Abstract
The primary objective of this study is to analyze the most up-to-date evidence regarding whether and how blood sugar regulation impacts cardiovascular health promotion and disease prevention by carrying out an umbrella review. Three separate, systematic literature searches identified a total of 2,343 articles. Overall, 44 studies were included for data extraction and analysis. The included systematic reviews and meta-analyses published between 1 January 2016 and 31 December 2017 were of good to very good quality (median OQAQ score = 17). Identified evidence suggests that cardiovascular prevention services should consider the regulation of blood glucose as a key target for intervention. Furthermore, CV prevention should adopt the recommendations for effective intervention and service development/training described in this review in existing evidence-based practice guidelines. Multidisciplinary teams should be formed to deliver multi-component interventions in community-based settings. There may be substantial opportunities for integrating CVD prevention and diabetes prevention services.
Keywords: blood sugar, cardiovascular health, prevention, umbrella review
Condensed Abstract
An umbrella review of up-to-date evidence regarding the role of blood sugar regulation in cardiovascular health promotion and disease prevention identified 44 systematic reviews and meta-analyses. Identified evidence suggests that cardiovascular prevention services should consider the regulation of blood glucose as a key target for intervention and adopt the recommendations for effective intervention and service development/training described in this review. These recommendations extend existing evidence-based practice guidelines. There may be substantial opportunities for integrating CVD prevention and diabetes prevention services, using multi-disciplinary teams to deliver intervention in community settings.
Background
The prevalence of cardiovascular diseases (CVD) is increasing worldwide (1). The absolute numbers of deaths caused by CVD has increased by more than 25% between 1990 and 2010 (2), while decreasing in higher income countries (3). As the number of patients with CVD is likely to increase further due to demographic changes, so will the costs of treatment (4). Hyperglycemia is a key modifiable risk factor for the development of CVD (5–8), with pre-diabetes being a major risk factor for the progression to type 2 diabetes (T2DM), which in turn increases CVD risk (9). As a part of a multifactorial risk factor intervention, effective reduction of hyperglycemia has a positive effect on CVD risk (10). This is true for both high and low levels of HbA1c at baseline (11–13). For reasons not well understood so far only non-fatal events could be reduced by intensive glucose lowering (14,15). Therefore, it is vital to understand the potential prevention and care pathways by which CVD risk is reduced by blood sugar regulation to guide prevention of CVD.
Established mechanisms in cardiovascular health promotion entail developing strategies for screening and preventive interventions tackling the mechanisms of disease development (16). Although trials of intensive glucose reduction via medication in the past decades showed no significant macrovascular benefits (17), recent studies of glucose lowering using glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors have shown sizable reductions in hard CVD endpoints compared to established glucose lowering strategies (18,19). This highlights the importance of the mechanisms tackled by these drugs in preventing CVD in DM patients. At the same time, lifestyle interventions focusing on improving diet, weight loss and physical activity are known to be highly effective in preventing progression to type 2 DM (T2DM) in persons with hyperglycemia and increased risk of DM and CVD (20,21). Risk factors for CVD overlap, as high blood pressure, weight, physical activity, cholesterol, diet, blood sugar are all interconnected. However, progression to T2DM adds considerable risk due to the micro and macrovascular complications of endocrine dysregulation (22). Indeed, nearly five decades of research in DM prevention and management have largely focused on interventions to identify risk, address behavioral risk factors, and improve management of blood sugars, BP, and lipids. There is, therefore, an opportunity for preventing CVD risk by focusing on the identification of mechanisms leading to CVD in people with hyperglycemia and taking steps to regulate blood glucose.
However, considerable debate remains in the medical and public health communities about whether individual-focused screen-and-treat interventions are a) sufficient and b) the most cost-effective approaches (23–26). The benefit from individual-focused interventions is potentially life-saving for individuals with high risk or established disease but reaches only a small fraction of the population (27). Since T2DM and downstream CVD are both associated with wider cultural and societal influences (28), there have been several calls for broader, more population-focused interventions that can reach larger groups of people. Some have suggested that policy interventions that use regulations, mass media or educational approaches, or environmental changes (29–33) will have broader, more cost-effective and sustainable impacts on DM and CVD. A whole-population approach aims to minimize the entire distribution of a risk factor, even if just by a small degree, to affect the proportion of those at risk (34).
Evidence-based guidance on individual- or population-based, as well as standardized care and prevention pathways are needed to integrate current evidence from trials into guidelines, practice and policies (35).
Objectives
The main objective of this study was to carry out an umbrella review (36) of recent evidence on interventions – both medical treatments and lifestyle modification – targeting blood sugar regulation. This will help to inform efforts to improve cardiovascular health via a more detailed consideration of blood glucose regulation pathways. Based on Andersen’s (37) Model of Health Services’ Use, the study group combined four perspectives in one overall review. The structure of the review covers medical treatments of blood sugar regulation contributing to CVD risk, factors influencing individual health behavior, and factors enabling population-based interventions. Based on this model, guidance for the development of future health promotion programs and competencies for training can be derived.
As illustrated by Figure 1, the specific objectives are:
To outline likely pathophysiological mechanisms by which blood glucose regulation affects CVD risk and evidence on pharmacological interventions targeting these pathways (1a and 1b)
To identify evidence-based individual level interventions for supporting effective behavior change to regulate blood sugar and thereby reduce CVD risk (1a and 1b)
To identify evidence-based population level interventions for supporting effective behavior change to regulate blood sugar and thereby reduce CVD risk (2a and 2b).
To translate key findings into applicable recommendations highlighting competencies for training for health promotion and for standardizing health promotion care pathways (3a and 3b).
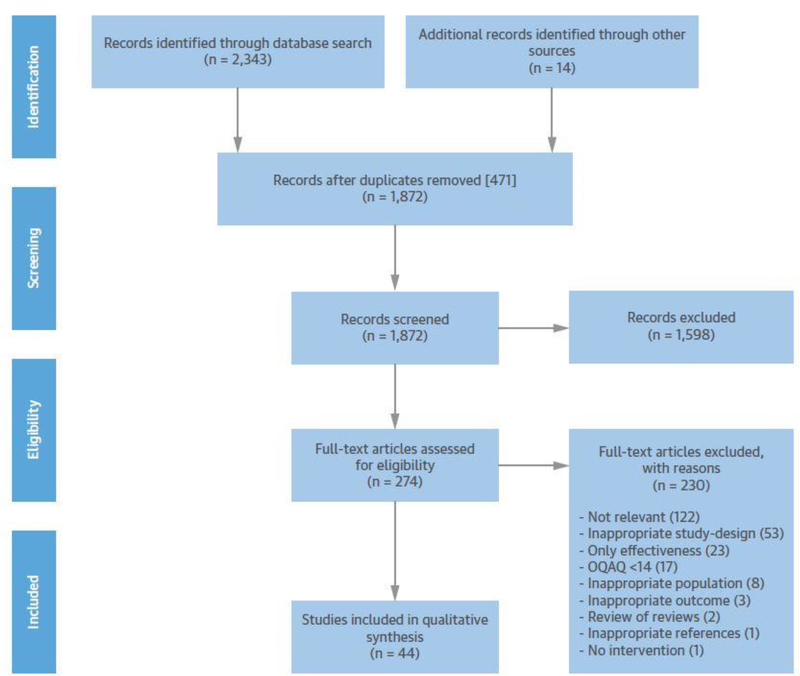
FIGURE 1: PRISMA Flow Chart of study selection process.
PRISMA Flow Chart shows the study selection process covering the single steps of identification via two-step-screening (title and abstract as well as full text base) for eligibility and inclusion into qualitative synthesis of this review.
Methods
In order to address the above aims, extensive automated and manual searches were performed to identify relevant systematic reviews of randomized controlled trials and meta-analyses. Database reviews were conducted by PT, LH, CG, JL, MMA and HM, duplicates were removed, and search results were triaged to the section writing teams (section 1 MMA, HM; section 2 CG, JL; section 3 MKA, MBW; section 4 PT, LH). Two reviewers completed screening of studies, data extraction, and quality assessments per section. Any discrepancies between the reviewers were resolved by discussion. Three core sections meant to answer the primary research question:
In what ways should blood sugar regulation be taken into account by future cardiovascular health promotion and prevention programs?
Search strategy and eligibility criteria
Comprehensive electronic searches were conducted in Medline and Embase for systematic reviews of RCTs or meta-analyses published between 1 January 2016 and 31 December 2017. The search areas were ‘MeSH terms’ or ‘title, abstract and key words’. To identify the main physiopathological mechanisms driving CV outcomes in T2DM patients (section 1), the search terms and synonyms covered the following criteria: study designs (systematic reviews and meta-analysis of RCTs), interventions (glucose lowering, in combination with BP and lipid lowering), mechanisms (physiopathological, pathophysiological), and measured outcomes (BP regulation, lipid profile regulation, CV events and mortality). To identify intervention components associated with effectiveness for individual level behavior change to address blood glucose regulation (section 2), search terms were identified and searches were constructed to combine each of the following areas: CVD risk factors, blood sugar regulation, behavioral intervention targets and specific study designs (systematic reviews and meta-analysis of RCTs). To investigate population-level interventions (section 3), a search for reviews of exposures like mandates, policies, programs, mass education, or built environmental changes that may be associated with widespread changes in blood sugar was conducted. Systematic reviews and meta-analyses reporting glucose-related outcomes, both actual blood glucoses (HbA1c, fasting blood glucose) or diabetes related outcomes (prevalence, incidence, or rates of acute or chronic complications) were included. Articles identified in any of the previous searches focusing on standardized care pathways, especially interventions in the primary care setting, were summarized in section 4.
The study groups hand-searched any systematic reviews and meta-analyses to include studies which may have been missed by the automated search. In order to provide a consistent picture of the current state of the art, single landmark studies of the past 15 years, as well as highlights of expected findings derived from current studies were also included.
After screening of title and abstracts all sections excluded reviews or meta-analysis which did not include humans, did not meet one of the four aims or were published before 1 January 2016. Although originally intended, a thorough focus on systematic reviews and meta-analyses primarily derived from RCTs was not feasible for population-level interventions (section 3) since their characteristics hardly allow for testing in an experimental setting. After full-text screening, studies were excluded if they did not inform the research question or were below the threshold of the OQAQ measurement (see below).
Quality assessment and standardized reporting
For quality assessment of the identified reviews, a modified version of Oxman and Guyatt’s Overview Quality Assessment Questionnaire (OQAQ) (38) was used. The OQAQ consists of nine quality items, each comprising the dimensions ‘yes’, ‘no’ or ‘partially/can’t tell’, carrying scores of 2, 1 and 0, resulting in an overall score of 0 to 18 points. To maximize the quality of evidence considered, we rejected studies with an OQAQ score less than 14.
Standardized reporting was ensured by applying the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting framework (39).
Data extraction and analysis
Although carried out independently, the three sections applied identical methods for data extraction, analysis and reporting. A revised template for extracting/summarizing empirical evidence from a prior systematic review of reviews (40) was applied. Not all included systematic reviews and meta-analyses referred to glycemic control as a primary outcome, or distinguished between primary, secondary and surrogate outcomes. Therefore a presentation of results according to the outcome levels is not provided. Finally, implications for standardizing health promotion care pathways and competencies for health care workers were extracted from each of the three sections.
Results
Section 1 –. Pathophysiological pathways of blood glucose regulation on CVD risk
Review characteristics
Overall 769 references were identified including 455 from our section-specific search string, 305 articles identified by the searches for sections 2 and 3, and 9 references from hand searching (Fig. 2). After reviewing the title and abstract, 685 studies were excluded. Details of the evidence extracted are provided in Online Table 1.
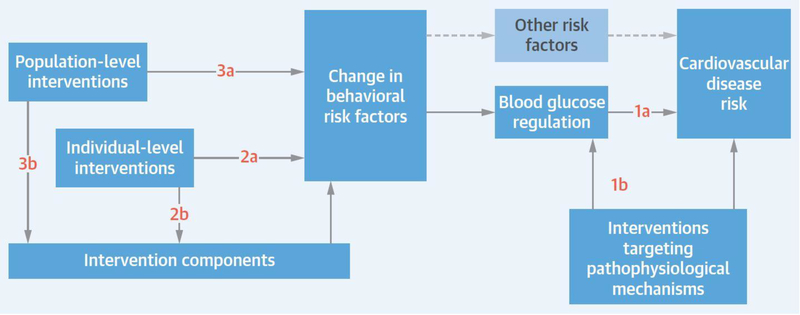
FIGURE 2: Blood Sugar Regulation: Logic Order and Interdependencies.
Cardiovascular disease (CVD) risk is influenced by blood glucose regulation, and both CVD risk and blood glucose regulation are influenced by interventions targeting pathophysiological mechanisms (1a, 1b). Other risk factors also affect CVD risk. Blood glucose regulation and other risk factors are, in turn, influenced by changes in behavioral risk factors. Population-level (3a, 3b) and individual-level (2a, 2b) interventions and intervention components influence the behavioral risk factors, influencing blood glucose regulation and, ultimately, CVD risk. Boxes represent the objectives of the individual review sections; arrows show the interdependencies of the objectives for their contribution to the explanation of CVD risk.
Study quality
On a scale of 0–18, the median OQAQ score of 17 included studies was 17 (IQR = 1.5), indicating that they were high quality systematic reviews and meta-analyses.
Outcomes and mechanisms
Individuals with T2DM have a 2 to 3-fold greater risk of CVD than people without T2DM, with women showing an increased elevated risk compared to men (2.5 fold risk increase in women compared to 1.7-fold in men) (41). Moreover, people with T2DM have an increased risk of long-term mortality following coronary revascularization in both stable ischemic and ACS populations (42–46). Through outlining the recently analyzed glucose lowering pharmacological interventions, the pathophysiological mechanisms leading to cardiovascular (CV) events in T2DM patients will be presented.
Intensive glucose lowering in landmark trials such as UKPDS (47), ACCORD (48), ADVANCE (27), and VADT (49) mainly utilizing insulin or sulfonylureas had improved microvascular but not macrovascular outcomes, suggesting that glucose lowering in itself does not reduce CV events. Hence, reduction of hyperglycemia using insulin therapy does not reduce the risk of CV events (50). Metformin may lower the incidence of CVD, but this is still not proven (51). Moreover, the latest analyses addressing intensive glucose lowering show that in comparison to less intensive therapy, intensive glucose lowering had no influence on CV mortality, but reduced the risk of myocardial infarction (52) in addition to a reduction in the risk of diabetic nephropathy and retinopathy (53). Hence, managing blood glucose alone in T2DM does not seem to reduce macrovascular CV events and targeting other mechanisms should be explored.
Using dipeptidylpeptidase 4 (DPP-4) inhibitors for glycemic control through augmenting the bioavailability of GLP-1 held the promise of improved CV outcomes from pre-clinical studies but it failed to be proven in clinical trials to date (54). Some DPP-4 inhibitors even increase the risk of hospitalization for heart failure (55). Given the broad physiological activity and distribution of DPP-4, speculation arose about the off-target effects of DPP-4 inhibitors including their impact on sympathetic nervous system leading to cardiotoxicity (56). Although GLP-1 RA reduce hyperglycemia through the same pathway as DPP-4 inhibitors, they are associated with improved CV outcomes and have less adverse effects.
Meta-analyses found that GLP-1 RA 1) reduced body weight, systolic BP, triglycerides (TG), and low-density lipoprotein cholesterol (LDL-c) better than insulin (57), 2) were associated with a lower risk of myocardial infarction after long-term treatment in comparison to sulfonylurea-based therapy (18), 3) reduced the risk of all-cause and CV mortality in comparison to placebo (58,59), 4) reduced the risk of severe hypoglycemia (58), and 5) did not increase the risk of heart failure, stroke, and microvascular complications including diabetic retinopathy (DR) and nephropathy (DN) (59). However, one GLP-1 RA, exenatide, was found to increase the risk of arrhythmias in T2DM patients (55). The mechanisms through which GLP-1 RA reduce CV risk beyond glucose-lowering effects possibly include reduction in body weight, lipids, blood pressure, inflammatory markers, oxidative stress, endothelial dysfunction, and subclinical atherosclerosis (60).
Similar to GLP-1 RA, SGLT2 inhibition reduces systolic and diastolic BP (61–63) besides reducing glucose reabsorption in the kidney, inducing glucosuria and thereby reducing blood glucose. Moreover, SGLT2 inhibition reduces all-cause and CV mortality and major CV events (19,54,63,64). Controversies arose, however, regarding the risk of stroke, which ranged from no effect (54,63,64) to increased risk (19). The most prominent explanation for cardioprotection is the diuretic effect of empagliflozin and consequently the reduction in plasma volume (65). Although conventional diuretics and thiazides show moderate or no reduction in risk of heart failure, the diuretic action of empagliflozin does not induce reflex activation of the sympathetic nervous system and the consequent neurohormonal cardiac dysregulation. Moreover, the proximal inhibition of glucose and sodium reabsorption has beneficial consequences on renal function as opposed to distal inhibition (66). Other cardioprotective mechanisms include regulation of metabolic processes such as increased plasma glucagon (67) and ketone (68) concentrations and reduced uric acid serum levels conferring protection against increased BP and vascular damage (69). Another theory is derived from data on reduced vascular oxidative stress and inflammation after treatment (70), conferring a reduction in aortic stiffness, an independent risk factor of CV mortality (71). Hence, the metabolic effects of SGLT2 inhibitors leading to weight loss, blood pressure reduction, natriuresis, and improved renal function reduce major adverse cardiac events, primarily CV death, independent of its glucose lowering activity. Whether use of these agents alone eliminates the need for BP and lipid lowering therapies needs to be addressed.
The landmark trials (27,47–49) investigated combining intensive glucose lowering with BP and lipid lowering therapies, termed multifactorial interventions. In recent analyses, multifactorial interventions reduced CV mortality in addition to improving CV outcomes (72). However, intensive BP lowering in (already well-controlled) T2DM patients had more risks than benefits. In one analysis, the renin–angiotensin system blockers were most effective in T2DM patients in reducing cardiovascular risk vs. placebo, but there was little or no further benefit in lowering systolic BP below 130 mm Hg (73). Furthermore, although antihypertensive treatment reduced the risk of all-cause mortality, myocardial infarction, and heart failure in T2DM patients with baseline systolic BP between 140–150 mm Hg, the risk of CV mortality increased if the baseline systolic BP was lower than 140 mm Hg (74).
Taken together, the analyses presented above suggest that pharmacological interventions improving glycemic control in people with T2DM can lead to improvements in CV outcomes. BP regulation seems to be a key pathway mediating this effect. Preventing progression to T2DM in those at risk is also a key risk-reduction pathway.
Strengths and limitations
The included reviews are of high quality and represent the latest evidence of intervention efficacy in relation to CVD risk reduction through blood glucose management in adults with T2DM. Although many studies of case-control or observational nature addressed the association of genetic polymorphisms with increased risk of CV outcomes in people with T2DM, the exclusion of non-RCT studies from our review minimized the comprehensiveness of mechanisms and interventions reviewed.
Section 2 –. Individual level interventions for supporting behavior change to regulate blood sugar
Evidence from landmark studies
Three “landmark” papers were identified in relation to making recommendations on the optimal content of individual lifestyle interventions. These were a prior systematic review of reviews of diet and physical activity interventions (40), US national standards for diabetes selfmanagement education and support (75) and public health guidance on diabetes prevention from the UK’s National Institute for Health and Clinical Excellence (NICE) (76). Existing recommendations from these sources are summarized in Table 1 and are supplemented with the main evidence from the current review.
Table 1:
Evidence-based recommendations on content for interventions to manage blood glucose and cardiovascular risk via changes in diet and physical activity.
| Existing Recommendation (from prior landmark studies /guidelines) | New evidence /updates |
|---|---|
| To maximize weight loss, interventions should promote changes in both diet and physical activity (40,76). | Evidence from the current review supports the extension of this recommendation to maximizing blood glucose reduction (86,90). The addition of physical activity to diet may have less effect on FPG or HOMA-IR (100) however. |
| Interventions should use established, well defined behavior change techniques (e.g. Motivational interviewing, actionplanning, relapse prevention) (40,76). NB: Such intervention strategies are defined in recent taxonomies of behavior change techniques (147). | Evidence from the current review supports the extension of this recommendation as follows:- The use of behavior change theory in designing interventions may help to increase effectiveness for reducing HbA1c (87,94). However, further evidence is needed on which specific theoretical elements are essential /optimal. Providing people with meals to ensure a balanced diet is effective for reducing HbA1c for up to 24 months (87). This strategy may be particularly suited for people with low problem-solving capabilities. More research is needed on cost-effectiveness and sustainability. Recent evidence on motivational interviewing for blood glucose reduction is mixed and this may reflect a need for high quality delivery /provider skills to ensure success (78,82). Including problem-solving and social comparison techniques were associated with increased effectiveness (87), as was the use of self-regulation techniques (see following row). |
| Interventions should encourage the use of “self-regulation” techniques, which are associated with increased effectiveness. Self-regulation includes specific and individualised goal setting; prompting self-monitoring; providing feedback on performance and review of behavioral goals (40,76). | Evidence from the current review supports the extension of this recommendation:- As well as selfmonitoring of weight or physical activity, selfmonitoring of blood glucose, may be useful for some people with T2DM to help regulate blood glucose (83). Due regard is needed to ensure ‘meaningful monitoring’ and avoiding possible anxiety or frustration in individual cases. |
| Intervention content and delivery should be tailored to individual needs, culture and capabilities (e.g. problem-solving skills, literacy) (75,76). | The current review identified no new evidence on this issue. |
| Interventions should encourage participants to engage social support for behavior changes (i.e. engage others who are important such as family, friends, and colleagues) (40,76). | The current review identified no new evidence on this issue. However, evidence on the benefits of problem solving (above) and expert opinion suggests that it may be important to address negative social influences as well as engaging positive social support. |
| Interventions may be delivered by a wide range of people /professions, subject to appropriate training. This may include doctors, nurses, dieticians/nutritionists, exercise specialists and lay people, often working within a multi-disciplinary team (40) At least one of the team members responsible for facilitating diabetes self-management services should be a suitably trained registered nurse, registered dietitian nutritionist, pharmacist or certified diabetes educator (75). |
Evidence from the current review was broadly consistent with the existing recommendation and also provided examples of effective glucose-reduction in interventions led by health professionals working alongside peer supporters and pharmacists (89,94,95). Including a dietitian in the provider team is specifically recommended for increasing effects on glucose and weight in interventions with a dietary component (87,92,97). Interventions led by peers were less effective for reducing blood sugar than those led by health professionals (94). |
| Interventions may be delivered in a wide range of settings, including healthcare settings, the workplace, the home, and in the community | The current update did not identify evidence on this issue |
| Interventions may be delivered using group, individual or mixed modes (individual and group). | Evidence from the current review is consistent with this recommendation. |
| Interventions should include a strong focus on maintenance. It is not clear how best to achieve behavior maintenance but techniques designed to address maintenance include: self-monitoring of progress, providing feedback, reviewing of goals, engaging social support, use of relapse management techniques and providing follow-up prompts. | The findings of this review were consistent with this recommendation (see above section on overall effectiveness) |
| Interventions should maximize the frequency or number of contacts with frequency or number of contacts with participants (40,76). The intensity of intervention should be matched to the level of risk for progression to (or of) T2DM (76) |
Evidence from the current review is consistent with the existing recommendations. For instance, interventions lasting 36–72 months reduced diabetes incidence and the Number Needed to Treat more than those lasting 624 months (26). |
| Intervention person-cente approach (75,76). | Evidence from the current review is consistent with this, although a need for high quality delivery was identified (see evidence on motivational interviewing above). |
| Offer follow-up sessions /support/reviews of progress at regular intervals following the initial intervention period to reinforce positive behavior change and provide support, in case of relapse (75,76). | Evidence from the current review was consistent with this recommendation. For example, contacting participants for the remainder of the follow-up period to promote continued adherence to healthy lifestyle principles was associated with increased maintenance of effects on weight and FPG (76). |
| Incorporating text messaging into diabetes self-management interventions improves engagement and outcomes. Along with other digital health technologies, it may be possible to establish a self-management feedback loop with four key elements to provide ongoing, realtime engagement in selfmanagement: (75) 1. Two-way communication 2. Analysis of patient-generated health data 3. Customized education 4. Individualized feedback |
The current review supports and extends this recommendation. Web based interventions, text messaging and mobile phone apps all had significant effects in reducing HbA1c at 3 to 12 months of follow up (81,84,86,88,98,99). Digital interventions were also effective for increasing physical activity (86,88) and reducing weight (79,86,88,98) in the short term (8–12 months). Further evidence, although mixed, suggests that the addition of in-person support increases effectiveness for weight loss (79,86,93). Hence, the use of „blended“ interventions (where patients collect and share data on target behaviors /health outcomes with intervention providers /health professionals to facilitate a tailored discussion) is recommended to support lifestyle behavior change. |
Left column indicates existing existing recommendations based on prior landmark studies and guidelines); right column shows what the current review adds
FPG = Fasting plasma glucose; HOMA-IR = Homeostatic Model Assessment for Insulin Resistance; T2DM = Type 2 diabetes mellitus; HbA1c = Glycated Hemoglobin
Review characteristics
Intervention characteristics
Our searches identified 861 articles, of which 24 met both the selection and quality criteria (26,77–100) (Figure 2). The characteristics of the included reviews are provided in Online Table 2.1. We identified 95 analyses relating intervention components to effectiveness (Online Tables 2.2 to 2.9) and 19 effectiveness analyses (Online Table 2.1). Full details of these 114 analyses are presented in Online Table 2.2 and a summary is provided below.
Study quality
The methodological quality of included reviews was generally good (median OQAQ score = 16; IQR = 3). The most common methodological weakness was the lack of use of study quality data to inform analyses (e.g. by excluding low quality trials).
Outcomes/ Effective components of interventions
Overall effectiveness
Evidence from seven systematic reviews (26,80,87,93,96,97,100) found that individual lifestyle interventions targeting physical activity and/or diet were effective in reducing blood glucose (HbA1c, fasting plasma glucose, fasting insulin, HOMA-IR and 2-hour blood glucose) for up to 54 months, reducing diabetes incidence in people at risk of developing type 2 diabetes for up to 72 months and in reducing weight at up to 52 months of follow up. However, there was considerable variation in outcomes between studies, indicating the importance of understanding what content and delivery factors are associated with increased effectiveness.
Evidence from six systematic reviews focusing on maintenance of effects (26,77,86,87,93,100) found a reduction in effects on both blood glucose and weight over time (for up to 60 months of follow up).
Components of interventions associated with increased effectiveness
Recommendations derived from both the current review and the landmark papers are presented in Table 1.
Strengths and limitations
The included reviews are of high quality and represent the latest evidence on components of interventions related to effectiveness in reducing blood sugar and the related risk factors of weight and physical activity. Our consideration of evidence relating to blood glucose regulation adds meaningful new recommendations to existing guidance on CV risk prevention (Table 1). Relating the findings to prior evidence-based recommendations helps to minimize sampling bias due to the time-limits on our search strategy. However, as most interventions targeted multiple behaviors, it was impossible to identify components associated with changes in specific behaviors.
Section 3 –. Population level interventions for supporting behavior change to regulate blood sugar
Review characteristics
Based on the electronic database searches, 85 articles were assessed for inclusion. Of these, 75 were excluded because the systematic review objectives were unrelated (34), the reviews evaluated individual-focused interventions (17), and/or assessed clinical care models (10) or competencies of personnel (14) to address diabetes prevention or management. Although an additional hand search was carried out, no additional systematic reviews that meeting the eligibility criteria were identified.
In total, 10 full-text articles were reviewed for inclusion. Of these, seven review articles were excluded for the following reasons: did not report glucose-related outcomes (n = 3); were not reviews of population level interventions (n = 2); or only included one study with both a population level intervention and a glucose outcome (n = 2). The three remaining articles included one systematic review (101) and two systematic review/meta-analyses (102,103).
Study quality
All included studies were of excellent quality scoring 17 or higher on an 18-point scale.
Outcomes/ Effective components of interventions
Each of the included studies did not focus on unique intervention strategies; instead, they summarized the results related to population-level efforts targeted at a particular disease-related outcome (recognizing early symptoms for type 1 diabetes (101) or a specific community setting (worksites (102,103)). Deylami and colleagues (101) summarized the evidence for awareness campaigns about early type 1 diabetes symptoms in children targeted to the population and primary healthcare professionals. The authors reported that the programs, which included educational posters, reusable shopping bags, and/or provision of glucose testing equipment to healthcare providers, reduced the rates of diabetic ketoacidosis (rates of diabetic ketoacidosis were reduced in 4/7 studies).
Two reviews (102,103) summarized the data around worksite-based changes and cardio-metabolic outcomes. Both reviews included studies using a variety of intervention models including one or more of the following: individual or group-based education, medical testing, electronic messaging, online programs, self-monitoring of weight and/or physical activity, physical activity prescriptions, educational materials, active workstations, and provision of healthy meals. Reed et al (102) reported on 24 studies (20 were included in the meta-analysis) that examined the effectiveness of workplace interventions for improving moderate-to-vigorous activity levels and cardio-metabolic outcomes among working adult women in high-income country settings. Two of the included studies reported changes in blood glucose; in the meta-analysis, these interventions were shown to significantly reduce blood glucose levels among participants (−0.18 mmol/L, 95% CI −0.29—0.07). In their meta-analysis, Shrestha and colleagues (103) concluded that workplace dietary interventions significantly reduced HbA1c and reduced fasting glucose among participants (pooled results from the meta-analysis: −0.18% reduction in HbA1c, 95% CI −0.29 to −0.06; −2.60 mg/dL fasting glucose, 95% CI −5.27 to 0.08).
Two of the excluded articles included very limited data (1 study in each review) around population-based interventions and glycemic outcomes. In their review of interventions to reduce ischemic heart disease in sub-Saharan Africa, Ebireri et al (104) included one study that showed a mass media education campaign had no effects on fasting blood glucose levels (105). When reviewing the associations between medication copays and both medication adherence and health outcomes, Gourzoulidis and colleagues (106) found only one study that reported HbA1c outcomes after a copay increase [a $5 copay increase was significantly associated with a 0.1 point increase in HbA1c (107)].
Large-scale dissemination and implementation research related to health policies for diabetes is still nascent. While randomized controlled trials (RCT) and especially meta-analysis of RCTs are considered the strongest research designs to infer causation (108), many environmental-, system-, employer-, or even government-level policy interventions are often not amenable ethically or physically possible to evaluate using RCTs. However, causal inference can be enhanced through the use of rigorous quasi-experimental study designs and sophisticated analytical techniques (109,110). Furthermore, many environmental changes (111) as well as regulatory, payment, employer, and health system policies and programs may be opportunities for natural experiment evaluations; here, the investigator does not control the intervention, but utilizes the opportunity and easily available data sources to assess whether these policies and programs had the intended effects (112) as well as any unintended societal consequences. Many of the studies assessing the impacts of policies to influence detection, prevention, and management of diabetes have tended to utilize quasi-experimental designs (113,114).
Many existing studies, as well as those included in this review, only include intermediate outcomes and not hard health outcomes – for example, a review of food policies seeking to reduce adult obesity and diabetes in the US reported that most studies focused on food purchasing patterns (54% of included studies) or self-reported food consumption (46%) with only 34% including body weight/composition as outcomes (115). Furthermore, studies with biomedical outcomes were more likely to be lower quality studies and less likely to show positive results (115).
Strengths and limitations
There is limited data on the effectiveness of population-based interventions for improving glucose outcomes, including both blood glucose levels (HbA1c, fasting blood glucose) or diabetes related outcomes (prevalence, incidence, or rates of acute or chronic complications). No evidence was found solely for T2DM. Among the studies identified for this review article, only three systematic reviews included sufficient data on glycemic outcomes to be included. Furthermore, much of the existing evidence for population-based interventions relies on observational studies and should be interpreted with caution.
Section 4 –. Competencies and health promotion care pathways
Analysis of studies relating to competencies and standardized health promotion care pathways
Review characteristics
Across the three searches, 12 systematic reviews and meta-analyses were identified as relating to health care worker involvement and task shifting towards health practitioners other than doctors while three other studies were found to be concentrating on integrated care, e.g. by applying care models or evaluating quality improvement. Integrated care is defined following Godwin’s health-system based definition, as a “continuum of health promotion, disease prevention, diagnosis, treatment, disease-management, rehabilitation and palliative care services, coordinated across the different levels […] of care” (116).
Study quality
The methodological quality of included reviews was generally good (median OQAQ score = 16; IQR = 2). The main methodological weaknesses were a lack of quality assessment and sensitivity analyses. Additional issues were not reporting the number of included patients in analyses, missing power analysis, and poor reporting on handling of dropouts/missing data/ use of intention to treat analysis.
Outcomes/ Effective components of interventions
The three studies focusing on integrated care using standardized approaches like the chronic care model were all concerned with the management of T2DM (117–119). However, there is a lack of good quality evidence on CV risk factor improvement via integrated care (120). While the chronic care model used in primary care was evaluated as highly effective in reducing mortality and HbA1c, standardized European approaches in multifaceted diabetes care showed only small improvements in clinical outcomes like HbA1c. However, one review reported higher potential for newly diagnosed patients (118). With respect to integrated care in general, improvement was not only shown in patient outcomes, but also for process costs and health service utilization rates (119).
Pharmacist interventions to reduce the burden of CVD and T2DM were especially effective when pharmacists performed a clinical decision making process as part of multi-disciplinary health care team (95). While improvements in clinical outcomes like HbA1c, DBP/SBP or cholesterol were only short term (120), the effect size compared well with care delivered by primary physicians (95,120,121) and was found in various settings, including primary care and hospitals (95).
Community-based approaches vary in the type of health care professional involved. The majority of the reviews (122–126) were concerned with the involvement of community-based pharmacists in the management of CVD or T2DM; two focused on community health worker involvement (123,127), while one involved community-based nurses (128). Regardless of the health practitioners involved, six studies reported improvements in clinical outcomes, such as HbA1c, DBP/SBP, glycemic as well as lipid control and all-cause mortality (122–125,127,128). Improvements in medication adherence were shown by three studies (124–126). For health-related outcomes of interventions delivered by community-based pharmacists, such as quality of life, adherence to treatment and/or medication and achievement of health goals, the results are more heterogeneous. Their effectiveness relies highly on patient-centeredness, i.e. whether they take into account their patients’ health beliefs, goals and literacy (124). Community-based health workers interventions were reported to be as effective in achieving lifestyle change in obese patients as similar interventions in other primary care settings (123,127). They were, however, inferior to commercial weight loss programs for generating weight loss (126). Mixed, yet overall positive results were found for the effect of community-based pharmacists on health resource usage including hospitalization rate, urgent care and emergency department visits (125). However, there is evidence of low strength on the effectiveness of chronic disease management supervised by pharmacists when studying clinical outcomes such as clinical CV events and all-cause mortality (125).
Two reviews reported pharmacist-led interventions to be cost-effective (125,126). It is worth noting that the positive effect of team approaches or at least strong connections between physicians and community-based health practitioners were reported as key factors in the success of such interventions (123,124).
One review found equally good glycemic outcomes for nurses prescribing glucose regulation medication on glycemic outcomes compared with physicians (129), but not when compared with multi-disciplinary teams. However, the lack of evidence for CV care, indicated by a low number of studies reporting adverse events (127) as well as missing long-term clinical outcomes (123), calls for more research on evidence-based health promotion care pathways.
Strengths and limitations
No individual search string was applied to identify relevant literature for this part of the review. Included studies were identified as a part of the search process identifying literature for individual and population-level interventions.
Implications derived from the sections 1 to 4
Recommendations for service design/delivery and the training of healthcare professionals involved in CVD prevention, drawn from the evidence in all four sections above are summarized in Table 2.
Table 2:
Summary of practical recommendations for service design /delivery and the training of healthcare professionals involved in CVD prevention
| Intervention level | Practical recommendations |
|---|---|
| Pathophysiological | 1 Consider baseline BP before initiating intensive BP lowering in combination with intensive glucose lowering. |
| 2 Consider the established patient CVD risk before deciding on pharmacological intervention. | |
| Individual-level | 1 Health promotion pathways should include lifestyle interventions targeting changes in diet, physical activity and weight loss (for those who are overweight or obese) for people with diabetes or non-diabetic hyperglycemia (prediabetes), as well as medication-adherence for those with diabetes. The intensity of interventions should be matched to the level of cardiovascular risk and the risk of T2DM (or its complications). |
| 2 Maximizing effectiveness of individual level interventions requires the commissioning of both interventions with evidence-based content (see Table 1) and the use of staff with the right competencies and training to effect high quality delivery. | |
| 3 It is worth considering the development of “blended” interventions, where mobile /internet based technologies are used to share information between the patient and the clinician. This can facilitate helpful dialogue and help to facilitate a therapeutic self-management feedback loop including self-monitoring, feedback and individually-tailored problem-solving. eHealth interventions may be effective as stand-alone interventions for some people (those who have the capacity to be pro-active, but should not be relied upon as a whole-population solution. | |
| 4 Although temporary changes in weight /lifestyle can have beneficial longer term effects on blood glucose (particularly in the context of diabetes prevention), greater efforts are required to address the (ongoing) problem of relapse /reversion to prior habits. This may involve the provision of ongoing, regular maintenance contacts beyond the initial intervention period. | |
| Population-level | 1 The workplace setting is a highly attractive environment for population-level interventions as it incorporates social support in a stable environment. |
| 2 There is a strong need for programs and trials providing longitudinal and more definitive data on the impact of population-level interventions on cardiometabolic risk factors and diseases. | |
| General recommendations on competencies for training and standardizing health promotion care pathways | 1 Integration of existing CVD prevention and diabetes prevention, or diabetes self-management programs have the potential to improve care pathways. |
| 2 Task-shifting in multidisciplinary care teams, especially involving community pharmacist and/or nurses, may improve clinical outcomes and may be of special benefit in regions where shortage of service limits availability of care. | |
| 3 CVD prevention service provider staff need to have /be trained to have the following competencies: Delivery of group or individual level behavior change interventions including: Facilitation of key behavior change techniques, including motivation-building using personcentered counselling techniques, making action plans, supporting self-monitoring (including the use of digital and blended care approaches), facilitating barrier identification and problem-solving and identifying and addressing social influences. |
|
| 4 CVD prevention service managers need to have /be trained to have the following competencies …. Understanding the evidence (as presented here) and being competent in commissioning /co-design of effective, evidence-based behavior change programs and training courses that will deliver the above competencies. Making the case to policy makers to acquire sufficient funding to deliver effective lifestyle interventions. Cutting corners when translating clinical intervention programs into real-world settings is likely to undermine effectiveness and cost-effectiveness (148). Designing stepped care pathways to ensure that the type / intensity of intervention is mapped to individual needs and capabilities. |
|
Left column shows the focus of the individual review sections (pathophysiological and lifestyle interventions targeting the individual and the population); right column shows practical recommendations derived from the evidence identified in each section; last row synthesizes the evidence into general recommendations on competencies for training and standardizing health promotion care pathways for the individual sections
BP = Blood pressure; CVD = Cardiovascular disease; T2DM = Type 2 diabetes mellitus; eHealth = electronic health
Discussion
This umbrella review summarizes recent high quality systematic reviews and meta-analyses, including evidence on pathophysiological, individual and population level interventions to regulate blood glucose in people at risk of cardiovascular disease. These findings may serve to help stakeholders develop recommendations for blood sugar regulation to reduce CV risk (Table 2).
An overview of the efficacy of interventions on the individual- or population-levels to regulate blood sugar is presented, with an emphasis on the need to reduce cardiovascular risk.
The latest analyses demonstrate that intensive glucose lowering, once thought to have no benefit in CV outcomes, can reduce micro- and macrovascular morbidities and CV mortality. Multifactorial interventions that combine intensive glucose lowering with BP and lipid lowering have also improved outcomes. However, the baseline BP of T2DM patients must be considered before initiating therapy as it can counteract the beneficial impact on CV outcomes (73,74). Analyses from recent trials of the glucose lowering drug classes GLP-1 RA and SGLT-2 inhibitors demonstrate improved glycemic control and CV outcomes in comparison to established therapies, with the common mechanism between the classes of drugs being BP lowering. Similarly, non-pharmaceutical interventions in T2DM patients, including physical activity and diet programs, not only efficiently reduce blood glucose but also BP and dyslipidemia, albeit with less side effects than pharmacological interventions.
The majority of the evidence on lifestyle interventions does not go beyond the regulation of blood sugar or focusses on relatively short-term effects on CV risk markers. Additionally, existing evidence is negatively affected by underpowered studies, borderline significant results or other methodological weaknesses (130,131). Consequently, there is a need for evidence on hard, longer-term CV outcomes resulting from lifestyle intervention in people with glucose dysregulation. The identified evidence on individual level behavioral interventions supported existing recommendations on the content and delivery of behavior change interventions for diabetes prevention and diabetes management (40,75,76), but also supported the use of behavior change theory in intervention design, the need for high quality delivery of person-centered delivery techniques, involvement of dietitians, self-monitoring of blood glucose (for people with diabetes) and the use of digital/mobile platforms to enhance intervention effectiveness. However, more research is needed to establish a) the cost-effectiveness of more or less intensive lifestyle interventions, b) the effectiveness and cost-effectiveness of interventions to improve the maintenance of lifestyle changes, and c) the effectiveness and cost-effectiveness of novel interventions to promote lifestyle changes (for instance, it would seem that most existing interventions focus on rational/planning and problem-solving strategies for supporting behavior change, rather than using novel methods to target impulsive, emotional or social pathways to change (132–134)). Future interventions should incorporate text messaging, social media networking and other digital health technology to encourage empowerment and self-management (135).
While evidence on interventions aiming at individual behavior change is vast (Tables 1 and 2), population-based approaches employing multiple mechanisms like mandates, policies, programs, mass education, or built environmental changes are scarcely tested for their impact on glycemic outcomes. For developing and maintaining new routines of healthy behavior the workplace setting provides a potentially supportive environment (136). There are on-going programs and larger trials of policy, taxation, built environment, and other interventions focused on the broader population (101,137,138). Assessments of these programs and trials should provide longitudinal data and more definitive data on the impact of these programs on cardiometabolic risk factors and diseases.
Population-based changes can be especially complicated to evaluate because of wider (and sometimes unintended) effects – for example, taxation programs might be considered socially regressive because they disproportionately impact lower socioeconomic classes (139). Other researchers recommend several possible areas for intervention at the policy and environmental level, such as introducing liability for adverse health effects of food and beverage products in combination with individual level prevention approaches (140).
The review identified evidence for the effectiveness of task shifting from medical doctors to community health workers and other community-based health practitioners like nurses and pharmacists (123,127). This finding may be of tremendous benefit in rural areas and settings where a shortage of services and resources requires a shifting of responsibilities, such as prescribing following algorithms and protocols (129). Additionally, the potential for community-based multi-disciplinary teams to increase effectiveness in both individual and community level interventions was observed (95). This is in line with current evidence highlighting significant improvements of multi-disciplinary interventions in other areas of chronic disease management (141,142). Recent findings also indicate emerging evidence to support policy for chronic care management in primary care and community settings (143), especially since integrated care has proven to be cost-effective (144).
Taken altogether, the outcomes from every section support the potential for integrating prevention services. On a pathophysiological level, our research uncovers the benefits of blood glucose lowering for patients with T2DM in order to lower CV risk and thereby prevent CVD. For lifestyle interventions targeting the individual behavior, the logic is less straightforward, yet nevertheless needs to be considered: While individual level interventions for behavior change directly reduce blood glucose, they indirectly target CVD risk. By promoting physical activity and diets, CVD risk factors such as increased blood glucose and weight are addressed. Population-wide strategies also reduce CVD risk both directly and indirectly: interventions seeking to reduce adult obesity in people with T2DM thereby also address a major risk factor for CVD. Yet, there is also evidence supporting the effectiveness of physical activity implemented in a work place setting in reducing hazardous cardio-metabolic outcomes. Despite of the identified lack of good quality evidence on CV risk factor improvement via integrated care (120), incorporating community-based pharmacists into multidisciplinary care teams can reduce the burden of CVD and T2DM (95).
Combining the results derived from the single sections suggests integrating future services and policy efforts to develop standardized care pathways covering diabetes prevention, diabetes management and CVD prevention. Focusing on the entire pathway of CVD and diabetes prevention, management and care is also supported by the OECD health policy studies on CVD and diabetes (145) as well as evidence-based recommendations given by the European guidelines on CVD prevention in clinical practice (146).
Strengths and Limitations
This study used robust, high quality systematic reviewing methods to generate an overview of evidence on glucose-regulation. Systematic umbrella reviews are helpful to summarize complicated and vast amounts of research to efficiently inform decision makers in the health sector, such as policy makers and clinicians (36).
A focus on this high quality data limits the risk of overlooking major trends on a given subject. However, there are some limitations that need to be acknowledged. Some relevant research may not have been included in meta-analyses, despite being of high quality. Such selection bias may have arisen from limitations in the search terms and range of databases searched. Further selection bias might arise from the choice of relevant primary studies by the authors of the reviews and meta-analyses included. As the search for systematic reviews and meta-analyses on population-wide interventions has shown, experimental trial designs are less commonly applied (and may be less appropriate) in the field of community based intervention, either due to ethical concerns or simple economic barriers of feasibility. Hence, including observational studies or quasi-experimental designs might have generated additional insights. To include the most up to date evidence, the search was limited to the latest systematic reviews and meta-analyses being published during a narrow period of time (early 2016 to late 2017). Finally, section four did not apply an individual search strategy but relied on reviews identified by the other three sections.
Conclusion
Overall, this review has outlined the state-of-the-art in relation to the field of blood glucose regulation and suggests that this evidence can be used to extend existing guidance for CVD prevention (Table 2).
CV prevention services should consider the regulation of blood glucose as a key target for intervention and adopt the recommendations for effective intervention and service delivery described in this review, as well as in existing evidence-based practice guidelines. Addressing the individual need of patients is key for CV prevention programs and should take into account pathophysiological mechanisms and aspects influencing individual behavior change, both on the individual as well as the environmental level. Multidisciplinary teams (including pharmacists, nurses, or community-health workers) should be formed to deliver multi-component interventions in community-based settings.
Supplementary Material
Abbreviations
- AMPK
Adenosine monophosphate-activated protein kinase
- BCT
Behavior change technique
- BMI
Body mass index
- BP
Blood Pressure
- CV
Cardiovascular
- CVD
Cardiovascular diseases
- DBP
Diastolic blood pressure
- DPP-4
Dipeptidyl peptidase-4
- FPG
Fasting plasma glucose
- GLP-1 RA
Glucagon-like peptide-1 receptor agonists
- HbA1c
Glycated hemoglobin
- HDL-c
High-density lipoprotein cholesterol
- IQR
Interquartile range
- KATP
ATP-sensitive potassium
- LDL-c
Low-density lipoprotein cholesterol
- MI
Myocardial infarction
- PA
Physical activity
- PPG
Postprandial blood glucose
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- SD
Standard deviation
- SGLT2
Sodium-glucose cotransporter 2
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglycerides
Footnotes
Disclosures
Peter E.H. Schwarz has no relevant financial or nonfinancial relationships to disclose.
Patrick Timpel has no relevant financial or nonfinancial relationships to disclose.
Lorenz Harst has no relevant financial or nonfinancial relationships to disclose.
Colin Greaves has no relevant financial or nonfinancial relationships to disclose.
Mohammed K. Ali has no relevant financial or nonfinancial relationships to disclose.
Jeffrey Lambert has no relevant financial or nonfinancial relationships to disclose.
Mary Beth Weber has no relevant financial or nonfinancial relationships to disclose.
Mohamad Musbah Almedawar has no relevant financial or nonfinancial relationships to disclose.
Henning Morawietz has no relevant financial or nonfinancial relationships to disclose.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 8th edn. Brussels, Belgium: International Diabetes Federation, 2017. [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Rayner M, Townsend N. European Cardiovascular Disease Statistics 2017. Brussels: European Heart Network, 2017. [Google Scholar]
- 4.Seuring T, Archangelidi O, Suhrcke M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. PharmacoEconomics 2015;33:811–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong KN, Fuster V, Rosenson RS, Rosendorff C, Bhatt DL. How Low to Go With Glucose, Cholesterol, and Blood Pressure in Primary Prevention of CVD. J Am Coll Cardiol 2017;70:2171–2185. [DOI] [PubMed] [Google Scholar]
- 6.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 2004;164:2147–2155. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–40. [DOI] [PubMed] [Google Scholar]
- 8.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol 2018;6:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. [DOI] [PubMed] [Google Scholar]
- 11.Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waugh N, Shyangdan D, Taylor-Phillips S, Suri G, Hall B. Screening for type 2 diabetes: a short report for the National Screening Committee. Health Technol Assess 2013;17:Chapter 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Emerging Risk Factors Collaboration, Angelantonio ED, Gao P et al. Glycated Hemoglobin Measurement and Prediction of Cardiovascular Disease. JAMA 2014;311:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray KK, Seshasai SR, Wijesuriya S et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–72. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull FM, Abraira C, Anderson RJ et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298. [DOI] [PubMed] [Google Scholar]
- 16.Labarthe DR, Dunbar SB. Global cardiovascular health promotion and disease prevention: 2011 and beyond. Circulation 2012;125:2667–76. [DOI] [PubMed] [Google Scholar]
- 17.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou CY, Chang YT, Yang JL et al. Effect of Long-term Incretin-Based Therapies on Ischemic Heart Diseases in Patients with Type 2 Diabetes Mellitus: A Network Meta-analysis. Sci Rep 2017;7:15795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JH, Foote C, Blomster J et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2016;4:411–9. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz PE, Greaves CJ, Lindstrom J, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:363–373. [DOI] [PubMed] [Google Scholar]
- 21.Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary Prevention of Cardiovascular Disease in Diabetes Mellitus. J Am Coll Cardiol 2017;70:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood) 2016;241:1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glumer C, Yuyun M, Griffin S et al. What determines the cost-effectiveness of diabetes screening? Diabetologia 2006;49:1536–44. [DOI] [PubMed] [Google Scholar]
- 24.Williamson DF, Narayan KMV. Identification of persons with dysglycemia: Terminology and practical significance. Prim Care Diabetes 2009. 3:211–217. [DOI] [PubMed] [Google Scholar]
- 25.Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KMV. Screening for Type 2 Diabetes and Dysglycemia. Epidemiol Revi 2011;33:63–87. [DOI] [PubMed] [Google Scholar]
- 26.Barry E, Roberts S, Oke J, Vijayaraghavan S, Normansell R, Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ 2017;356:i6538. [DOI] [PubMed] [Google Scholar]
- 27.The ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 28.Colagiuri R, Colagiuri S, Yach D, Pramming S. The answer to diabetes prevention: science, surgery, service delivery, or social policy? Am J Public Health 2006;96:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977–1998. JAMA 2003;289:450–3. [DOI] [PubMed] [Google Scholar]
- 30.Brownell KD, Farley T, Willett WC et al. The public health and economic benefits of taxing sugar-sweetened beverages. N Engl J Med 2009;361:1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak NL, Brownell KD. Taxation as prevention and as a treatment for obesity: the case of sugar-sweetened beverages. Curr Pharm Des 2011;17:1218–22. [DOI] [PubMed] [Google Scholar]
- 32.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med 2007;357:370–9. [DOI] [PubMed] [Google Scholar]
- 33.Elbel B, Kersh R, Brescoll VL, Dixon LB. Calorie labeling and food choices: a first look at the effects on low-income people in New York City. Health Aff (Millwood) 2009;28:w1110–21. [DOI] [PubMed] [Google Scholar]
- 34.Rose G Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981;282:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin GP, Kocman D, Stephens T, Peden CJ, Pearse RM. Pathways to professionalism? Quality improvement, care pathways, and the interplay of standardisation and clinical autonomy. Sociol Health Illn 2017;39:1314–1329. [DOI] [PubMed] [Google Scholar]
- 36.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132–140. [DOI] [PubMed] [Google Scholar]
- 37.Andersen RM. Revisiting the Behavioral Model and Access to Medical Care: Does it Matter? J Health Soc Behav 1995;36:1–10. [PubMed] [Google Scholar]
- 38.Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol 1991;44:1271–1278. [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greaves CJ, Sheppard KE, Abraham C et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong X, Cai R, Sun J et al. Diabetes as a risk factor for acute coronary syndrome in women compared with men: a meta-analysis, including 10 856 279 individuals and 106 703 acute coronary syndrome events. Diabetes Metab Res Rev 2017;33. [DOI] [PubMed] [Google Scholar]
- 42.Bundhun PK, Bhurtu A, Yuan J. Impact of type 2 diabetes mellitus on the long-term mortality in patients who were treated by coronary artery bypass surgery: A systematic review and meta-analysis. Medicine 2017;96:e7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malmberg K, Yusuf S, Gerstein HC et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 2000;102:1014–9. [DOI] [PubMed] [Google Scholar]
- 44.Straumann E, Kurz DJ, Muntwyler J et al. Admission glucose concentrations independently predict early and late mortality in patients with acute myocardial infarction treated by primary or rescue percutaneous coronary intervention. Am Heart J 2005;150:1000–6. [DOI] [PubMed] [Google Scholar]
- 45.Chen PC, Chua SK, Hung HF et al. Admission hyperglycemia predicts poorer short‐ and long‐term outcomes after primary percutaneous coronary intervention for ST‐elevation myocardial infarction. J Diabetes Invest 2014;5:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin K, Goldberg RJ, Spencer F, et al. Implications of diabetes in patients with acute coronary syndromes: The global registry of acute coronary events. Arch Intern Med 2004;164:1457–1463. [DOI] [PubMed] [Google Scholar]
- 47.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 48.Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duckworth W, Abraira C, Moritz T et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Tong Y, Zhang Y et al. Effects on All-cause Mortality and Cardiovascular Outcomes in Patients With Type 2 Diabetes by Comparing Insulin With Oral Hypoglycemic Agent Therapy: A Meta-analysis of Randomized Controlled Trials. Clin Ther 2016;38:372–386.e6. [DOI] [PubMed] [Google Scholar]
- 51.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017;60:1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang H-J, Zhou Y-H, Tian Y-J, Du H-Y, Sun Y-X, Zhong L-Y. Effects of intensive glucose lowering in treatment of type 2 diabetes mellitus on cardiovascular outcomes: A meta-analysis of data from 58,160 patients in 13 randomized controlled trials. Int J Cardiol 2016;218:50–58. [DOI] [PubMed] [Google Scholar]
- 53.Zoungas S, Arima H, Gerstein HC et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:431–437. [DOI] [PubMed] [Google Scholar]
- 54.Savarese G, D’Amore C, Federici M et al. Effects of Dipeptidyl Peptidase 4 Inhibitors and Sodium-Glucose Linked coTransporter-2 Inhibitors on cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis. Int J Cardiol 2016;220:595–601. [DOI] [PubMed] [Google Scholar]
- 55.Wang T, Wang F, Zhou J, Tang H, Giovenale S. Adverse effects of incretin-based therapies on major cardiovascular and arrhythmia events: meta-analysis of randomized trials. Diabetes Metab Res Rev 2016;32:843–857. [DOI] [PubMed] [Google Scholar]
- 56.Packer M Do DPP-4 Inhibitors Cause Heart Failure Events by Promoting Adrenergically Mediated Cardiotoxicity? Clues From Laboratory Models and Clinical Trials. Circ Res 2018;122:928–932. [DOI] [PubMed] [Google Scholar]
- 57.Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab 2017;19:216–227. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Chen X, Lu P et al. Incretin-based agents in type 2 diabetic patients at cardiovascular risk: compare the effect of GLP-1 agonists and DPP-4 inhibitors on cardiovascular and pancreatic outcomes. Cardiovasc Diabetol 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gargiulo P, Savarese G, D’Amore C et al. Efficacy and safety of glucagon-like peptide-1 agonists on macrovascular and microvascular events in type 2 diabetes mellitus: A meta-analysis. Nutr Metab Cardiovasc Dis 2017. 27:1081–1088. [DOI] [PubMed] [Google Scholar]
- 60.Rizzo M, Nikolic D, Patti AM et al. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim Biophys Acta 2018. [DOI] [PubMed] [Google Scholar]
- 61.Baker WL, Buckley LF, Kelly MS et al. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on 24-Hour Ambulatory Blood Pressure: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnston R, Uthman O, Cummins E et al. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2017;21:1–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of Sodium-Glucose Cotransport-2 Inhibitors on Blood Pressure in People With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of 43 Randomized Control Trials With 22 528 Patients. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monami M, Dicembrini I, Mannucci E. Effects of SGLT-2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol 2017;54:19–36. [DOI] [PubMed] [Google Scholar]
- 65.Sha S, Polidori D, Heise T et al. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014;16:1087–95. [DOI] [PubMed] [Google Scholar]
- 66.Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. [DOI] [PubMed] [Google Scholar]
- 67.Bonner C, Kerr-Conte J, Gmyr V et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–7. [DOI] [PubMed] [Google Scholar]
- 68.Ferrannini E, Muscelli E, Frascerra S et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherney DZ, Perkins BA, Soleymanlou N et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–90. [DOI] [PubMed] [Google Scholar]
- 72.Seidu S, Achana FA, Gray LJ, Davies MJ, Khunti K. Effects of glucose-lowering and multifactorial interventions on cardiovascular and mortality outcomes: a meta-analysis of randomized control trials. Diabet Med 2016;33:280–9. [DOI] [PubMed] [Google Scholar]
- 73.Thomopoulos C, Parati G, Zanchetti A. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10 - Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens 2017;35:922–944. [DOI] [PubMed] [Google Scholar]
- 74.Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 2016;352:i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beck J, Greenwood DA, Blanton L et al. 2017 National Standards for Diabetes Self-Management Education and Support. Diabetes Care 2017. [DOI] [PubMed] [Google Scholar]
- 76.National Institute for Health and Clinical Excellence - NICE. Preventing type 2 diabetes: risk identification and interventions for individuals at high risk. NICE public health guidance; 38 Last updated: September 2017. ed, 2012. [Google Scholar]
- 77.Cai X, Qiu SH, Yin H et al. Pedometer intervention and weight loss in overweight and obese adults with Type 2 diabetes: a meta-analysis. Diabet Med 2016;33:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ekong G, Kavookjian J. Motivational interviewing and outcomes in adults with type 2 diabetes: A systematic review. Patient Educ Couns 2015;99:944–52. [DOI] [PubMed] [Google Scholar]
- 79.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev Med 2017;100:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Modesti PA, Galanti G, Cala P, Calabrese M. Lifestyle interventions in preventing new type 2 diabetes in Asian populations. Intern Emerg Med 2015;11:375–84. [DOI] [PubMed] [Google Scholar]
- 81.Porter J, Huggins CE, Truby H, Collins J. The Effect of Using Mobile Technology-Based Methods That Record Food or Nutrient Intake on Diabetes Control and Nutrition Outcomes: A Systematic Review. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thepwongsa I, Muthukumar R, Kessomboon P. Motivational interviewing by general practitioners for Type 2 diabetes patients: a systematic review. Family practice 2017;34:376–383. [DOI] [PubMed] [Google Scholar]
- 83.Zhu H, Zhu Y, Leung SW. Is self-monitoring of blood glucose effective in improving glycaemic control in type 2 diabetes without insulin treatment: a meta-analysis of randomised controlled trials. BMJ open 2016;6:e010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arambepola C, Ricci-Cabello I, Manikavasagam P, Roberts N, French DP, Farmer A. The Impact of Automated Brief Messages Promoting Lifestyle Changes Delivered Via Mobile Devices to People with Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Controlled Trials. J Med Internet Res 2016;18:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baskerville R, Ricci-Cabello I, Roberts N, Farmer A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with Type 2 diabetes: a systematic review and meta-analysis. Diabetic medicine : a journal of the British Diabetic Association;34:612–620. [DOI] [PubMed] [Google Scholar]
- 86.Beishuizen CR, Stephan BC, van Gool WA et al. Web-Based Interventions Targeting Cardiovascular Risk Factors in Middle-Aged and Older People: A Systematic Review and Meta-Analysis. J Med Internet Res 2016;18:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cradock KA, G OL, Finucane FM et al. Diet Behavior Change Techniques in Type 2 Diabetes: A Systematic Review and Meta-analysis. Diabetes Care 2017;40:1800–1810. [DOI] [PubMed] [Google Scholar]
- 88.Cui M, Wu X, Mao J, Wang X, Nie M. T2DM Self-Management via Smartphone Applications: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0166718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daly B, Tian CJL, Scragg RKR. Effect of nurse-led randomised control trials on cardiovascular risk factors and HbA1c in diabetes patients: A meta-analysis. Diabetes Res Clin Pract 2017;131:187–199. [DOI] [PubMed] [Google Scholar]
- 90.Fu S, Li L, Deng S, Zan L, Liu Z. Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: a systematic review and meta-analysis. Sci Rep 2016;6:37067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev Med 2017;100:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moller G, Andersen HK, Snorgaard O. A systematic review and meta-analysis of nutrition therapy compared with dietary advice in patients with type 2 diabetes. Am J Clin Nutr 2017;106:1394–1400. [DOI] [PubMed] [Google Scholar]
- 93.Mudaliar U, Zabetian A, Goodman M et al. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis. PLoS Med 2016;13:e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Odgers-Jewell K, Ball LE, Kelly JT, Isenring EA, Reidlinger DP, Thomas R. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med 2017;34:1027–1039. [DOI] [PubMed] [Google Scholar]
- 95.Pousinho S, Morgado M, Falcao A, Alves G. Pharmacist Interventions in the Management of Type 2 Diabetes Mellitus: A Systematic Review of Randomized Controlled Trials. J Manag Care Spec Pharm 2016;22:493–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor J, Stubbs B, Hewitt C et al. The Effectiveness of Pharmacological and Non-Pharmacological Interventions for Improving Glycaemic Control in Adults with Severe Mental Illness: A Systematic Review and Meta-Analysis. PLoS One 2017;12:e0168549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun Y, You W, Almeida F, Estabrooks P, Davy B. The Effectiveness and Cost of Lifestyle Interventions Including Nutrition Education for Diabetes Prevention: A Systematic Review and Meta-Analysis. J Acad Nutr Diet 2017;117:404–421.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, Xue H, Huang Y, Huang L, Zhang D. A Systematic Review of Application and Effectiveness of mHealth Interventions for Obesity and Diabetes Treatment and Self-Management. Adv Nutr 2017;8:449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yasmin F, Banu B, Zakir SM, Sauerborn R, Ali L, Souares A. Positive influence of short message service and voice call interventions on adherence and health outcomes in case of chronic disease care: a systematic review. BMC Med Inform Decis Mak 2016;16:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X, Imperatore G, Thomas W et al. Effect of lifestyle interventions on glucose regulation among adults without impaired glucose tolerance or diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract 2016;123:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deylami R, Townson J, Mann M, Gregory JW. Systematic review of publicity interventions to increase awareness amongst healthcare professionals and the public to promote earlier diagnosis of type 1 diabetes in children and young people. Pediatr Diabetes 2017. [DOI] [PubMed] [Google Scholar]
- 102.Reed JL, Prince SA, Elliott CG et al. Impact of Workplace Physical Activity Interventions on Physical Activity and Cardiometabolic Health Among Working-Age Women: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017;10. [DOI] [PubMed] [Google Scholar]
- 103.Shrestha A, Karmacharya BM, Khudyakov P, Weber MB, Spiegelman D. Dietary Interventions to Prevent and Manage Diabetes in Worksite Settings: a Meta-Analysis. J Occup Health 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ebireri J, Aderemi AV, Omoregbe N, Adeloye D. Interventions addressing risk factors of ischaemic heart disease in sub-Saharan Africa: a systematic review. BMJ Open 2016;6:e011881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grace JM, Wilder CJ, Strydom GI. The effect of a physical and a combined health promotion intervention programme on some selected health indicators on South African colliery executives. South Afr J Res Sports, Phys Educ Recreation 2009;31:9–18. [Google Scholar]
- 106.Gourzoulidis G, Kourlaba G, Stafylas P, Giamouzis G, Parissis J, Maniadakis N. Association between copayment, medication adherence and outcomes in the management of patients with diabetes and heart failure. Health Policy 2017;121:363–377. [DOI] [PubMed] [Google Scholar]
- 107.Hunt J, Rozenfeld Y, Shenolikar R. Effect of patient medication cost share on adherence and glycemic control. Managed care 2009;18:47–53. [PubMed] [Google Scholar]
- 108.Soumerai SB, Starr D, Majumdar SR. How Do You Know Which Health Care Effectiveness Research You Can Trust? A Guide to Study Design for the Perplexed. Prev Chronic Dis 2015;12:E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soumerai SB, Ceccarelli R, Koppel R. False Dichotomies and Health Policy Research Designs: Randomized Trials Are Not Always the Answer. J Gen Intern Med 2017;32:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Basu S, Meghani A, Siddiqi A. Evaluating the Health Impact of Large-Scale Public Policy Changes: Classical and Novel Approaches. Ann Rev Public Health 2017;38:351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Humphreys DK, Panter J, Sahlqvist S, Goodman A, Ogilvie D. Changing the environment to improve population health: a framework for considering exposure in natural experimental studies. J Epidemiol Community Health 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Craig P, Katikireddi SV, Leyland A, Popham F. Natural Experiments: An Overview of Methods, Approaches, and Contributions to Public Health Intervention Research. Annu Rev Public Health 2017;38:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hill JO, Galloway JM, Goley A et al. Scientific Statement: Socioecological Determinants of Prediabetes and Type 2 Diabetes. Diabetes Care 2013;36:2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ackermann RT, Kenrik Duru O, Albu JB et al. Evaluating diabetes health policies using natural experiments: the natural experiments for translation in diabetes study. Am J Prev Med 2015;48:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Freudenberg N, Franzosa E, Sohler N, Li R, Devlin H, Albu J. The State of Evaluation Research on Food Policies to Reduce Obesity and Diabetes Among Adults in the United States, 2000–2011. Prev Chronic Dis 2015;12:E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goodwin N Understanding Integrated Care. Int J Integr Care 2016;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baptista DR, Wiens A, Pontarolo R, Regis L, Reis WC, Correr CJ. The chronic care model for type 2 diabetes: a systematic review. Diabetol Metab Syndr 2016;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bongaerts BWC, Müssig K, Wens J et al. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and meta-analysis. BMJ Open 2017;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Busetto L, Luijkx KG, Elissen AMJ, Vrijhoef HJM. Context, mechanisms and outcomes of integrated care for diabetes mellitus type 2: a systematic review. BMC Health Serv Res 2016;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Babar ZU, Kousar R, Murtaza G, Azhar S, Khan SA, Curley L. Randomized controlled trials covering pharmaceutical care and medicines management: A systematic review of literature. Res Social Adm Pharm 2017:521–539. [DOI] [PubMed] [Google Scholar]
- 121.Aguiar PM, Brito Gde C, Lima Tde M, Santos AP, Lyra DP Jr., Storpirtis S Investigating Sources of Heterogeneity in Randomized Controlled Trials of the Effects of Pharmacist Interventions on Glycemic Control in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0150999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asayut N, Sookaneknun P, Chaiyasong S, Saramunee K. Outcomes, costs and stakeholders’ perspectives associated with the incorporation of community pharmacy services into the National Health Insurance System in Thailand: a systematic review. Int J Pharm Pract 2018:16–27. [DOI] [PubMed] [Google Scholar]
- 123.Seidu S, Walker NS, Bodicoat DH, Davies MJ, Khunti K. A systematic review of interventions targeting primary care or community based professionals on cardio-metabolic risk factor control in people with diabetes. Diabetes Res Clin Pract 2016;113:1–13. [DOI] [PubMed] [Google Scholar]
- 124.Deters MA, Laven A, Castejon A et al. Effective Interventions for Diabetes Patients by Community Pharmacists: A Meta-analysis of Pharmaceutical Care Components. Ann Pharmacother 2017:1060028017733272. [DOI] [PubMed] [Google Scholar]
- 125.Greer N, Bolduc J, Geurkink E et al. Pharmacist-led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared With Usual Care. Ann Intern Med 2016. [DOI] [PubMed] [Google Scholar]
- 126.Brown TJ, O’Malley C, Blackshaw J et al. Exploring the evidence base for Tier 3 weight management interventions for adults: a systematic review. Clin Obes 2017;7:260–272. [DOI] [PubMed] [Google Scholar]
- 127.Jeet G, Thakur JS, Prinja S, Singh M. Community health workers for non-communicable diseases prevention and control in developing countries: Evidence and implications. PLoS One 2017;12:e0180640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Massimi A, De Vito C, Brufola I et al. Are community-based nurse-led self-management support interventions effective in chronic patients? Results of a systematic review and meta-analysis. PLoS One 2017;12:e0173617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tabesh M, Magliano DJ, Koye DN, Shaw JE. The effect of nurse prescribers on glycaemic control in type 2 diabetes: A systematic review and meta-analysis. Int J Nurs Stud 2017. [DOI] [PubMed] [Google Scholar]
- 130.Li G, Zhang P, Wang J et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–9. [DOI] [PubMed] [Google Scholar]
- 131.The Look Ahead Research Group. Long Term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes: Four Year Results of the Look AHEAD Trial. Arch Intn Med 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Beurden SB, Greaves CJ, Smith J, Abraham C. Techniques for Modifying Impulsive Processes Associated with Unhealthy Eating: A Systematic Review. Health Psychol 2016;35:793–806. [DOI] [PubMed] [Google Scholar]
- 133.Kim DA, Hwong AR, Stafford D et al. Social network targeting to maximise population behaviour change: a cluster randomised controlled trial. Lancet 2015;386:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strack F, Deutsch R. Reflective and Impulsive Determinants of Social Behavior. Pers Soc Psychol Rev 2004;8:220–247. [DOI] [PubMed] [Google Scholar]
- 135.Alicia A-M, Josep MS-S, Gemma M-M, Medina FX, Ramon C-C, Francesc S-R. Use of mobile phones as a tool for weight loss: a systematic review. J Telemed Telecare 2014;20:339–349. [DOI] [PubMed] [Google Scholar]
- 136.Hafez D, Fedewa A, Moran M, O’Brien M, Ackermann R, Kullgren JT. Workplace Interventions to Prevent Type 2 Diabetes Mellitus: a Narrative Review. Curr Diab Rep 2017;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pomeranz JL, Wilde P, Huang Y, Micha R, Mozaffarian D. Legal and Administrative Feasibility of a Federal Junk Food and Sugar-Sweetened Beverage Tax to Improve Diet. Am J Public Health 2018;108:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malambo P, Kengne AP, De Villiers A, Lambert EV, Puoane T. Built Environment, Selected Risk Factors and Major Cardiovascular Disease Outcomes: A Systematic Review. PLoS One 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wright A, Smith KE, Hellowell M. Policy lessons from health taxes: a systematic review of empirical studies. BMC Public Health 2017;17:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwarz PE, Riemenschneider H. Slowing Down the Progression of Type 2 Diabetes: We Need Fair, Innovative, and Disruptive Action on Environmental and Policy Levels! Diabetes Care 2016;39 Suppl 2:S121–6. [DOI] [PubMed] [Google Scholar]
- 141.Minneboo M, Lachman S, Snaterse M et al. Community-Based Lifestyle Intervention in Patients With Coronary Artery Disease: The RESPONSE-2 Trial. J Am Coll Cardiol 2017;70:318–327. [DOI] [PubMed] [Google Scholar]
- 142.McMurray JJ, Adamopoulos S, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 143.Smith SM, Wallace E, O’Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016;3:Cd006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cronin J, Murphy A, Savage E. Can chronic disease be managed through integrated care cost-effectively? Evidence from a systematic review. Ir J Med Sci 2017;186:827–834. [DOI] [PubMed] [Google Scholar]
- 145.OECD. Cardiovascular Disease and Diabetes: Policies for Better Health and Quality of Care, 2015.
- 146.Piepoli MF, Hoes AW, Agewall S et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Michie S, Richardson M, Johnston M et al. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann Behav Med 2013;46:81–95. [DOI] [PubMed] [Google Scholar]
- 148.Dunkley AJ, Bodicoat DH, Greaves CJ et al. Diabetes Prevention in the Real World: Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes and of the Impact of Adherence to Guideline Recommendations: A Systematic Review and Meta-analysis. Diabetes Care 2014;37:922–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.