Abstract
Objectives
This study aims to compare the efficiency of conventional motorized traction (CMT) with non-surgical spinal decompression (NSD) using the DRX9000™ device in patients with low back pain associated with lumbar disc herniation (LDH).
Patients and methods
Between March 2009 and September 2009, a total of 48 patients (29 females, 19 males; mean age 43.1±9.8 years; range, 18 to 65 years) were randomized into two groups. The first group (n=24) underwent CMT and the second group (n=24) underwent NSD for a total of 20 sessions over six weeks. The patients were evaluated before and after the treatment. Pain was assessed using the Visual Analog Scale (VAS), functional status using the Oswestry Disability Index (ODI), quality of life using the Short Form-36 (SF-36), state of depression mood using the Beck Depression Inventory (BDI), and the global assessment of the illness using the Patient's Global Assessment of Response to Therapy (PGART) and Investigator's Global Assessment of Response to Therapy (IGART) scales.
Results
There was no significant difference in the evaluation outcomes before the treatment between the groups. However, a statistically significant decline was found in the VAS, ODI, and BDI scores after the treatment in both groups (all p<0.001). Except for two subgroups, no significant changes were observed in the SF-36 form. Assessment of “marked improvement” was globally most frequently reported one in both groups. No significant difference was observed in the evaluation outcomes after treatment between the groups.
Conclusion
Our study results show that both CMT and NSD are effective methods in pain management and functional status and depressive mood improvement in patients with LDH, and NSD is not superior to CMT in terms of pain, functionality, depression and quality of life.
Keywords: DRX9000™, Low back pain, spinal decompression, traction
Introduction
Low back pain (LBP) is defined as the pain, muscle tension and stiffness with or without an accompanying leg pain in the region between the 12th rib and gluteal fold at the proximal thigh.[1] Low back is the location where the highest incidence of musculoskeletal pain is observed. Approximately 80% of individuals living in the industrialized countries suffer from LBP during a part of their active lives.[2] For most authors, acute LBP refers to LBP lasting for less than six weeks, subacute LBP to LBP lasting for 6 to 12 weeks, and chronic LBP to LBP lasting for more than 12 weeks.[3] In general, LBP is considered non-specific; however, lumbar disc herniation (LDH) is a frequent cause of LBP.[4] Lumbar disc herniation is a clinical entity characterized by low back and leg pain caused by the compression of the lumbar spinal nerve root by a degenerative disc.[2] The majority of patients respond to conservative treatment. Conservative treatment involves resting, drug therapy, physical therapy, exercise, manipulation, epidural injections, bracing, and back school exercises.[2] One of the physical therapy modalities used in the treatment of LDH is traction, which can also be combined with other modalities. Traction in physiatry practice is usually applied to the neck and back spine, and it can ensure to achieve separation of the joint surfaces, decreased disc protrusion, elongation in the soft tissues, relaxation in muscles, and mobilization in the joints.[5] As a result of separation of the joint surfaces, the compression in the surrounding tissues may be removed. Meanwhile, an improvement in the line-up of the bony structures as well as relaxation in other nervous tissues can be also achieved. All of these outcomes are useful for the relief of pain due to spinal dysfunction.[5] Traction can be classified as continuous, static (fixed), or intermittent according to the application period, and as autotraction, gravity-assisted, manual, inversion, aquatic, positional, mechanical, and motorized traction according to the force applied.[5-8] For LBP of a discogenic origin, some evidence indicates that both simple and motorized traction can expand the intervertebral space and reduce disk protrusion and intradiscal pressure.[9,10] However, systematic reviews of clinical trials of traction for LBP with or without sciatica have shown that traction is probably not effective in relieving pain, compared to placebo, sham, or other treatment modalities.[11-13] The most recent incarnation of traction has been a form of intermittent motorized traction commonly referred to as non-surgical spinal decompression (NSD) therapy. Developers and manufacturers of the equipment along with clinicians often consider it a unique form of traction.[14] Specifically, DRX9000™ (Axiom Worldwide, Tampa, FL, USA) is a novel, expensive, computerized traction device for treating pain caused by discogenic origin. It is a non-surgical procedure designed to alleviate pressure on the anatomical structures which cause LBP.[15] The DRX9000™ uses a split-table design to reduce friction between the patient and the device. The patient lays supine; a chest and shoulder support system controls the upper body, and a knee rest is used to eliminate pelvic rotation. The apparatus has built-in air bladders, disc-angle-pull adjusters, and harnesses and can increase the decompression force more slowly in the latter part of the therapy. The DRX9000™ uses a motor pulley to deliver the mechanic segmental distraction, which can be delivered in a static or an oscillatory fashion for a preselected duration. The traction force is maximized logarithmically. In this fashion, a traction force can be applied effectively on a specific disc without causing paraspinal muscle spasm reflexes. The device may also perform level-specific decompression. With the specific axial angular traction force applied by the device, L1-L5 lumbar vertebrae are specifically treated. Level specificity is made thanks to the angular gradient, as reported in previous studies, and based on the air sac supporting the lordotic curve.[16] Although several papers relating to intermittent and static traction have been published, there is very limited evidence in the scientific literature to support the effectiveness of non-surgical spinal decompression therapy defined as motorized traction utilizing variable force, variable traction/relaxation times, and variable angles of pull. To the best of our knowledge, this intervention has not been compared to other less expensive conservative treatment options such as conventional motorized traction (CMT), yet. Therefore, in the present study, we aimed to compare the effects of CMT method and of NSD performed by a DRX9000™ device on pain, functional status, depression, and quality of life in patients with LBP associated with LDH.
Patients and Methods
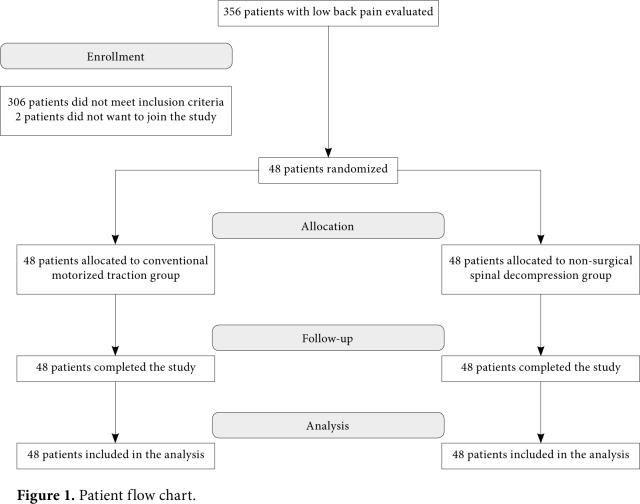
Patients at a tertiary physical therapy and rehabilitation hospital with low back and leg pain were evaluated, and a total of 48 patients (19 males, 29 females; mean age 43.1±9.8 years; range: 18 to 65 years) with the diagnosis of LDH were included in the study between March 2009 and September 2009. A written informed consent was obtained from each patient. The study protocol was approved by the Ethics Committee of the Turkish Ministry of Health, Ankara Physical Medicine and Rehabilitation Training and Research Hospital. The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients with a LBP associated with LDH of longer than three months without lumbar spinal injection or lumbar surgery history and without previous physical therapy and rehabilitation session during the past six months were included in the study. To exclude other causes which can lead to low back - leg pain, laboratory and radiological examinations were carried out prior to treatment, including complete blood count, routine biochemistry, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), urinalysis, four-way lumbar spine radiographs, and magnetic resonance imaging (MRI). Based on the MRI findings, asymmetric focal prolongation of the disc into spinal canal or neural foramen by crossing the vertebral bodies through the incomplete defects in the annulus fibrosus was assessed as a protrusion; projection of the herniated disc into the spinal canal by tearing posterior longitudinal ligament as an extrusion; and complete break of the projecting part and becoming free in the epidural space as a sequestration. Patients with a protruding disc herniation according to the MRI results were included in the study. Exclusion criteria were as follows: cognitive dysfunction, neurological deficits, an extruded and/or sequestrated LDH, spinal fusion, pregnancy, malignancy, spinal compression fracture, spondylolisthesis, aortic aneurysms, severe peripheral neuropathy, vertebral infection, rheumatic diseases, and moderate to severe depression, as assessed by a Beck Depression Inventory (BDI) score of ≥19. Treatment procedure Patients were randomized into two groups using the method of sealed envelopes. The first group (n=24) underwent CMT and the second group (n=24) underwent NSD using the DRX9000™ device. All patients included in the study completed the study (Figure 1).
Figure 1. Patient flow chart.
The CMT procedure was performed using a motorized traction device of the Elettronica Pagani (Paderno Dugnano MI, Italy) brand. The table of this device has separable segments to reduce the friction forces. The patients' body weights were taken with a weighing scale before treatment. Traction was applied to the patient lying on the table in the supine position. The chest strap was fitted over the lower ribs, and the waist strap on anterior iliac crests. A stool was placed below the patient's legs in such a way that the hip and knees flexed to 90 degrees to reduce the patient's lumbar lordosis. Traction was applied by starting with a force corresponding to 25% up to a maximum 50% of the patient's body weight, by increasing the force gradually according to the patient's tolerance. Traction was applied for a total period of 30 min in an intermittent pattern, consisting of 40 sec of traction and 20 sec of relaxation. The patients received treatment with the DRX9000™ in accordance with the manufacturer's instructions. The DRX9000™ NSD system is controlled by a computer. First, the demographic data of 24 patients was input into the computer. The level of the disc to be subjected to the treatment was identified according to the results of the patient's clinical status and MRI findings. Upper and lower body harnesses suitable to the patient's body were placed on the body. The waist harness was positioned to correspond with the iliac crest. The treatment bed was put into the upright position, and the patient was lead to the platform at the base of the bed and his/her body weight was measured with the electronic scales in pound unit. The armpit supports and lumbar support of the bed were adjusted to fit appropriately according to patient's height. The bed was set with the patient to the treatment position and the head supports were placed. A support was also placed under the knee. The lower body harness was tightened and attached to the traction rope. The air sac located on the bed was inflated in a manner to support the lordotic curve. The upper body harness was tightened and its strap was attached to the hook at the head part of the bed. The armpit supports were adjusted according to the patient. For the initial session, the traction force was set to 10 pounds below half of the patient's weight. The force to be applied during each session was increased by 5 to 10 pounds depending on the patient's tolerance; however, the maximum traction force was not allowed to exceed 10 pounds over half of the patient's weight. Each treatment session took 30 min in total. A total of 20 treatment sessions were administered to both patient groups during a period of six weeks, consisting of sessions on five days a week during the first two weeks (2x5), three days a week during the following two weeks (2x3), and two days a week (2x2) during the last two weeks. The patients in both groups received a hot pack for 20 min and transcutaneous electrical nerve stimulation (TENS) (Chattanooga Intelect TENS Standard, Elsa, USA) (the conventional analgesic mode was used) of the treatment for a period of 20 min before the treatment. At the second week, the patients were instructed on isometric exercises to strengthen low back and abdominal muscles. The patients in both groups were allowed to take only paracetamol as an analgesic during the treatment. Measurement parameters Patient data including age, sex, body mass index (BMI) (kg/m2), smoking, duration of the illness, and occupational status were recorded. Occupational status of the patients was classified as deskwork, heavy-lifting work, stands up at work, and works as a driver, retired, or not working. The patients were also asked, if they had any referred pain and about the location of the pain such as the hip, thigh, or leg pain, if any. History of analgesic use was recorded. Drugs were classified as paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), and anticonvulsants. A 10 cm Visual Analog Scale (VAS) was used to evaluate the pain severity.[17] A horizontal ruler of 10 cm long was used. The patients were asked to mark the score corresponding to their pain level on the pain scale, which is between 0=no pain and 10=most severe pain. The patients were asked to mark pain at rest, pain during movement, and pain at night separately. The minimum clinically important difference (MCID) was reported to be between 1 and 1.9 points after treatment for chronic LBP.[18,19] The patient's global assessment was made using the Patient's Global Assessment of Response to Therapy (PGART) scale, while the physician's global assessment was made using the Investigator's Global Assessment of Response to Therapy (IGART) scale.[20] Using these scales, patients and physicians separately made global assessments of the illness by assigning a value ranging from -1 to 3 (3=almost complete relief, 2=marked improvement, 1=slight improvement, 0=no change, and -1=worsening of symptoms). The Oswestry Disability Index (ODI) was used for the assessment of functional status in patients with LBP.[21] This scale consists of 10 questions, each with six options, and each option requiring a score ranging between 0 and 5. The minimum score to be obtained from the scale is 0 and the maximum score is 50, a score of 50 indicating the highest level of functional impairment. The Turkish reliability and validity study of the ODI was done by Yakut et al.[22] The Cronbach's alpha ranged from 0.71 to 0.87. The test-retest reliability was also shown to be high with values ranging from r=0.83 to 0.99. The intraclass correlation coefficient values from 0.84 to 0.94 were reported. The MCID was reported to be between 4 and 10.5 points. The consensus called for a minimal change of 10 points to be clinically significant.[23] The Short Form-36 (SF-36) was used to assess quality of life of the patients.[24] The Turkish reliability and validity study of the SF-36 was done by Kocyigit et al.[25] The SF-36 is a 36-item questionnaire filled by the patients themselves. It has eight subgroups, including physical function, physical role restriction, pain, general health, vitality, social function, emotional role difficulty, and mental health. The score of this form ranges from 0 to 100. Zero score indicates the worst health status, while 100 reflects the best health status. The internal consistency of the SF-36 dimensions was assessed with the Cronbach's alpha statistic. Alpha values of >0.8 were gained for all dimensions of the SF-36; therefore, the internal consistency was good. The internal reliability was calculated by dividing the data into five subgroups of overall self-rated general health. All values were >0.7. The internal reliability was shown to be high, suggesting that SF-36 should be an appropriate measure for use in subgroups of the population with generally poor overall health, as well as groups with generally good overall health.[26] The MCID of SF-36 was also reported to be between 4.9 and 18 points after treatment for chronic LBP.[19,27] As LBP of longer duration may cause depression, the patients were also assessed for depression using the BDI.[28] The BDI is composed of 21 items, and the patients are asked to choose the most appropriate answers for their situations. Each answer is assigned a score ranging from 0 to 3, resulting in a total score ranging from 0 to 63 for this assessment. The results are evaluated as follows: a score of 0-9 indicates no depression/minimal depression; 10-18, mild depression; 19-29, moderate depression; and 30-63 indicates severe depression. The Turkish reliability and validity study of the BDI was done by Hisli.[29] The Cronbach's alpha ranged from 0.55 to 0.96 (mean: 0.72) for psychiatric patients. For non-psychiatric patients, the correlation was between 0.55 and 0.73 (mean: 0.60). The test-retest reliability was shown to be 0.74.[30] In the study written by Button et al.[31] to calculate the MCID on the BDI, about 14 to 17% increase in the BDI scores was associated with feeling worse, compared to 36 to 45% improvement was associated with feeling better. The patients were evaluated twice, once before treatment and once after treatment. The pre-treatment assessment was performed by a single investigator, while the sealed envelope containing a written description of the treatment protocol was handed over to the patients by another investigator. The envelope was opened by the physiotherapist who, then, applied the appropriate treatment according to his/her group. Following a six-week treatment, an evaluation was made by a third investigator. Therefore, the physicians remained blinded to the group assignment. Due to the nature of the study, the patients were aware of the device used for the treatment and, therefore, the patients were unable to be blinded. Statistical analysis Statistical analysis was performed using the SPSS for Windows versiyon 15.0 software (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed in the form of cross tabulations for categorical variables, and in mean, median, standard deviation (SD), minimum, and maximum for numeric variables. The chi-square test was used to compare independent categorical variables, while the Monte Carlo simulation method was done in multiple comparisons, where the chi-square criteria were not satisfied. The Fisher's exact test was used to compare exactly two groups, while the Mann-Whitney U test was applied to compare abnormally distributed two groups. In addition, t-test statistics were used to compare normally distributed two numerical variables. The Wilcoxon test was done for double comparisons of dependent abnormally distributed numerical variables, while a paired t-test was used for dependent normally distributed numerical variables. A p value of <0.05 was considered statistically significant. The study population consisted of 48 patients. The sample size was unable to be calculated at the beginning of the study. However, the post-hoc power analysis revealed 86.11% power within the 0.8 effect size G*power program 3.1.9.2 (Heinrich Heine University, Dusseldorf, Germany). In addition, the most recent literature data were used for the traction and DRX9000™.
Results
In this study, a total of 24 patients received CMT, while another 24 patients received NSD. Demographic and clinical characteristics of the patient groups are presented in Table 1. There was no statistically significant difference in these variables between the two groups, except for sex (p=0.008).
Table 1. Demographic and clinical characteristics of patient groups.
| Treatment applied | |||||||
| Conventional motorized | Non-surgical spinal | ||||||
| traction (n=24) | decompression (n=24) | ||||||
| Variable | n | % | Mean±SD | n | % | Mean±SD | p |
| Sex | 0.008*† | ||||||
| Female | 19 | 79,2 | 10 | 41,7 | |||
| Male | 5 | 20,8 | 14 | 58,3 | |||
| Age (year) | 43.4±8.5 | 42.8±11.1 | 0.817‡ | ||||
| Body mass index (kg/m2) | 27.0±5.1 | 26.8±3.8 | |||||
| Occupation | 0.586† | ||||||
| Does not work | 13 | 54,2 | 10 | 41,7 | |||
| Desk work | 7 | 29,2 | 10 | 41,7 | |||
| Heavy lifting work | 1 | 4,2 | 0 | 0,0 | |||
| Driver | 0 | 0,0 | 2 | 8,3 | |||
| Retired | 2 | 8,3 | 1 | 4,2 | |||
| Stands up at work | 1 | 4,2 | 1 | 4,2 | |||
| Smoking | 0.379‡ | ||||||
| Smoker | 9 | 37,5 | 4 | 16,7 | |||
| Ex-smoker | 1 | 4,2 | 2 | 8,3 | |||
| Duration of smoking (packs/year) | 14.8±8.3 | 16.6±6.5 | 0.681‡ | ||||
| Period of illness (years) | 5.4±5.5 | 7.4±6.6 | 0.244§ | ||||
| Previous treatment, if any | 0.151† | ||||||
| Medical treatment | 13 | 54,2 | 8 | 33,3 | |||
| Physical therapy and rehabilitation | 11 | 45,8 | 16 | 66,7 | |||
| Reflected pain | 1.000† | ||||||
| Hip | 2 | 8,3 | 3 | 12,5 | |||
| Thigh | 1 | 4,2 | 1 | 4,2 | |||
| Leg | 21 | 87,5 | 20 | 83,3 | |||
| Use of analgesic | 0.741† | ||||||
| Paracetamol | 2 | 8,7 | 3 | 13 | |||
| Non-steroidal anti-inflammatory drug | 18 | 78,3 | 19 | 82,6 | |||
| Anticonvulsant | 3 | 13 | 1 | 4,3 | |||
| * p<0.05 is considered statistically significant; SD: Standard deviation; † Pearson Chi-Square test; ‡ t-test; § Mann Whitney-U test. | |||||||
The levels of LDH were evaluated in two groups. In the NSD group, the herniation level was at L3-4 in two patients (8.3%), at L4-5 in six patients (25%), at L5-S1 in nine patients (37.5%), and both at L4-L5 and L5-S1 levels in seven patients (29.2%). These patients were level-specifically treated by inputting these levels into the computer software. In the CMT group, the herniation level was at L3-L4 in three patients (12.5%), at L4-L5 in six patients (25%), at L5-S1 in nine patients (37.5%), and at both L4-L5 and L5-S1 in six patients (25%). There was no statistically significant difference in the level of LDH between the two groups (p=0.924). The patients' and physician's global assessments after the treatment were made using the PGART and IGART scales, respectively. In both groups, the patients and the physicians globally made the most common assessment as marked improvement; however, there was no statistically significant difference between the groups (p=0.804, 0.454, respectively) (Table 2).
Table 2. Patients' and physician's global assessment of response to therapy.
| Treatment applied | |||||
| Conventional motorized | Non-surgical spinal | ||||
| traction (n=24) | decompression (n=24) | ||||
| n | % | n | % | p | |
| Patients’ assessment | 0.804* | ||||
| No change | 2 | 8,3 | 1 | 4,2 | |
| Slight improvement | 5 | 20,8 | 7 | 29,2 | |
| Marked improvement | 16 | 66,7 | 16 | 66,7 | |
| Complete relief-normal | 1 | 4,2 | 0 | 0,0 | |
| Physician’s assessment | 0.454* | ||||
| No change | 1 | 4,2 | 0 | 0,0 | |
| Slight improvement | 3 | 12,5 | 6 | 25,0 | |
| Marked improvement | 20 | 83,3 | 18 | 75,0 | |
| Complete relief-normal | 0 | 0,0 | 0 | 0,0 | |
| * Pearson chi-square test. | |||||
Before the treatment, no statistically significant differences were observed in the pain scores as assessed by the VAS, functional status as assessed by the ODI, the quality of life as assessed by the SF-36, and depression scores as assessed by the BDI between the groups (Table 3). However, after the treatment, there was a statistically significant decline in the VAS (at rest, during movement, and at night), ODI, and BDI scores in both groups as well (p<0.001). Except for the physical role restriction (p=0.028) subgroup of the SF-36 in the NSD group and the pain subgroup (p=0.036) of the SF-36 in the CMT group, there was no statistically significant change in the other subgroups of the SF-36 form.
Table 3. The Visual Analog Scale, Oswestry Disability Index, Beck Depression Index and Short Form-36 scores of patient groups .
| Treatment applied | |||||
| Conventional motorized | Non-surgical spinal | ||||
| traction | decompression | ||||
| Mean±SD | p1 | Mean±SD | p1 | p2 | |
| VAS at rest pre-treatment | 3.9±1.6 | <0.001* | 3.9±1.9 | <0.001* | 0.776‡ |
| VAS at rest post-treatment | 1.6±1.9 | 1.4±1.6 | 0.708‡ | ||
| VAS at night pre-treatment | 2.9±2.3 | <0.001* | 2.9±2.7 | <0.001* | 0.851‡ |
| VAS at night post-treatment | 1.1±1.5 | 0.9±1.3 | 0.617‡ | ||
| VAS during movement pre-treatment | 7.3±1.4 | <0.001* | 6.8±1.8 | <0.001* | 0.317‡ |
| VAS during movement post-treatment | 3.9±1.9 | 3.3±1.7 | 0.342‡ | ||
| BDI pre-treatment | 12.8±10.0 | <0.001* | 10.5±10.0 | <0.001* | 0.339‡ |
| BDI post-treatment | 8.3±8.0 | 7.3±7.6 | 0.665‡ | ||
| ODI pre-treatment | 38.9±26.0 | <0.001* | 31.8±20.6 | <0.001* | 0.445‡ |
| ODI post-treatment | 22.6±15.3 | 18.1±12.2 | 0.445‡ | ||
| SF-36 Physical Function pre-treatment | 45.6±19.4 | 0.135† | 52.5±21.2 | 0.078† | 0.247§ |
| SF-36 Physical Function post-treatment | 37.9±24.0 | 39.8±27.5 | 0.852‡ | ||
| SF-36 Physical Role Difficulty pre-treatment | 69.8±46.0 | 0.076* | 68.8±45.6 | 0.028* | 0.835‡ |
| SF-36 Physical Role Difficulty post-treatment | 51.0±49.2 | 40.6±48.2 | 0.527‡ | ||
| SF-36 Pain pre-treatment | 38.6±17.0 | 0.036† | 35.2±20.2 | 0.167† | 0.531§ |
| SF-36 Pain post-treatment | 48.0±17.3 | 42.2±22.5 | 0.325§ | ||
| SF-36 General Health pre-treatment | 39.9±10.9 | 0.925† | 39.0±13.4 | 0.890† | 0.940‡ |
| SF-36 General Health post-treatment | 38.9±15.5 | 39.6±16.0 | 0.881‡ | ||
| SF-36 Vitality pre-treatment | 48.3±19.9 | 0.232† | 51.3±21.0 | 0.900† | 0.623§ |
| SF-36 Vitality post-treatment | 56.3±19.2 | 52.1±24.3 | 0.513§ | ||
| SF-36 Social Function pre-treatment | 51.6±13.4 | 0.436* | 58.9±12.5 | 0.079* | 0.072‡ |
| SF-36 Social Function post-treatment | 49.0±11.0 | 53.6±9.4 | 0.125‡ | ||
| SF-36 Emotional Role Difficulty pre-treatment | 41.7±50.4 | 0.108* | 36.1±46.0 | 0.463* | 0.722‡ |
| SF-36 Emotional Role Difficulty post-treatment | 23.6±42.3 | 29.2±46.4 | 0.679‡ | ||
| SF-36 Mental Health pre-treatment | 59.0±20.4 | 0.381† | 61.0±18.7 | 0.457† | 0.725§ |
| SF-36 Mental Health post-treatment | 64.7±20.1 | 57.0±21.2 | 0.268‡ | ||
| p1: Comparison within the group (pre-treatment vs post-treatment); p2: Comparison between groups (conventional motorized traction vs non-surgical spinal decompression); * Wilcoxon test; † paired t-test; ‡ Mann-Whitney U test; § t-test; p value of <0.05 is considered statistically significant (shown in bold); VAS: Visual Analog Scale; BDI: Beck Depression Index; ODI: Oswestry Disability Index; SF-36: Short Form-36. | |||||
In addition, there were no statistically significant differences after the treatment between the CMT and NSD groups (Table 3). None of the patients developed any treatment-related complication.
Discussion
Traction is a technique used to stretch soft tissues and to separate joint surfaces or bone fragments by the use of a pulling force.[32] Several physicians have recommended traction for conditions including protruded intervertebral discs, spinal muscle spasm, and general pain and stiffness.[33] Various types of traction are used to treat LBP patients, often in combination with other treatments. The most commonly used traction techniques are mechanic or motorized traction where the traction is exerted by a motorized pulley, and manual traction in which the traction is exerted by the therapist, using his or her body weight to alter the force and direction of the pull.[6] Cyriax used traction treatment in LDH in 1950s, and argued that decompression could be provided by a traction process to relieve the pressure on the joints.[9] In 1985, Onel et al.,[10] in a computed tomography scan, performed a lumbar traction procedure, and reported that continuous traction with a force of 45 kg expanded the disc gap, retarded disk herniation, and opened the facet joint spacing, neural foramen, and spinal canal. The authors concluded that lumbar traction was effective on spinal structures. The DRX9000™ is a relatively new device for motorized traction therapy. In a retrospective study conducted by Apfel et al.,[34] computed tomography was used before and after DRX9000™ NSD in 30 patients with LBP associated with disc herniation, and significant increases were observed in the disc heights after the treatment. In another study including 219 patients with herniated or degenerative disc disease, the authors found that, using DRX9000™, spinal decompression relieved symptoms and restored mechanical function and normalized the range of motion in 86% of the patients who were previously thought to be surgical candidates.[14] In a randomized-controlled study conducted by El-Gendy,[35] experimental group received DRX9000™ treatment, exercises, and ice packs, while the control group received only exercises and ice packs. The authors concluded that DRX9000™ had an effect in pain reduction, although this effect was not statistically significant. In a review by Daniel,[36] the following question was asked: “Does the scientific literature support the efficacy claims made in the advertising media?” This paper opened a debate on the efficacy of spinal decompression therapy and very limited evidence to support the efficacy of NSD therapies, particularly VAX-DTM (Vax-D Medical Technologies LLC Palm Harbor, FL, USA), another trademark of the NSD equipment, was found. Considering the cost-benefit relationship, most of the comprehensively studied and less expensive treatment options were available to the clinician.[36] In the industrialized countries, LBP and sciatica represent one of the leading causes of work unproductivity and disability before the age of 45.[37] Therefore, we did not include patients over the age of 65 in the study. This was because the degenerative changes in the disc, vertebrae, and in the facet joints would worsen the clinical situation associated with LDH in elderly. It is also demonstrated that there is a relationship between disc herniation, disc narrowing visualized by imaging, and work-related physical factors, such as carrying heavy loads, flexion of the trunk, or whole-body vibrations.[37] In the aforementioned study, 54.2% of the patients in the CMT group and 41.7% of the patients in the NSD group did not work at all. This is not consistent with the existing literature.[1-4,37] This can be explained by a relatively small sample size of the aforementioned study and by the fact that 60.4% of the patients in the study were females who were mostly housewives. Each patient included in our study was subjected to MRI examination to support the diagnosis and to classify the disc pathology radiologically, documenting the level of LDH. However, there were no statistically significant differences in the MRI findings including the localization and pathology of LDH between the CMT and NSD groups. Consistent with the literature, the majority of disk herniations were localized at the level of L4-L5 and L5-S1.[16,34] In addition, as our study population consisted of patients with LBP, the primary endpoint of this study was the pain complaint as measured by the VAS. In a retrospective study including 94 patients with chronic discogenic LBP, Macario et al.[16] performed NSD using a DRX9000™ device and, in addition to decompression, all patients received a physical therapy program consisting of hot pack application before treatment and ice application and stretching exercises after treatment. At the end of the study, the VAS scores were reduced from 6.1±2.3) to 0.9±1.2). The amount of analgesics also decreased, and an improvement in their activities of daily life was reported. Sherry et al.[38] evaluated the effectiveness of VAX-DTM, another trade mark of a NSD equipment, in a controlled study in which one group of patients received VAX-DTM treatment and the other group received TENS treatment. The authors defined the treatment success as a reduction of more than 50% in the VAS scores. While a reduction of 68.4% was observed in the VAS in the VAX-DTM group, treatment success in the TENS group was reported to be 0%. Another end point of the present study was the functional status as evaluated by the ODI. Leslie et al.[39] found a significant reduction in the ODI scores after NSD treatment using a DRX9000™ device in 18 patients with chronic LBP. However, in a single- blind, randomized-controlled trial conducted by Schimmel et al.[40] using an Accu-SPINATM (Steadfast Corporation Ltd, Essex, UK) device, which is a similar NSD equipment, a significant reduction was found in the ODI scores; however, there was no statistically significant difference between the treatment and placebo groups. In the present study, the ODI scores after treatment in both treatment groups decreased, compared to the baseline values; however, there was no significant difference between the groups. Patients with LBP are more mentally distressed. Self- reported symptoms of somatization, anxiety, phobic anxiety, obsessive-compulsive disorder, hostility, and depression are all more common among patients with LBP, compared to the general population.[41] In a study conducted by Hung et al.[42] including 225 patients with chronic LBP, depression was the strongest factor associated with the disability. In another study by Hong et al.[43] including the patients with chronic LBP, the BDI scores were found to be higher than in the healthy controls, which adversely affected the quality of life of the patients. Similarly, in the present study, the BDI scores before the treatment were 12.8±10.0 in the CMT group and 10.5±10.0 in the NSD group, indicating mild depression. However, a statistically significant reduction was observed in the BDI scores in both groups after the treatment, although there was no statistically significant difference between the groups. Based on these findings, we believe that this study is valuable, as it assessed the effect of traction therapy on depression. Furthermore, certain improvements were achieved in the present study in pain scores measured by the VAS, in functional status as evaluated by the ODI, and in state of depression as assessed by the BDI. However, no statistically significant differences were found in the quality of life, as measured by the SF-36 in both groups before and after the treatment, except for a physical role limitation subgroup of the SF-36 in the NSD group and a pain subgroup of the SF-36 in the CMT group. We consider that decreased pain, increased functionality, and improved depression would actually lead to a sense of well-being in the quality of life. We also consider that this non-achievement was due to the fact that post-treatment measurements were made too early, immediately after the treatment. In the long- term follow-up, we believe that improved well-being in the daily life activities would reduce pain and increase the functionality. In the global assessment of the illness, 66.7% of the patients in both groups reported marked improvement. In the physician's assessment, 83.3% in the CMT group and 70% in the NSD group reported marked improvement. Therefore, we consider that the reduction in pain and the increase in functional status have been reflected in these global assessment findings. However, there was no statistically significant difference in the patient's and physician's global assessments between the two groups. One of the limitations of this study is the lack of a control group. A control group receiving only hot pack and TENS would have been absolutely necessary, if the primary objective was to establish the effects of either CMT or NSD. However, the primary objective was to compare the effects of these two methods. In addition, hot pack and TENS application to the patients before traction and instructing them in exercise therapy may have led to failure in achieving accuracy in the effectiveness assessment. Nonetheless, we considered that applying traction alone to the patients with chronic pain would not be ethical. In accordance with the manufacturer guidelines, treatment protocol included the instruction on lumbar stretching exercises, myofascial release or heat and/or muscle stimulation prior to DRX9000™ treatment. In addition, there was neither sham CMT group nor sham NSD group in this study, which can be regarded as another limitation. However, blinding of the patients by using sham traction with reduced weights was difficult. At least 26% of the patient's body weight is required to overcome friction. However, sham traction with low weights may provide some relief in addition to the placebo effect.[44] Blinding the assessor after treatment may be the simplest part of the protocol to achieve practical outcomes. Therefore, in the present study, the assessor was blinded. Third limitation of this study is the inability to perform long-term follow-up of the patients; therefore, our next goal would be to obtain long-term results to investigate whether treatment effects are long-lasting. Finally, one other limitation of the present study is that there were no control MRI scans after the treatment sessions due to financial reasons. As the work was done independently of the patient's social insurance, there was no research budget to obtain control MRI scans. On the other hand, despite certain limitations in the present study, we believe that it may prove valuable in that it compares the conventional form of traction treatment employed for many years with the more recent DRX9000™ device system. In conclusion, our study findings show that both CMT and NSD treatments are effective methods in controlling pain, in enhancing functional status, and in reducing depressive mood in patients with chronic LBP associated with LDH. However, the NSD does not appear to be any superior to the CMT in terms of pain, functionality, depression, and quality of life. To the best of our knowledge, this study is the first to compare two different traction modalities; therefore, we believe that it will contribute to the existing literature.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.İnanıcı F. Fiziksel Tıp ve Rehabilitasyon. 2. Ankara: Güneş Tıp Kitabevleri; 2011. Bel ağrısı nedenleri ve muayenesi; pp. 2053–2066. [Google Scholar]
- 2.Oğuz H. Tıbbi Rehabilitasyon. 2. İstanbul: Nobel Tıp Kitabevleri; 2004. Bel ağrıları; pp. 1131–1171. [Google Scholar]
- 3.Ramond-Roquin A, Bouton C, Bègue C, Petit A, Roquelaure Y, Huez JF. Psychosocial Risk Factors, Interventions, and Comorbidity in Patients with Non-Specific Low Back Pain in Primary Care: Need for Comprehensive and Patient- Centered Care. Front Med (Lausanne) 2015;2:73–73. doi: 10.3389/fmed.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun J, Baraliakos X, Regel A, Kiltz U. Assessment of spinal pain. Best Pract Res Clin Rheumatol. 2014;28:875–877. doi: 10.1016/j.berh.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Keleş I. Fiziksel Tıp ve Rehabilitasyon. 2. Ankara: Güneş Tıp Kitabevleri; 2011. Spinal traksiyon; pp. 1091–1108. [Google Scholar]
- 6.Sarı H. Tıbbi Rehabilitasyon. 2. İstanbul: Nobel Tıp Kitabevleri; 2004. Traksiyon; pp. 363–373. [Google Scholar]
- 7.Sarıoğlu S, Dinçer G. Spinal traksiyon. Archives of Rheumatology. 2003;18:116–122. [Google Scholar]
- 8.Duyur B, Erdem H. Traksiyon Tedavisi. Fiziksel Tip. 1999;2:47–52. [Google Scholar]
- 9.Crisp EJ, Cyriax JH, Christie BG. Discussion on the treatment of backache by traction. Proc R Soc Med. 1955;48:805–814. [PMC free article] [PubMed] [Google Scholar]
- 10.Onel D, Tuzlaci M, Sari H, Demir K. Computed tomographic investigation of the effect of traction on lumbar disc herniations. Spine (Phila Pa 1976) 1989;14:82–90. doi: 10.1097/00007632-198901000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Wegner I, Widyahening IS, van Tulder MW, Blomberg SE, de Vet HC, Brønfort G, et al. Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev. 2013;8:003010–003010. doi: 10.1002/14651858.CD003010.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gay RE, Brault JS. Evidence-informed management of chronic low back pain with traction therapy. Spine J. 2008;8:234–242. doi: 10.1016/j.spinee.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 14.Gionis TA, Eric DC. Surgical alternatives: Spinal decompression. Orthopedic Technology Review. 2003;6:36–39. [Google Scholar]
- 15.O'Hara DA. Traction and the DRX9000. Topics in Pain Management. 2009;24:1–6. [Google Scholar]
- 16.Macario A, Richmond C, Auster M, Pergolizzi JV. Treatment of 94 outpatients with chronic discogenic low back pain with the DRX9000: a retrospective chart review. Pain Pract. 2008;8:11–17. doi: 10.1111/j.1533-2500.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- 17.Hong CZ. Physical Medicine and Rehabilitation. 4. Philadelphia: Elsevier Saunders; 2011. Muscle pain syndromes; pp. 971–1001. [Google Scholar]
- 18.Hägg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 19.Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82–82. doi: 10.1186/1471-2474-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutbeyaz ST, Sezer N, Koseoglu F, Kibar S. Low-frequency pulsed electromagnetic field therapy in fibromyalgia: a randomized, double-blind, sham-controlled clinical study. Clin J Pain. 2009;25:722–728. doi: 10.1097/AJP.0b013e3181a68a6c. [DOI] [PubMed] [Google Scholar]
- 21.Fairbank J. Use of Oswestry Disability Index (ODI) Spine (Phila Pa 1976) 1995;20:1535–1537. [PubMed] [Google Scholar]
- 22.Yakut E, Düger T, Oksüz C, Yörükan S, Ureten K, Turan D, et al. Validation of the Turkish version of the Oswestry Disability Index for patients with low back pain. Spine (Phila Pa 1976) 2004;29:581–585. doi: 10.1097/01.brs.0000113869.13209.03. [DOI] [PubMed] [Google Scholar]
- 23.Vianin M. Psychometric properties and clinical usefulness of the Oswestry Disability Index. J Chiropr Med. 2008;7:161–163. doi: 10.1016/j.jcm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Koçyiğit H, Aydemir Ö, Ölmez N, Memiş A. Kısa Form- 36 (KF-36)'nın Türkçe Versiyonunun Güvenilirliği ve Geçerliliği. İlaç ve Tedavi Dergisi. 1999;12:102–106. [Google Scholar]
- 26.Jenkinson C, Wright L, Coulter A. Criterion validity and reliability of the SF-36 in a population sample. Qual Life Res. 1994;3:7–12. doi: 10.1007/BF00647843. [DOI] [PubMed] [Google Scholar]
- 27.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8:968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Hisli N. Beck Depresyon Envanteri'nin geçerliliği üzerine bir çalışma. Psikoloji Dergisi. 1988;6:118–122. [Google Scholar]
- 30.Kılınç S, Torun F. Türkiye'de klinikte kullanılan depresyon değerlendirme ölçekleri. Dirim Tıp Gazetesi. 2011;186:39–47. [Google Scholar]
- 31.Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, et al. Minimal clinically important difference on the Beck Depression Inventory--II according to the patient's perspective. Psychol Med. 2015;45:3269–3279. doi: 10.1017/S0033291715001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brault JS, Kappler RE, Grogg BE. Physical Medicine and Rehabilitation. 4. Philadelphia: Elsevier Saunders; 2011. Manipulation, traction and massage; pp. 427–447. [Google Scholar]
- 33.Madson TJ, Hollman JH. Lumbar Traction for Managing Low Back Pain: A Survey of Physical Therapists in the United States. J Orthop Sports Phys Ther. 2015;45:586–595. doi: 10.2519/jospt.2015.6036. [DOI] [PubMed] [Google Scholar]
- 34.Apfel CC, Cakmakkaya OS, Martin W, Richmond C, Macario A, George E, et al. Restoration of disk height through non-surgical spinal decompression is associated with decreased discogenic low back pain: a retrospective cohort study. BMC Musculoskelet Disord. 2010;11:155–155. doi: 10.1186/1471-2474-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Gendy SR. Impact of spinal decompression on pain in patients with chronic lumbar disc prolapse. Int J Physiother. 2015;2:819–823. [Google Scholar]
- 36.Daniel DM. Non-surgical spinal decompression therapy: does the scientific literature support efficacy claims made in the advertising media. Chiropr Osteopat. 2007;15:7–7. doi: 10.1186/1746-1340-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petit A, Roquelaure Y. Low back pain, intervertebral disc and occupational diseases. Int J Occup Saf Ergon. 2015;21:15–19. doi: 10.1080/10803548.2015.1017940. [DOI] [PubMed] [Google Scholar]
- 38.Sherry E, Kitchener P, Smart R. A prospective randomized controlled study of VAX-D and TENS for the treatment of chronic low back pain. Neurol Res. 2001;23:780–784. doi: 10.1179/016164101101199180. [DOI] [PubMed] [Google Scholar]
- 39.Leslie JB, Pergolizzi JV, Macario A, Apfel JJ, Clair D, Richmond C, et al. Prospective Evaluation of the Efficacy of Spinal Decompression via the DRX9000 for Chronic Low Back Pain. The Journal of Medicine. 2008;1:2–8. [Google Scholar]
- 40.Schimmel JJ, de Kleuver M, Horsting PP, Spruit M, Jacobs WC, van Limbeek J. No effect of traction in patients with low back pain: a single centre, single blind, randomized controlled trial of Intervertebral Differential Dynamics Therapy. Eur Spine J. 2009;18:1843–1850. doi: 10.1007/s00586-009-1044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christensen J, Fisker A, Mortensen EL, Olsen LR, Mortensen OS, Hartvigsen J, et al. Comparison of mental distress in patients with low back pain and a population-based control group measured by Symptoms Check List--A case-referent study. Scand J Public Health. 2015;43:638–647. doi: 10.1177/1403494815581697. [DOI] [PubMed] [Google Scholar]
- 42.Hung CI, Liu CY, Fu TS. Depression: An important factor associated with disability among patients with chronic low back pain. Int J Psychiatry Med. 2015;49:187–198. doi: 10.1177/0091217415573937. [DOI] [PubMed] [Google Scholar]
- 43.Hong JH, Kim HD, Shin HH, Huh B. Assessment of depression, anxiety, sleep disturbance, and quality of life in patients with chronic low back pain in Korea. Korean J Anesthesiol. 2014;66:444–450. doi: 10.4097/kjae.2014.66.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macario A, Pergolizzi JV. Systematic literature review of spinal decompression via motorized traction for chronic discogenic low back pain. Pain Pract. 2006;6:171–178. doi: 10.1111/j.1533-2500.2006.00082.x. [DOI] [PubMed] [Google Scholar]