ||||||
| Unemployed | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Employed | 1.5 (−0.7, 3.8) | 2.0 (−0.5, 4.5) | −4.8 (−9.1, −0.3) | −4.7 (−12.8, 4.1) | −5.8 (−14.3, 3.6) | 10.1 (−9.0, 33.1) |
| Family income: poverty line | 0.3 (−0.8, 1.3) | 0.1 (−0.9, 1.2) | 1.3 (−2.2, 4.9) | 2.2 (−2.1, 6.7) | 2.6 (−1.9, 7.3) | −8.4 (−18.1, 2.5) |
| Health insurance status | ||||||
| No current coverage | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Current private or public coverage | 1.0 (−1.2, 3.2) | 1.0 (−1.2, 3.4) | −4.4 (−11.1, 2.9) | 1.0 (−8.1, 11.0) | 2.0 (−7.4, 12.4) | −23.0 (−42.1, 2.4) |
| Marital status | ||||||
| Single | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Married or living with partner | 0.4 (−1.9, 2.8) | −0.1 (−2.6, 2.4) | 4.3 (−2.0, 10.9) | −5.3 (−14.0, 4.3) | −6.9 (−15.6, 2.7) | 25.8 (−2.1, 61.7) |
| Nativity | ||||||
| Born in the US | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Born in a US territory or another country | 3.8 (0.9, 6.8)* | 4.0 (0.9, 7.2)* | 1.1 (−7.7, 10.7) | 4.9 (−6.4, 17.6) | 2.8 (−8.8, 15.9) | 22.7 (−5.7, 59.8) |
| Lifestyle Factors | ||||||
| Alcohol z-score | 1.2 (0.1, 2.4) | 1.4 (0.2, 2.6) | 0.4 (−2.1, 3.0) | −0.3 (−4.4, 3.9) | 0.3 (−4.1, 4.9) | 0.2 (−7.7, 8.7) |
| Serum cotinine z-score | −0.5 (−1.7, 0.6) | −0.4 (−1.6, 0.8) | −1.6 (−3.2, 0.0) | 17.8 (13.3, 22.4)* | 17.9 (13.1, 23.0)* | 8.4 (−0.2, 17.7) |
| Prenatal supplement use3 | ||||||
| No | 0.0 (ref.) | -- | 0.0 (ref.) | 0.0 (ref.) | -- | 0.0 (ref.) |
| Yes | −3.0 (−7.5, 1.8) | -- | 2.2 (−3.6, 8.4) | −19.3 (−30.5, −6.2)* | -- | −27.4 (−42.8, −7.7) |
| Other multi-vitamin/multi-mineral use3 | ||||||
| No | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) | 0.0 (ref.) |
| Yes | 1.1 (−1.0, 3.3) | 0.0 (−2.3, 2.4) | 10.6 (2.3, 19.6) | −1.5 (−10.2, 8.1) | −4.4 (−13.4, 5.5) | 10.4 (−15.2, 43.7) |
| Gravidity z-score | 0.3 (−0.8, 1.3) | 0.1 (−0.9, 1.2) | 0.6 (−2.4, 3.6) | 1.3 (−1.9, 4.6) | 0.7 (−2.7, 4.2) | −2.3 (−11.4, 7.9) |
| BMI z-score | −0.7 (−1.5, 0.1) | −0.7 (−1.7, 0.2) | −0.5 (−2.4, 1.6) | −1.9 (−5.4, 1.7) | −1.8 (−5.3, 1.9) | −4.3 (−13.0, 5.2) |
| Dietary Factors | ||||||
| Calories z-score | −0.8 (−2.4, 0.8) | −1.2 (−2.9, 0.6) | 3.5 (−1.8, 9.0) | 3.8 (−3.0, 11.1) | 1.2 (−5.7, 8.6) | 31.6 (9.4, 58.3)* |
| Seafood z-score | 1.4 (0.7, 2.1)* | 1.4 (0.6, 2.2)* | 3.0 (0.7, 5.3) | 2.4 (−0.2, 5.2) | 2.5 (−0.2, 5.2) | 1.9 (−10.9, 16.4) |
| Meat z-score | −0.1 (−1.0, 0.8) | −0.2 (−1.1, 0.8) | 1.0 (−1.2, 3.2) | −0.2 (−3.7, 3.3) | −0.3 (−3.9, 3.5) | −1.0 (−9.3, 8,1) |
| Poultry z-score | 0.1 (−0.9, 1.0) | 0.2 (−0.8, 1.1) | −0.6 (−3.7, 2.6) | −0.3 (−4.4, 3.9) | −0.2 (−4.3, 4.1) | 7.4 (−2.9, 18.8) |
| Vegetables z-score | 0.6 (−0.2, 1.4) | 0.7 (−0.1, 1.6) | −1.0 (−3.9, 2.0) | 0.4 (−3.4, 4.4) | 0.7 (−3.1, 4.7) | −1.3 (−11.6, 10.2) |
| Legumes z-score | 0.6 (−0.1, 1.3) | 0.5 (−0.2, 1.3) | 1.5 (−0.5, 3.5) | 0.0 (−2.9, 3.1) | −0.3 (−3.2, 2.8) | 4.9 (−4.8, 15.7) |
| Nuts and seeds z-score | 0.6 (−0.1, 1.3) | 0.8 (0.1, 1.5) | −2.2 (−4.4, 0.1) | −1.9 (−6.0, 2.4) | −1.4 (−5.8, 3.2) | −0.2 (−7.8, 7.9) |
| Fruit z-score | 1.1 (0.3, 1.9)* | 0.7 (−0.2, 1.6) | 3.9 (0.9, 7.1) | −0.6 (−3.6, 2.5) | −1.9 (−5.1, 1.4) | 10.5 (−1.1, 23.5) |
| Eggs z-score | 0.5 (−0.3, 1.4) | 0.7 (−0.2, 1.6) | −1.2 (−3.8, 1.4) | −0.8 (−4.1, 2.6) | −0.3 (−3.8, 3.5) | −6.0 (−16.4, 5.7) |
| Dairy z-score | 0.0 (−1.5, 1.6) | 0.2 (−0.9, 1.3) | −2.0 (−4.9, 1.0) | 1.0 (−6.1, 8.6) | 1.1 (−3.8, 6.2) | −5.6 (−13.7, 3.2) |
| Whole grains z-score | 0.2 (−0.7, 1.1) | 0.2 (−0.8, 1.1) | −0.4 (−3.1, 2.4) | −0.6 (−4.4, 3.5) | 0.1 (−3.7, 4.1) | −6.2 (−14.7, 3.3) |
| Refined grains z-score | 1.4 (0.3, 2.6) | 1.6 (0.4, 2.8)* | −2.2 (−6.5, 2.4) | −3.8 (−8.6, 1.3) | −3.2 (−8.0, 2.0) | −12.2 (−23.6, 0.9) |
| Public water z-score | 0.2 (−0.6, 1.0) | 0.2 (−0.6, 1.0) | 0.5 (−1.3, 2.4) | 0.6 (−3.6, 5.0) | 0.2 (−4.2, 4.9) | −2.9 (−12.3, 7.5) |
| Private water z-score | 0.2 (−0.9, 1.3) | 0.3 (−0.5, 1.0) | −1.2 (−5.5, 3.3) | −0.8 (−4.5, 2.9) | −0.2 (−4.5, 4.1) | −8.2 (−16.8, 1.3) |
| Model R2 | 0.45 | 0.45 | 0.60 | 0.58 | 0.58 | 0.75 |
Adjusted for all variables listed in the Table in addition to survey cycle (2003-2004, 2005-2006, 2007-2008, 2009-2010, 2011-2012, or 2013-2014) and urinary creatinine (mg/dL)
For all continuous variables, the adjusted percent difference in biomarker concentration corresponds to a 1-standard deviation increase
Because women who were not pregnant or breastfeeding rarely used prenatal supplements (2.4%), we combined prenatal with other multi-vitamin/multi-mineral supplement use for this sub-group
Q-value < 0.05
Older age, Hispanic ethnicity, other race/ethnicity, and serum cotinine levels were each positively related to urinary cadmium concentrations, whereas prenatal supplement use was inversely associated with urinary cadmium concentrations (Table 4). These results mostly were largely consistent across analyses stratified by reproductive status. Notably, greater caloric intakes were significantly associated with higher urinary cadmium concentrations among pregnant and/or breastfeeding women only, suggesting diet may be a potential source of exposure for this sub-group. The sociodemographic, lifestyle, and dietary variables assessed explained 58-75% of variation in urinary cadmium levels (Table 4).
Metal Mixtures
Of the 1,267 women of reproductive age with blood and urinary metal measures available from NHANES 2003-2012, 10.5% had levels of blood lead, blood mercury, urinary inorganic arsenic, and urinary cadmium above their respective geometric means. We again observed a declining temporal trend for those classified as having combined high exposures (i.e., concentrations of all four biomarkers above the geometric mean), with a prevalence of 23.3% during 2003-2004, 29.2% during 2005-2006, 24.0% during 2007-2008, 19.8% during 2009-2010, and only 3.7% during 2011-2012. Pairwise Spearman correlations between most of the metal biomarkers were weakly positive (rs < 0.20) with urinary inorganic arsenic and cadmium concentrations displaying the strongest correlation at rs = 0.47. Results from the multiple metal index model suggested older age, non-Hispanic black race/ethnicity, a birthplace in a US territory or another country, greater alcohol consumption, and lower BMI were significant predictors of having high concentrations of all four metal biomarkers (Table 5). The model discriminated fairly well with a C-statistic of 0.86.
Table 5.
Adjusted odds of high concentrations of blood lead, blood mercury, urinary inorganic arsenic, and urinary cadmium levels among women of reproductive age, NHANES 2003-2012.
| Characteristic1 | Overall (N=1,267) |
|---|---|
| Sociodemographics | OR (95% CI)2 |
| Age z-score | 1.6 (1.2-2.1)* |
| Race/Ethnicity | |
| Non-Hispanic white | 1.0 (ref.) |
| Non-Hispanic black | 2.1 (1.2-3.8)* |
| Hispanic | 0.9 (0.4-1.9) |
| Other | 3.2 (1.2-8.7) |
| Educational attainment | |
| Less than high school diploma | 1.0 (ref.) |
| High school diploma/GED | 0.7 (0.3-1.3) |
| At least some college | 0.8 (0.4-1.8) |
| College graduate or above | 1.1 (0.4-2.8) |
| Employment status | |
| Unemployed | 1.0 (ref.) |
| Employed | 1.7 (1.0-2.8) |
| Family income: poverty line | 1.1 (0.8-1.5) |
| Health insurance status | |
| No current coverage | 1.0 (ref.) |
| Current private or public coverage | 1.0 (0.6-1.9) |
| Marital status | |
| Single | 1.0 (ref.) |
| Married or living with partner | 1.2 (0.7-1.9) |
| Nativity | |
| Born in the US | 1.0 (ref.) |
| Born in a US territory or another country | 5.8 (2.8-11.7)* |
| Lifestyle Factors | |
| Alcohol z-score | 1.4 (1.1-1.9)* |
| Serum cotinine z-score | 1.2 (1.0-1.6) |
| Prenatal supplement use | |
| No | 1.0 (ref.) |
| Yes | 0.7 (0.2-2.0) |
| Other multi-vitamin/multi-mineral use | |
| No | 1.0 (ref.) |
| Yes | 1.3 (0.7-2.3) |
| Gravidity z-score | 1.1 (0.8-1.3) |
| BMI z-score | 0.7 (0.6-0.9)* |
| Dietary Factors | |
| Calories z-score | 1.3 (0.8-2.0) |
| Seafood z-score | 1.2 (0.9-1.5) |
| Meat z-score | 0.9 (0.7-1.1) |
| Poultry z-score | 1.0 (0.8-1.2) |
| Vegetables z-score | 1.0 (0.7-1.3) |
| Legumes z-score | 1.0 (0.8-1.2) |
| Nuts and seeds z-score | 0.9 (0.6-1.3) |
| Fruit z-score | 0.9 (0.7-1.2) |
| Eggs z-score | 1.1 (0.9-1.3) |
| Dairy z-score | 0.8 (0.6-1.1) |
| Whole grains z-score | 0.8 (0.7-1.1) |
| Refined grains z-score | 0.8 (0.6-1.2) |
| Public water z-score | 1.1 (0.9-1.4) |
| Private water z-score | 1.0 (0.8-1.3) |
| Model C-statistic | 0.86 |
For all continuous variables, the adjusted odds ratio corresponds to a 1-standard deviation increase
Adjusted for all variables listed in the Table in addition to survey cycle (2003-2004, 2005-2006, 2007-2008, 2009-2010, or 2011-2012) and urinary creatinine (mg/dL)
Q-value < 0.05
Sensitivity Analysis
When creatinine-corrected urinary inorganic arsenic and cadmium concentrations were used to derive the multiple metal index, 7.7% of women were classified as having high concentrations of all four metal biomarkers (i.e., creatinine-corrected inorganic arsenic, creatinine-corrected urinary cadmium, blood lead, and blood mercury). As in the original analysis, older age and a birthplace outside of the 50 United States were found to be positively associated with higher toxic metal exposures although non-Hispanic black race/ethnicity, alcohol consumption, and BMI were no longer significant (Supplemental Table 1). Greater seafood intake and a public drinking water source also emerged as significant predictors of high exposures to all four metals.
Discussion
We sought to evaluate toxic metal exposures among US women of reproductive age in terms of temporal trends and in relation to individual-level characteristics. In cross-sectional analyses, sociodemographic factors tended to be the most strongly related to biomarkers of lead, mercury, inorganic arsenic, and cadmium exposure. Single-metal models, as well as the metal mixtures model, indicated older age, racial/ethnic minorities, and being born outside of the 50 United States were related to higher metals exposures. Compared to non-Hispanic white women, non-Hispanic black women had higher concentrations of blood lead and mercury; Hispanic women had higher concentrations of blood mercury, urinary inorganic arsenic, and urinary cadmium; and women of another race/ethnicity (including Asian women) had higher concentrations of blood lead, blood mercury, urinary inorganic arsenic, and urinary cadmium. Certain lifestyle factors, namely alcohol intake and serum cotinine levels (an integrated measure of active and secondhand tobacco smoke exposure) were positively associated with blood lead, urinary cadmium, and the multiple metal index (reflecting high levels of exposure to all four metals). Lower BMIs were associated with lower concentrations of blood lead and mercury. Use of multi-vitamins/multi-minerals, including prenatal formulations, was inversely associated with blood lead and urinary cadmium concentrations.
Associations for dietary intakes were mostly null, although we did observe some important associations for certain toxic metals. Specifically, a public drinking water source was associated with higher blood lead levels; seafood, meat, poultry, nuts and seeds, and egg intakes with higher blood mercury levels; and seafood, fruit, and refined grain intakes with higher urinary inorganic arsenic levels. Finally, there was evidence of a positive association between caloric intakes with higher urinary cadmium levels only among pregnant/breastfeeding women. Some of these findings are consistent with prior research. The link between seafood intake and mercury exposure has long been recognized.(25) Studies have also shown seafood, particularly seaweed, fruits and fruit juices, and rice may be important sources of inorganic arsenic exposure.(26–28) Our findings regarding a public drinking water source with blood lead levels and egg, meat, poultry, and nuts and seed intakes with blood mercury levels appear to be previously unrecognized. Finally, although dietary intakes were not found to be related to urinary cadmium concentrations, the significant association seen for caloric intake among pregnant and breastfeeding women might be reflective of distinct dietary patterns (as opposed to specific foods) within this sub-group.(29, 30)
Levels of all four toxic metals assessed in this study decreased over time. Biomarker concentrations tended to be lower among women who were currently pregnant or breastfeeding, with the exception of inorganic arsenic. There are several potential explanations for differences in metal biomarker concentrations by reproductive status, including hemodilution during gestation, dietary modifications, and lifestyle changes such as quitting smoking.(29, 31–33) Nevertheless, the overall decline in biomarker levels among women of reproductive age are encouraging and indicate ongoing efforts, such as the Safe Drinking Water Act, fish consumption advisories, smoking cessation programs, and removal of lead from gasoline have been effective at reducing environmental exposures.(22, 33–35) Still, toxic metals remain a critical health concern for women of reproductive age as the long-term impacts of metal mixture exposures on reproductive health, birth outcomes, and offspring health have not yet been elucidated.
Data from this analysis and others suggest lifestyle modifications targeted at decreasing alcohol use and tobacco smoke exposure may translate to reductions in toxic metal exposures.(33, 36) Of these, smoking cessation programs, particularly those focused on establishing smoke-free homes, may be most effective given the strong associations observed between serum cotinine and levels of metal biomarkers and the high prevalence of cigarette smoke exposure in this population.(37) In fact, we found that as many as one in four women of reproductive age were active smokers (according to their serum cotinine concentrations) while another two in four women were exposed to cigarette smoke secondhand. Our findings also suggest adopting certain dietary changes or promoting wider use of multi-mineral/multi-vitamin supplements might be other options for lowering exposures. One area ripe for future research is the optimization of intakes of micronutrients known to interact with toxic metals. For example, the essential metals copper, iron, and zinc, can compete with toxic metals for absorption and possess antioxidant properties to protect from toxic metal-induced oxidative stress.(38) Animal studies have demonstrated supplementation with essential metals can reduce toxic metal body burdens for mothers and their offspring.(39, 40)
Our study has many strengths, including the use of six consecutive cycles of nationally-representative data from the National Health and Nutrition Examination Survey, assessment of a wide variety of sociodemographic, lifestyle, and dietary factors, and reliance on objective exposure biomarkers. Furthermore, we provide descriptive statistics regarding potential sources of toxic metal exposures amongst women of reproductive age such as the prevalence of active and secondhand cigarette smoking and average daily intakes of food groups that may be contaminated. However, there are several limitations worth noting. Blood and urinary metal biomarkers were measured in sub-samples, which, although randomly selected, resulted in small sample sizes with especially small numbers of pregnant and/or breastfeeding women. We may therefore have been underpowered to detect important predictors of toxic metal exposures for this sub-group. Additionally, dietary assessments were conducted using two 24-hour recalls, the second of which was conducted after the collection of blood and urine samples. Despite the out-of-order temporal sequence, averaging across repeated recalls approximates “usual” or “long-term” dietary intakes more accurately than a single recall because of daily intra-individual variation.(41) We analyzed a large number of variables, however, data on some factors known to be associated with toxic metal exposures were unavailable. For instance, the National Center for Health Statistics does not publicly release geographic locations of NHANES participants, which could be used to incorporate information regarding air pollution or drinking water sources that may serve as important sources of metals exposure. Similarly, the NHANES dropped questions from their housing questionnaire in recent years regarding the age of the home and time lived in the home, which may have been useful for ascertaining lead-based paint exposure.(42) Rather than evaluating individual foods, we assessed intakes of food groups which could have masked specific sources of toxic metal exposures. Moreover, the cross-sectional nature of the data may have resulted in reverse causality. Mice exposed to lead, for example, have responded by losing weight, which may explain the inverse association we observed between body mass index and blood lead levels.(43) Finally, the selected biomarkers have different half-lives — approximately 3 days for urinary arsenic, over 10 years for urinary cadmium, 30 days for blood lead, and 80 days for blood mercury – representing different time points of exposure.(16, 44–46)
In summary, we found that US women of reproductive age who are older, non-Hispanic black, Hispanic, other race/ethnicity, born in a US territory, or foreign-born have higher exposures to the toxic metals inorganic arsenic, cadmium, lead, and mercury. Our findings are consistent with prior studies of NHANES data and highlight some important determinants of toxic metal exposures amongst this vulnerable population.(13, 14, 47) As evidence of the health effects of toxic metal mixture exposures continues to accumulate, public health efforts should prioritize the high-risk sub-populations of women of reproductive age identified in this study for further assessment of exposure sources and interventions to reduce such exposures. In particular, future prospective epidemiologic studies may want to focus on modifiable risk factors for toxic metal exposures including lifestyle and dietary characteristics.
Supplementary Material
Acknowledgments
We would like to thank the staff and participants of the National Health and Nutritional Examination Survey. Paige A. Bommarito was supported by the National Institute of Environmental Health Sciences (NIEHS) T32ES007018.
Footnotes
Supplementary information is available at Journal of Exposure Science and Environmental Epidemiology website.
Conflicts of Interest
None to declare.
References
- 1.Agency of Toxic Substances and Disease Registry. ATSDR’s Substance Priority List Atlanta, GA: 2017. [Available from: https://www.atsdr.cdc.gov/spl/. [Google Scholar]
- 2.Milton AH, Hussain S, Akter S, Rahman M, Mouly TA, Mitchell K. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. Int J Environ Res Public Health. 2017;14(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamadrid-Figueroa H, Tellez-Rojo MM, Hernandez-Avila M, Trejo-Valdivia B, Solano-Gonzalez M, Mercado-Garcia A, et al. Association between the plasma/whole blood lead ratio and history of spontaneous abortion: a nested cross-sectional study. BMC Pregnancy Childbirth. 2007;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Chen W, Wang D, Jin Y, Chen X, Xu Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere. 2014;108:33–9. [DOI] [PubMed] [Google Scholar]
- 5.Murcia M, Ballester F, Enning AM, Iniguez C, Valvi D, Basterrechea M, et al. Prenatal mercury exposure and birth outcomes. Environ Res. 2016;151:11–20. [DOI] [PubMed] [Google Scholar]
- 6.Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit. 2009;11(7):1322–30. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Esquinas E, Perez-Gomez B, Fernandez MA, Perez-Meixeira AM, Gil E, de Paz C, et al. Mercury, lead and cadmium in human milk in relation to diet, lifestyle habits and sociodemographic variables in Madrid (Spain). Chemosphere. 2011;85(2):268–76. [DOI] [PubMed] [Google Scholar]
- 8.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115(4):609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freire C, Amaya E, Gil F, Fernandez MF, Murcia M, Llop S, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ. 2018;621:340–51. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson JL, Muckle G, Ayotte P, Dewailly E, Jacobson SW. Relation of Prenatal Methylmercury Exposure from Environmental Sources to Childhood IQ. Environ Health Perspect. 2015;123(8):827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20-40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect.2017;125(6):067015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott S, Wu J, Cai B, Lawson A, Marjorie Aelion C. Probability of intellectual disability is associated with soil concentrations of arsenic and lead. Chemosphere. 2011;84(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007-2012. J Toxicol Environ Health A. 2017;80(9):502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. Blood mercury levels in US children and women of childbearing age, 1999-2000. JAMA. 2003;289(13):1667–74. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Toxic Substances and Disease Registries. Toxicological Profile for Mercury. Atlanta, GA; 1999. [Google Scholar]
- 16.Agency for Toxic Substances and Disease Registries. Toxicological Profile for Lead. Atlanta, GA; 2007. [PubMed] [Google Scholar]
- 17.Agency for Toxic Substances and Disease Registries. Toxicological Profile for Cadmium. Atlanta, GA; 2012. [PubMed] [Google Scholar]
- 18.Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, et al. Estimation of Inorganic Arsenic Exposure in Populations With Frequent Seafood Intake: Evidence From MESA and NHANES. Am J Epidemiol. 2016;184(8):590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275(16):1233–40. [PubMed] [Google Scholar]
- 20.Bailey RL, Fakhouri TH, Park Y, Dwyer JT, Thomas PR, Gahche JJ, et al. Multivitamin-mineral use is associated with reduced risk of cardiovascular disease mortality among women in the United States. J Nutr. 2015;145(3):572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Department of Agriculture; Food Patterns Equivalents Database In: Agriculture Research Service, editor. Beltsville, Maryland: 2018. [Google Scholar]
- 22.Nigra AE, Sanchez TR, Nachman KE, Harvey D, Chillrud SN, Graziano JH, et al. The effect of the Environmental Protection Agency maximum contaminant level on arsenic exposure in the USA from 2003 to 2014: an analysis of the National Health and Nutrition Examination Survey (NHANES). Lancet Public Health. 2017;2(11):e513–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environmental health perspectives. 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y YH Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Statistical Methodology Series B. 1995;57:289–300. [Google Scholar]
- 25.Bradley MA, Barst BD, Basu N. A Review of Mercury Bioavailability in Humans and Fish. Int J Environ Res Public Health. 2017;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, et al. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS One. 2014;9(9):e104768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mania M, Rebeniak M, Szynal T, Wojciechowska-Mazurek M, Starska K, Ledzion E, et al. Total and inorganic arsenic in fish, seafood and seaweeds--exposure assessment. Rocz Panstw Zakl Hig. 2015;66(3):203–10. [PubMed] [Google Scholar]
- 28.Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. Probabilistic Modeling of Dietary Arsenic Exposure and Dose and Evaluation with 2003-2004 NHANES Data. Environ Health Perspect. 2010;118(3):345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes LE, Graham JE, Berglund C, Bell RC. Dietary Change during Pregnancy and Women’s Reasons for Change. Nutrients. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Ding N, Tucker KL, Weisskopf MG, Sparrow D, Hu H, et al. A Western Diet Pattern Is Associated with Higher Concentrations of Blood and Bone Lead among Middle-Aged and Elderly Men. J Nutr. 2017;147(7):1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere. 2016;163:270–82. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y, McCullough LE, Tzeng JY, Darrah T, Vengosh A, Maguire RL, et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health. 2017;17(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Hansen AR, McGalliard Z, Gover L, Yan F, Zhang J. Trends in Smoking and Smoking Cessation During Pregnancy from 1985 to 2014, Racial and Ethnic Disparity Observed from Multiple National Surveys . Matern Child Health J. 2018;22(5):685–93. [DOI] [PubMed] [Google Scholar]
- 34.Cusack LK, Smit E, Kile ML, Harding AK. Regional and temporal trends in blood mercury concentrations and fish consumption in women of child bearing Age in the united states using NHANES data from 1999-2010. Environ Health. 2017;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991-1994. Environ Health Perspect. 1998;106(11):745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrington CD, Montwill B, Bolger PM. An intervention analysis for the reduction of exposure to methylmercury from the consumption of seafood by women of child-bearing age. Regul Toxicol Pharmacol. 2004;40(3):272–80. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Tong EK, Mao Z, Hu TW, Lee AH. A Clustered Randomized Controlled Trial to Reduce Secondhand Smoke Exposure Among Nonsmoking Pregnant Women in Sichuan Province, China. Nicotine Tob Res. 2016;18(5):1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhai Q, Narbad A, Chen W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients. 2015;7(1):552–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasanthi RP, Devi CB, Basha DC, Reddy NS, Reddy GR. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int J Dev Neurosci. 2010;28(2):161–7. [DOI] [PubMed] [Google Scholar]
- 40.Enli Y, Turgut S, Oztekin O, Demir S, Enli H, Turgut G. Cadmium intoxication of pregnant rats and fetuses: interactions of copper supplementation. Arch Med Res. 2010;41(1):7–13. [DOI] [PubMed] [Google Scholar]
- 41.Freudenheim JL, Johnson NE, Wardrop RL. Misclassification of nutrient intake of individuals and groups using one-, two-, three-, and seven-day food records. Am J Epidemiol. 1987;126(4):703–13. [DOI] [PubMed] [Google Scholar]
- 42.Caldwell KL, Cheng PY, Jarrett JM, Makhmudov A, Vance K, Ward CD, et al. Measurement Challenges at Low Blood Lead Levels. Pediatrics. 2017; 140(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donald JM, Bradley M, O’Grady JE, Cutler MG, Moore MR. Effects of low-level lead exposure on 24 h activity patterns in the mouse. Toxicol Lett. 1988;42(2):137–47. [DOI] [PubMed] [Google Scholar]
- 44.Buchet JP, Lauwerys R, Roels H. Urinary excretion of inorganic arsenic and its metabolites after repeated ingestion of sodium metaarsenite by volunteers. Int Arch Occup Environ Health. 1981;48(2):111–8. [DOI] [PubMed] [Google Scholar]
- 45.Suwazono Y, Kido T, Nakagawa H, Nishijo M, Honda R, Kobayashi E, et al. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers. 2009;14(2):77–81. [DOI] [PubMed] [Google Scholar]
- 46.Jo S, Woo HD, Kwon HJ, Oh SY, Park JD, Hong YS, et al. Estimation of the Biological Half-Life of Methylmercury Using a Population Toxicokinetic Model. Int J Environ Res Public Health. 2015;12(8):9054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsoi MF, Cheung CL, Cheung TT, Cheung BM. Continual Decrease in Blood Lead Level in Americans: United States National Health Nutrition and Examination Survey 1999-2014. Am J Med. 2016;129(11):1213–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.