Abstract
Glycated hemoglobin A1c (A1C) levels have traditionally been the gold standard for assessing glycemic control and treatment efficacy in patients with type 2 diabetes. However, A1C does not take into account fluctuations in blood glucose levels known as glycemic variability (GV). In recent years, GV has become increasingly clinically relevant, because of a better understanding of the need to reach target A1C while avoiding hypoglycemia. GV relates to both hyperglycemia and hypoglycemia, and has been associated with poorer quality of life. Diabetes treatments targeting multiple pathophysiological mechanisms are most beneficial in controlling A1C and reducing GV. In clinical trials, a number of metrics are used to measure GV, many of which are not well understood in the clinical practice. Until a gold standard metric for GV is established, the variety of measurements available may confound the choice of an optimal treatment for an individual patient.
INTRODUCTION
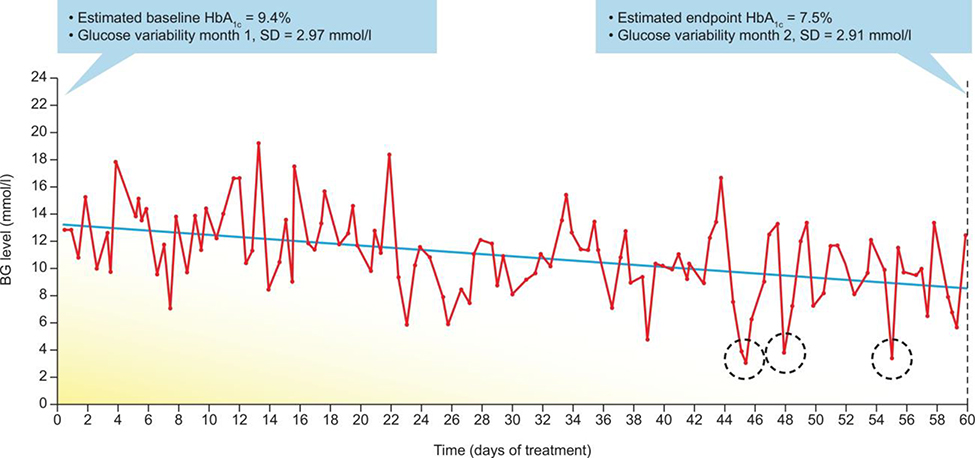
While the measurement of glycated hemoglobin A1c (A1C) is considered the gold standard for assessing glycemic control in patients with diabetes, this measure does not take into account fluctuations in blood glucose levels known as glycemic variability (GV). Optimization of glycemic control requires a careful balance that allows patients to reach target A1C while avoiding hypoglycemia.1 In recent years, the importance of considering GV, when optimizing treatment regimens has been receiving increasing interest from researchers and physicians. Fluctuations in blood glucose can produce within-day (intra-day) and day-to-day (inter-day) variations, which can increase both the risk of hypoglycemia (particularly at target A1C levels or lower) and glycemic excursions into the hyperglycemic range (particularly at higher A1C levels (Figure 1).2, 3 However, regardless of reduction in A1C levels, blood glucose fluctuations can still be present and, if not attenuated concurrently with A1C, can contribute to hypoglycemic episodes (Figure 1). There is evidence to suggest that GV can have an impact on patients’ psychological well-being and quality of life (QoL),4, 5 and furthermore, improvements in GV with diabetes treatment result in improved QoL measures.6
FIGURE 1.
Risk of hypoglycemia and improved glycemic control. Self-monitored blood levels of glucose recorded over 60 days. A downward trend in blood levels of glucose is evident; levels of HbA1c (estimated by the use of a linear formula) decreased from 9.4% at baseline to 7.5% by the end of the observation period. However, glucose variability remained relatively unchanged from the first to the second month of observation, which resulted in 3 hypoglycemic episodes (<3.9 mmol/L) registered by self-monitoring blood glucose at days 45, 48 and 55 (dotted circles). Reproduced with permission from Springer Nature: Nature Reviews Endocrinology, Metrics for glycaemic control—from HbA1c to continuous glucose monitoring, Kovatchev, 2017.3Abbreviations: HbA1c, hemoglobin A1c; SD, standard deviation
EFFECTS OF GV ON PATIENTS
Glycemic Control
The relationship between GV and hypoglycemia has been elucidated in a number of studies, with hypoglycemia being more common in patients with increased GV.8, 9 In a pooled analysis of 6 randomized controlled trials of 1,699 adult patients with T2D who received 24 weeks of insulin glargine or comparators, all measures of GV were significantly associated with poor glycemic control (A1C ≥ 7.0%) and on-trial development of hypoglycemia.10 In addition, a reduction in GV correlated strongly with reductions in both hypoglycemia and hyperglycemia episodes.11 Furthermore, it has been shown that intra-day GV is associated with an increased risk of overall and nocturnal hypoglycemia.12 Given that the risk of hypoglycemia increases with treatment intensification,9 it is useful to understand that hypoglycemia can be predicted based on both intra-day and inter-day GV in insulin-treated patients.8, 13,14
Both postprandial hyperglycemia and fasting hyperglycemia contribute to overall hyperglycemia and A1C levels, with postprandial glucose (PPG) being the main contributor to hyperglycemia at A1C levels closer to control (A1C < 7.0%).15 Although, basal insulin therapy is effective in reducing fasting plasma glucose (FPG) and preprandial blood glucose levels, Riddle et al showed that insulin glargine also reduces PPG.16 In addition, the FLuctuATion reduction with inSUlin and Glp-1 Added togetheR (FLAT-SUGAR) trial showed that the use of a glucagon-like peptide-1 receptor agonist (GLP-1 RA), rather than prandial insulin, may have a greater effect in reducing GV while maintaining similar A1C levels.17 The FLAT-SUGAR trial enrolled 102 patients with T2D, with increased risk for cardiovascular disease, and with A1C between 7.5% and 8.5%.17 After an initial run-in period on metformin and basal-bolus therapy with rapid-acting insulin, patients were randomized to either continue with basal-bolus therapy or change to metformin and basal insulin plus a twice-daily GLP-1 RA (exenatide). Continuous glucose monitoring (CGM) was used to assess GV. At 26 weeks, and despite similar A1C levels being attained in both treatment groups, the coefficient of variation (CV) of glucose measurements (a measure of variation in glucose levels around the mean level)18 was significantly reduced in patients treated with basal insulin plus a GLP-1 RA compared with those who received basal- bolus therapy.17 A range of other GV metrics also showed greater reductions in the basal insulin plus GLP-1 RA group. This study demonstrates that although different diabetes treatments may reduce A1C to a similar extent, their effectiveness in reducing GV can differ significantly.17Furthermore, even patients with optimal A1C control can experience significant daily fluctuations in blood glucose levels,19 and high rates of GV have been observed in patients with T2D, despite management with diet and exercise, oral antidiabetes drugs (OADs) and insulin therapy.20
Glucose excursions can differ from patient to patient, with factors such as gender and current treatment strategies being known to influence GV in patients with T2D.21 For example, younger age and a higher body mass index can contribute to wider glucose fluctuations. Moreover, patients on intensive therapy (triple combination or insulin therapy) and with higher A1C levels are at a greater risk of experiencing extreme glucose excursions than those with lower A1C levels.21
Diabetes-Related Complications
GV has also been shown to be associated with markers of endothelial and cardiovascular damage—even in patients with diabetes of short duration accompanied by optimal glycemic control.22 In patients with T2D and acute myocardial infarction, GV has been shown to predict mortality,23 with increased risk of mortality being observed in patients with increased visit-to-visit GV.24
Cardiovascular risk factors in T2D have been shown to be directly related to PPG levels.25 The beneficial cardiovascular effects of diabetes therapy may relate to the type of therapeutic agent used, with GLP-1 RAs, in particular, having a PPG-lowering effect.26 GLP-1 RAs, as well as sodium glucose cotransporter 2 inhibitors, have demonstrated significant improvements in GV.27 In people with T2D, high GV has been associated with cognitive impairment.28, 29 Furthermore, it has been demonstrated that diabetes treatment with any class of drug that reduces GV, such as dipeptidyl peptidase (DPP-4) inhibitors, may have an associated protective benefit on cognition in older patients with T2D.30
Mortality
Recently, the link between GV and the occurrence of severe hypoglycemia and subsequent mortality has been highlighted by secondary analyses of data from the double-blind Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Patients with Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE). These analyses show that patients with doubled day-to-day fasting GV have an increased risk of severe hypoglycemia and all-cause mortality,31 and that those who experience severe hypoglycemia have a more than two- fold higher risk of all-cause mortality and cardiovascular death, even after adjusting for potential confounding factors.32
QoL
Several studies have demonstrated that GV has implications for patients’ psychological well-being and QoL. In insulin-treated patients, high GV has been associated with poor QoL, particularly in response to treatment-related and diabetes-related emotional problems.4, 5 Increased GV has also been associated with increased length of hospital stays among inpatients with diabetes.33 In addition, sleep and nocturnal respiratory disturbances have been shown to be related to disturbances in glucose metabolism.34 Nocturnal hypoglycemia was detected in 39% of 24-hour CGM readings taken from 83 insulin-treated elderly people (65–80 years of age) over a 6-month period.35 Conversely, when diabetes treatment is associated with the perception of reduced GV, there is a corresponding improvement in QoL measures, such as work productivity, absenteeism and treatment satisfaction.6
HOW TO MEASURE GV
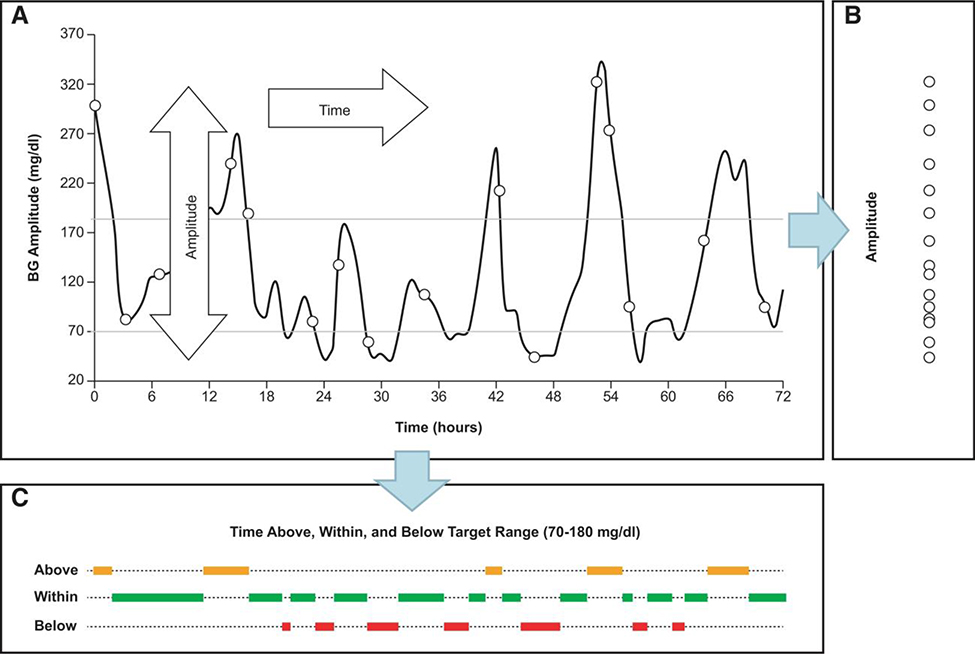
With increasing interest in the clinical importance of GV in T2D, there are a growing number of publications describing the metrics for assessing GV. This wealth of information is often confusing for both physicians and patients,7 with physicians being faced with as many as 20 different metrics for assessing GV.36 The lack of a uniformly accepted standard of measurement (a gold standard) for GV poses a challenge for those who want to compare studies or use this information in clinical practice. While detailed accounts of available GV metrics are presented elsewhere,3 here, we emphasize 2 principal measures: the amplitude of glucose excursions and time spent outside a target range (i.e., time spent in the hypoglycemic or hyperglycemic ranges) (Figure 2).37
FIGURE 2.
Principal components of GV. Glucose fluctuations are a process in time that has 2 dimensions—amplitude and time (A). Projected along its amplitude axis, this process is measured by metrics such as SD or MAGE (B). Projected along its time axis, this process is assessed by temporal characteristics, such as time within target range and time spent in hypoglycemia or hyperglycemia (C). Reproduced with permission from Kovatchev et al.37 Glucose variability: timing, risk analysis and relationship to hypoglycemia in diabetes, American Diabetes Association, 2016. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association. Abbreviations: BG, blood glucose; MAGE, mean amplitude of glycemic excursions; SD, standard deviation.
Amplitude of GV
The traditional approach to measuring GV consists of assessing the amplitude of glycemic excursions (Figure 2),37 which relies on self-monitored blood glucose (SMBG) data or on CGM. Some of the key metrics for GV amplitude are summarized in Table 1.18,38, 39, 40 Mean amplitude of glycemic excursions was the first to be developed, primarily to capture mealtime-related glucose excursions,41 and has been used widely for assessing GV.38, 42 While most physicians in clinical practice are familiar with the use of standard deviation (SD; total SD, intra-day SD and inter-day SD),7 GV-related research utilizes CV (which is the SD divided by the mean)18 as the preferred amplitude measure.41 CV is a metric related to mean blood glucose, and it is easier to describe hypoglycemic excursions using CV (compared with using SD alone) as GV is significantly influenced by mean blood glucose.18, 41 A recent international consensus statement for CGM recommends that when measuring GV, CV should be used as the primary measure, with SD as a secondary measure because of its familiarity to physicians.18
TABLE 1.
Metrics used to describe GV parameters.
| Metric | Description |
|---|---|
| Amplitude of GV (temporal resolution range: hours to days) | |
| MAGE | Diabetes-specific metric of the amplitude of glucose excursions. Mean of glycemic excursions from nadir to peak blood glucose level and vice versa that are >1 SD of blood glucose mean (it takes into account glycemic peaks and nadirs occurring daily, but does not account for the total number of fluctuations; it depends on sampling frequency; ambiguity as to where peaks and nadirs begin and end)38 |
| SD | Variation around the mean blood glucose (intra-day or inter-day)2 |
| CV = SD/mean | Magnitude of variability relative to mean blood glucose18, 39 |
| LBGI | Measure of frequency and magnitude of hypoglycemia (amplifies hypoglycemic excursions without accounting for hyperglycemia)13 |
| HBGI | Measure of frequency and magnitude of hyperglycemia (amplifies hyperglycemic excursions without accounting for hypoglycemia)40 |
| Timing of GV (based on CGM; temporal resolution range: minutes to hours) | |
| Time within, above or below target range | Quantitative measure of time spent in an individual’s target glucose range, time spent below this range, and time spent above this range. All values are needed to provide an assessment of overall glycemic control18 |
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation; GV, glycemic variability; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude of glycemic excursions; SD, standard deviation.
Other important aspects to consider, when measuring the amplitude component of GV is that the hyperglycemic range is much broader than the hypoglycemic range, and that the risk for hyperglycemia and hypoglycemia are clinically independent.37 Therefore, a 20 mg/dL decline in blood glucose in the hypoglycemia range (e.g., a drop from 70 to 50 mg/dL) is clinically more relevant than a 20 mg/dL rise in the hyperglycemia range (e.g., an increase from 160 to 180 mg/dL).37 Consequently, other metrics (such as low blood glucose index and high blood glucose index) that specifically assess hypoglycemia and hyperglycemia, respectively, may be more useful measures in this regard.3
Timing of Glucose Excursions
Including time as an additional component of GV results in greater complexity, which may be a reason why metrics relying on time are not widely used in clinical practice. However, with the increasing availability of CGM in recent years, such time-related GV metrics have become more well-known.7 Perhaps, the most clinically important of these metrics is the measure of time the patient spends within, above and below the target blood glucose range (Figure 2).37 Time in targeted blood glucose range (TIR) provides valuable information on the level of glycemic control, but gives only a partial picture of overall glycemic control18; time spent outside of the target range (both below and above target) also needs to be assessed separately, because they show different trends toward hypoglycemia and hyperglycemia, respectively, which is important information for therapy adjustment.18Clinically, an assessment of TIR can help patients understand how hypoglycemia or hyperglycemia improves with treatment over time. If the amount of time spent in hypoglycemic or hyperglycemic ranges is categorized into “action levels,” it can guide the urgency and degree of clinical response required.18 Reflecting this, a recent international consensus statement on interpretation of CGM data recommends that time spent outside of the target range be assessed and reported as a key diabetes control metric in clinical studies.18 With an increase in the use of CGM devices in the management of T2D, these metrics may also become increasingly important in routine practice.
CGM Devices
There are 3 basic types of CGM devices: “real-time” CGM devices, which continuously track glucose concentrations in the interstitial fluid; intermittently viewed CGM devices, which show continuous glucose measurements retrospectively at the time the patient or physician checks the data; and diagnostic CGM, which the patient is blinded to, and is intended to inform the physician about the patient’s blood glucose levels in their day-to-day lives.18, 43 All 3 types of CGM provide detailed information about glucose variability. While both real-time and intermittently viewed CGM devices monitor the TIR, real-time CGM can also warn users in real-time if their blood glucose is trending toward the hypoglycemic or hyperglycemic ranges.18 Various CGM devices of these types are commercially available, each of which has certain advantages and disadvantages. Overall, the new-generation CGM devices are becoming simpler and less expensive to use. For example, the FreeStyle Libre “flash” CGM device (Abbott Diabetes Care) is associated with lower daily costs compared with other systems, and does not require daily finger pricks for calibration with a SMBG device. However, this is an intermittently viewed CGM device, with data being stored and downloaded later, and so it does not provide hypoglycemia or hyperglycemia alarms.44 Another example, Dexcom’s CGM system, is a real-time device which can communicate directly with patient, care-giver and physician smart devices, and can send high and low blood glucose alerts. While the older versions of this device needed to be calibrated against SMBG at least twice a day; the new G6 generation is now finger-prick free using a factory calibration.45 Finally, a diagnostic CGM system, such as the iPro CGMS (Medtronic Diabetes, Northridge, CA)43 is capable of sensing blood glucose at 5-minute intervals throughout the course of the day, and is used over a 3-day period to obtain information regarding blood glucose levels. Patients are advised to maintain a log of daily activities, such as mealtimes and therapy administration. Patients are unable to view their blood glucose values during the time of recording, however, this information is downloaded later to provide a report to the physician regarding the 3-day time period. This data, alongside the patient log, can provide valuable information regarding the effectiveness of the patient’s diabetes management. Currently, CGM, used in conjunction with A1C monitoring, is recommended for determining glycemic status and as a basis for adjusting therapy in all patients with type 1 diabetes and certain patients with T2D, such as those failing to achieve target A1C on intensive insulin therapy (particularly if the patient has significant hypoglycemia).18
In general, at this time, uptake of CGM systems for T2D in primary care is low; however, as CGM system technology continues to improve and simplify, the assessment of GV may become more widespread, ultimately being used alongside A1C monitoring as a means of helping patients with T2D to achieve optimal glycemic control. For instance, the information provided by diagnostic CGM devices43 could assist the physician and patient to make informed choices regarding therapy and lifestyle modification. It is important to bear in mind that while CGM provides the most comprehensive overview of GV, there are a number of relevant GV metrics that can also be obtained from SMBG measurements as discussed above, and can be used to assess glycemic control and potentially guide treatment decisions.
EFFECTS OF DIABETES MEDICATIONS ON GV
Given the impact of PPG on intra-day GV, treatments that have effects on dysglycemia beyond reductions in A1C and FPG (such as DPP-4 inhibitors and GLP-1 RAs)46 may have potential benefits in patients with T2D. In comparative clinical studies, significant reductions in GV have been demonstrated with the GLP-1 RAs exenatide, liraglutide and lixisenatide when added to background therapy of oral antidiabetes drugs or high-dose basal-bolus therapy (Table 2).47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 The DPP-4 inhibitors sitagliptin53 and vildagliptin54significantly reduced GV compared with glimepiride and pioglitazone, respectively, in patients with T2D not adequately controlled on metformin monotherapy,53, 54 while saxagliptin showed no difference in GV reductions compared with the alpha-glucosidase inhibitor acarbose,52 which has known PPG-lowering effects (Table 2).47, 48,49, 50, 51, 52, 53, 54, 55, 56, 57, 58
TABLE 2.
Effects of antidiabetes drugs on glycemic variability in patients with type 2 diabetes.47, 48, 49, 50, 51, 52, 53, 54,55, 56, 57, 58
| Drug class | Study design (duration) | Comparator | Results |
|---|---|---|---|
| Drug | |||
| Alpha glucosidase inhibitor | |||
| Acarbosea | Double-blind, RCT (1 week) | Placeboa | Decrease in intra-day GV, especially postprandial variability with acarbose vs. placebo, but no significant change in inter-day GV51 |
| DPP-4 inhibitor | |||
| Saxagliptinb | RCT (1 year) | Acarboseb | Both saxagliptin and acarbose decreased GV to a similar extent. Greater reductions in A1C and FBG with saxagliptin vs. acarbose52 |
| Sitagliptinc | Double-blind, RCT (4 weeks) | Glimepiridec | Both sitagliptin and glimepiride reduced A1C, however, RCT significant reduction observed in the amplitude of GV (MAGE) with sitagliptin, but not with glimepiride. No difference in SD (4 weeks) between treatment groups53 |
| Vildagliptind | OL, RCT (16 weeks) | Pioglitazoned | Both vildagliptin and pioglitazone significantly reduced A1C and mean plasma glucose, but reduction in GV was only seen with vildagliptin54 |
| GLP-1 RA | |||
| Liraglutidee | OL, RCT (6 months) | Insulin uptitrationf | Addition of liraglutide to intensive high-dose (basal-bolus) insulin therapy resulted in significantly greater reductions in A1C and GV vs. insulin therapy alone48 |
| Sodium glucose co-transporter 2 inhibitor | |||
| Dapagliflozinf | OL, RCT (20 weeks)g | Saxagliptin | Dapagliflozin was associated with a significant reduction in mean glucose levels and in indicators of hypoglycemia during Ramadan. However there were no significant differences between dapagliflozin and saxagliptin in GV pre-Ramadan and during Ramadan55 |
| Rapid-acting basal insulin analogs | |||
| Insulin aspart, glulisine or lispro | OL, RCT (26 weeks) | Exenatide | Reduced coefficient of variation with metformin + insulin glargine + exenatide compared with metformin + insulin glargine + RAI. Other GV indices showed nonsignificant trends toward improvement with exenatide17 |
| Insulin lispro | Post hoc | Insulin glargine | Premixed insulin glargine plus insulin lispro was associated with reduced glycemic variability in metformin treated patients compared with insulin glargine alone60 |
| Long-acting basal insulin analogs | |||
| Insulin detemirh | OL, RCT (52 weeks) | Insulin glarginei | Both insulins resulted in clinically important reductions in A1C, with no significant difference between insulin detemir and insulin glargine in within-subject variability for self-monitored FPG and premeal prandial glucose, or risk of hypoglycemia56 |
| Insulin glargine | OL, RCT (2 years) | Standard care | Insulin glargine reduced PPG excursions. but did not increase risk of hypoglycemia, and was associated with similar SD and MAGE at 2 years compared with standard care57 |
| Combination | |||
| IDegLirag | Post hocanalysis of RCT (52 weeks) | Insulin degludec orliraglutide aloneg | IDegLira treatment reduced glucose fluctuations andpostprandial glucose excursions compared to insulin degludec58 |
| iGlarLii | Post hocanalysisof RCT (30 weeks) | Insulinglarginealonei | iGlarLixi treatment (vs. insulininsulin glargine) significantly reduced GV compared to insulininsulin glargine47 |
Acarbose or placebo, both added to existing medication of metformin and vildagliptin.
Saxagliptin or acarbose, both added to existing metformin.
Sitagliptin or glimepiride, both added to existing metformin.
Vildagliptin or pioglitazone, both added to existing metformin.
Liraglutide plus high-dose basal-bolus insulin vs. insulin uptitration alone in patients requiring >100 units of insulin/day, with or without metformin.
Dapagliflozin or saxagliptin, both in addition to metformin.
IDegLira or insulin degludec or liraglutide alone, all in addition to OADs.
Insulin detemir or insulin glargine, both as add-on to OADs.
iGlarLixi or insulin glargine alone, in patients previously on basal insulin ± OADs. Abbreviations: CV, coefficient of variation; DPP-4, dipeptidyl peptidase-4; FBG, fasting blood glucose; FPG, fasting plasma glucose; MAGE, mean amplitude of glycemic excursions; OAD, oral antidiabetes drug; OL, open-label; PPG, postprandial glucose; RAI, rapid acting insulin; RCT, randomized controlled trial; SD, standard deviation.
Rapid acting insulin analogs such as insulin lispro, aspart and glulisine may be used to reduce GV by minimizing periods of acute hyperglycemia, while being associated with lower rates of hypoglycemia than regular human insulin.59 In patients treated with metformin and basal-bolus insulin, those who swapped the rapid acting insulin component for a GLP-1 RA had significantly reduced CV of glucose compared with those who continued with original therapy. Other GV indices were not significantly different between treatment groups.17 In metformin-treated patients, a premixed basal insulin glargine and rapid-acting insulin lispro was shown to result in reduced GV compared with insulin glargine alone.60
Unlike GLP-1 RAs, the main glycemic effect of basal insulin is via its influence on A1C and FPG levels. So, unsurprisingly, basal insulin analogs do not have a pronounced impact on intra-day GV, with insulin glargine having a similar effect to metformin therapy (standard care group) on GV and risk of hypoglycemia.57 Similarly, insulin detemir did not differ in terms of effect on GV compared with insulin glargine (Table 2).47, 48, 49, 50, 51, 52, 53,54, 55, 56, 57, 58
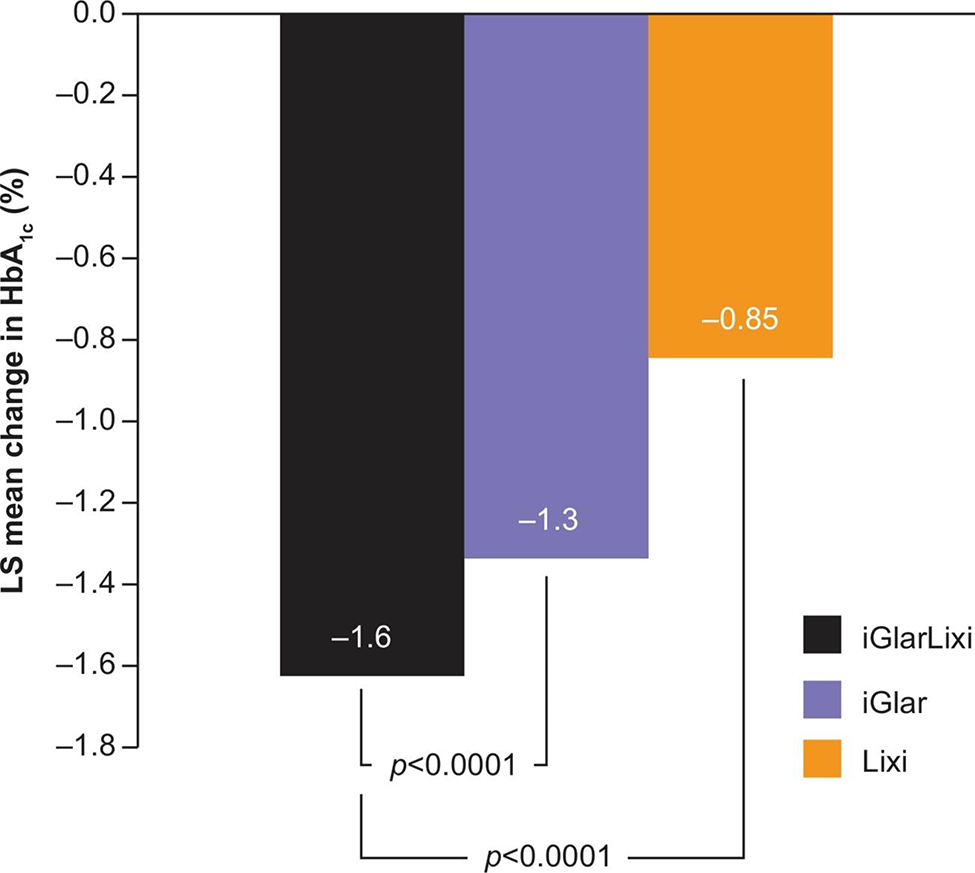
Given the complementary mechanisms of action of insulin and incretin mimetics, titratable fixed-ratio combination regimens consisting of basal insulin and a GLP-1 RA have been developed. iGlarLixi, a titratable fixed-ratio combination of insulin glargine plus lixisenatide resulted in greater improvements in overall glycemic control, and thereby, A1C levels, than the components alone in patients with suboptimal glycemic control despite oral and/or basal insulin background therapy (Figure 3).61, 62 In addition, iGlarLixi resulted in significant reductions in multiple GV metrics compared with insulin glargine alone.47In contrast, IDegLira, the combination of insulin degludec and liraglutide, yielded no significant differences in GV (as measured by SD) after 52 weeks of treatment compared with either insulin degludec or liraglutide alone.58 The difference in GV-lowering effect in the 2 trials likely reflects the fact that short-acting GLP-1 RAs such as lixisenatide delay gastric emptying, and thus have greater effects on PPG levels than longer-acting agents such as liraglutide.63
FIGURE 3.
Change in A1C with the iGlarLixi fixed ratio combination. Reproduced with permission from Rosenstock et al.61 Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial, American Diabetes Association, 2016. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association. LS mean change in HbA1c. Data are mean ± SD. Abbreviations: iGlar, insulin glargine; iGlarLixi, insulin glargine/lixisenatide; LS, least squares; lixi, lixisenatide; mITT, modified intent-to-treat.
SIGNIFICANCE OF GV IN CLINICAL PRACTICE
GV is emerging as an important metric to consider when assessing glycemic control in clinical practice. Although it remains controversial,7 some evidence has suggested that GV, especially in the hyperglycemic range, is associated with an increased risk of microvascular and macrovascular complications24, 64,65 linked to glucose peaks, fluctuations in oxidative stress and endothelial dysfunction. Specifically, some evidence points to a potential link between GV and the development of complications such as diabetic peripheral neuropathy,66cardiovascular autonomic neuropathy67 and stroke.68 Increased GV has also been associated with poor control and increased risk of hypoglycemia in patients with types 1 and 2 diabetes. Minimizing GV, especially PPG excursions, is an important aspect of overall glycemic management, and a primary barrier to optimal diabetes control. Patients should be evaluated for treatment on a case-by-case basis, ensuring that the selection of diabetes medication and blood glucose targets are likely to benefit the individual, while also reducing the risks of hypoglycemia.9 Emerging research suggests that the addition of an incretin mimetic with prominent PPG-lowering effects to basal insulin (such as a GLP- 1 RA)69 could help patients achieve optimal glycemic control while minimizing the potential for hypoglycemia.
Importantly, sulfonylureas have been associated with significant GV, and can lead to an increase in hypoglycemia and mortality.70 With the availability of CGM, it is now possible to rapidly assess glucose fluctuations and relate their dynamics to clinically relevant outcomes. Importantly, the reduction of GV, other than having an impact on clinical outcomes, is also associated with improved QoL and treatment satisfaction, and therefore likely to result in improved treatment persistence. While there is a multitude of evidence to suggest that GV is an important metric in clinical practice, the lack of studies directly measuring the impact of therapeutic interventions on minimizing GV and resultant outcomes enforces that there is a need for additional research to validate GV as a marker of risk factors for long-term complications. Fortunately, such efforts are under way and results should be expected shortly.
CLINICAL BARRIERS TO GV ASSESSMENT
Despite the potential advantages of measuring GV, there are barriers to its use in real-world clinical practice. One major factor is likely to be costs and reimbursement, both in terms of the technology involved and the additional time needed from physicians and other healthcare providers and support staff.71, 72, 73
Coverage of CGM by insurance companies is variable; around a quarter have positive coverage policies for T2D patients on insulin, with variable eligibility criteria.74 CGMs are usually considered “durable medical equipment” (DME), and are subject to the same deductibles and copays as other types of DME. Medicare and Medicaid cover only those CGM devices approved by the U.S. Food and Drug Administration as a replacement for fingerstick blood glucose testing for diabetes treatment decisions (i.e., “therapeutic” use), and only if patients are receiving intensive insulin therapy and require frequent insulin adjustment based on at least 4 blood glucose checks per day.74, 75 At the time of writing, this includes only the Dexcom G5, which is being replaced by the calibration-free Dexcom G6 (available as of June 2018, and currently under review by Medicare), and Abbott’s FreeStyle Libre. These devices educate patients on the impact of food on GV and allow patients and physicians to understand if they run on the high or low side of the average glucose range associated with a given A1C, and would have a similar impact on patients only administering oral therapies. Eligible patients who are covered by Medicare pay 20% of costs for CGM systems, although this additional amount may be covered by secondary insurance. As an example, the Abbott system, which is distributed through the pharmacy chain, is expected to have an annual reimbursement rate of $3000, with patients paying $600.75 Insurance limitations mean that patients who do not fit criteria for CGM coverage must either buy their own systems or rely on SMBG. Costs associated with personal CGM systems can be high; Abbott FreeStyle Libre costs in pharmacies ranged from around $70-$97 for the reader and $108-$159 for a month’s supply of sensors,76 while Dexcom G5 users typically spend around $2,500-$4,000 annually without insurance coverage.77 The newer Dexcom G6 has a longer sensor life than the G5 (10 days vs. 7 days), which should translate to lower costs for the newer version as a box of sensors costs the same for both systems but lasts longer overall with the G6. However, some patients have expressed concerns that the G6 sensor has a hard shut-off, while the FreeStyle Libre sensor recently received 14-day approval,78 with both modalities achieving calibration-free status. Currently, although such a process is “off label,” some self-pay patients extend sensor life by restarting their G5 sensors after 7 days, reducing out of pocket costs. This is not possible with the current G6 sensor.79 Even where CGM is a viable option for an individual patient, lack of experience or confidence on behalf of healthcare providers in interpreting data, together with a lack of support and/or reimbursement for the extra time required to train and monitor patients and interpret results, may impact GV assessment. At the time of writing, the Medicare physician fee for a participating physician working from his/her office to cover sensor placement, hook-up, calibration of monitor, patient training and printout of recording is $156.60 for physician-provided CGM (CPT code 95250, billable no more than once monthly), $56.16 for patient-provided CGM (CPT code 95249; billable only once over the time the patient owns the device) and $36.72 for analysis, interpretation and recording of CGM data by ambulatory CGM of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours (CPT code 95251, billable no more than once monthly).80 SMBG is also associated with considerable costs, not only in terms of reagent strips, but also lancets, meters, batteries and calibration.81, 82, 83 Insurance companies generally cover some, but not all related costs for people with diabetes; for example, Medicare-eligible patients pay 20% of the approved costs of meters, test strips and lancets plus deductibles.84 However, this may not cover multiple daily measurements, which limits the assessment of GV in these patients. Despite these limitations however, given the potential benefits, broadening more intensive monitoring of GV by CGM or increased SMBG for particular patients, such as those at risk of hypoglycemia and hyperglycemia, is warranted in primary care and is likely to be cost-effective in the long term.85, 86
CONCLUSIONS
For decades, A1C level has been the dominant metric in assessing glycemic control. The A1C level is used by physicians and patients to evaluate treatment responses and optimize diabetes therapy, and in clinical T2D research, it is the primary outcome of efficacy. However, A1C level has certain shortcomings as a measure of treatment benefit—the most prominent being its limited responsiveness to blood glucose fluctuations. As a result, glycemic goals set on lowering A1C alone could result in unbalanced treatment adjustments, potentially increasing the risk for hypoglycemia; GV has been shown to be associated with this risk. Fluctuations in blood glucose represented by GV metrics may provide a better predictor of such complications. Recommended metrics could include CV as a measure of overall GV, and low blood glucose index/high blood glucose index as metrics of the risk for hypoglycemia and hyperglycemia. Additionally, when CGM is used, TIR is a good measure of overall glycemic control. The measurement of GV using SMBG or CGM data has the potential to complement A1C data, and to provide a more comprehensive assessment of glycemic control in order to better inform treatment decisions.
ACKNOWLEDGMENTS
The contents of this article and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. The authors took responsibility for the writing of this manuscript, including critical review and editing of each draft, and approval of the submitted version. The authors received writing/editorial support in the preparation of this manuscript, provided by Georgina Bowden, PhD, and Ila Karve, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Author Disclosures: G.E.U. is partially supported by the Atlanta Clinical and Translational Science Award Program NIH/NATS UL1 TR002378 and 1P30DK111024–01 from the National Institutes of Health and National Center for Research Resources, has served on advisory boards for Sanofi, Merck and Intarcia and has received grant support (to Emory University) from Sanofi, Merck, Boehringer Ingelheim, Astra Zeneca, and Novo Nordisk
B.P.K. is partially supported by grants from the National Institutes of Health/NIDDK and by the University of Virginia project Precision Individualized Medicine for Diabetes; has served on an advisory panel for Sanofi, had speaking engagements for Dexcom and Sanofi; received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Sanofi, and Tandem.
Contributor Information
Guillermo E. Umpierrez, Division of Endocrinology, Diabetes, and Metabolism, Emory University School of Medicine, 69 Jesse Hill Jr Dr., Atlanta, GA 30303.
Boris P. Kovatchev, Center for Diabetes Technology, University of Virginia Health System, Charlottesville, Virginia.
REFERENCES
- 1.Cryer P Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188–2195. [DOI] [PubMed] [Google Scholar]
- 2.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol. 2017;13:425–436. [DOI] [PubMed] [Google Scholar]
- 4.Cox DJ, McCall A, Kovatchev B, et al. Effects of blood glucose rate of changes on perceived mood and cognitive symptoms in insulin-treated type 2 diabetes. Diabetes Care. 2007;30:2001–2002. [DOI] [PubMed] [Google Scholar]
- 5.de Ranitz-Greven W, Beulens J, Biesma D, et al. Is higher glycemic variability in type 2 diabetes patients associated with reduced quality of life? Endocr Rev. 2015;36(Suppl 2):2015. [Google Scholar]
- 6.Genovese S, Tedeschi D. Effects of vildagliptin/metformin therapy on patient-reported outcomes: work productivity, patient satisfaction, and resource utilization. Adv Ther. 2013;30:152–164. [DOI] [PubMed] [Google Scholar]
- 7.Bergenstal RM. Glycemic variability and diabetes complications: does it matter? simply put, there are better glycemic markers!. Diabetes Care. 2015;38:1615–1621. [DOI] [PubMed] [Google Scholar]
- 8.Cox DJ, Gonder-Frederick L, Ritterband L, et al. Prediction of severe hypoglycemia. Diabetes Care. 2007;30:1370–1373. [DOI] [PubMed] [Google Scholar]
- 9.Tschope D, Bramlage P, Schneider S, et al. € Incidence, characteristics and impact of hypoglycaemia in patients receiving intensified treatment for inadequately controlled type 2 diabetes mellitus. Diabetes Vasc Dis Res. 2016;13:2–12. [DOI] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Umpierrez G, DiGenio A, et al. Association of measures of glycemic variability with glycemic control and hypoglycemic events. Diabetes. 2012;61(Suppl 1):A291. [Google Scholar]
- 11.Jangam S, Hayter G, Dunn T. Reduction in glycemic variability is correlated with reductions in both hypoglycemia and hyperglycemia risk in type 1 and type 2 subjects. Diabetes Technol Ther. 2016;18(Suppl 1):A42. [Google Scholar]
- 12.Bailey TS, Bhargava A, De Vries JH, et al. Within-day variability based on 9-point profiles correlates with risk of overall and nocturnal hypoglycemia in adults with type 1 (T1D) and type 2 diabetes (T2D). Diabetes. 2017;66(Suppl 1):A107. [Google Scholar]
- 13.Kovatchev BP, Cox DJ, Gonder-Frederick LA, et al. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21:1870–1875. [DOI] [PubMed] [Google Scholar]
- 14.Qu Y, Jacober SJ, Zhang Q, et al. Rate of hypoglycemia in insulintreated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14:1008–1012. [DOI] [PubMed] [Google Scholar]
- 15.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881–885. [DOI] [PubMed] [Google Scholar]
- 16.Riddle M, Umpierrez G, DiGenio A, et al. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care. 2011;34:2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FLAT-SUGAR Trial Investigators. Glucose variability in a 26-week randomized comparison of mealtime treatment with rapid-acting insulin versus GLP-1 agonist in participants with type 2 diabetes at high cardiovascular risk. Diabetes Care. 2016;39:973–981. [DOI] [PubMed] [Google Scholar]
- 18.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chon S, Lee YJ, Fraterrigo G, et al. Evaluation of glycemic variability in well-controlled type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon C, Rodenzo W, Agrawal P, et al. Glycometrics in 70,167 patients with type 2 diabetes derived from retrospective continuous glucose monitoring (CGM). Diabetes Technol Ther. 2016;18(Suppl 1):A28. [Google Scholar]
- 21.Noyes JD, Soto-Pedre E, Donnelly LA, et al. Characteristics of patients with low and high HbA(1c) variability in type 2 diabetes. Diabetologia. 2016;59(Suppl 1):S61–S62. [Google Scholar]
- 22.Di Flaviani A, Picconi F, Di Stefano P, et al. Impact of glycemic and blood pressure variability on surrogate measures of cardiovascular outcomes in type 2 diabetic patients. Diabetes Care. 2011;34:1605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beliaeva N, Strongin L, Baranov E. The impact of glycemic variability on local contractility of the left ventricular in acute period of myocardial infarction. Cardiology (Switzerland). 2013;126(Suppl 2):93. [Google Scholar]
- 24.Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and allcause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37:2359–2365. [DOI] [PubMed] [Google Scholar]
- 25.Varanauskiene E Can blood glucose self-monitoring improve treatment outcomes in type 2 diabetes? Diabetes Res Clin Pract. 2008;82(Suppl 2): S112–S117. [DOI] [PubMed] [Google Scholar]
- 26.Owens DR, Monnier L, Bolli GB. Differential effects of GLP-1 receptor agonists on components of dysglycaemia in individuals with type 2 diabetes mellitus. Diabetes Metab. 2013;39:485–496. [DOI] [PubMed] [Google Scholar]
- 27.Dandona P Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol Ther. 2017;19:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels AM, Rombouts SARB, Simsek S, et al. Microvascular disease in type 1 diabetes alters brain activation. A functional magnetic resonance imaging study. Diabetes. 2006;55:334–340. [DOI] [PubMed] [Google Scholar]
- 29.Kim C, Sohn J-H, Jang MU, et al. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS ONE. 2015;10 e0132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo MR, Barbieri M, Boccardi V, et al. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol. 2014;69:1122–1131. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieber TR, Marso SP, McGuire DK, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe J, Bruce E, Kiss A. Glucose variability as a predictor of poor clinical outcomes among hospital in patients with diabetes mellitus. J Diabetes Sci Technol. 2012;6:A16. [Google Scholar]
- 34.Tatti P, Strollo F, Passali D. Sleep apnea, sleep disturbance, and fasting glucose variability: a pilot study. J Diabetes Sci Technol. 2013;7:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klimontov VV, Myakina NE. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab Syndr Clin Res Rev. 2017;11:119–124. [DOI] [PubMed] [Google Scholar]
- 36.Rodbard D Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med. 2011;123:107–118. [DOI] [PubMed] [Google Scholar]
- 37.Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016;39:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Service FJ. Glucose variability. Diabetes. 2013;62:1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Vries JH. Glucose variability: where it is important and how to measure it. Diabetes. 2013;62:1405–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovatchev BP, Otto E, Cox D, et al. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. [DOI] [PubMed] [Google Scholar]
- 41.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;3:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozzilli P CSII and other technologies for preventing beta cell failure in type 2 diabetes. Diabetes Technol Ther. 2016;18(Suppl 1):A1–A2.26836419 [Google Scholar]
- 43.Harrell RM, Orzeck EA. Coding guidelines for continuous glucose monitoring. Endocr Pract. 2010;16:151–154. [DOI] [PubMed] [Google Scholar]
- 44.Petrie JR, Peters AL, Bergenstal RM, et al. Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care. 2017;40:1614–1621. [DOI] [PubMed] [Google Scholar]
- 45.Introducing the new Dexcom G6 CGM System. Experience the power of what Dexcom G6 can do for you. (https://www.dexcom.com/en-GB/ukdexcom-g6-cgm-system). Accessed August 17, 2018.
- 46.Gerich J Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med. 2013;6:877–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovatchev B, De Climens AR, Dex T, et al. The association between iglarlixi and patient satisfaction with their treatment’s ability to control type 2 diabetes (T2D) is mediated by reduced glycemic variability (GV). Diabetes. 2017;66(Suppl 1):A624. [Google Scholar]
- 48.Lane W, Weinrib S, Rappaport J, et al. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16:827–832. [DOI] [PubMed] [Google Scholar]
- 49.Goldenberg R, Umpierrez G, Digenio A, et al. Lixisenatide reduces glycemic variability when added to basal insulin in patients with type 2 diabetes mellitus. Can J Diabetes. 2014;38(Suppl 5):S40. [Google Scholar]
- 50.Frias JP, Nakhle S, Ruggles JA, et al. Exenatide once weekly improved 24-hour glucose control and reduced glycaemic variability in metformin-treated participants with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2016;19:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derosa G, Franzetti I, Querci F, et al. Effect of acarbose on glycemic variability in patients with poorly controlled type 2 diabetes mellitus receiving stable background therapy: a placebo-controlled trial. Pharmacotherapy. 2015;35:983–990. [DOI] [PubMed] [Google Scholar]
- 52.Wang MM, Lin S, Chen YM, et al. Saxagliptin is similar in glycaemic variability more effective in metabolic control than acarbose in aged type 2 diabetes inadequately controlled with metformin. Diabetes Res Clin Pract. 2015;108:e67–e70. [DOI] [PubMed] [Google Scholar]
- 53.Kim H-S, Shin J-A, Lee S-H, et al. A comparative study of the effects of a dipeptidyl peptidase-iv inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metformin. Diabetes Technol Ther. 2013;15:810–816. [DOI] [PubMed] [Google Scholar]
- 54.Kim NH, Kim D-L, Kim KJ, et al. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, pilot study. Endocrinol Metab. 2017;32: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yusoff Y Glycaemic variability with dapagliflozin compared to saxagliptin in type 2 diabetic patients during Ramadan fasting. Endocr Rev. 2017;38 (3 Suppl). [Google Scholar]
- 56.Rosenstock J, Davies M, Home PD, et al. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanefeld M, Koehler C, Hoffmann C, et al. Effect of targeting normal fasting glucose levels with basal insulin glargine on glycaemic variability and risk of hypoglycaemia: a randomized, controlled study in patients with early Type 2 diabetes. Diabet Med. 2010;27:175–180. [DOI] [PubMed] [Google Scholar]
- 58.Vilsboll T, Philis-Tsimikas A, Kilpatrick ES, et al. Assessment of glycaemic control by continuous glucose monitoring in patients with type 2 diabetes treated with IDegLira. Diabetologia. 2015;58:S401. [Google Scholar]
- 59.Bode BW. Glycemic variability and the role it should play in diabetes management and blood glucose monitoring. US Endocrinol. 2009;4(2):67–70. [Google Scholar]
- 60.Hirsch I, Yuan H, Campaigne B, et al. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract. 2009;15:343–348. [DOI] [PubMed] [Google Scholar]
- 61.Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39:2026–2035. [DOI] [PubMed] [Google Scholar]
- 62.Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–1980. [DOI] [PubMed] [Google Scholar]
- 63.Madsbad S Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Folli F, Corradi D, Fanti P, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7:313–324. [DOI] [PubMed] [Google Scholar]
- 65.Chang CM, Hsieh CJ, Huang JC, et al. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49(Suppl 1):S171–S177. [DOI] [PubMed] [Google Scholar]
- 66.Xu F, Zhao L-H, Su J-B, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jun JE, Jin SM, Baek J, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin CC, Yang CP, Li CI, et al. Visit-to-visit variability of fasting plasma glucose as predictor of ischemic stroke: competing risk analysis in a national cohort of Taiwan Diabetes Study. BMC Med. 2014;26:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajaj H, Venn K, Ye C, et al. Least glucose variability is observed with the combination of a GLP-1 receptor agonist and basal insulin among four commonly used insulin regimens in type 2 diabetes. Can J Diabetes. 2014;38(Suppl 5):S41. [DOI] [PubMed] [Google Scholar]
- 70.Sainsbury CAR, Cunningham SG, Jones GC. Sulphonylurea use is positively and metformin inversely associated with an increased rate of hypoglycaemia, glucose variability and 5 year mortality amongst inpatients with Type 2 diabetes in a retrospective matched cohort study. Diabet Med. 2016;33(Suppl 1):193. [Google Scholar]
- 71.Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3:992–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinemann L, DeVries JH. Reimbursement for continuous glucose monitoring. Diabetes Technol Ther. 2016;18(Suppl 2):S248–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodbard D Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(Suppl 2):S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graham C Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol Ther. 2017;19(S3):S60–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caffrey M Abbott’s Freestyle Libre CGM Gains Medicare Coverage. American Journal of Managed Care Website. https://www.ajmc.com/newsroom/abbotts-freestyle-libre-cgm-gains-medicare-coverage. Published January 5, 2018. Accessed July 11, 2018.
- 76.Dmowska A, Brown A. FreeStyle Libre Now Available in Major US Pharmacies. Diatribe Website. https://diatribe.org/freestyle-libre-now-availablemajorus-pharmacies. Published December 11, 2017. Accessed July 11, 2018. [Google Scholar]
- 77.Doheny K Medicare to Cover Therapeutic CGM, Sets Criteria. Endocrine Web Website. https://www.endocrineweb.com/news/diabetes/57179-medicare-cover-therapeutic-cgm-sets-criteria. Published November 7, 2017. Accessed July 11, 2018.
- 78.Abbott. Freestyle Libre 14 Day, Now FDA Approved. Abbot Website. http://www.abbott.com/corpnewsroom/product-and-innovation/freestylelibre-14-day.html Published August 1, 2018. Accessed August 10, 2018.
- 79.Howe D Comparing the Dexcom G6 to the G5. Beyond Type 1 Website. https://beyondtype1.org/comparing-the-dexcom-g6-to-the-g5/. Published April 30, 2018. Accessed August 1, 2018. [Google Scholar]
- 80.Centers for Medicaid and Medicare services; 2018. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed July 11, 2018.
- 81.Davidson MB. Counterpoint: self-monitoring of blood glucose in type 2 diabetic patients not receiving insulin: a waste of money. Diabetes Care. 2005;28:1531–1533. [DOI] [PubMed] [Google Scholar]
- 82.Yeaw J, Lee WC, Aagren M, et al. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm. 2012;18:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie Y, Agiro A, Bowman K, et al. Lowering cost share may improve rates of home glucose monitoring among patients with diabetes using insulin. J Manag Care Spec Pharm. 2017;23:884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medicare. Diabetes supplies & services; 2018. https://www.medicare.gov/coverage/diabetes-supplies-and-services.html. Accessed July 11, 2018.
- 85.Fonda SJ, Graham C, Munakata J, et al. The cost-effectiveness of realtime continuous glucose monitoring (RT-CGM) in Type 2 diabetes. J Diabetes Sci Technol. 2016;10:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of The American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22:1008–1021. [DOI] [PubMed] [Google Scholar]