Abstract
Background
While live imaging of embryonic development over long periods of time is a well established method for embryos of the frog Xenopus laevis, once development has progressed to the swimming stages (e.g. Stage 40 and beyond), continuous live imaging becomes more challenging because the tadpoles must be immobilized. Current imaging techniques for these advanced stages generally require bringing the tadpoles in and out of anesthesia for short imaging sessions at selected time points, severely limiting the resolution of the data.
Results
Here we demonstrate that creating a constant flow of diluted tricaine methanesulfonate (MS-222) over a tadpole greatly improves their survival under anesthesia. Based on this result, we describe a new method for imaging X. laevis tadpoles in this anesthetic using a peristaltic pump to support the tadpole during continuous live imaging sessions of up to 48 hours. The addition of a stable optical window allows for high quality imaging through the anesthetic solution.
Conclusions
This automated imaging system provides for the first time a method for continuous observations of developmental and regenerative processes in advanced Xenopus tadpoles for at least 48 hours.
Keywords: time-lapse, anesthesia, MS-222, microscopy
Introduction
The larval form of the African clawed frog, Xenopus laevis, provides an ideal system to study developmental and regenerative processes in vivo (Beck & Slack, 2001; Henry et al., 2008). Embryos are relatively large and can be genetically manipulated through the use of Morpholinos, synthetic mRNAs, and transgenes (Henry et al., 2008; Mimoto & Christian, 2011; Takagi et al., 2013). In order to better understand changes in gene expression and function using these techniques, methods of imaging live embryos in real-time have been established and are now invaluable tools in many laboratories (Keller 1978; Kieserman et al., 2010; Wallingford, 2010; Danilchik, 2011). However, these techniques are limited to the early stages of development, before the tadpole gains locomotion. It is during these more advanced stages of development that Xenopus larvae display remarkable regenerative abilities in the limb, tail, spinal cord, and parts of the eye (Henry et al., 2008; Slack et al., 2008; Beck et al., 2009). In addition, many other important developmental processes continue during these advanced stages, such as those of the limb, heart, and nervous system (Nieuwkoop & Faber, 1956; Tschumi, 1957; Warkman & Krieg, 2007). Currently, to monitor expression and functional changes during these important developmental and regenerative processes, one must either fix specimens at a few selected time points, or repeatedly bring the animals in and out of anesthesia for brief individual imaging sessions. In either case, this greatly reduces the resolution of these experiments.
The current anesthetic of choice for X. laevis is tricaine methanesulfonate (commonly known as MS-222), which is widely accepted and used for procedures in fish and amphibians (Popovic et al, 2012; Guénette et al., 2013). When Xenopus tadpoles are immersed in a buffered solution of MS-222 (typically a 1:2,000 dilution), the anesthetic is absorbed quickly and acts to inhibit sensory function, motor function, and consciousness (Lalonde-Robert et al., 2012). While this quick activity makes it an ideal anesthetic agent for short procedures, X. laevis larvae are not able to live when kept in this anesthetic for long periods of time, also making it a widely used choice as a euthanizing agent at higher concentrations (Leary et al., 2013). The consequence is that any live imaging sessions of anesthetized tadpoles are limited to a short period of time before their health begins to fail. One question that can be raised is whether this failing is due to the direct effect of the anesthetic agent itself, or is instead the result of poor respiration due to immobilization. This question is raised from observations in fish, where it is thought that one of the primary causes of death for adult fish kept in continuous MS-222 is asphyxiation due the cessation of buccal pumping and gill irrigation (Soivio et al., 1977; Cornish & Moon, 1986). As a result, fish are often supported using an artificial gill irrigation system during long anesthesia to promote gas exchange with the gills (Carter et al., 2011). Since Xenopus tadpoles also use buccal pumping for gill irrigation, it is possible that they also become hypoxic when left in MS-222 for long periods of time. Additionally, since frogs are bimodal breathers and are able to undergo gas exchange through their skin (Emilio & Shelton, 1974; Boutilier & Shelton, 1986), it is possible that being immobilized in static anesthetic also affects their ability to properly exchange gas through these tissues.
In this study, we found the simple act of rocking Xenopus tadpoles in a dilute anesthetic solution of MS-222 (1:7,000) significantly increases their longevity from hours up to days. By using a peristaltic pump to create a similar flow over the animals, tadpoles can remain anesthetized and immobilized to allow for live imaging over long periods of time. Here we describe the construction of an apparatus that accomplishes this task, and we demonstrate the power of this technique by showing the development of the hindlimb over the course of 48 hours. Additionally, we demonstrate the usefulness of this technique when combined with transgenesis by imaging cellular divisions in the cornea epithelium using a transgenic histone H2B-mCherry nuclear reporter. This technique opens the door for new imaging of a variety of developmental and regenerative processes in Xenopus and should provide new insights into these key areas of research.
Results
Rocking tadpoles in MS-222 significantly increases survivability
We hypothesized that part of the reason tadpoles expire in the continuous presence of MS-222 is due to poor oxygenation resulting from immobilization in the anesthetic. In order to test this hypothesis, we set up a simple experiment where tadpoles were placed in an anesthetic solution of MS-222 on a common lab rocker in order to aerate and circulate the solution to promote better gas exchange. All of the control cases (n=8) kept stationary in the anesthetic expired within 8 hours, with 50% expiring within the first 4 hours. Those that did survive past a few hours were opaque and pale in color, and often displayed signs of heart failure such as arrhythmia, slow blood flow, and signs of fluid accumulation and swollen tissue, particularly noticeable over the heart. Remarkably, 75% of the rocked cases (n=8) survived for 48 hours. This difference represents a statistically significant increase in survivability (p-value < 0.0001; Fig. 1A). It is important to note that some tadpoles naturally open their mouths when anesthetized, which could allow for some flow into the buccal cavity and over the gills while rocking. However, this did not appear to be necessary as it was observed that animals with closed mouths were also able to survive for the full 48 hours. This observation suggests that it is cutaneous gas exchange that is sufficient to increase survivability.
Fig. 1.
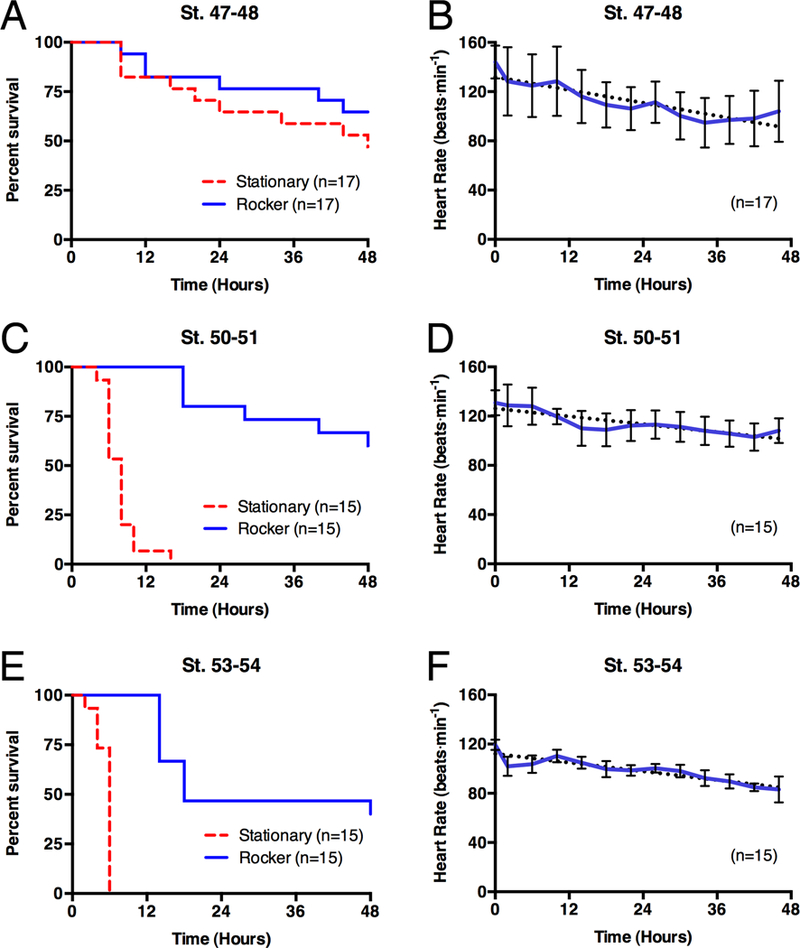
Survivability of anesthetized tadpoles. (A) Kaplan-Meier survival curve of tadpoles anesthetized in 1:7,000 MS-222. Tadpoles kept either stationary (blue; n=8) or on the rocker (red; n=8) demonstrated a statistically significant difference (p<0.0001; Mantel-Cox test) between the groups. The rocking effect can be recreated using a circulating pump (green; n=5; described below). (B) Mean heart rates of rocking tadpoles that survived for 48 hours, either stages 50–51 (solid; n=3) or stages 53–54 (dashed; n=3). Error Bars show standard deviation. Dotted lines are linear regression lines with equations of y=−0.6712x+113.8 (st. 53–54) and y=−0.7575x+137.3 (st. 50–31).
Throughout the experiments the heart rate of each animal was determined as a quantitative assessment of the vitality of the tadpoles in the anesthetic solution. Of the six cases that survived in the anesthetic solution for the full 48 hours, a slight decrease in the heart rate over time was observed (Fig. 1B,D,F). This was true regardless of the stages used. While the baseline heart rate of the older and larger tadpoles was lower than that of the younger tadpoles (as described by Hou & Burggren, 1995), the trend between the two groups was very similar with an approximate reduction of 4 to 4.5 beats per minute every 6 hours.
Prolonged anesthesia of immobilized tadpoles
In order to see if gill irrigation could be used to support a tadpole held in a fixed position, we created an artificial gill irrigation system (similar to those used in fish), using a small funnel to direct flow through the open mouth and over the gills; however, this method produced variable survivability results (data not shown). Since directed flow through the open mouth did not appear to be necessary to promote effective gas exchange as observed in the rocker experiments, we designed a more general system to create flow over the entire animal in order to more closely recreate those conditions (Fig. 2). A custom manifold was created from plexiglass and placed into a petri dish along with three pedestals to hold a coverslip as the solution current impacts the ability to clearly view the animal (Fig. 3 and 4A). Non-toxic modeling clay was placed between the pedestals to hold tadpoles gently, but securely, in place. Minimal contact was maintained between the animals and clay to promote flow of the anesthetic solution around the tadpoles. However, it is necessary to provide enough contact to prevent the animals from moving in the flow. It is also important to note that great care was taken to specifically avoid pushing any clay against the heart or abdomen, so as not to interfere with heart contractions or peristalsis of the gut.
Fig. 2.
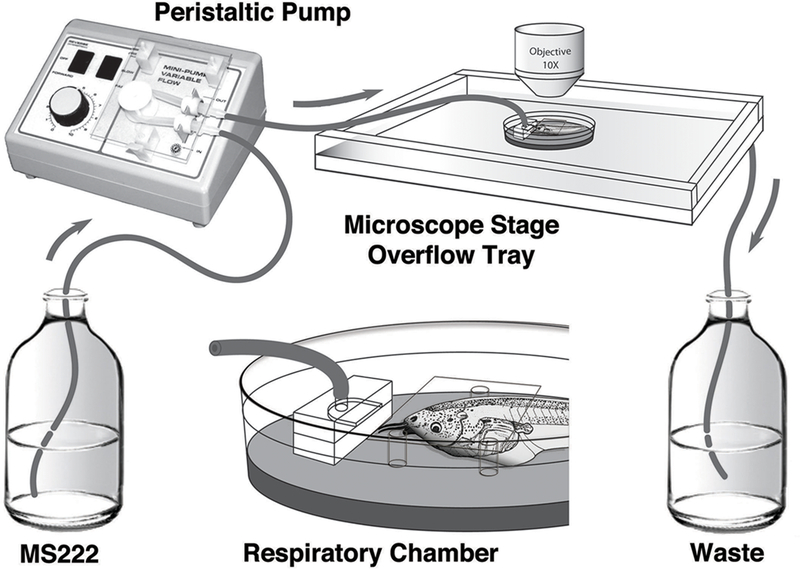
Schematic drawing of the respiratory chamber and pump system. An anesthetic solution of MS-222 is pumped to the respiratory chamber via a peristaltic pump. The respiratory chamber contains a manifold that provides continuous flow of anesthetic solution over the tadpole, which is held in place with clay. Three fixed pedestals hold a coverslip to provide a transparent and flat surface for imaging. The respiratory chamber is placed into a shallow plexiglass overflow tray to catch the anesthetic solution. The overflow tray collects anesthetic runoff to a waste container that can be discarded or recirculated, as needed by the experiment.
Fig. 3.
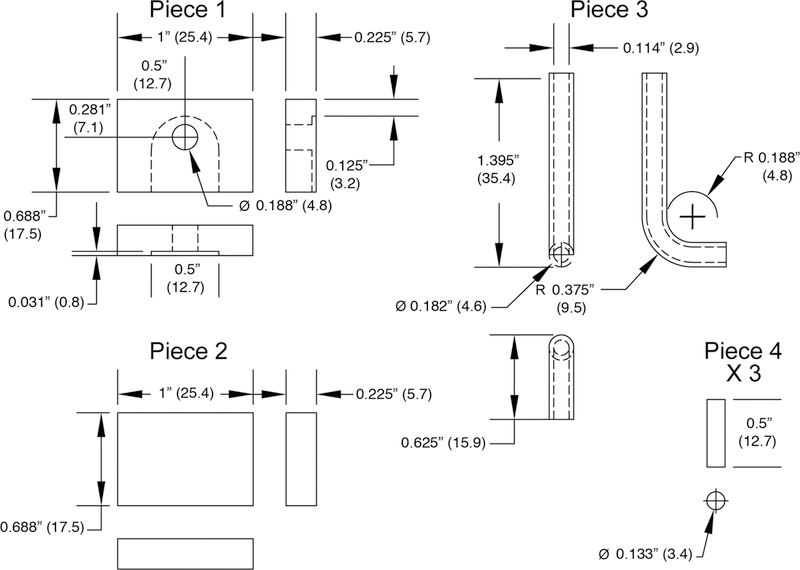
Specifications of manifold pieces. A 0.5 inch endmill was used to create a slot in a piece of 0.25 inch plexiglass (Piece 1), and an inlet hole was drilled into the top to connect with the slot, as shown. Piece 1 was fixed on top of Piece 2 using 1,2-dichloroethane. A piece of a 1 ml plastic serological pipette (Falcon 7506, Becton Dickinson Labware, Lincoln Park, NJ) was bent at approximately 90° (Piece 3) over a flame and was fixed (using dichloroethane) into the hole of Piece 1. Three pedestals (Piece 4) were cut from plexiglass rod, to be used to hold a coverslip. The assembled manifold and the three plexiglass rods were then affixed to a 100×15 mm petri dish to create the respiratory chamber using dichloroethane (see Figure 4A). Specification diagrams were generated in AutoCad. Measurements are in inches, with millimeters provided in parenthesis.
Fig. 4.
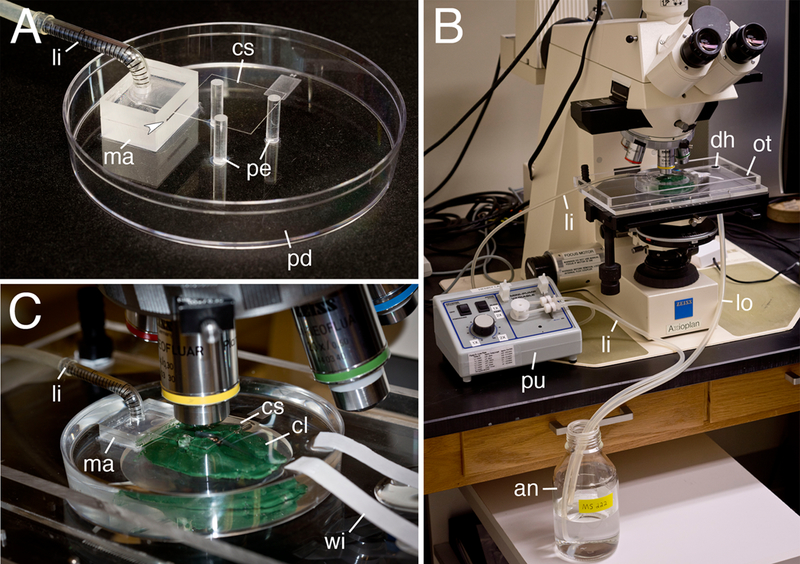
Xenopus Live Imaging Setup. A) The Xenopus respiratory chamber. Anesthetic solution is pumped into the manifold to generate flow across the tadpole. Three pedestals are used to hold a coverslip to allow for a smooth optical surface for imaging. Arrowhead indicates the slot that the anesthetic solution flows through. B) The microscopy setup. Anesthetic solution is pumped using a peristaltic pump to the manifold. The overflow tray collects the run-off and either returns it to the source for a recirculating setup, or can go to separate waste collection bottle (not shown). C) Clay is used to hold the tadpole in place underneath the coverslip. Filter paper wicks are used to accelerate drainage and maintain a low water level at the top of the dish. Abbreviations used are: anesthetic solution (an), clay (cl), coverslip (cs), drain hole (dh), line-in (li), line-out (lo), manifold (ma), overflow tray (ot), petri dish (pd), pedestal (pe), pump (pu), and filter paper wick (wi).
Once the tadpole was properly placed in the clay, the respiratory chamber was placed into an overflow tray to catch the runoff from the continuously pumped anesthetic solution. This tray drained into a reservoir bottle containing the anesthetic solution. A variable-speed peristaltic pump was used to pump the anesthetic solution from the reservoir bottle through the slot on the manifold and over the tadpole at a rate of 20 ml/min, creating a recirculating system. While we did not carry out extensive trials, we found that lower flow rates of approximately 10 ml/min were insufficient, as tadpoles did not survive past six hours at these rates (n=2). However, a flow rate of 20 ml/min worked well, supporting tadpoles ranging from stage 48 to stage 55 for 48 hours (n=5; Fig. 1A). For younger and older tadpoles, one may need to empirically determine an appropriate flow rate that works well.
In vivo Imaging
Once it was demonstrated that tadpoles survive well while kept stationary in the respiratory chamber, we adapted the system to be used with an upright microscope for time-lapse imaging (Fig. 4). A custom plexiglass overflow tray was built to attach to the microscope stage for collection of the anesthetic runoff to be recirculated (Fig. 4B). The respiratory chamber was then placed underneath the objective, with the tadpoles mounted in clay and a stationary coverslip secured on top of three pedestals using clay (Fig. 4C). The coverslip is necessary to maintain an optically transparent and flat surface for imaging, as without it, the circulating air-water interface distorts the image due to water tension and subtle changes in fluid volume. It is also important to make the top surface of the coverslip hydrophobic, to prevent anesthetic solution from flowing over the top of the coverslip. However, if a water immersion objective were to be used, then the coverslip would no longer be necessary.
Using this setup, we anesthetized wild-type tadpoles and imaged them for up to 48 hours. As an example, we imaged 48 hours of hindlimb development. This is made possible by using the automatic image acquisition capabilities of the SPOT Flex microscope camera and software (Diagnostic Instruments, Sterling Heights, MI). Figure 5 shows the development of a St. 53–54 left hindlimb. During this time interval we were able to observe the emergence and migration of melanophores in the limb, vascular development in the digital plate, and elongation of the digits. Particularly noticeable is the elongation of digit 4, which corresponds to the protrusion of the marginal vein at that digit (Fig. 5). The full time-lapse video of these events can be viewed in Supplemental Movie 1. This video also shows melanophores on the tail and limb as they respond to changes in illumination and other physiological conditions. In addition, one can see continued peristalsis of the gut and the expulsion of waste products. These events continue throughout the anesthetic treatment, indicating that these physiological events appear to be unaffected by the prolonged anesthesia.
Fig. 5.
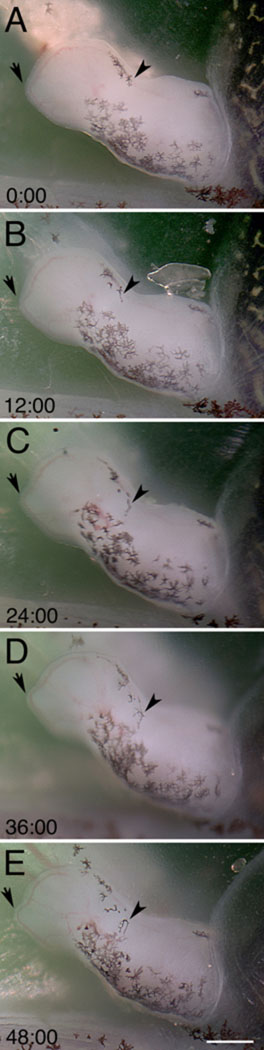
Time-lapse imaging of hindlimb development. (A-E) Growth of a stage 53–54 left hindlimb at 12 hour intervals over 48 hours. During this time melanophores can be seen responding to light by changing the distribution of their melanosomes. A melanophore (arrowhead) can be seen migrating in the limb, in addition to vascularization and digit development in the digital plate. A protrusion of the marginal vein can be seen developing as digit IV elongates (arrow). This plate corresponds to Supplemental Movie 1. Relative time is denoted in hours:minutes. Scale bar in E is 250 μm.
One of the powerful uses of this technique is in combination with transgenic expression of fluorescently labeled proteins in living tissues. We generated F0 transgenic tadpoles, expressing the mG-HGEM transgene (Fig. 6A), which carries a heat-shock inducible histone H2B-mCherry fusion protein, in order to fluorescently label cell nuclei (Beck et al., 2003; Takagi et al., 2013). This nuclear labeling allows for monitoring divisions and tracking cellular movements within a tissue. Using the in vivo imaging setup, we imaged fluorescently labeled nuclei in the cornea epithelium. Figure 6 (B–G) follows an individual cell of the cornea epithelium through the phases of mitotic division and the migration of the daughter cells away from each other. The entire division can be viewed as a time-lapse movie in Supplemental Movie 2.
Fig. 6.
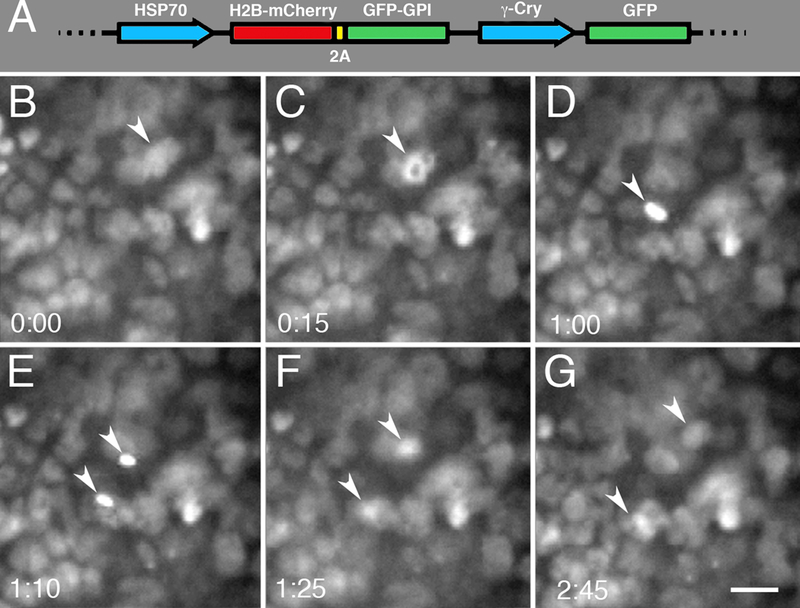
Micrographs of cell division in Xenopus cornea epithelium. A) The mG-HGEM transgenic construct carrying a heat-shock inducible histone H2B-mCherry protein. It also carries a membrane GFP protein separated by a 2A peptide sequence (not imaged in this particular application). As a screening tool the construct uses the lens-specific gamma-crystallin promoter to drive GFP expression. (B-G) Imaging of a cornea epithelial cell going through mitosis (arrowheads). (B) Pre-mitotic cell. (C) Prophase. (D) Metaphase. (E) Anaphase. (F) Telophase. (G) The resulting daughter cells. This particular division occurred over an unusually long distance. A movie of this division can be seen in Supplemental Movie 2. Relative time is denoted in hours:minutes. Scale bar in G is 20 μm.
Discussion
One of the most significant advantages of this system is the relative ease with which one can set up imaging experiments and collect very complete sets of data. It takes only a few minutes to prepare the specimen, and the time interval and focal planes between successive images can be customized as required by the experiment. Currently, the only manual work that must be done during the imaging session is daily changes of the anesthetic solution and adjustment of the microscope focus to compensate for any possible drift or growth of the specimen. In setups where one has an automated motorized stage, focal adjustment is less of a concern since multiple focal planes can be captured as a stack and flattened into a single image using appropriate software. This ensures that the area of interest remains in focus for the duration of the time-lapse experiment.
One concern with this technique is the potential physiological effect of the MS-222 on the anesthetized animal. In both the leopard frog Rana pipiens and adult zebrafish, it has been reported that MS-222 decreases heart rates during anesthesia (Cakir & Strauch, 2005; Sun et al., 2009). However, recent work in X. laevis reports very little effect on heart rates during anesthesia of adults in MS-222 (Lalonde-Robert et al., 2012), so it was unclear whether or not MS-222 would affect heart rates in larval Xenopus. In an attempt to minimize any potential effects, we used a 1:7,000 dilution of MS-222, which is sufficient to anesthetize the tadpole. While the concentrations used for surgical applications are generally higher than this (e.g. 1:2,000 in Perry et al., 2013), dilutions as low as 1:10,000 have been reported as effective (Hou & Burggren, 1995; Sakaguchi et al., 1997). At the concentrations used here, we did observe a gradual reduction in heart rate over time (Fig 1B), suggesting that MS-222 does have some effect on the cardiovascular system of larval Xenopus. However, the heart rates of these tadpoles never deviated from what would otherwise be considered a normal range for Xenopus larvae of those sizes and stages of development (Hou and Burggren, 1995).
One limitation with this technique is the length of time that the imaging can be carried out. We terminated our experiments after a predetermined 48 hours, at which time tadpoles still appeared to be in good health. This means it may be possible for even longer imaging sessions to be carried out using this method; however, care must be taken to consider experimental artifacts that may be introduced in studies greater than 48 hours. For instance if the heart rate continues to decline, at some point there may not be enough blood circulation to maintain the health of the tissues of interest. Also, the lack of food for multiple days will likely affect metabolism, and potentially the observed results.
In summary, this technique offers a new approach to imaging developmental and regenerative processes in living tissues. Until now, this has not been feasible due to the inability to maintain X. laevis tadpoles for long periods of time in an anesthetic solution. For instance, the development of the limb, eye, heart, and nervous system are all easily imaged through the transparent larval skin and can now be more effectively studied in vivo. It is anticipated that the adoption of this imaging system by other laboratories will help provide new insight into the development of many systems and tissues, and aid our current understanding of cellular biology in the context of the living organism.
Experimental Procedures
Animals
Xenopus laevis adults were acquired from Nasco (Fort Atkinson, WI). Larvae were generated and reared as previously described (Henry & Grainger, 1987; Schaefer et al., 1999). All larvae were developmentally staged according to Nieuwkoop and Faber (1956). The anesthetic used in all experiments was MS-222 (Sigma, St. Louis, MO) diluted 1:7,000 (0.14 mg/ml) in 1/20X normal amphibian media (NAM; Slack, 1984); and all animals were euthanized at the end of the designated 48-hour time interval. Transgenic animals were generated following the protocol described by Smith et al. (2006). The mG-HGEM transgenic construct was created by adding the sequences for histone H2B-mCherry and membrane bound GFP (mG) into the HGEM (heat-shock green-eyed monster; Beck et al., 2003) construct downstream of a heat-shock inducible hsp70 promoter (the membrane bound GFP signal was not utilized in this particular study). This transgenic cassette uses a gamma-crystallin promoter to drive GFP expression in the lens as a method for screening transgenic animals. Heat shocks were carried out on the whole animals by bath immersion in water at 34°C for 30 minutes. The animal care and use in this work has been overseen by the University of Illinois Institutional Animal Care and Use Committee and monitored by the staff of the University of Illinois Division of Animal Resources (DAR).
Rocking vs. Stationary Anesthesia
Tadpoles were fully anesthetized in 1:7,000 MS-222 for 2 minutes before being individually transferred into small glass bowls containing 50 ml of the anesthetic solution. Cases were placed either onto a Labnet Rocker 25 (Labnet International, Edison, NJ) set to 20 rocks per minute (rpm) with a tilt range of +/− 7°, or sibling controls were left stationary beside the rocker. Anesthetic solution was changed every 12 hours. Heart rates were determined by manually counting heartbeats viewed through a dissecting microscope (Stemi 2000, Zeiss) to determine heart rate per minute. Death was defined at the point at which the calculated heart rate was observed to be zero beats per minute (bpm). Statistical tests and figures were generated using Prism software (GraphPad Software, Inc., La Jolla, CA).
Respiratory Chamber
The respiratory chamber was constructed as detailed in Figure 3. 1:7,000 MS-222 solutions were made in 350 ml batches (a quantity sufficient to recirculate in our system) and changed every 24 hours. Silastic tubing was used to connect the anesthetic solution source to the respiratory chamber (Fig. 4A) via a variable speed medium flow peristaltic pump (Cat No. 73160–32, Cole-Parmer, Vernon Hills, IL) set to a flow rate of 20 ml/min (tubing of 127 mm length and 4.8 mm inner diameter that was capable of an adjustable range of 17.0–60.0 ml/min was used). Tadpoles were held in place using non-toxic modeling clay (Van Aken International, Rancho Cucamonga, CA). To create a recirculating system, the respiratory chamber was then placed into a shallow plexiglass overflow tray, which drained back into the anesthetic source solution. In order to maintain a constant fluid level, wicks cut from Whatman filter paper were used to pull solution out of the respiratory chamber.
Imaging
All images were taken using a Zeiss Axioplan microscope. In order to protect the microscope and to drain the overflow of anesthetic solution, a custom overflow tray was built out of plexiglass to fit precisely onto the microscope stage. Tadpoles were placed into the respiratory chamber as previously described, and the respiratory chamber was placed into the overflow tray. To provide a consistent optical surface for imaging, a coverslip was then placed on the pedestals with small pieces of clay. The top of the coverslip was treated with Rain-X Original Glass Treatment (Rain-X, Huston, TX) to create a hydrophobic surface to prevent anesthetic solution from spilling over the top of the coverslip. Images were taken automatically at 5 minute intervals using a SPOT Flex camera and the SPOT Advanced software (Diagnostic Instruments, Sterling Heights, MI). A shutter was used to control the light source via the TTL output on the SPOT Flex camera, so that the animal was only exposed to light while the image was being captured. For hindlimb imaging, a motorized stage (Prior Scientific, Rockland, MA) was used to take multiple focal planes, which were then flattened into a single image using Helicon Focus (Helicon Soft Ltd). During imaging sessions, heart rates were occasionally checked to monitor the health of the animal. This was accomplished by switching to a lower power objective (2.5x) during the interval between captured images. Time-lapse movies (Supplemental Movies 1 and 2) were generated in ImageJ (NIH, Bethesda, MD).
Supplementary Material
Movie of left hindlimb development. Relative time is denoted in hours:minutes. Frame rate is 21 frames per second. Scale bar = 250 μm
Movie of a mitotic division in cornea epithelium. Relative time is denoted in hours:minutes. Frame rate is 7 frames per second. Scale bar = 20 μm
Main Points:
Constant flow of anesthetic solution over tadpoles permits efficient aeration and greatly increases survival time in diluted MS-222.
Anesthetic flow permits continuous live imaging of Xenopus tissues for at least 48 hours.
Addition of a stable optical window allows for high quality imaging.
We demonstrate applications of this technique by imaging hindlimb development for 48 hours, as well as show mitotic division and cell migration obtained from transgenic animals expressing fluorescently tagged proteins.
Acknowledgements
The authors would like to thank A. G. Thomas for his help checking heart rates, and K. J. Perry for her work generating the transgenic mG-HGEM construct. Additionally, the authors would like to thank Dr. Jonathan Slack (University of Minnesota) for providing the HGEM construct, and Dr. John Wallingford (University of Texas at Austin) for providing the mG construct. This work was supported by NIH-NEI, EY09844.
Grant support: NIH-NEI, EY09844
References
- Beck CW, Slack JW. 2001. An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol 2:reviews1029.1-reviews1029.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JMW. 2003. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell 5:429–439. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisúa Belmonte JC, Christen B. 2009. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn 238:1226–1248. [DOI] [PubMed] [Google Scholar]
- Boutilier RG, Shelton G. 1986. The effects of forced and voluntary diving on ventilation, blood gases and pH in the aquatic amphibian, Xenopus laevis. J Exp Biol 122:209–222. [DOI] [PubMed] [Google Scholar]
- Cakir Y, Strauch SM. 2005. Tricaine (MS-222) is a safe anesthetic compound compared to benzocaine and pentobarbital to induce anesthesia in leopard frogs (Rana pipiens. Pharmacol Rep 57:467–474. [PubMed] [Google Scholar]
- Carter KM, Woodley CM, Brown RS. 2011. A review of tricaine methanesulfonate for anesthesia of fish. Rev Fish Biol Fisheries 21:51–59. [Google Scholar]
- Cornish IME, Moon TW. 1986. The glucose and lactate kinetics of American eels, Anguilla rostrata (LeSueur), under MS 222 anaesthesia. J Fish Biol 28:1–8. [Google Scholar]
- Danilchik MV. 2011. Manipulating and imaging the early Xenopus laevis embryo. Methods Mol Biol 770:21–54. [DOI] [PubMed] [Google Scholar]
- Emilio MG, Shelton G. 1974. Gas exchange and its effect on blood gas concentrations in the amphibian, Xenopus laevis. J Exp Biol 60:567–579. [DOI] [PubMed] [Google Scholar]
- Guénette SA, Giroux MC, Vachon P. 2013. Pain perception and anaesthesia in research frogs. Exp Anim 62:87–92. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. 1987. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev Biol 124:200–214. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Wever JA, Veragara MN, Fukui L. 2008. Xenopus, An Ideal Vertebrate System for Studies of Eye Development and Regeneration In Animal Models for Eye Research (Ed. Tsonis PA), pp. 57–92. Academic Press, Massachusetts. [Google Scholar]
- Hou PC, Burggren WW. 1995. Blood pressures and heart rate during larval development in the anuran amphibian Xenopus laevis. Am J Physiol 269:R1120–1125. [DOI] [PubMed] [Google Scholar]
- Keller RE. 1978. Time-lapse cinemicrographic analysis of superficial cell behavior during and prior to gastrulation in Xenopus laevis. J Morphol 157:223–247. [DOI] [PubMed] [Google Scholar]
- Kieserman EK, Lee C, Gray RS, Park TJ, Wallingford JB. 2010. High-magnification in vivo imaging of Xenopus embryos for cell and developmental biology. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5427. [DOI] [PubMed] [Google Scholar]
- Lalonde-Robert V, Beaudry F, Vachon P. 2012. Pharmacologic parameters of MS222 and physiologic changes in frogs (Xenopus laevis) after immersion at anesthetic doses. J Am Assoc Lab Anim Sci 51:464–468. [PMC free article] [PubMed] [Google Scholar]
- Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre CB, Gwaltney-Bran S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. 2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Available at: http://www.avma.org/kb/policies/documents/euthanasia.pdf. [Google Scholar]
- Mimoto MS, Christian JL. 2011. Manipulation of gene function in Xenopus laevis. Methods Mol Biol 770:55–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. 1956. Normal table of Xenopus laevis (Daudin). North-Holland Publishing Company, Amsterdam. [Google Scholar]
- Perry KJ, Thomas AG, Henry JJ. 2013. Expression of pluripotency factors in larval epithelia of the frog Xenopus laevis: evidence for the presence of cornea epithelial stem cells. Dev Biol 374:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic NT, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Berakovic AP, Klobucar RS. 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol 28:553–564. [Google Scholar]
- Sakaguchi DS, Janick LM, Reh TA. 1997. Basic fibroblast growth factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: generation of retinal neurons and glia. Dev Dyn 209:387–398. [DOI] [PubMed] [Google Scholar]
- Schaefer JJ, Oliver G, Henry JJ. 1999. Conservation of gene expression during embryonic lens formation and cornea-lens transdifferentiation in Xenopus laevis. Dev Dyn 215:308–318. [DOI] [PubMed] [Google Scholar]
- Slack J 1984. Embryonic development. Cell communication in early embryos. Nature 311:107–108. [DOI] [PubMed] [Google Scholar]
- Slack JM, Lin G, Chen Y. 2008. The Xenopus tadpole: a new model for regeneration research. Cell Mol Life Sci 65:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Fairclough L, Latinkic BV, Sparrow DB, Mohun TJ. 2006. Xenopus laevis transgenesis by sperm nuclear injection. Nat Protoc 1:2195–2203. [DOI] [PubMed] [Google Scholar]
- Soivio A, Nyholm K, Huhti M. 1977. Effects of anaesthesia with MS 222, neutralized Ms 222 and benzocaine on the blood constituents of rainbow trout (Salmo gairdneri). J Fish Biol 10:91–101. [Google Scholar]
- Sun P, Zhang Y, Yu F, Parks E, Lyman A, Wu Q, Ai L, Hu CH, Zhou Q, Shung K, Lien CL, Hsiai TK. 2009. Micro-electrocardiograms to study post-ventricular amputation of zebrafish heart. Ann Biomed Eng 37:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi C, Sakamaki K, Morita H, Hara Y, Suzuki M, Kinoshita N, Ueno N. 2013. Transgenic Xenopus laevis for live cell imaging in cell and developmental biology. Dev Growth Differ 55:422–433. [DOI] [PubMed] [Google Scholar]
- Tschumi PA. 1957. The growth of the hindlimb bud of Xenopus laevis and its dependence upon the epidermis. J Anat 91:149–173. [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB. 2010. Low-magnification live imaging of Xenopus embryos for cell and developmental biology. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5425. [DOI] [PubMed] [Google Scholar]
- Warkman AS, Krieg PA. 2007. Xenopus as a model system for vertebrate heart development. Semin Cell Dev Biol 18:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie of left hindlimb development. Relative time is denoted in hours:minutes. Frame rate is 21 frames per second. Scale bar = 250 μm
Movie of a mitotic division in cornea epithelium. Relative time is denoted in hours:minutes. Frame rate is 7 frames per second. Scale bar = 20 μm


