Abstract
Background:
Body mass index (BMI), sarcopenia and obesity-related comorbidities have been associated with head and neck squamous cell carcinoma (HNSCC) progression.
Methods:
We conducted a retrospective analysis of 441 normal-weight, overweight and obese HNSCC patients treated at Montefiore Medical Center (NY). Patients were grouped by BMI prior to treatment and assessed for differences in survival adjusting for comorbid conditions (cardiovascular disease and diabetes). Evidence of sarcopenia was also assessed using pre-treatment abdominal CT scans in a subset of 113 patients.
Results:
Prior to treatment, 55% of HNSCC patients were overweight or obese. Overweight/obese patients had significantly better overall survival (hazard ratio [HR]=0.4, 95%CI:0.3–0.6) compared to normal-weight patients, independent of comorbid conditions. Patients with sarcopenia had significantly poorer survival (HR=2.1, 95%CI:1.1–3.9) compared to non-sarcopenic patients, with the strongest association seen among overweight/obese patients.
Conclusions:
Our data support the importance of sarcopenia assessment, in addition to BMI, among patients with HNSCC.
Keywords: Epidemiology, Sarcopenia, Survival, Body Mass Index, Obesity
INTRODUCTION
With nearly 60,000 new cases every year, head and neck cancer accounts for 3.6% of cancer incidence in the United States.1 Head and neck squamous cell carcinoma (HNSCC) is by far the most common type. Treatment modality of choice for HNSCC is dependent on American Joint Committee on Cancer staging and primary site.2,3 Since the 1990s, prognosis has substantially improved for head and neck cancers,4 but individual prognostic factors are still being elucidated, with body mass index (BMI) and muscle depletion gaining attention.5
Although the biological mechanism is not known, cancer incidence is higher with increases in body mass index, regardless of sex and race,6 and worse outcomes have been reported among overweight patients in studies of several types of cancer.2,7,8 However, several studies have shown improved prognosis in overweight patients with esophageal, head and neck, and gastric cancers.9,10,11,12,13 Furthermore, growing evidence suggests sarcopenia, the age-related, progressive loss of muscle quality and function, may be a predictor of poor cancer survival 14,15,16 and increased chemotoxicity.15
In addition to the possible link to cancer risk, increasing BMI is also a known risk factor for comorbid conditions such as cardiovascular disease (CVD)17 and diabetes18. Conversely, commonly used medications for dyslipidemia and diabetes, such as statins and metformin, have been independently associated with improved overall survival in reports examining patients with head and neck cancers.19,20 Due to conflicting data and the complex relationship of BMI and body composition with overall health and cancer outcomes, further investigation of the independent relationship between pretreatment BMI and survival, while considering confounding factors, is warranted. We therefore set out to assess the association between pretreatment BMI, body composition as assessed by presence of sarcopenia, comorbidities, and survival in a diverse inner-city population of patients with HNSCC.
MATERIALS AND METHODS
We conducted a retrospective review of 776 patients with HNSCC diagnosed and treated at Montefiore Medical Center (MMC) in Bronx, NY between 2004 and 2014. The study sample included patents with histologically confirmed invasive primary squamous cell carcinomas of the oropharynx, nasopharynx, larynx, hypopharynx, and oral cavity identified through the MMC cancer registry. The Institutional Review Board at MMC and Albert Einstein College of Medicine approved the study protocol and waiver of informed consent (No.2015–4483).
Clinical and pathologic factors were abstracted from electronic medical records where available and checked by manual chart review (G.C.). Missing clinico-pathologic data, including information on comorbid conditions and medication history prior to treatment, were also obtained through a review of medical records. Tumor stage was determined based on American Joint Committee on Cancer criteria (6th and 7th Editions) applied based on the standard for the date of diagnosis. The primary clinical outcome assessed was overall survival, defined as time from diagnosis (in months) to death from all causes.
Measurement of body mass index
Body mass index was derived from height and weight measurements collected prior to primary treatment (average 1.7 months prior) and calculated using average weight in kilograms divided by the square of height in meters. Patients were grouped by adult BMI status: underweight (BMI<18.5 kg/m2), normal-weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) or obese (BMI≥30.0 kg/m2).5
Sub-study to assess muscle depletion
To assess whether evidence of muscle depletion (sarcopenia) prior to treatment was also associated with survival in overweight/obese and normal-weight HNSCC patients, we conducted a nested case-control study of 113 patients with pretreatment abdominal computerized tomography (CT) scans collected within 6 months prior to treatment and up to 10 days after start of treatment using a measurement protocol outlined by Prado et al.13 HNSCC patients who died within 5 years of diagnosis were matched to surviving HNSCC patients on BMI group and tumor site using a nest-case control approach. This allowed us to efficiently sample cases with CT scans for assessment of sarcopenia.
Measurements were performed on abdominal CT scans that were obtained as part of whole-body CT obtained in conjunction with diagnostic positron emission tomography (PET). Two consecutive axial CT scan images from the level of the patient’s L3 vertebra were assessed by a single rater (M.F.) using the Aquarius software (TeraRecon, Foster City, CA) to measure the relative area of lean muscle mass. Skeletal muscle was identified on the CT scans using Hounsfield Unit thresholds of −29 to +150. Total muscular cross-sectional area (in cm2) was estimated by summing overall visible lower lumbar muscles. The mean of the total muscular cross-sectional areas from the two cuts was used and standardized to the patient’s height, providing the lumbar skeletal muscle index (in cm2/m2) normalized to patient stature. Evidence of sarcopenia was determined using pre-established cut-offs for cancer patients (<52.4 cm2/m2 for men and <38.5 cm2/m2 for women).16,18
Statistical analyses
Differences in BMI distribution and sarcopenia by clinical and pathological characteristics were examined by contingency tables and chi-square tests. To evaluate whether BMI was associated with clinical outcome, we generated Kaplan-Meier plots comparing the BMI groups of normal-weight vs. overweight and obese HNSCC patients stratified on clinico-pathologic factors including comorbid conditions (i.e., history of CVD, diabetes and HIV). Given weight loss may be an indicator of cachexia in HNSCC patients,21,22 we excluded underweight patients from survival analyses. We generated similar plots for muscle depletion comparing sarcopenic vs. non-sarcopenic HNSCC patients stratified by BMI group, clinico-pathologic factors and comorbid conditions.
To evaluate whether BMI or sarcopenia were independently associated with overall survival, we ran multivariable Cox proportional hazards regression models adjusting for age, gender and prognostic indicators. The potential for confounding was examined using a change in point estimate criterion for all socio-demographic and clinico-pathologic factors, including: race, ethnicity, primary treatment modality (surgery vs. chemo-radiotherapy, and unimodal vs. multi-modal treatment), tumor stage, anatomic site, smoking history (ever vs. never; current, former vs. never, and by pack-years), alcohol consumption, HIV status, history of CVD and diabetes, and tumor p16 or human papillomavirus (HPV) status available on a sub-cohort of patients followed prospectively as part of an ongoing study.23 Statistical analyses were conducted with the Stata v.14.2 statistical software package (Stata Inc., College Station, TX), and all tests were two-sided with a threshold for significance defined as p<0.05.
RESULTS
Seven hundred seventy-six adults in our cohort met our study inclusion criteria for invasive HNSCC treated at MMC, and pre-treatment weight data measured on average within 1.7 months of primary treatment (median=26 days, interquartile range:5 to 43 days) was available for 501 of the HNSCC patients. The median pretreatment BMI was 25.8 kg/m2, with 23% obese (N=115), 32% overweight (N=161), 33% normal-weight (N=165), and 12% underweight (N=60). After exclusion of underweight patients, the resulting analytical cohort included 441 normal-weight, overweight and obese HNSCC patients.
We found significant differences in distributions of normal-weight, overweight and obese patients by age, race, tumor stage, CVD and diabetes (Table 1). No significant differences in clinico-pathologic factors were observed between patients in the study cohort and those excluded due to lack of height and pre-treatment weight data (Supplemental Table 1).
Table 1.
Study population characteristics stratified by body mass index prior to treatment and clinico-pathologic characteristics in head and neck cancer patients
| Clinical variables | Normal-weight No. of patients (%) |
Overweight No. of patients (%) |
Obese No. of patients (%) |
p- value* |
Pathological variables |
Normal-weight No. of patients (%) |
Overweight No. of patients (%) |
Obese No. of patients (%) |
p- value* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis | Tumor site‡ | ||||||||||||||
| ≤60 years | 73 | (44.24%) | 53 | (32.92%) | 60 | (52.17%) | Larynx | 74 | (44.85%) | 64 | (39.75%) | 47 | (40.87%) | ||
| >60 years | 92 | (55.76%) | 108 | (67.08%) | 55 | (47.83%) | 0.005 | Oral cavity | 32 | (19.39%) | 38 | (23.60%) | 22 | (19.13%) | |
| Gender | Oropharynx | 51 | (30.91%) | 53 | (32.92%) | 37 | (32.17%) | 0.838 | |||||||
| Female | 50 | (30.30%) | 40 | (24.84%) | 38 | (33.04%) | T classification | ||||||||
| Male | 115 | (69.70%) | 77 | (75.16%) | 121 | (66.96%) | 0.301 | T1T2 | 73 | (44.24%) | 98 | (60.87%) | 70 | (60.87%) | |
| Race | T3T4 | 88 | (53.33%) | 59 | (36.65%) | 43 | (37.39%) | 0.018 | |||||||
| African American | 55 | (33.33%) | 30 | (18.63%) | 25 | (21.74%) | N classification | ||||||||
| Caucasian/Other | 85 | (51.52%) | 100 | (62.11%) | 75 | (65.22%) | 0.007 | N0 | 69 | (41.82%) | 67 | (41.61%) | 60 | (52.17%) | |
| Ethnicity | N+ | 96 | (58.18%) | 93 | (57.76%) | 55 | (47.83%) | 0.490 | |||||||
| Hispanic | 56 | (33.94%) | 62 | (38.51%) | 39 | (33.91%) | Tumor stage | ||||||||
| Non-Hispanic | 93 | (56.36%) | 76 | (47.20%) | 69 | (60.00%) | 0.117 | I/II | 39 | (23.64%) | 49 | (30.43%) | 41 | (35.65%) | |
| Smoking | III/IV | 126 | (76.36%) | 112 | (69.57%) | 74 | (64.35%) | 0.086 | |||||||
| Never | 25 | (15.15%) | 36 | (22.36%) | 31 | (26.96%) | Primary treatment | ||||||||
| Former | 66 | (40.00%) | 70 | (43.48%) | 40 | (34.78%) | Chemo/Radiotherapy | 75 | (46.58%) | 70 | (44.59%) | 50 | (43.48%) | ||
| Current | 69 | (41.82%) | 49 | (36.52%) | 42 | (36.52%) | 0.063 | Surgery+ | 86 | (53.42%) | 87 | (55.41%) | 65 | (56.52%) | 0.869 |
| Diabetes | HIV∥ | ||||||||||||||
| No | 119 | (81.51%) | 111 | (79.29%) | 52 | (57.78%) | Negative | 33 | (20.00%) | 22 | (13.66%) | 28 | (24.35%) | ||
| Yes | 27 | (18.49%) | 29 | (20.71%) | 38 | (42.22%) | <0.001 | Positive | 15 | (9.09%) | 4 | (2.48%) | 7 | (6.09%) | 0.108 |
| CVD† | P16β | ||||||||||||||
| No | 112 | (73.20%) | 86 | (55.84%) | 45 | (41.67%) | Negative | 46 | (75.41%) | 43 | (70.49%) | 23 | (65.71%) | ||
| Yes | 41 | (26.80%) | 68 | (44.16%) | 63 | (58.33%) | <0.001 | Positive | 15 | (24.59%) | 18 | (29.51%) | 12 | (34.29%) | 0.368 |
Chi-square p-values comparing normal weight to overweight/obese patients.
History of cardiovascular disease (CVD) including MI, CHF, CAD, VT, PAD and stroke.
Excluding (n=23) nasopharynx cancer cases.
HIV results available for 109 cases.
Clinical p16 staining results available for 157 cases.
Pre-treatment BMI is associated with survival in head and neck cancer patients
Univariate analyses showed poorer overall (Figure 1) and cancer-specific survival (Supplemental Figure 1) for normal-weight HNSCC patients compared to overweight and obese patients. No difference in risk of loco-regional recurrence or distant metastasis was seen between normal-weight and overweight/obese patients.
FIGURE 1.
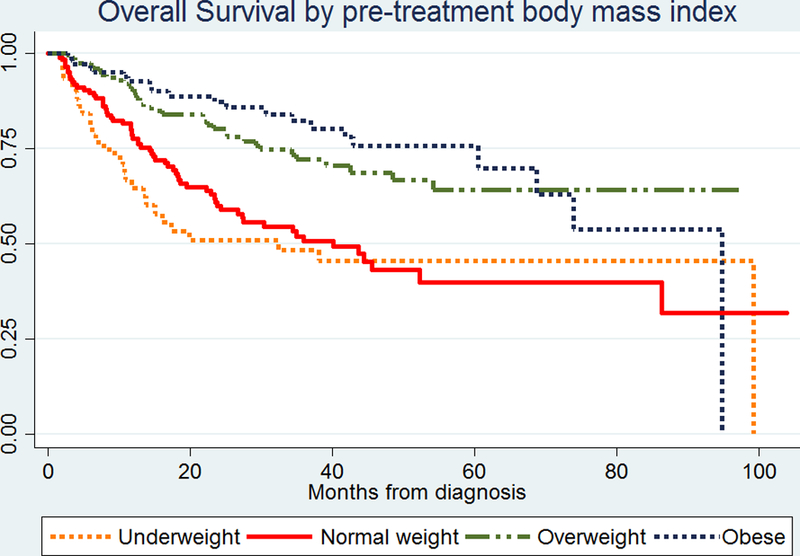
Kaplan-Meier plot for overall survival by body mass index (BMI) prior to treatment in patients with head and neck cancer.
With respect to other clinico-pathologic factors assessed at diagnosis, normal-weight patients who received chemotherapy and/or radiation had significantly worse overall survival, as did male normal-weight patients, and those with evidence of nodal spread at diagnosis (Supplemental Figure 2). The relative differences in survival between normal and overweight patients were unchanged across other clinico-pathologic factors including age, race/ethnicity, site, tumor stage, smoking, and alcohol history. No significant differences were observed either by BMI among patients who tested positive or negative for HPV or p16 (N=99 and 157 patients, respectively).
Association of body mass index with survival remains significant after stratification by common obesity related comorbidities
Given the significant associations observed between BMI and history of CVD and diabetes, we also assessed for differences in survival by these comorbid conditions (Figure 2). No differences in survival were observed between HNSCC patients with and without a diagnosis of CVD when stratified by BMI status, nor by statin use among those with CVD (N=172). Overweight patients with CVD however, had improved survival compared to normal-weight patients with CVD irrespective of statin use (Log-rank p=0.0086). Whereas HNSCC patients with a history of diabetes had poorer survival than those without, the relative difference in survival between normal-weight and overweight patients was the same. Among diabetics (N=94), overweight patients had improved survival compared to normal-weight patients irrespective of metformin use (Log-rank p=0.0032). Similarly, no significant differences in survival were observed between patients taking or not taking metformin when stratified by BMI.
FIGURE 2.
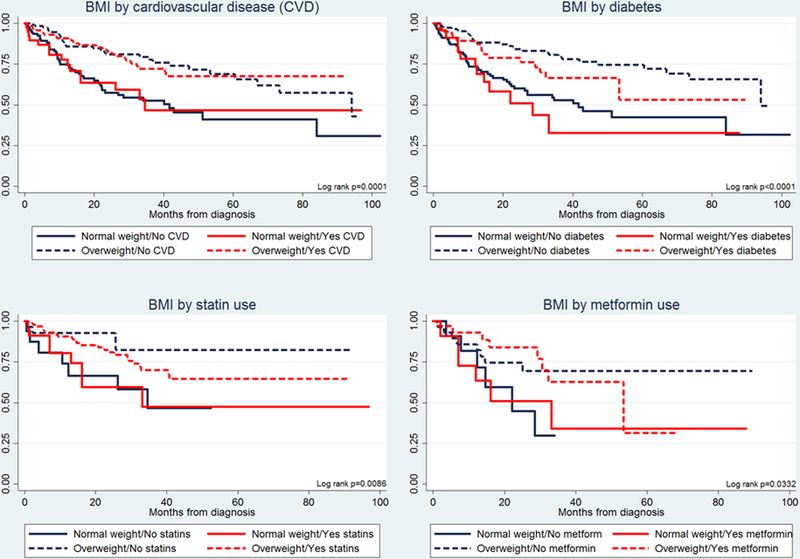
Kaplan-Meier plots for overall survival by pretreatment body mass index (BMI), cardiovascular disease (CVD), and diabetes in patients with head and neck cancer.
Association of body mass index with survival is not mediated by post-treatment weight loss
To investigate the potential for effect modification by post-treatment weight loss, we stratified the analyses comparing normal and overweight patients that lost >10% of their weight within three months of treatment (Supplemental Figure 3). Normal-weight patients were not more likely to lose weight after treatment than overweight and obese patients (Chi2 p=0.720). Nonetheless, among overweight HNSCC patients who lost >10% of their weight following treatment, survival remained better, albeit not significantly, compared to normal-weight patients. In addition, overweight patients who maintained, or even gained weight, following treatment had significantly better overall survival than those who lost >10% of their weight. Survival was similar for normal-weight patients regardless of weight change post-treatment.
Pre-treatment sarcopenia may be associated with survival independent of body mass index
To investigate the association between muscle depletion and overall survival, we assessed 62 HNSCC overweight/obese and 52 normal-weight patients in our nested case-control study subcohort for whom we had evaluable pre-treatment abdominal CT scans. We observed a significant inverse association between sarcopenia and BMI with 48.4% (n=30) of overweight/obese patients vs. 82.7% (n=43) of normal-weight patients presenting with evidence of sarcopenia (Chi2 p<0.0001).
Kaplan-Meier analyses revealed a significant (Log-rank p=0.004) association between sarcopenia and overall survival, with sarcopenic patients having poorer survival compared to patients without sarcopenia. When we stratified the analysis by BMI group, sarcopenic patients had significantly poorer overall survival compared to non-sarcopenic patients, regardless of BMI group prior to treatment (Figure 3).
FIGURE 3.
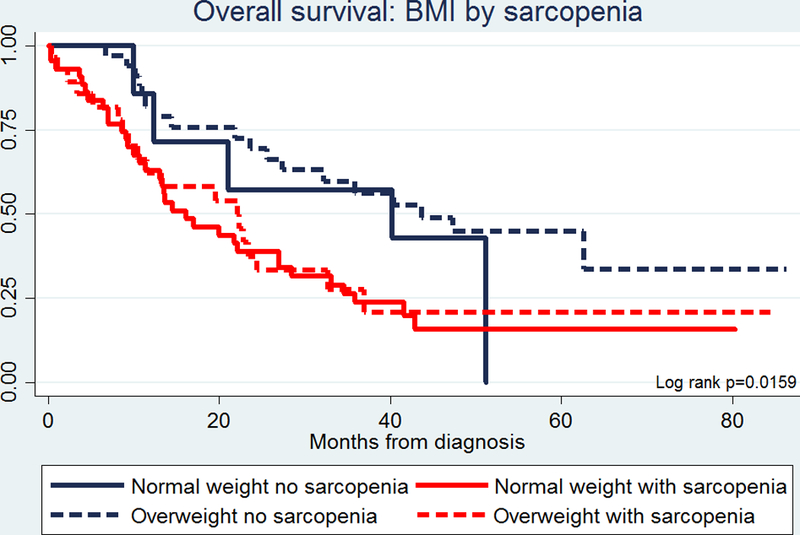
Kaplan-Meier plot for overall survival by body mass index (BMI) and sarcopenia assessed prior to treatment in patients with head and neck cancer.
When assessed by history of CVD or diabetes, sarcopenic patients had significantly poorer overall survival compared to non-sarcopenic patients independent of history of CVD (Supplemental Figure 4). The association was not significant when survival was examined for sarcopenia stratified by diabetes diagnosis. With respect to other clinico-pathologic variables including: age, ethnicity, gender, nodal classification, and treatment modality; sarcopenic patients consistently showed poorer overall survival compared to non-sarcopenic patients.
Multivariable analyses confirm independent associations between pre-treatment body mass index, sarcopenia and survival in head and neck cancer patients
We ran multivariable cox regression analyses to test for the independent associations between BMI, sarcopenia and survival. Overweight and obese HNSCC patients had significantly better overall survival (adjusted hazard ratio [HR]=0.44, 95%CI:0.3–0.6) compared to patients who were normal-weight prior to treatment, adjusting for tumor stage, age, gender and primary treatment (Table 2). Additional adjustment for tumor site, smoking history, HIV status, history of CVD, and diabetes did not change the association with BMI (Supplemental Table 2), nor did race, ethnicity, regular alcohol consumption, other comorbidities, HPV or p16 status. Similar associations with BMI were observed across the different HNSCC sites, including oral cavity, laryngeal and oropharyngeal sites (Supplemental Table 3). We confirmed that the proportional hazards assumptions were met for all covariates included in the final models.
Table 2.
Multivariable regression models showing independent associations between body mass index, sarcopenia and overall survival in head and neck cancer patients
| BMI cohort* | Sarcopenia subcohort* | |||||
|---|---|---|---|---|---|---|
| Clinical variables | HR | 95%CI | p-value | HR | 95%CI | p-value |
| Pre-treatment BMI | ||||||
| Normal-weight | 1.0 | Ref | 1.0 | Ref | ||
| Overweight | 0.44 | 0.3–0.6 | 0.000 | 0.89 | 0.5–1.5 | 0.673 |
| Sarcopenia | ||||||
| No | ||||||
| Yes | 2.08 | 1.1–3.9 | 0.021 | |||
| Age at diagnosis | ||||||
| ≤60 years | ||||||
| >60 years | 1.20 | 0.8–1.7 | 0.330 | 0.87 | 0.5–1.4 | 0.601 |
| Gender | ||||||
| Female | ||||||
| Male | 1.34 | 0.9–2.0 | 0.179 | 1.02 | 0.5–1.9 | 0.954 |
| Tumor stage | ||||||
| I/II | ||||||
| III/IV | 2.65 | 1.5–4.5 | 0.000 | 2.05 | 1.0–4.3 | 0.059 |
| Primary Treatment | ||||||
| Surgery+ | ||||||
| Chemo/Radiotherapy | 1.65 | 1.1–2.4 | 0.009 | 1.51 | 0.9–2.5 | 0.099 |
Body mass index (BMI). Hazard ratio (HR) and 95% confidence intervals (CI) by multivariable Cox proportional hazards regression for overall survival mutually adjusting for all variables in table. P-value for HR estimates derived by two-sided Wald test.
Among the (n=113) HNSCC patients assessed for evidence of sarcopenia, we observed a significant association between overall survival and sarcopenia (HR=2.08, 95%CI:1.1–3.9; Table 2) that attenuated the association with BMI; the HR for overweight vs. normal-weight patients changed from 0.64 to 0.89 after adjustment for sarcopenia, whereas the HR for sarcopenia increased (to 2.21, 95%CI:1.3–3.8) after removal of BMI, suggesting mediation. Additional adjustment for tumor site, smoking history, HIV status, history of CVD, and diabetes further strengthened the association between survival and sarcopenia (Supplemental Table 2). When stratified by BMI status, the association with sarcopenia was stronger among overweight patients (adjusted HR=2.71, 95%CI:1.3–5.8) compared to normal-weight patients (adjusted HR=1.25, 95%CI:0.4–3.9), although the test statistic for effect modification was not significant (p-value for effect modification=0.366).
DISCUSSION
Our study identified increased pretreatment BMI to be independently associated with improved cancer survival in HNSCC patients. However, we found evidence of muscle depletion (sarcopenia) to be inversely associated with BMI group (i.e., occurred more often among normal-weight HNSCC patients). Sarcopenia was also independently associated with reduced cancer survival regardless of BMI group, suggesting that sarcopenia may be a better prognostic indicator compared to BMI in HNSCC patients.
Across a wide variety of cancer types, obesity is significantly associated with greater cancer incidence, as well as with higher risk of death from cancer.3,5,6 However, this relationship is not consistent for all malignancies.5,8,9,11 Furthermore, survival analyses have been conducted that contradict our understanding of obesity being coupled with poorer outcomes.8,9,10,11 Albergotti et al. conducted a retrospective review of 300 patients with HPV positive oropharyngeal HNSCC and found that patients with a BMI less than 25 kg/m2 had significantly shorter overall and disease-specific survival than their overweight counterparts.24
Sarcopenia, however, may be a mediating factor for these findings, as studies suggest that sarcopenia may be associated with poor cancer survival.13,14,15 The prevalence of sarcopenia varies based on a number of factors, including race, gender and BMI.13,14,25 Prevalence has been shown to be higher among normal-weight adults (40%) compared to obese adults (8%).26 A study by Prado et al.13 of patients with gastro-intestinal and respiratory tumors found evidence of sarcopenia in 15% of obese patients using pretreatment CT scans, with an average lean muscle mass comparable to patients who are severely underweight. In other studies of breast and colorectal cancer including normal-weight, overweight and obese patients, the prevalence of sarcopenia varied from 16% to 19%.14,22 An inverse association between BMI and sarcopenia was reported for breast cancer patients with mean BMI increasing in patients with sarcopenia versus without.22
This inverse correlation between BMI and sarcopenia may explain in part the apparent decrease in survival observed in normal-weight versus overweight and obese cancer populations.9,27 Whereas loss of lean muscle mass can occur independently of BMI, the association between sarcopenia and poor overall survival may increase with BMI in cancer patients. Van Vledder et al.14 detected almost a three-fold increase in risk of overall mortality (HR=2.9) among normal and overweight breast cancer patients with sarcopenia compared to those without. Prado et al.13 detected a HR of 4.2 among obese gastro-intestinal and respiratory cancer patients with sarcopenia. This group was defined as “sarcopenic obesity,” and the study showed reduced survival in this group compared to their non-sarcopenic obese counterparts.13
Grossberg et al.28 showed that skeletal muscle depletion, both before and after radiotherapy, was associated with poorer overall survival in HNSCC patients. The study concluded, however, that pretreatment BMI was a better prognostic indicator than skeletal muscle index. Our analysis suggests that there is interplay between skeletal muscle quality, which we assessed via the measure of sarcopenia and BMI. Although being overweight may be protective, having sarcopenia appears to be associated with reduced survival, thus making the survival in overweight and obese patients with sarcopenia comparable to that in normal-weight patients with sarcopenia.
Comorbidities are another factor that may affect survival in cancer patients. A previous study by Piccirillo29 showed comorbidity to be an independent prognostic factor in head and neck cancer. However, studies have shown improved outcomes in patients taking medications for cardiovascular conditions; with statins reducing cancer-related mortality,19 and metformin improving survival in patients with laryngeal HNSCC18. Others have suggested an association with anemia.30 Our study focused on two comorbidities highly prevalent in obese patients – CVD and diabetes – and showed that overweight patients had better overall survival compared to normal-weight patients regardless of CVD or diabetes diagnoses. However, diabetes may also be associated with poorer survival and may be a confounder in the association between BMI and survival. Our sub-group analyses of diabetes and CVD patients showed that stratifying by metformin or statin use, respectively, did not change the observed association of improved overall survival in overweight versus normal-weight HNSCC patients. The associations also held true in our analysis of sarcopenia and survival. These results suggest that obesity-related comorbidities and their associated treatment medications do not explain the association between improved survival and higher BMI, or the association between reduced survival and sarcopenia.
Our study has some limitations. The study was conducted at a single health center located in a diverse urban setting, so the results may not be generalizable to institutions with different patient populations; our patient population from the Bronx represents a more racially and ethnically diverse cohort than examined in previous reports.15,24 Our analysis of comorbidities and medication history was limited by documentation in the electronic medical records. Although our classification of patients as normal-weight, overweight, and obese uses conventional cut-offs, BMI is an imperfect estimation of obesity and nutritional status. Sarcopenia is another method of assessing body composition, but its definition and role has also been controversial.12,24 While the use of abdominal CT scans to determine evidence of sarcopenia has shown clinical relevance for cancer prognosis in multiple studies,13–16,31 abdominal CT assessment is not routinely obtained for patients with newly diagnosed HNSCC, unless it has been acquired in conjunction with diagnostic whole-body PET scanning.
Despite these limitations, increased pretreatment BMI was consistently associated with improved cancer survival among HNSCC patients, independent of clinical and pathological indicators. Comorbid conditions commonly associated with increased BMI, such as diabetes and cardiovascular disease, and their associated treatment medications of metformin and statins, did not affect the association. Normal-weight patients were also more likely to present with evidence of muscle depletion or sarcopenia at diagnosis, which may be a mediating factor. Overall, the consideration of BMI and sarcopenia may aid in prognostication among patients with HNSCC.
Supplementary Material
Acknowledgments
Funding: This project is supported in part by NIH grants CA115243 and DE023941 (to Nicolas F. Schlecht), National Cancer Institute P30 grants to the Einstein Cancer Research Center (CA013330) and to Roswell Park Comprehensive Cancer Institute (CA016056), and the Departments of Otorhinolaryngology-Head & Neck Surgery, Radiology and Pathology, Albert Einstein College of Medicine, Montefiore Medical Center. Thomas J. Ow’s contribution was supported by a NIH K12 grant (CA132783) and a National Center for Advancing Translational Science (NCATS) grant (UL1TR001073; to the Einstein-Montefiore CTSA).
Results from this study were presented at the annual Combined Otolaryngology Spring Meetings (April 26-30, 2017, San Diego, CA).
Footnotes
This manuscript is submitted posthumously on behalf of Ms. Gina Chang, a medical student who contributed significantly to the study, including collecting data, conducting statistical analyses and helping draft the manuscript. The coauthors dedicate this publication to honor her memory.
REFERENCE
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65(1): 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 2007; 110(7): 1429–1435. [DOI] [PubMed] [Google Scholar]
- 3.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2013; 11(8): 917–923. [DOI] [PubMed] [Google Scholar]
- 4.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010; 15(9): 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollander D, Kampman E, van Herpen C. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hematol 2015; 96(2): 323–338. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371(9612): 569–578. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003; 348(17): 1625–1638. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373(9669): 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith M, Zhou M, Whitlock G, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int J Cancer 2008; 122(7): 1604–1610. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer 2013; 109(11): 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol 2006; 24(31): 5017–5024. [DOI] [PubMed] [Google Scholar]
- 12.Trivers KF, De Roos AJ, Gammon MD, et al. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol 2005; 3(3): 225–230. [DOI] [PubMed] [Google Scholar]
- 13.Gama RR, Song Y, Zhang Q, et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck 2017; 39(6):1226–1233. [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9(7): 629–635. [DOI] [PubMed] [Google Scholar]
- 15.Van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012; 99(4): 550–557. [DOI] [PubMed] [Google Scholar]
- 16.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009; 15(8): 2920–2926. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162(16): 1867–1872. [DOI] [PubMed] [Google Scholar]
- 18.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289(1): 76–79. [DOI] [PubMed] [Google Scholar]
- 19.Sandulache VC, Hamblin JS, Skinner HD, Kubik MW, Myers JN, Zevallos JP. Association between metformin use and improved survival in patients with laryngeal squamous cell carcinoma. Head Neck 2014; 36(7): 1039–1043. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367(19): 1792–1802. [DOI] [PubMed] [Google Scholar]
- 21.Couch ME, Dittus K, Toth MJ, et al. Cancer cachexia update in head and neck cancer: pathophysiology and treatment. Head Neck 2015; 37(7): 1057–72. [DOI] [PubMed] [Google Scholar]
- 22.Martin L1, Birdsell L, Macdonald N, et al. Cancer Cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013; 31(12): 1539–1547. [DOI] [PubMed] [Google Scholar]
- 23.Salazar CR, Smith RV, Garg MK, et al. Human papillomavirus-associated head and neck squamous cell carcinoma survival: a comparison by tumor site and initial treatment. Head Neck Pathol 2014; 8(1): 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albergotti WG, Davis KS, Abberbock S, et al. Association of pretreatment body mass index and survival in human papillomavirus positive oropharyngeal squamous cell carcinoma. Oral Oncol 2016; 60; 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villaseñor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv 2012; 6(4): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delmonico MJ, Harris TB, Lee JS, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007; 55(5): 769–774. [DOI] [PubMed] [Google Scholar]
- 27.Takenaka Y, Takemoto N, Nakahara S, et al. Prognostic significance of body mass index before treatment for head and neck cancer. Head Neck 2015; 37(10): 1518–1523. [DOI] [PubMed] [Google Scholar]
- 28.Grossberg AJ, Chamchod S, Fuller CD, et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA Oncol 2016; 2(6): 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope 2000; 110(4):593–602. [DOI] [PubMed] [Google Scholar]
- 30.Te Riele RJLM Dronkers EAC, Wieringa MH, et al. Influence of anemia and BMI on prognosis of laryngeal squamous cell carcinoma: Development of an updated prognostic model. Oral Oncol 2018; 78: 25–30. [DOI] [PubMed] [Google Scholar]
- 31.Swartz JE, Pathen AJ, Wegner I, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol 2016; 62:28–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


