Abstract
Background
Chronic venous insufficiency (CVI) is a progressive and common disease that affects the superficial and deep venous systems of the lower limbs. CVI is characterised by valvular incompetence, reflux, venous obstruction, or a combination of these with consequent distal venous hypertension. Clinical manifestations of CVI include oedema, pain, skin changes, ulcerations and dilated skin veins in the lower limbs. It can result in a large financial burden on health systems. There is a wide variety of treatment options or therapies for CVI, ranging from surgery and medication to compression and physiotherapy. Balneotherapy (treatments involving water) is a relatively cheap option and potentially efficient way to deliver physical therapy for people with CVI.
Objectives
To assess the efficacy and safety of balneotherapy for the treatment of people with chronic venous insufficiency (CVI).
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, AMED and CINAHL databases, the World Health Organization International Clinical Trials Registry Platform and the Clinical Trials.gov trials register to August 2018. We searched the LILACS and IBECS databases. We also checked references, searched citations and contacted study authors to identify additional studies.
Selection criteria
We included randomised and quasi‐randomised controlled trials comparing balneotherapy with no treatment or other types of treatment for CVI. We also included studies that used a combination of treatments.
Data collection and analysis
Two review authors independently reviewed studies retrieved by the search strategies. Both review authors independently assessed selected studies for complete analysis. We resolved conflicts through discussion. We attempted to contact trial authors for missing data, obtaining additional information. For binary outcomes (leg ulcer incidence and adverse events), we presented the results using odds ratio (OR) with 95% confidence intervals (CI). For continuous outcomes (disease severity, health‐related quality of life (HRQoL), pain, oedema, skin pigmentation), we presented the results as a mean difference (MD) with 95% CI.
Main results
We included seven randomised controlled trials with 891 participants (outpatients in secondary care). We found no quasi‐randomised controlled trials. Six studies (836 participants) evaluated balneotherapy versus no treatment. One study evaluated balneotherapy versus a phlebotonic drug (melilotus officinalis) (55 participants). There was a lack of blinding of participants and investigators, imprecision and inconsistency, which downgraded the certainty of the evidence.
For the balneotherapy versus no treatment comparison, there probably was no improvement in favour of balneotherapy in disease severity signs and symptom score as assessed using the Venous Clinical Severity Score (VCSS) (MD –1.66, 95% CI –4.14 to 0.83; 2 studies, 484 participants; moderate‐certainty evidence). Balneotherapy probably resulted in a moderate improvement in HRQoL as assessed by the Chronic Venous Insufficiency Questionnaire 2 (CVIQ2) at three months (MD –9.38, 95% CI –18.18 to –0.57; 2 studies, 149 participants; moderate‐certainty evidence), nine months (MD –10.46, 95% CI –11.81 to –9.11; 1 study; 55 participants; moderate‐certainty evidence), and 12 months (MD –4.99, 95% CI –9.19 to –0.78; 2 studies, 455 participants; moderate‐certainty evidence). There was no clear difference in HRQoL between balneotherapy and no treatment at six months (MD –1.64, 95% CI –9.18 to 5.89; 2 studies, 445 participants; moderate‐certainty evidence). Balneotherapy probably slightly improved pain compared with no treatment (MD –1.23, 95% CI –1.33 to –1.13; 1 study; 390 participants; moderate‐certainty evidence). There was no clear effect related to oedema between the two groups at 24 days (MD 43.28 mL, 95% CI –102.74 to 189.30; 2 studies, 153 participants; very‐low certainty evidence). There probably was no improvement in favour of balneotherapy in the incidence of leg ulcers (OR 1.69, 95% CI 0.82 to 3.48; 2 studies, 449 participants; moderate‐certainty evidence). There was probably a reduction in incidence of skin pigmentation changes in favour of balneotherapy at 12 months (pigmentation index: MD –3.59, 95% CI –4.02 to –3.16; 1 study; 59 participants; low‐certainty evidence). The main complications reported included erysipelas (OR 2.58, 95% CI 0.65 to 10.22; 2 studies, 519 participants; moderate‐certainty evidence), thromboembolic events (OR 0.35, 95% CI 0.09 to 1.42; 3 studies, 584 participants; moderate‐certainty evidence) and palpitations (OR 0.33, 95% CI 0.01 to 8.52; 1 study; 59 participants; low‐certainty evidence), with no clear evidence of an increase in reported adverse effects with balneotherapy. There were no serious adverse events reported in any of the studies.
For the balneotherapy versus a phlebotonic drug (melilotus officinalis) comparison, we observed no clear difference in pain symptoms (OR 0.29, 95% CI 0.03 to 2.87; 1 study; 35 participants; very low‐certainty evidence) and oedema (OR 0.21, 95% CI 0.02 to 2.27; 1 study; 35 participants; very low‐certainty evidence). This single study did not report on the other outcomes of interest.
Authors' conclusions
We identified moderate‐ to low‐certainty evidence that suggests that balneotherapy may result in a moderate improvement in pain, quality of life and skin pigmentation changes and has no clear effect on disease severity signs and symptoms score, adverse effects, leg ulcers and oedema when compared with no treatment. For future studies, measurements of outcomes such as disease severity sign and symptom score, quality of life, pain and oedema and choice of time points during follow‐up must be standardised for adequate comparison between trials.
Plain language summary
Balneotherapy for chronic venous insufficiency (CVI)
Background
Chronic venous insufficiency is a disease caused by abnormal transport of blood into the veins of the lower limbs, which means the veins cannot pump enough blood back to the heart. This condition is defined by several signs, with gnarled and enlarged veins being the most common and venous ulcers being the most severe. There is a wide variety of management options or therapies for chronic venous insufficiency, ranging from surgery and medicine, to compression (applying force) and physical therapies. Balneotherapy is a possible way to deliver physical therapy for people with chronic venous insufficiency. Balneotherapy is a traditional medical technique that involves water and is usually practiced in spas. It consists of the immersion in mineral water or mud loaded with minerals. It may or may not include exercise. Alone or combined with usual care, balneotherapy may provide a significant improvement in the quality of life of people with chronic venous insufficiency when compared with usual care alone.
Study characteristics
We identified seven randomised controlled trials (studies in which the participants were divided between treatment groups through random method) (most recent search August 2018). Six studies compared balneotherapy versus no treatment, and one study compared balneotherapy versus a medicine called melilotus officinalis. The studies used different types of balneotherapy and different treatment times.
Key results and certainty of the evidence
For the balneotherapy versus no treatment comparison there probably is no improvement in favour of balneotherapy in disease severity signs and symptoms score (moderate‐certainty evidence). Balneotherapy probably improves health‐related quality of life and pain (moderate‐certainty evidence). There probably is no improvement in favour of balneotherapy for leg ulcers (moderate‐certainty evidence). There is no clear effect related to oedema (swelling caused when fluid leaks out of the body's tiny blood vessels) between balneotherapy and no treatment (very low‐certainty evidence). Balneotherapy probably reduces skin pigmentation changes (low‐certainty evidence). None of the studies reported any serious adverse events. There were fewer side effects (infection and blood clots in the legs) in people receiving balneotherapy compared to no treatment.
When comparing balneotherapy with melilotus officinalis, there were insufficient data to detect clear differences between the two treatments for pain and oedema in the single small study. There were no data available for the other outcomes of interest such as disease severity signs and symptoms score, quality of life, leg ulcers and skin pigmentation.
The certainty of the evidence was affected by the small number of trials with few participants and the impossibility of blinding of participants and physicians conducting the balneotherapy treatment, which could have led to bias.
Summary of findings
Background
See Appendix 1 for a glossary of terms.
Description of the condition
Chronic venous insufficiency (CVI) occurs when the normal transport of superficial or deep venous blood is disturbed, causing venous hypertension and haemodynamic disturbances. This resultant inability to maintain pressure and flow to the heart in the venous system is largely responsible for the symptoms of the disease. This health condition is defined by several signs, with varicose veins the most common, and venous ulcers the most severe. Oedema, venous eczema, hyperpigmentation of the ankle skin, atrophie blanche and lipodermatosclerosis may also be seen (Bergan 2006; Perrin 2016). It is thought that valve reflux plays a role in the aetiology of CVI, with chronic endothelial inflammation and subsequent localised dysfunction reducing the synthesis of anti‐inflammatory agents, and potentially increasing the expression of proinflammatory molecules and cytokines, which also contribute to the disease (Beebe‐Dimmer 2005; Castro‐Ferreira 2018; Lee 2016). In CVI, the increase in ambulatory venous hypertension, with subsequent activation of endothelial cells, extravasation of macromolecules and erythrocytes, leukocyte diapedesis, tissue oedema and chronic inflammatory changes, may result in oedema, hyperpigmentation, lipodermatosclerosis, eczema or venous ulcers (Gloviczki 2011).
CVI is progressive, and has a high prevalence in the economically active population, but its impact on the quality of life of an affected individual is poorly understood (Rossi 2015). Prevalence of varicose veins in the UK is between 20% and 40% in adults (Carroll 2013). The prevalence of venous ulcers in the general population is between 1% and 1.5%, rising to 5% in people over 80 years old. Venous ulcers can be extremely long‐lasting, with about 20% of ulcers failing to heal after two years, and 8% failing to heal after five years (Carroll 2013). Nicolaides 2014 reported a prevalence of varicose veins between 25% and 33% in women, and between 10% and 20% in men. The prevalence of more severe stages of CVI, such as oedema and cutaneous alterations, ranged from 3% to 11% (Nicolaides 2014).
The most commonly used classification in CVI is called CEAP, which was adopted worldwide to facilitate communication on CVI, and serve as a basis for a scientific analysis of the alternatives for treating the disease. It is based on clinical manifestations (C), aetiological factors (E), anatomical distribution (A) and pathophysiological findings (P). This classification assists in the systematic approach and orientation in the daily clinical investigation of people with CVI, as a system of ordered documentation, and basis for decisions regarding the appropriate treatment (Eklöf 2004).
There is a wide variety of management options or therapies for CVI, ranging from surgery and medication, to compression, balneotherapy and physical therapies.
Traditional CVI treatments include varicose vein surgery, foam sclerotherapy, and endovenous laser and radiofrequency ablation. Surgical removal is a common procedure for the problem, but has been associated with neuropathy, scarring, infection, bruising, deep venous thrombosis, pain and prolonged postoperative recovery. Foam sclerotherapy is considered faster but less effective than the conventional surgical option. Because they are minimally invasive, ablative techniques are increasingly used, and offer potential benefits, such as reduced complications, faster recovery and fewer physical limitations, with lower recurrence rates, compared to conventional surgical techniques (Carroll 2013).
Medical treatments for the management of CVI include phlebotonic or venoactive drugs (e.g. flavonoids, such as horse chestnut, rutosides, and hesperidin; Gloviczki 2011; Martinez‐Zapata 2016; Pittler 2012).
Compression, balneotherapy (treatment involving water) and physical therapy can improve blood flow by increasing tissue pressure and improved local lymphatic drainage. This decreases venous hypertension, with an improvement in inflammation and stasis (Wong 2012). There are several different types of vascular compression therapy for use in CVI, ranging from simple wraps to graduated elastic stockings, which can be knee or thigh length (Konschake 2016; Motykie 1999).
Bathing in natural mineral or thermal waters appears to have positive effects related to specific properties of immersion in water, which are due to hydrostatic pressure, osmotic pressure and temperature (Caggiati 2018a). The effects of balneotherapy are based on both the chemical and physical properties of the agents (Gutenbrunner 2010).
Physical therapy is aimed at restoring the function of the calf muscle pump and improving health‐related quality of life (HRQoL), and offers a useful adjunct treatment (Carpentier 2009). Reduced mobility of the ankle and decreased function of the calf muscle pump are associated with the progressive severity of CVI. The aim of these therapies is to obtain a persistent increase in the efficacy of the mechanisms facilitating venous return (Caggiati 2018a). Structured fitness to improve limb muscle strength and ankle mobility may improve venous haemodynamics, mobility and well‐being by improving muscle pump function (Padberg 2004). The strengthening of the lower limb muscles may lead to beneficial changes in venous haemodynamics, allowing the reduction of blood flow, functional venous volume and residual volume fraction, and increased blood ejection fraction (Da Silva 2010).
Adherence to physical and compression therapy is not always good, especially in the hot season (Gloviczki 2011). These techniques are considered successful, but the recurrence rates are high, ranging from 21% to 67% in compression therapy, which suggests factors beyond patient education in non‐use (Raju 2007).
Physical therapies or other types of non‐conventional therapies have not been mentioned in recent guidelines on CVI. However, there is increasing evidence of the role of these modalities in preventing disease progression and in optimising the results of surgical and pharmacological treatments (Caggiati 2018a).
Description of the intervention
Treatments involving water (balneotherapy) have been used for centuries and are widely used today (Blain 2016). In people with severe diseases, such as rheumatoid arthritis and osteoarthritis, balneotherapy helps to improve physical function and pain relief (Verhagen 2007; Verhagen 2015).
The aims of balneotherapy in people with CVI are to improve range of joint motion, relieve muscle spasm and maintain or improve functional mobility (Carpentier 2014; Gutenbrunner 2010).
In some countries, balneotherapy is a popular way of treating CVI, but its efficacy has not yet been fully evaluated (Angoules 2014). For example, in France, more than 60,000 people are treated annually in this way (Carpentier 2009). The term balneotherapy is classically used to mean bathing in thermal or mineral waters, and differs from hydrotherapy in some contexts. However, since the beginning of the 20th century, both terms have been accepted for all forms of water treatment (Johnson 1990; Verhagen 2007). Balneotherapy is also defined as the use of natural mineral waters, gases and peloids (natural organic‐mineral products formed in the course of geological processes) (Pasek 2010). Equivalent terms are hydrotherapy and crenobalneotherapy (Forestier 2014). The substances used for balneotherapy are medical mineral waters (hypothermal (less than 35 °C), isothermal (35 °C to 36 °C) and hyperthermal (more than 36 °C)), medical peloids (including peat, fango (of volcanic origin), mud (from sea, lakes or river beds)), clay and natural gases (carbon dioxide, hydrogen sulphide and radon) (Gutenbrunner 2010). Treatment is based on specific properties of the mineral water, such as hydrostatic pressure, osmotic pressure and water temperature.
Traditionally, the treatment is delivered as a three‐week course in a spa resort specialising in the treatment of people with CVI. Treatment regimens usually consist of four balneotherapy sessions per day, six days a week, for three weeks. The types of balneotherapy sessions are chosen by spa physicians for each patient, according to his or her needs and capabilities, and combines active and intensive balneotherapy, using mineral waters with a dedicated patient education programme (Carpentier 2009).
In the literature, different researchers use different types of balneotherapy; there is no consensus on a rigid session therapy protocol or treatment sequence.
For example, a complete balneotherapy treatment may include one or more of the following regimens (Blain 2016; Carpentier 2009):
whirlpool bath with automatic air and water massage;
controlled walking in semi‐deep water (to increase mobility and balance of joints, walking on a carpet of small air bubbles to stimulate proprioception and microcirculation, walking against water flow to increase the venous return by pump calf muscles);
balance therapy, using an irregular sloping surface (promoting stimulation of the plantar arch and venous pump, relaxation of the ankles and improvement of limb physical perception, with improvement of venous pumping);
bath with strong underwater massaging jets;
massage by physiotherapist under a light spray shower;
simple bath;
massage by physiotherapist with limbs underwater;
application of thermal mud;
gymnastics in deep water.
How the intervention might work
Balneotherapy combines many procedures using mineral water; movement within the pool aims to restore muscle pump action, and the hydrostatic pressure may decrease oedema. Underwater massages and Kneipp technique (alternate hot and cold showers) stimulate the cutaneous vasomotor response, and underwater exercises may benefit aggravating locomotor factors, including knee or ankle ankyloses (Forestier 2014).
Hydrostatic pressure acts on the tissues and exerts a compression of blood vessels, which may aid venous return and reduction of oedema and pain (Becker 2009; Forestier 2014). Underwater sonography of legs has shown that immersion in water reduces the diameter of normal and varicose veins, increases spontaneous flow and decreases reflux when present (Caggiati 2018b). Heat and the buoyancy of water can block pain signals, by acting on thermal and mechanoreceptor receptors and increasing blood flow. There is also the mental relaxation associated with hydrotherapy that promotes pain improvement (Bender 2005), and underwater exercises improve aggravating locomotor factors and restore muscle pump (Forestier 2014). It has been shown that calf strengthening improves muscle endurance, and may even restore proper muscle pump function, with increased ejection fraction and reduced residual fraction (Caggiati 2018a).
The largest randomised controlled trial (RCT) in the field to date has shown that balneotherapy provides a significant improvement in clinical symptoms and quality of life for people with advanced CVI, for at least one year of follow‐up (Carpentier 2014).
Why it is important to do this review
There is a high prevalence of varicose veins and other signs of CVI, such as oedema, skin changes or venous ulcerations, which result in a large financial burden on health systems (Gloviczki 2011). Balneotherapy is a relatively cheap and efficient way to deliver physical therapy (Klick 2008). Balneotherapy, either alone or combined with usual care, may provide a significant improvement in the quality of life of people with CVI, when compared with usual care alone. This treatment is usually well tolerated, especially for those who do not consistently wear their compression stockings, or those for whom there is no surgical solution (Forestier 2014). This type of therapy may also be of great value in people with CVI with few available therapeutic options (Blain 2016). This review reports the available evidence of the effectiveness and safety of balneotherapy to allow healthcare professionals and consumers to make informed decisions on treatment methods for CVI, and highlights any uncertainties about this treatment.
Objectives
To assess the efficacy and safety of balneotherapy for the treatment of people with chronic venous insufficiency (CVI).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs that compared balneotherapy for CVI with no treatment or other types of treatment.
Types of participants
We included adults, who were at least 18 years of age, diagnosed with CVI (primary or post‐thrombotic), with evidence of venous incompetence, demonstrated by ultrasound duplex examination, with at least a significant reflux.
We excluded people with a contraindication to spa treatment (cardiac or renal failure, immunodeficiency, psychiatric disorders, limited walking ability). We also excluded people with oedema of non‐venous origin (clinical lymphoedema, cardiac failure, hypoalbuminaemia), symptomatic neurological diseases of the lower limbs (neurogenic pain or abnormal neurological examination of the lower limbs), or with significant peripheral arterial disease (ankle‐brachial index (ABI) less than 0.90).
Types of interventions
We included studies that evaluated balneotherapy treatment, defined as bathing in natural mineral or thermal waters. Because of its many treatment options, combinations and duration, there is currently no detailed definition of balneotherapy. Therefore, we included any type of balneotherapy treatment described by the study authors. We included studies that compared balneotherapy versus placebo or no treatment, and compared treatment methods against each other. We included comparisons with other treatments such as:
placebo or no treatment;
compression therapy (including elastocompression, mechanical compression);
phlebotonic drugs (including flavonoids or synthetic products in any dose or frequency);
any other treatment.
Treatments may have been used in combination, as long as the comparison treatments were balanced across groups and balneotherapy was the differentiating treatment.
Types of outcome measures
Primary outcomes
Disease severity signs and symptoms score (measured using any validated instrument, such as the Venous Clinical Severity Score (VCSS; Rutherford 2000). See Table 3.
Health‐related quality of life (HRQoL, measured using any validated instrument, such as the Chronic Venous Insufficiency Questionnaire 2 (CVIQ2) or EuroQol (EQ‐5D); Brooks 1996; Launois 1996).
Adverse events of treatment (including palpitations, superficial thrombosis, infection or erysipelas, risk of falling).
1. Venous Clinical Severity Score (VCSS).
| Clinical descriptor | Absent (0) | Mild (1) | Moderate (2) | Severe (3) |
| Pain | None | Occasional | Daily not limiting | Daily limiting |
| Varicose veins | None | Few | Calf or thigh | Calf and thigh |
| Venous oedema | None | Foot and ankle | Below knee | Knee and above |
| Skin pigmentation | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Inflammation | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Induration | None | Limited perimalleolar | Diffuse lower 1/3 calf | Wider above lower 1/3 calf |
| Number of active ulcers | None | 1 | 2 | ≥ 3 |
| Ulcer duration | None | < 3 month | 3–12 month | > 1 year |
| Active ulcer size | None | < 2 cm | 2–6 cm | > 6 cm |
| Compression therapy | None | Intermittent | Most days | Fully comply |
Secondary outcomes
Pain (measured using validated visual analogue scales (VAS); patient‐graded pain from no discomfort at 0, to unbearable at 10).
Oedema (measured by validated scales, such as VAS, perimeter or volume of the leg).
Incidence of leg ulcer.
Skin pigmentation changes (measured using validated methods, including skin chromametry).
We reported the time points presented in the studies.
Search methods for identification of studies
We applied no restrictions on language of publication.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web searched from inception to 7 August 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2018, Issue 7);
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) (searched from 1 January 2017 to 7 August 2018);
Embase Ovid (searched from 1 January 2017 to 7 August 2018);
AMED Ovid (searched from 1 January 2017 to 7 August 2018);
CINAHL Ebsco (searched from 1 January 2017 to 7 August 2018).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 2.
The Information Specialist searched the following trials registries on 7 August 2018:
the World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch);
ClinicalTrials.gov (clinicaltrials.gov).
The review authors searched LILACS (Latin American and Caribbean Health Science Information database) and IBECS (Indice Bibliográfico Españ̃ol de Ciencias de la Salud), both at lilacs.bvsalud.org/ on 15 August 2018. See Appendix 3 for details of the search strategy used. We did not used a filter, but selected the RCTs manually in the LILACS and IBECS databases. Three review authors (MAMS, LCUN and FM) configured this search strategy. The review authors, in collaboration with the Cochrane Brazil Information Specialist, searched these databases.
Searching other resources
We checked the bibliographies of included trials for further references to relevant trials. We contacted specialists in the field and authors of the included trials for any possible unpublished data.
Data collection and analysis
Selection of studies
Two review authors (MAMS, LCUN) independently reviewed studies retrieved by the search strategies and assessed if the trials met the selection criteria, based on title, abstract, or both. Both review authors independently assessed selected studies for complete analysis. We resolved conflicts through discussion, and if necessary, by involving a third review author, who had the final vote (LLC or FM). We reviewed all studies without an abstract in full text. We included studies only published as an abstract if sufficient data were available to determine study eligibility. We attempted to contact the authors of the abstract for further information. We presented a PRISMA flow diagram to show the process of trial selection.
Data extraction and management
Two review authors (MAMS and LCUN) independently extracted the data, transcribing it onto pre‐established collection forms. We resolved disagreements by discussion within the review team. We collected the following information.
Characteristics of the study: details of the publication (e.g. year, country, authors, journal), study design, population data (e.g. age, comorbidities, CEAP classification of venous disease, duration of disease, history of previous treatments), details of intervention (e.g. type of therapy, duration of therapy), adverse events (palpitations, superficial thrombosis, erysipelas, risk of falling), number of participants allocated to each treatment group, duration of follow‐up, cost of treatment.
Results: outcomes measured, time points at which outcomes were assessed, HRQoL measurement (e.g. CVIQ2 or EQ‐5D).
Assessment of risk of bias in included studies
Two review authors (LLC and FM) independently assessed all the included trials using Cochrane's 'Risk of bias' tool, described in Section 8.5 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011). We evaluated the following sources of risk: random sequence generation, concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other bias. We assessed each item according to what was reported in each individual study, and decided if they were at high, low or unclear risk of bias. We contacted the study author(s) to seek clarification in cases of uncertainty over methodology or data.
Measures of treatment effect
For binary outcomes (leg ulcer incidence and adverse events), we presented the results using odds ratio (OR) with 95% confidence intervals (CI). For the continuous outcomes (disease severity, HRQoL, pain, oedema, skin pigmentation), we presented the results as a mean difference (MD) with 95% CI. If studies have not used the same scales, we presented the results as a standardised mean difference (SMD) with 95% CI.
Unit of analysis issues
We considered each participant as the unit of analysis. For trials that considered multiple interventions in the same group, we only analysed the data of interest.
Dealing with missing data
We noted partial and incomplete data on the data collection form, and took this into account when assessing the overall quality of the study. We also tried to contact the study authors for further information. We reported missing data in the Characteristics of included studies tables, and we used intention‐to‐treat analysis.
Assessment of heterogeneity
We quantified inconsistency among the pooled estimates using the I² statistic, which examines the percentage of total variation across trials due to heterogeneity rather than variation due to chance (Higgins 2011). We interpreted the thresholds for the I² statistic as follows: less than 30% = low heterogeneity, 30% to 60% = moderate heterogeneity, 60% to 90% = substantial heterogeneity and more than 90% = considerable heterogeneity (Higgins 2011). If studies differ methodologically and clinically, it may be preferable not to pool the results.
Assessment of reporting biases
If, for futures updates, we are able to include more than 10 studies in the meta‐analysis, we intend to assess the presence of publication bias and other reporting bias using funnel plots. If asymmetry is present, we intend to explore possible causes, including publication bias, poor methodological quality and true heterogeneity (Higgins 2011).
Data synthesis
We carried out statistical analysis using Review Manager 5 software (Review Manager 2014). We used a random‐effects model to synthesise the data because of the complexity of the intervention and differences in existing balneotherapy regimens. We used OR if the data were dichotomous, or a difference between means if the data were continuous. In cases where it was not possible to pool data using the meta‐analysis, we described the data narratively.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses to consider the following.
Age.
Gender.
Severity of CVI.
Duration of treatment.
Diabetes.
Obesity.
Osteomuscular diseases.
Post‐thrombotic syndrome.
Sensitivity analysis
If sufficient studies were identified, we planned to conduct sensitivity analysis, depending on the study characteristics identified during the review process. We planned to carry out sensitivity analyses by excluding trials at high risk of bias for all domains (Higgins 2011).
'Summary of findings' table
Using GRADEpro GDT software, we prepared 'Summary of findings' tables to present the key information for balneotherapy versus other treatments in participants with CVI (GRADEpro GDT). We created one table for each treatment comparison. We included the following outcomes in each table.
Disease severity signs and symptoms score.
HRQoL.
Adverse events of treatment.
Pain.
Oedema.
Incidence of leg ulcer.
Skin pigmentation changes.
We assessed the certainty of the evidence for each outcome as high, moderate, low or very low, based on the criteria of risk of bias, inconsistency, indirectness, imprecision and publication bias, using the GRADE approach (GRADE 2004). We based the tables on methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions, and planned to justify any departures from the standard methods (GRADE 2004; Higgins 2011).
Results
Description of studies
Results of the search
The search identified 621 records after duplicates removed. After reading the titles and abstracts, we excluded 595 irrelevant records and considered 26 full‐text articles for eligibility. We excluded 12 studies (13 articles), with reasons given in the Characteristics of excluded studies table. We included seven studies (nine articles) which met the protocol inclusion criteria (see Figure 1 for PRISMA flow diagram).
1.
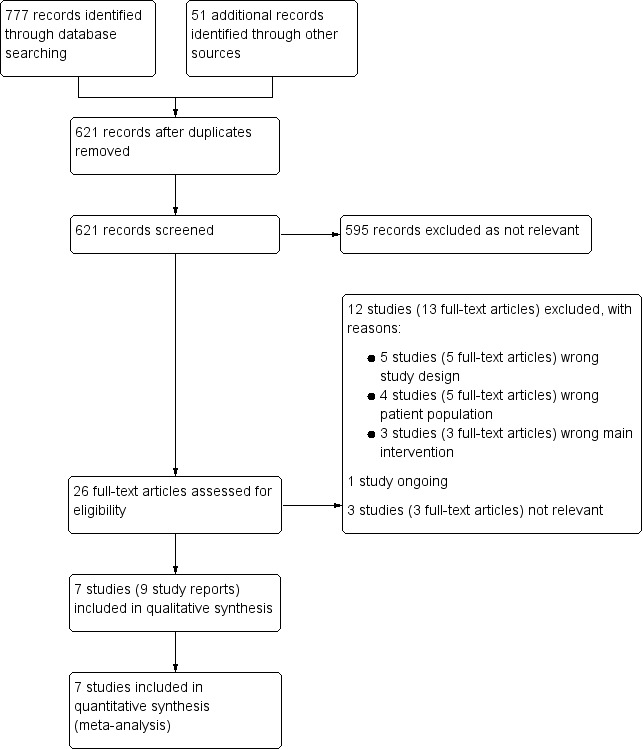
Study flow diagram.
Included studies
Type of study
Final selection, based on consensus, resulted in inclusion of seven studies. All selected studies were RCTs published between 1991 and 2014 (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Forestier 2014; Mancini 2003; Stefanini 1996). We identified no quasi‐RCTs. All seven included studies evaluated people with CVI. Only three studies calculated the sample size (Carpentier 2009; Carpentier 2014; Forestier 2014). See Characteristics of included studies table.
Setting
Two studies were conducted in Austria (Ernst 1991; Ernst 1992), two in Italy (Mancini 2003; Stefanini 1996), and three in France (Carpentier 2009; Carpentier 2014; Forestier 2014).
Unit of analysis
All studies used participants as a unit of analysis.
Study participants
The seven studies provided data for 891 participants. The number of participants varied from 59 (Carpentier 2009) to 425 (Carpentier 2014). All studies evaluated people with CVI. Inclusion and exclusion criteria of the included studies varied widely. Most of the studies excluded people with serious comorbidities that would compromise a balneotherapy programme.
Carpentier 2009 and Carpentier 2014 evaluated people with skin changes, but no active ulcer (CEAP C4 and C5). Forestier 2014 evaluated people diagnosed with CVI CEAP C3 or C4. Mancini 2003 evaluated people with CVI C1 to C5. The other studies did not mention the CEAP classification.
All studies evaluated women and men, with women being the majority.
Mancini 2003 reported the age range without the mean (range 19 to 78 years). The mean age of the other studies ranged from 55 to 65.1 years.
Intervention
Studies involved different types of balneotherapy treatments for both legs such as walking in the pool or basin, underwater massage, bath in a tub and showering the legs, continuous cold water, intermittent cold and warm water, associated with lower limb exercises, administered gel onto the skin or even educational workshops.
Six studies compared balneotherapy with no treatment (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Forestier 2014; Mancini 2003). One study compared a phlebotonic drug (melilotus officinalis) versus balneotherapy (Stefanini 1996).
The duration of the follow‐up ranged from 15 days (Stefanini 1996) to 18 months (Carpentier 2014).
We found no eligible studies of mechanical compression or any other treatment listed in our protocol (de Moraes Silva 2018).
Outcomes
Two studies measured disease severity signs and symptoms score using the VCSS (Carpentier 2014; Forestier 2014). Four studies evaluated HRQoL and adverse events of treatment (including palpitations, superficial thrombosis and erysipelas (Carpentier 2009; Carpentier 2014; Forestier 2014; Mancini 2003). One study described pain using a score from 0 to 3 (Stefanini 1996). Two studies reported pain measured using VAS, ranging 0 to 10 (Carpentier 2009; Carpentier 2014). Two studies reported oedema measured using leg volume or leg circumference (Ernst 1991; Ernst 1992), and one study reported the number of people with oedema (Stefanini 1996). Two studies mentioned the incidence of ulcers (Carpentier 2009; Carpentier 2014). One study mentioned skin pigmentation changes (Carpentier 2009).
Funding
Three trials obtained funding or support from spas or thermes associations (Carpentier 2014; Forestier 2014; Stefanini 1996). We were unclear if this could have influenced the conduct and results of the studies.
Excluded studies
In total, we excluded 12 studies because: they were not RCTs (Aquino 2016; Blain 2016; Coccheri 2002; Costantino 2003; Roques 2012); they did not include people with CVI (Carpentier 2002; Hartmann 1993; Hartmann 1995; NCT00348907); and participants did not receive balneotherapy as the main treatment (Brock 2001; Hartmann 1991; Schumann 2011). See Characteristics of excluded studies table.
Ongoing studies
We identified one ongoing study (NCT02553720). See Characteristics of ongoing studies table.
Risk of bias in included studies
Figure 2 and Figure 3 provide an overall summary of bias present within each of the included studies (see also Characteristics of included studies table for further details).
2.
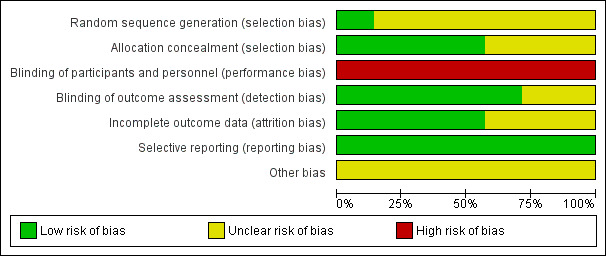
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
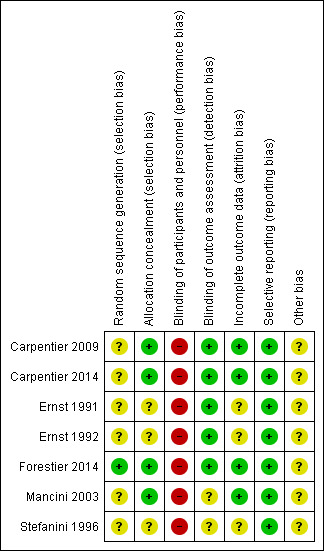
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All studies reported were RCTs, but only one was at low risk of bias (Forestier 2014). The remaining six studies did not provide details about the generation of random sequences in addition to a statement of 'randomised' (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Mancini 2003; Stefanini 1996).
Allocation concealment
Only four included studies were at low risk of bias (Carpentier 2009; Carpentier 2014; Forestier 2014; Mancini 2003). The other three studies were considered at unclear risk of bias due to lack of information (Ernst 1991; Ernst 1992; Stefanini 1996).
Blinding
Because the nature of exercise‐based studies involves performing an activity versus standard care, medication or another intervention, it is impossible to blind the participants and the professionals who perform the therapeutic approach. All included studies were therefore at high risk of performance bias.
However, five studies were blinded to the evaluators of the results obtained with the proposed treatments, and were therefore at low risk of detection bias (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Forestier 2014). The other two included studies had an unclear risk of bias due to the lack of reporting of blinding of outcome assessors (Mancini 2003; Stefanini 1996).
For self‐assessment outcomes (e.g. HRQoL and pain), the outcome assessments were not blinded as blinding of the participants was not possible due to the nature of the intervention.
Incomplete outcome data
Three studies provided no details about incomplete outcome data (Ernst 1991; Ernst 1992; Stefanini 1996). We considered them at unclear risk of bias. In the other four studies, all exclusions were reported with reasons and by study group (Carpentier 2009; Carpentier 2014; Forestier 2014; Mancini 2003). We considered these at low risk of attrition bias.
Selective reporting
All seven included studies reported all their planned outcomes and were at low risk of bias (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Forestier 2014; Mancini 2003; Stefanini 1996).
Other potential sources of bias
Three trials obtained funding or support from spas or a thermes associations (Carpentier 2009; Forestier 2014; Stefanini 1996). In this way, it was unclear if this could have influenced the studies. The four remaining studies were also at unclear risk of bias as we identified no other potential sources of bias (Carpentier 2009; Ernst 1991; Ernst 1992; Mancini 2003).
Effects of interventions
Summary of findings for the main comparison. Balneotherapy compared to no treatment for chronic venous insufficiency.
| Balneotherapy compared to no treatment for chronic venous insufficiency | ||||||
| Patient or population: people with chronic venous insufficiency Setting: outpatient Intervention: balneotherapy Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with balneotherapy | |||||
| Disease severity signs and symptoms score –VCSS Scale: 0–27 follow‐up: range 3–12 months | The mean disease severity sign and symptom score was 8.21 | MD 1.66 lower (4.14 lower to 0.83 higher) | — | 484 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,b | — |
| Health‐related quality of life –CIVIQ2 Scale: 20–100 follow‐up: mean 3 months | The mean health‐related quality of life score was 53.8 | MD 9.38 lower (18.18 lower to 0.57 lower) | — | 149 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,b | — |
|
Adverse events of treatment –thromboembolic event follow‐up: range 3–12 months |
Study population | OR 0.35 (0.09 to 1.42) | 584 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | No serious adverse events documented. | |
| 40 per 1000 | 15 per 1000 (4 to 56) | |||||
| Pain assessed with: VAS Scale: 0–10 follow‐up: mean 3 months | The mean pain score was 4.96 | MD 1.23 lower (1.33 lower to 1.13 lower) | — | 390 (1 RCT) | ⊕⊕⊕⊝ Moderatea,b | — |
| Oedema follow‐up: 24 days | The mean oedema was 3065.5 mL | MD 43.28 mL higher (102.74 lower to 189.30 higher) | — | 153 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | — |
|
Incidence of leg ulcer follow‐up: 12 months |
Study population | OR 1.69 (0.82 to 3.48) | 449 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,b | — | |
| 58 per 1000 | 94 per 1000 (48 to 175) | |||||
|
Skin pigmentation changes ‐ pigmentation index follow‐up: 12 months |
The mean skin pigmentation changes – pigmentation index was 6.57 | MD 3.59 lower (4.02 lower to 3.16 lower) | — | 59 (1 RCT) | ⊕⊕⊝⊝ Lowb,d | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CIVIQ2: Chronic Venous Disease Quality of life questionnaire; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; VCSS: Venous Chronic Severity Score. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe number of studies was small but no unexplained inconsistency was detected. bDowngraded one level for lack of blinding of participants and investigators. cDowngraded one level for inconsistency. dDowngraded one level for imprecision (number of participants fewer than 400).
Summary of findings 2. Balneotherapy compared to melilotus officinalis for chronic venous insufficiency.
| Balneotherapy compared to melilotus officinalis for chronic venous insufficiency | ||||||
| Patient or population: people with chronic venous insufficiency Setting: outpatient Intervention: balneotherapy Comparison:melilotus officinalis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with melilotus officinalis | Risk with balneotherapy | |||||
| Disease severity signs and symptoms score | See comment | See comment | — | — | — | The single study in this comparison did not assess this outcome. |
| Health‐related quality of life | See comment | See comment | — | — | — | The single study in this comparison did not assess this outcome. |
| Adverse events of treatment | See comment | See comment | — | — | — | The single study in this comparison did not assess this outcome. No serious adverse events were documented. |
| Pain assessed with: % of participants follow‐up: median 15 days | Study population | OR 0.29 (0.03 to 2.87) | 35 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 200 per 1000 | 68 per 1000 (7 to 418) | |||||
|
Oedema
assessed with: % participants follow‐up: median 15 days |
Study population | OR 0.21 (0.02 to 2.27) | 35 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | — | |
| 200 per 1000 | 50 per 1000 (5 to 362) | |||||
| Incidence of leg ulcer | See comment | See comment | — | — | — | The single study in this comparison did not assess this outcome. |
| Skin pigmentation changes | See comment | See comment | — | — | — | The single study in this comparison did not assess this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision (number of participants fewer than 400). bDowngraded one level for lack of blinding of participants and investigators.
The data presented in the study papers, even after communication with authors of some of the studies, were too scarce to allow analysis 'between groups' in a global way. In addition, the studies used a wide variety of outcome measures; therefore, interventions and outcome measures were considered heterogeneous.
Balneotherapy versus no treatment
Six studies included a control group receiving no treatment (Carpentier 2009; Carpentier 2014; Ernst 1991; Ernst 1992; Forestier 2014; Mancini 2003). In all of the studies, the participants and the investigators could not be blinded.
Primary outcomes
Disease severity signs and symptoms score
Carpentier 2014 showed that after one year of treatment the improvement in the VCSS score was greater in the balneotherapy group (MD –1.2, 95% CI –1.6 to –0.8) compared to the control group (MD –0.6, 95% CI –1.0 to –0.2). Forestier 2014 also reported improvement in the VCSS in data in the balneotherapy group compared with the control group (P < 0.0001). However, the time points were different between both trials: 12 months in Carpentier 2014 and three months in Forestier 2014. For Carpentier 2014, we used Plot Digitizer software (Plot Digitizer) to extract the relevant data from the study publication.
Pooling the two studies showed there probably was no improvement in favour of balneotherapy in disease severity signs and symptoms score (MD –1.66, 95% CI –4.14 to 0.83; I² = 94%; 2 studies, 484 participants; moderate‐certainty evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 1 Disease severity signs and symptom score (VCSS).
Health‐related quality of life
Three studies used the CIVIQ2 HRQoL analysis tool (Carpentier 2009; Carpentier 2014; Forestier 2014). The questions of the CIVIQ2 result in a global score with higher scores reflecting more severe impairment. For Carpentier 2009, we used Plot Digitizer software (Plot Digitizer) to extract the relevant data from the study publication.
Pooled results of these studies showed that there was probably moderate improvement in HRQoL with balneotherapy compared with no treatment at three months (MD –9.38, 95% CI –18.18 to –0.57; I² = 88%; 2 studies, 149 participants; moderate‐certainty evidence), at nine months (MD –10.46, 95% CI –11.81 to –9.11; 1 study, 55 participants; moderate‐certainty evidence) and 12 months (MD –4.99, 95% CI –9.19 to –0.78; I² = 97%; 2 studies, 445 participants; moderate‐certainty evidence). There was no clear difference between balneotherapy and no treatment at six months (MD –1.64, 95% CI –9.18 to 5.89; I² = 100%; 2 studies, 445 participants; moderate‐certainty evidence) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 2 Health‐related quality of life (CIVIQ2).
One study reported HRQoL using EQ‐5D (Carpentier 2014). Data presented in the paper showed the differences between baseline and follow‐up values for the balneotherapy and no treatment groups in favour of balneotherapy at six months (MD 0.030, 95% CI 0.026 to 0.034; 1 study; 390 participants; low‐certainty evidence) and 12 months (MD 0.040, 95% CI 0.037 to 0.044; 1 study; 390 participants; low‐certainty evidence) (Analysis 1.3; Figure 4).
1.3. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 3 Health‐related quality of life (EQ‐5D).
4.
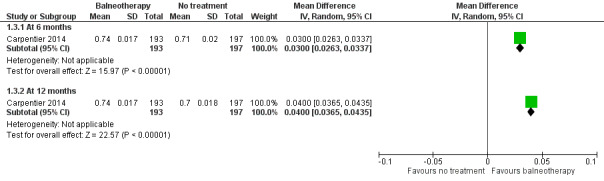
Forest plot of comparison: 1 Balneotherapy versus no treatment, outcome: 1.3 Health‐related quality of life (EQ‐5D).
Mancini 2003 analysed HRQoL at six months using SF‐36 (Wood‐Dauphinee 1999). It showed perception of "physical role" and "social functioning" were improved in both groups. The study authors also reported an improvement in the value related to "bodily pain" only in group balneotherapy in this study. Only "social functioning" may have improved HRQoL in favour of balneotherapy (MD 22.50, 95% CI 11.96 to 33.04; 1 study; 37 participants; low‐certainty evidence), but it was just one study with few participants (Analysis 1.4).
1.4. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 4 Health‐related quality of life (SF‐36).
Adverse events of treatment
No studies reported serious adverse events.
Four studies reported adverse events (Carpentier 2009; Carpentier 2014; Forestier 2014; Mancini 2003). These included erysipelas (OR 2.58, 95% CI 0.65 to 10.22; 2 studies, 519 participants; moderate‐certainty evidence), thromboembolic events (OR 0.35, 95% CI 0.09 to 1.42; 3 studies, 584 participants; moderate‐certainty evidence) and palpitations (OR 0.33, 95% CI 0.01 to 8.52; 1 study; 59 participants; low‐certainty evidence) (Analysis 1.5). There was no clear evidence of an increase in reported adverse effects with balneotherapy.
1.5. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 5 Adverse events of treatment.
Secondary outcomes
Pain
Two studies reported on pain (Carpentier 2009; Carpentier 2014). We were unable to pool the data as there was insufficient information.
Carpentier 2014 presented the differences between the VAS scores of baseline and six months and between six months and 12 months. We used the data reported in the study publication to extract three‐month data (complete statistical data with mean and standard deviations (SD)) using Plot Digitizer software (Plot Digitizer). Carpentier 2014 showed that pain probably improved slightly with balneotherapy compared with no treatment at three months' follow‐up (MD –1.23, 95% CI –1.33 to –1.13; 1 study; 390 participants; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 6 Pain.
Carpentier 2009 reported that weekly self‐evaluation VAS assessments showed improvement in leg symptoms from week 4 to week 52 in the balneotherapy group (P < 0.001).
Oedema
Two studies analysed oedema through leg volumetry at 12 days (MD 91.46 mL, 95% CI –0.65 to 183.58; 2 studies, 182 participants; very low‐certainty evidence) and 24 days (MD 43.28 mL, 95% CI –102.74 to 189.30; 2 studies, 153 participants; very low‐certainty evidence) (Analysis 1.7), or through minimal ankle circumference measures of the leg at 24 days (SMD 0.76, 95% CI –0.90 to 2.42; I² = 96%; 2 studies, 182 participants; low‐certainty evidence; Analysis 1.8) (Ernst 1991; Ernst 1992). There was no clear effect due to imprecision and differences in care between the two studies.
1.7. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 7 Oedema (leg volume).
1.8. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 8 Oedema (leg circumference) (24 days).
Incidence of leg ulcer
Two studies showed the incidence of leg ulcer at 12 months (Carpentier 2009; Carpentier 2014). There was probably no improvement in favour of balneotherapy in the incidence of leg ulcers (OR 1.69, 95% CI 0.82 to 3.48; 2 studies, 449 participants; moderate‐certainty evidence; Analysis 1.9).
1.9. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 9 Incidence of leg ulcer.
Skin pigmentation changes
One study analysed skin pigmentation changes (Carpentier 2009). There was a small difference in favour of balneotherapy at 12 months in erythema index (MD –1.42, 95% CI –1.60 to –1.24; 1 study, 59 participants; low‐certainty evidence) and pigmentation index (pigmentation index: MD –3.59, 95% CI –4.02 to –3.16; 1 study, 59 participants; low‐certainty evidence; Analysis 1.10).
1.10. Analysis.
Comparison 1 Balneotherapy versus no treatment, Outcome 10 Skin pigmentation changes.
Balneotherapy versus melilotus officinalis
One study compared balneotherapy versus melilotus officinalis versus balneotherapy plus melilotus officinalis (Stefanini 1996). For this study, we used the data from the melilotus officinalis group and balneotherapy groups only.
Primary outcomes
Disease severity signs and symptoms score
The study did not report disease severity signs and symptoms score.
Health‐related quality of life
The study did not report HRQoL.
Adverse events of treatment
The study reported no serious adverse events.
Secondary outcomes
Pain
Meta‐analysis found no clear difference in pain (OR 0.29, 95% CI 0.03 to 2.87; 1 study, 35 participants; very low‐certainty evidence; Analysis 2.1). However, Stefanini 1996 did not use standardised scales and reported only the percentage of participants reporting the presence of the symptom at a 15‐day endpoint.
2.1. Analysis.
Comparison 2 Balneotherapy versus melilotus officinalis, Outcome 1 Pain.
Oedema
Meta‐analysis found no clear difference in oedema (OR 0.21, 95% CI 0.02 to 2.27; 1 study, 35 participants; very low‐certainty evidence; Analysis 2.2). However, Stefanini 1996 did not use standardised scales and reported only the percentage of participants reporting the presence of the symptom at a 15‐day endpoint.
2.2. Analysis.
Comparison 2 Balneotherapy versus melilotus officinalis, Outcome 2 Oedema.
Incidence of leg ulcer
The study did not report incidence of leg ulcer.
Skin pigmentation changes
The study did not report skin pigment changes.
Subgroup and sensitivity analysis
We were unable to perform subgroup due to lack of information of the trials and the small number of studies identified.
Discussion
Summary of main results
We studied balneotherapy in the treatment of CVI in seven trials. Unfortunately, most studies had moderate power and had methodological flaws.
Low‐ to moderate‐certainty evidence showed that balneotherapy was probably more beneficial when compared with no treatment for pain, HRQoL and skin pigmentation changes. There was probably no improvement in favour of balneotherapy on disease severity signs and symptoms score, leg ulcers, oedema and adverse events.
When comparing balneotherapy with the phlebotonic drug melilotus officinalis, there were insufficient data to detect clear differences between treatment groups for pain and oedema in the single study with 35 participants. There were no data available for the other outcomes of interest such as disease severity signs and symptoms score, HRQoL, leg ulcers and skin pigmentation.
The certainty of evidence was affected by the small number of trials with few participants and the impossibility of blinding of participants and physicians conducting the balneotherapy treatment. These limitations weakened the applicability of the evidence and should be considered in interpreting the results.
Overall completeness and applicability of evidence
We included seven studies in this review. Many studies reported on one or two outcomes of interest for this review. The studies ranged from 15 days to 18 months, with outcomes reported at different time points. In addition, studies used different scales to assess HRQoL and pain. Together with the small number of included studies and the impossibility of blinding of participants and physicians conducting the balneotherapy treatment, the applicability of the evidence was weakened and should be considered when interpreting the results.
Quality of the evidence
We judged the overall certainty of the evidence to be very low to moderate according to the GRADE approach, as described in the Cochrane Handbook for Systematic Reviews of Interventions (GRADE 2004; Higgins 2011).
Balneotherapy versus no treatment
The certainty of the evidence for the outcomes disease signs and symptom score, HRQoL, pain and leg ulcer was decreased by one level to moderate for lack of blinding of participants and investigators. The certainty of evidence for thrombotic events, erysipelas and palpitation was also downgraded by one level to moderate for lack of blinding of participants and investigators. The certainty of evidence for oedema was downgraded by three levels to very low certainty because of lack of blinding of participants and investigators, inconsistency (differences in care) and imprecision. The certainty of the evidence for erythema index and pigmentation index was downgraded by two levels to low because of lack of blinding of participants and investigators and imprecision. See Table 1.
Balneotherapy versus melilotus officinalis
The certainty of the evidence for pain and oedema was downgraded three levels for lack of blinding of participants and investigators (one level) and imprecision (two levels). See Table 2.
In addition, small samples and lack of information in some trials limited the analysis of subgroups based on age, gender, severity of CVI, diabetes, obesity, osteomuscular diseases and post‐thrombotic syndrome. It was not possible to evaluate with confidence the variation in the effectiveness of balneotherapy in relation to these parameters. But it was possible to perform subgroup analysis based on follow‐up time.
Older studies have been reported more poorly, as journal reporting criteria were previously less rigorous, resulting in greater inherent bias.
Potential biases in the review process
The study publications of Carpentier 2009 and Carpentier 2014 showed that mean and SDs values relating to the outcomes disease severity signs and symptoms (VCSS) (Carpentier 2014), quality of life (CIVIQ2) (Carpentier 2009), and pain (Carpentier 2014) were not extensively reported in the text. Therefore, we used the data extraction tool Plot Digitizer to extract the relevant data for these outcomes.
Ernst 1991 did not report SDs for oedema (minimal ankle difference) for the balneotherapy group. Following the Cochrane Handbook for Systematic Reviews of Interventions, we used the SD from the other study in this comparison to allow us to pool the data (Ernst 1992; Higgins 2011).
Agreements and disagreements with other studies or reviews
We are not aware of previous reviews of balneotherapy for CVI.
Our review showed similar results relating to quality of life and pain as non‐randomised studies evaluating balneotherapy in people with CVI (Aquino 2016; Coccheri 2002). Aquino 2016 analysed the effects of aquatic exercises on quality of life in 16 participants with CVI. Aquino 2016 observed that the balneotherapy improved quality of life and reduced pain. Coccheri 2002 analysed 70 people with CVI divided into two groups in a non‐randomised trial of treatment with balneotherapy or compression stockings, showing improvement in quality of life in the group receiving balneotherapy.
Authors' conclusions
Implications for practice.
This review provided evidence of low to moderate certainty in the choice of balneotherapy in selected people with chronic venous insufficiency (CVI) in quality of life, pain and changes in skin pigmentation. The effects were demonstrated after two to three weeks of balneotherapy. Although there is no standardisation of balneotherapy exercises and therapies adopted, all trials cite similar therapeutic methodologies. Most studies report positive results, but provide insufficient evidence to support data, with small numbers of participants and limited data. The scientific evidence is insufficient due to the high risk of bias in most studies and the lack of adequate statistical analysis.
Implications for research.
We believe that it is essential and possible to carry out randomised trials assessing the effectiveness of balneotherapy with low risk of bias in order to provide solid evidence for the treatment of CVI. High‐quality research is needed, focusing on appropriate allocation concealment, blinding and an adequate data presentation and analysis. The design and reporting of future trials should conform to the CONSORT statement and include:
clearer definitions of sample size calculation and randomisation of groups;
blinding of the outcome assessor who will perform the analysis of the data. It should be noted that due to the treatment methodology it is not possible to blind participants and spa physicians;
evaluation of specific target groups such as people with severe forms of CVI and post‐thrombotic syndrome;
assessment after the balneotherapy session (on average three weeks), medium‐term periods of three to six months and long‐term periods (12 months);
standardise and clearly describe the therapeutic sequence of balneotherapy (types of activities performed, frequency and duration of treatment);
the cost implications for the balneotherapy, and comparison with other methods;
use quality of life scores directed to the CVI (e.g. Aberdeen Varicose Vein Questionnaire score) in outcomes;
evaluation of adherence and compliance to rehabilitation protocols during and after balneotherapy treatment.
Notes
Parts of the methods section of this review were based on a standard template established by Cochrane Vascular.
Acknowledgements
We would like to thank Cochrane Vascular for support in the preparation of the review, and for the designing and running the search strategy.
We like to thank Cochrane Brazil, the Coordination of Superior Level Staff Improvement – Brazil (CAPES), the Division of Interdisciplinary Surgery and Vascular Surgery of Universidade Federal de São Paulo, Brazil and Division of Vascular Surgery of Hospital de Clínicas de Itajubá, Minas Gerais, Brazil for their methodological support.
We would like to thank Seleno Glauber de Jesus‐Silva for assistance in data extraction with Plot Digitizer software.
We would like to thank Lydia Jones and Larissa Rackl for the German translation and Henrique Jorge Guedes Neto for the Italian translation of trials.
We would like to thank Dr Patrick H Carpentier and Dr Roman Jacques Forestier for the availability and answers related to the trials in which they were responsible.
The review authors, and the Cochrane Vascular editorial base, are grateful to the following peer reviewers for their time and comments: Dr Alberto Caggiati, Sapienza University of Rome, Rome, Italy, and Dr Arianne P Verhagen, University of Technology Sydney, Sydney, Australia.
Appendices
Appendix 1. Glossary of terms
| Term | Definition |
| Atrophie blanche | Small smooth ivory‐white areas on the skin with hyperpigmented borders and telangiectasias. |
| Ankyloses | Stiffness of a joint due to abnormal adhesion and rigidity of the bones of the joint. |
| CEAP | Comprehensive classification system developed to allow uniform diagnosis and comparison of patient populations with chronic venous disorders; created by an international ad hoc committee of the American Venous Forum in 1994. CEAP stands for clinical manifestations (C), aetiological factors (E), anatomical distribution (A) and pathophysiological findings (P). |
| Chronic venous insufficiency | Medical condition in which the veins cannot pump enough blood back to the heart. |
| Compression therapy | Application of an elastic garment around the leg. |
| Diapedesis | Passage of blood cells through unruptured walls of a vein or artery into the tissues. |
| Erysipelas | Acute infection, typically with a skin rash, usually on any of the legs and toes, face, arms or fingers. |
| Erythema | Superficial reddening of the skin. |
| Fibrosis | The thickening and scarring of connective tissue. |
| Hyperpigmentation | Increased pigmentation of an area of the skin. |
| Lipodermatosclerosis | Inflammation caused by fibrosis of subcutaneous fat. |
| Lymphoedema | Collection of fluid that causes swelling (oedema) in the arms and legs. |
| Oedema | Excess of watery fluid collecting in the tissue of the body, swelling caused when fluid leaks out of the body's capillaries. |
| Placebo | Substance or treatment with no active therapeutic effect. |
| Salso‐bromojodinated | Type of thermal water |
| Superficial thrombosis | Inflammatory thrombotic disorder in which a thrombus develops in a vein located near the surface of the skin. |
| Thrombosis | Local coagulation or clotting of the blood in a part of the circulatory system. |
| Ultrasound duplex | Non‐invasive evaluation of blood flow through your arteries and veins. |
| Varicose veins | Gnarled, enlarged veins |
| Vascular | Relating to blood vessels |
| Venoactive drugs | Heterogeneous group of medicinal products, which have effects on symptoms related to chronic venous disease. |
| Venous | Relating to a vein |
| Venous eczema | Long‐term skin condition that affects the lower legs. |
Appendix 2. Search strategies
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Venous Insufficiency EXPLODE ALL TREES 505 #2 MESH DESCRIPTOR Varicose Veins EXPLODE ALL TREES 997 #3 MESH DESCRIPTOR Saphenous Vein EXPLODE ALL TREES 643 #4 ((varicos* near3 (vein* or veno*))):TI,AB,KY 981 #5 ((tortu* near3 (vein* or veno*))):TI,AB,KY 10 #6 ((incomp* near3 (vein* or veno* or saphenous or valv*))):TI,AB,KY 113 #7 ((insuffic* near3 (vein* or veno* or saphenous))):TI,AB,KY 181 #8 (((saphenous or vein* or veno*) near3 reflux)):TI,AB,KY 180 #9 GSV:TI,AB,KY 167 #10 CVI:TI,AB,KY 186 #11 CVD:TI,AB,KY 3668 #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 6139 #13 MESH DESCRIPTOR Hydrotherapy EXPLODE ALL TREES 1419 #14 MESH DESCRIPTOR Balneology EXPLODE ALL TREES 500 #15 aqua*:TI,AB,KY 1273 #16 Balneo*:TI,AB,KY 311 #17 Bath:TI,AB,KY 1651 #18 bathe*:TI,AB,KY 127 #19 bathing:TI,AB,KY 475 #20 Baths:TI,AB,KY 531 #21 Hydrotherap*:TI,AB,KY 349 #22 spa:TI,AB,KY 752 #23 thalasso*:TI,AB,KY 8 #24 water:TI,AB,KY 18907 #25 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 23427 #26 #12 AND #25 113 |
113 |
| Clinicaltrials.gov | venous insufficiency OR Varicose Veins OR Saphenous Vein | Balneotherapy OR Hydrotherapy OR Balneology OR spa OR bathe OR water | 14 |
| ICTRP Search Portal | venous insufficiency OR Varicose Veins OR Saphenous Vein | Balneotherapy OR Hydrotherapy OR Balneology OR spa OR bathe OR water | 14 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to 2017 and 2018 only | 1 exp Venous Insufficiency/ 7108 2 exp Varicose Veins/ 17218 3 exp Saphenous Vein/ 14727 4 (varicos* adj3 (vein* or veno*)).ti,ab. 7082 5 (tortu* adj3 (vein* or veno*)).ti,ab. 370 6 (incomp* adj3 (vein* or veno* or saphenous or valv*)).ti,ab. 2733 7 (insuffic* adj3 (vein* or veno* or saphenous)).ti,ab. 5254 8 ((saphenous or vein* or veno*) adj3 reflux).ti,ab. 1688 9 GSV.ti,ab. 826 10 CVI.ti,ab. 2042 11 CVD.ti,ab. 28771 12 or/1‐11 70067 13 exp HYDROTHERAPY/ 19186 14 exp BALNEOLOGY/ 12057 15 aqua*.ti,ab. 70303 16 Balneo*.ti,ab. 2194 17 Bath.ti,ab. 28050 18 bathe*.ti,ab. 3306 19 bathing.ti,ab. 9584 20 Baths.ti,ab. 5410 21 Hydrotherap*.ti,ab. 919 22 spa.ti,ab. 9699 23 thalasso*.ti,ab. 594 24 water.ti,ab. 674200 25 or/13‐24 786154 26 12 and 25 857 27 randomised controlled trial.pt. 465683 28 controlled clinical trial.pt. 92537 29 randomized.ab. 417870 30 placebo.ab. 190650 31 drug therapy.fs. 2036014 32 randomly.ab. 294778 33 trial.ab. 434925 34 groups.ab. 1819498 35 or/27‐34 4252204 36 26 and 35 186 37 from 36 keep 1‐186 186 |
186 |
| Embase 1974 to 2017 and 2018 only | 1 exp vein insufficiency/ 9339 2 exp varicosis/ 43945 3 exp saphenous vein/ 12097 4 (varicos* adj3 (vein* or veno*)).ti,ab. 7947 5 (tortu* adj3 (vein* or veno*)).ti,ab. 549 6 (incomp* adj3 (vein* or veno* or saphenous or valv*)).ti,ab. 3468 7 (insuffic* adj3 (vein* or veno* or saphenous)).ti,ab. 7302 8 ((saphenous or vein* or veno*) adj3 reflux).ti,ab. 2361 9 GSV.ti,ab. 1191 10 CVI.ti,ab. 2767 11 CVD.ti,ab. 42140 12 or/1‐11 109127 13 exp hydrotherapy/ 3234 14 exp balneotherapy/ 9146 15 aqua*.ti,ab. 75281 16 Balneo*.ti,ab. 1890 17 Bath.ti,ab. 33110 18 bathe*.ti,ab. 3781 19 bathing.ti,ab. 10647 20 Baths.ti,ab. 5906 21 Hydrotherap*.ti,ab. 1165 22 spa.ti,ab. 13945 23 thalasso*.ti,ab. 539 24 water.ti,ab. 745033 25 or/13‐24 849677 26 12 and 25 1268 27 randomised controlled trial/ 486960 28 controlled clinical trial/ 459266 29 random$.ti,ab. 1251195 30 randomisation/ 79315 31 intermethod comparison/ 218636 32 placebo.ti,ab. 262317 33 (compare or compared or comparison).ti. 439023 34 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1677534 35 (open adj label).ti,ab. 61506 36 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 200501 37 double blind procedure/ 143969 38 parallel group$1.ti,ab. 20831 39 (crossover or cross over).ti,ab. 89611 40 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 271025 41 (assigned or allocated).ti,ab. 319194 42 (controlled adj7 (study or design or trial)).ti,ab. 280127 43 (volunteer or volunteers).ti,ab. 217129 44 trial.ti. 233839 45 or/27‐44 3854663 46 26 and 45 344 47 from 46 keep 1‐344 344 |
344 |
| AMED | 1 exp Venous insufficiency/ 52 2 exp Varicose veins/ 67 3 exp Veins/ 119 4 (varicos* adj3 (vein* or veno*)).ti,ab. 27 5 (tortu* adj3 (vein* or veno*)).ti,ab. 0 6 (incomp* adj3 (vein* or veno* or saphenous or valv*)).ti,ab. 1 7 (insuffic* adj3 (vein* or veno* or saphenous)).ti,ab. 45 8 ((saphenous or vein* or veno*) adj3 reflux).ti,ab. 1 9 GSV.ti,ab. 1 10 CVI.ti,ab. 14 11 CVD.ti,ab. 115 12 or/1‐11 386 13 exp Hydrotherapy/ 743 14 aqua*.ti,ab. 351 15 Balneo*.ti,ab. 81 16 Bath.ti,ab. 267 17 bathe*.ti,ab. 14 18 bathing.ti,ab. 195 19 Baths.ti,ab. 104 20 Hydrotherap*.ti,ab. 226 21 spa.ti,ab. 148 22 thalasso*.ti,ab. 9 23 water.ti,ab. 2835 24 or/13‐23 4100 25 12 and 24 9 26 exp CLINICAL TRIALS/ 3766 27 RANDOM ALLOCATION/ 314 28 DOUBLE BLIND METHOD/ 661 29 Clinical trial.pt. 1212 30 (clinic* adj trial*).tw. 5410 31 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2849 32 PLACEBOS/ 590 33 placebo*.tw. 3118 34 random*.tw. 17631 35 PROSPECTIVE STUDIES/ 1109 36 or/26‐35 22657 37 25 and 36 3 |
3 |
| CINAHL 2017 and 2018 only | S42 S26 AND S41 79 S41 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 344,225 S40 MH "Random Assignment" 39,141 S39 MH "Triple‐Blind Studies" 86 S38 MH "Double‐Blind Studies" 24,907 S37 MH "Single‐Blind Studies" 8,017 S36 MH "Crossover Design" 11,250 S35 MH "Factorial Design" 921 S34 MH "Placebos" 8,370 S33 MH "Clinical Trials" 92,987 S32 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,528 S31 TX crossover OR "cross‐over" 14,627 S30 AB placebo* 28,502 S29 TX random* 220,471 S28 TX trial* 252,003 S27 TX "latin square" 143 S26 S12 AND S25 221 S25 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 61,911 S24 TX water 32,549 S23 TX thalasso* 17 S22 TX spa 17,585 S21 TX Hydrotherap* 1,287 S20 TX Baths 6,384 S19 TX bathing 3,318 S18 TX bathe* 396 S17 TX Bath 6,384 S16 TX Balneo* 715 S15 TX aqua* 3,962 S14 (MH "Balneology") 379 S13 (MH "Hydrotherapy+") 4,233 S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 9,839 S11 TX CVD 5,527 S10 TX CVI 468 S9 TX GSV 17 S8 TX (saphenous or vein* or veno*) n3 reflux 74 S7 TX insuffic* n3 (vein* or veno* or saphenous) 887 S6 TX incomp* n3 (vein* or veno* or saphenous or valv*) 112 S5 TX tortu* n3 (vein* or veno*) 18 S4 TX varicos* n3 (vein* or veno*) 736 S3 (MH "Saphenous Vein") 548 S2 (MH "Varicose Veins+") 2,495 S1 (MH "Venous Insufficiency+") 686 |
79 |
Appendix 3. LILACS and BECS search strategy
(mh: (hydrotherapy) OR mh: (hidroterapia) OR (bath* whirlpool) OR (hydrotherapies) OR (e02.779.492*) OR (e02.831.535.492*) OR (hp3.018.148*) OR mh: (balneology) OR mh: (balneología) mh: (balneologia) OR (balneotherapy) OR (e02.056*) OR (hp3.018.091*) OR mh: (physical therapy modalities) OR mh: (modalidades de fisioterapia) OR (modalidades de fisioterapia) OR (neurological physiotherapy) OR (neurophysiotherapy) OR (physical therapy techniques) OR (modalit* physical therapy) OR (physical therapy technique*) OR (physiotherap* techniques) OR (physiotherapy neurological) OR (e02.779*) OR (e02.831.535*)) AND (mh: (venous insufficiency) OR mh: (insuficiencia venosa) OR mh: (insuficiência venosa) OR (insufficienc* venous) OR (c14.907.952*) OR mh: (varicose veins) OR mh: (várices) OR mh: (varizes) OR (varix) OR (varicose vein*) OR (varices) OR (c14.907.927*) OR mh: (edema) OR (anasarca) OR (dropsy) OR (hydrops) OR (c23.888.277*) OR mh: (venous thrombosis) OR mh: (trombosis de la vena) OR mh: (trombose venosa) OR (deep venous thrombosis) OR (deep‐vein thrombos*) OR (deep‐venous thrombos*) OR (deep vein thrombos*) OR (deep venous thrombos*) OR (phlebothrombos*) OR (thrombos* venous) OR (c14.907.355.830.925*) OR mh: (postthrombotic syndrome) OR mh: (síndrome postrombótico) mh: (síndrome pós‐trombótica) OR (venous stasis syndrome) OR (syndrome postthrombotic) OR (syndrome venous stasis) OR (c14.907.355.830.925.462*) OR (c14.907.952.880*) OR mh: (venous thromboembolism) OR mh: (tromboembolia venosa) OR mh: (tromboembolia venosa) OR (thromboembolism venous) OR (c14.907.355.590.700*) OR mh: (embolism AND thrombosis) OR mh: (embolia y trombosis) OR mh: (embolia e trombose) OR (thrombosis AND embolism) OR (c14.907.355*)) AND (instance:"regional") AND ( db:("LILACS" OR "IBECS"))
Data and analyses
Comparison 1. Balneotherapy versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity signs and symptom score (VCSS) | 2 | 484 | Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐4.14, 0.83] |
| 2 Health‐related quality of life (CIVIQ2) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 2 | 149 | Mean Difference (IV, Random, 95% CI) | ‐9.38 [‐18.18, ‐0.57] |
| 2.2 At 6 months | 2 | 445 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐9.18, 5.89] |
| 2.3 At 9 months | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐10.46 [‐11.81, ‐9.11] |
| 2.4 At 12 months | 2 | 445 | Mean Difference (IV, Random, 95% CI) | ‐4.99 [‐9.19, ‐0.78] |
| 3 Health‐related quality of life (EQ‐5D) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 1 | 390 | Mean Difference (IV, Random, 95% CI) | 0.03 [0.03, 0.03] |
| 3.2 At 12 months | 1 | 390 | Mean Difference (IV, Random, 95% CI) | 0.04 [0.04, 0.04] |
| 4 Health‐related quality of life (SF‐36) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Physical functioning | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐22.48, 17.48] |
| 4.2 Physical role | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐18.35, 16.95] |
| 4.3 Bodily pain | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 8.75 [‐3.94, 21.44] |
| 4.4 General health | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.23 [‐6.47, 12.93] |
| 4.5 Vitality | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.73 [‐8.82, 16.28] |
| 4.6 Social functioning | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 22.5 [11.96, 33.04] |
| 4.7 Emotional role | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 12.36 [‐14.09, 38.81] |
| 4.8 Mental health | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 5.11 [‐6.91, 17.13] |
| 5 Adverse events of treatment | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Erysipelas | 2 | 519 | Odds Ratio (M‐H, Random, 95% CI) | 2.58 [0.65, 10.22] |
| 5.2 Thromboembolic event | 3 | 584 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.09, 1.42] |
| 5.3 Palpitations | 1 | 59 | Odds Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.52] |
| 6 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 At 3 months (visual analogue scale) | 1 | 390 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.33, ‐1.13] |
| 7 Oedema (leg volume) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 At 12 days | 2 | 182 | Mean Difference (IV, Random, 95% CI) | 91.46 [‐0.65, 183.58] |
| 7.2 At 24 days | 2 | 153 | Mean Difference (IV, Random, 95% CI) | 43.28 [‐102.74, 189.30] |
| 8 Oedema (leg circumference) (24 days) | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.76 [‐0.90, 2.42] |
| 9 Incidence of leg ulcer | 2 | 449 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.82, 3.48] |
| 10 Skin pigmentation changes | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Pigmentation index (12 months) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐3.59 [‐4.02, ‐3.16] |
| 10.2 Erythema index (12 months) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.60, ‐1.24] |
Comparison 2. Balneotherapy versus melilotus officinalis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carpentier 2009.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 59 participants (29 in balneotherapy group and 30 control group) Start date: no details given Duration of participation: 15 months End date: no details given Method of randomisation: centralised randomisation was performed after the inclusion visit, and its result kept hidden from the investigators. Blinding: participants – no; spa physician – yes; outcome assessors – yes Power calculation: calculated the number of participants required as 20 in each group (using a bilateral test, with α = 0.05, β = 0.15). This hypothesis was based on poorly documented potential outcomes (worsening by 15% of the pigmentation in the control group vs 5% in the treated group, with a 10% standard deviation). The authors decided to include 60 participants in order to accommodate potential problems of underestimation of variability and possible dropouts. Losses to follow‐up: 4 withdrew soon after randomisation and refused the follow‐up. 1 late refusal (woman from the treatment group because of personal difficulties in the organisation of her stay in the spa resort). The 3 others came from participants from the control group: 1 for an unexplained personal reason; 1 because of the onset of family problems and 1 because he expected an immediate spa treatment and did not accept the result of the randomisation. Unit of allocation: participants Source of funding: no details given |
|
| Participants |
Setting: outpatients (second care) Country: France Gender: 19 men and 40 women Age: mean age 59.3 years in balneotherapy group and 62.5 years in control group Inclusion criteria: people with primary or post‐thrombotic CVI with skin changes but no active ulcer (C4 or C5), and the evidence of a venous incompetence demonstrated by ultrasound duplex examination with at least a significant reflux (> 1‐second duration in standing position) in the superficial, deep, perforator veins, or a combination of these. Aged ≥18 years, living in Grenoble area and willing to participate (written informed consent) in the study (to perform a 3‐week course of spa treatment of in La Léchère resort and a follow‐up of 15 months' duration including a weekly self‐administered questionnaire and a medical examination every 3 months). Exclusion criteria: prior spa treatment course, if a surgical or endovascular treatment of the venous disease was planned for the study time course or had been performed < 6 months prior to the inclusion visit. People with contraindication of spa treatment (life‐threatening disease, cardiac or renal failure, immunodeficiency, psychiatric disorders, strong limitation of ambulation), oedema of non‐venous origin (clinical lymphoedema, cardiac failure, hypoalbuminaemia), symptomatic neurological diseases of the lower limbs (neurogenic pain or abnormal neurological examination of the lower limbs), or significant peripheral arterial disease (ankle‐brachial index < 0.90). |
|
| Interventions |
Technique: 4 balneotherapy sessions per day, 6 days per week for 3 weeks. During the stay, the participant also participated in 2 or 3 × 90‐minute educational workshops performed according to a previously published method. Balneotherapy group: 3‐week spa treatment course in La Léchère soon after randomisation. Control group: during comparison period no treatment; followed by spa treatment after the comparison period (i.e. starting soon after day 365) Duration of follow‐up: comparison period 1 year; total duration of follow‐up: 15 months Cointerventions: during the whole study time course, participants of both groups remained attended by their usual physicians, who provided them with any care they thought useful for their participants. |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
All outcomes were measured every 6 months, except pain, which was measured every 5 weeks. |
|
| Identification |
Authors name: Patrick H Carpentier Institution: Clinique Universitaire de Médecine Vasculaire, Pôle Pluridisciplinaire de Médecine, Centre Hospitalier Universitaire de Grenoble, and the Centre de Recherche Universitaire de la Léchère. email: patrick.carpentier@ujf‐grenoble.fr |
|
| Notes | The study authors confirmed this trial used a different population from the study reported in Carpentier 2014 (personal communication by email, 5 February 2019). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Low risk | Central allocation (results kept hidden from investigator). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data management performed blindly, including a final blind review regarding protocol deviations and missing data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons and by study group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No evidence of other bias. |
Carpentier 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 425 participants (214 in balneotherapy group and 211 control group) Start date: no details given Duration of participation: 18 months End date: no details given Method of randomisation: centralised randomisation. Blinding: participant – no; spa physician – yes; assessor outcomes – yes Power calculation: the number of participants to be included was calculated from the expected reduction in the incidence of leg ulcers. The hypothesis was based on a yearly incidence of 20% in the control group compared with 10% in the treated group, with an alpha risk of 5% and a power of 80%. 199 participants were needed for the comparison, and the authors decided to include 440 participants to allow for dropouts. Total number of participants randomised: 425 Total number of participants analysed: 390 Losses to follow‐up: 35 losses (14 in control group; 21 in spa treatment group) Number of withdrawals and reasons: control group – 11 withdrew consent, 2 dropped out, 1 early stop. Spa treatment – 19 withdrew consent, 1 dropped out, 1 early stop Unit of allocation: participants Source of funding: no details given |
|
| Participants |
Setting: outpatients (second care) Country: France (multicentre) Gender: 186 men and 239 women Age (mean): 63.5 years in balneotherapy group and 65.1 years in control group Inclusion criteria: people with primary or post‐thrombotic CVI with skin changes but no active ulcer (C4a, C4b, or C5) and evidence of venous incompetence demonstrated by duplex ultrasound examination with ≥ 1 significant reflux (of > 1‐second duration in a standing position) in the superficial veins, deep veins, perforator veins, or a combination of these. Aged ≥ 18 years and willing to participate (written informed consent) in the study (to attend a 3‐week course of spa treatment in 1 of the 12 French spa resorts treating people with CVI) and to accept a follow‐up of 18 months' duration including a monthly self‐administered questionnaire and a medical examination every 6 months. Exclusion criteria: people with surgical or endovascular treatment of the venous disease planned at any time during the study period (18 months) or had been performed < 6 months prior to the inclusion visit. Spa treatment in the previous 6 months or a contraindication to spa treatment (life‐threatening disease, cardiac or renal failure, immunodeficiency, psychiatric disorders, severe difficulty walking), oedema of non‐venous origin (clinical lymphoedema, cardiac failure, hypoalbuminaemia), symptomatic neurological diseases of the lower limbs (neurogenic pain or abnormal neurological examination of the lower limbs) or significant peripheral arterial disease (ankle‐brachial index < 0.70) |
|
| Interventions |
Technique: 4 balneotherapy sessions per day, 6 days per week for 3 weeks, and educational activities that were differently organised in each resort. The balneotherapy sessions included a 15‐minute walking session in a specially designed pool with tracks in semi‐deep (80 cm) cool (28 °C) water (training of muscle pump function under water compression); a 20‐minute whirlpool bath session with automatic air and water massage cycles (aimed at relaxation and mobilisation of the superficial skin volume flow); a 10‐minute bath session with customised underwater strong massaging jets (mobilisation and softening of the sclerotic subcutaneous tissues); and a 10‐minute massage session of the leg and ankle skin areas by a registered physiotherapist under a light spray shower (softening of the sclerotic subcutaneous tissues) or a 15‐minute joint mobilisation session in a deep (150 cm) warm (34 °C) pool under the supervision of a physiotherapist (improvement of ankle, and also knee and hip joint mobility for better ambulation and muscle‐pump functioning) Balneotherapy group: 3‐week spa treatment course in the spa resort closest to their home, soon after randomisation. Control group: during comparison period no treatment; followed by spa treatment after the comparison period (i.e. starting soon after day 365); Duration of follow‐up: comparison period 1 year; total duration of follow‐up: 18 months |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
All outcomes were measured every 6 months. |
|
| Identification |
Authors name: Patrick H Carpentier Institution: Clinique Universitaire de Médecine Vasculaire, Pôle Pluridisciplinaire de Médecine, Centre Hospitalier Universitaire, Grenoble email: pcarpentier@chu‐grenoble.fr |
|
| Notes | The study authors confirmed this trial used a different population from the study reported in Carpentier 2009 (personal communication by email, 5 February 2019). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data managers blinded to randomisation, including the final data review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons and by study group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Funding by Association Française de Researche Thermale. Sessions customised according the participant's needs and capabilities by specialist spa physician. |
Ernst 1991.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 61 participants (30 in balneotherapy group and 31 control group) Start date: no details given Duration of participation: 24 days End date: no details given Methods of randomisation: no details given Blinding: mentioned "double‐blind" Power calculation: no details given Losses to follow‐up: no details given Unit of allocation: participants Source of funding: no details given |
|
| Participants |
Setting: outpatients (second care) Country: Austria Gender: 16 men and 45 women Age (mean): 58.1 (SD 7.4) years Inclusion criteria: people with primary varicosity Exclusion criteria: people with post‐traumatic or post‐thrombotic CVI, lymphoedema, hereditary vascular abnormalities, venous compression syndromes, congestive heart disease, liver and kidney diseases, malignancies, inflammatory diseases, haematological abnormalities, peripheral arterial occlusive disease. |
|
| Interventions |
Technique: treatment comprised intermittent cold and warm water according to Kneipp therapy (Forestier 2014), or continuous cold‐water treatments, mostly in region of lower legs up to the knees. It was carried 5 days per week and lasted about 12 minutes. Hydrotherapy group: hydrotherapy Control group: no hydrotherapy Duration of follow‐up: 24 days Cointerventions: participants already wearing compression stockings continued to do so. |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
All outcomes were measured at 12 and 24 days |
|
| Identification |
Authors name: Edzard Ernst Institution: Department Physical Medicine and Rehabilitation, University of Vienna email: no details given |
|
| Notes | We assumed Ernst 1991 and Ernst 1992 were separate studies but were unable to confirm this with the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and spa physicians not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators uninformed as to which study group given participant belonged. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No evidence of other bias. |
Ernst 1992.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 122 participants (60 in balneotherapy group and 62 control group) Start date: no details given Duration of participation: 24 days End date: no details given Methods of randomisation: no details given Blinding: mentioned "double‐blind" Power calculation: no details given Losses to follow‐up: no details given Unit of allocation: participants Source of funding: no details given |
|
| Participants |
Setting: outpatients (secondary care) Country: Austria Gender: 32 men and 90 women Age (mean): 60.6 years Inclusion criteria: people with varicose veins Exclusion criteria: people with post‐traumatic or post‐thrombotic CVI, lymphoedema, hereditary vascular abnormalities, venous compression syndromes, congestive heart disease, liver disease, kidney disease, malignancies, inflammatory diseases, haematological abnormalities, peripheral arterial occlusive disease. |
|
| Interventions |
Technique: treatment consist of daily therapy with alternating warm and cold water. It was administered to both legs at 7 a.m. 5 days per week Hydrotherapy group: hydrotherapy Control group: no hydrotherapy Duration of follow‐up: 24 days Cointerventions: all concomitant therapy was left constant during the period study |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
All outcomes were measured at 12 and 24 days |
|
| Identification |
Authors name: Edzard Ernst Institution: Department Physical Medicine and Rehabilitation, University of Vienna email: no details given |
|
| Notes | We assumed Ernst 1991 and Ernst 1992 were separate studies but were unable to confirm this with the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and spa physician not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on. |
| Other bias | Unclear risk | No evidence of other bias. |
Forestier 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 99 participants (51 in balneotherapy group and 48 control group) Start date: July 2010 Duration of participation: 18 days End date: May 2012 Methods of randomisation: block randomisation generated by 1 of the authors. It was carried out with block sizes of 6, 8 and 10 participants with a random order which was determined by dice rolls. Every block comprised 50% treatment and 50% control. Concealed allocation was performed by the same author who was not in contact with the participants of the study. Blinding: participant – no; investigators – yes; outcome assessors – yes Power calculation: Bonferroni correction indicated that a difference was significant for a P value below 0.005 Losses to follow‐up: 5 (3 in balneotherapy group; 2 in control group) Unit of allocation: participant Source of funding: Thermes Nationaux d'Aix‐ Les‐Bains, France |
|
| Participants |
Setting: outpatients (second care) Country: France Gender: 13 men and 81 woman Age (mean): 58 (SD 13) years in balneotherapy group; 60 (SD 13) years in control group Inclusion criteria: aged 18–80 years and diagnosed with CVI stage 3 or 4 according to the CEAP classification regardless of aetiology. People were included if they accepted to participate in a 3‐week treatment in the spa centre and to be followed‐up for 6 months. Exclusion criteria: pregnant women, contraindication for spa treatment (chronic infectious diseases, cancer, heart failure, serious liver or kidney disease, open leg ulcer, psychiatric disorders, immune deficiency, phlebitis, erysipelas or history of erysipelas), planned surgery in the next 3 months, spa treatment in the previous 6 months, professional involvement in the spa centre. |
|
| Interventions |
Technique: 4 different spa techniques daily: Kneipp therapy, walking in the pool, underwater massage and bath in a tub. After termination of the daily programme, participants would rest 20 minutes in the Trendelenburg position. Kneipp therapy is an alternating warm (28 °C) and cold (14 °C) shower on the legs of 10 minutes' duration. The walking pool is 60 cm deep with an underwater shower jet at 23 °C and participants walk in it for 10 minutes without stopping. Underwater massage is performed under a 38 °C shower by a senior physiotherapist, beginning at the feet and gradually proceeding to the thighs, lasting 10 minutes. The bathtub contains an underwater shower at 30 °C, which also works from the feet and gradually proceeds to the thighs over a period of 20 minutes. Balneotherapy group: 18 days of balneotherapy in 3 weeks Control group: usual care Duration of follow‐up: 3 months Cointerventions: all concomitant therapy was left constant during the period study. |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
|
|
| Identification |
Authors name: Romain Jacques Forestier Institution: Centre de Recherche Rheumatologique et Thermal, Aix‐les‐Bains, France email: romain.forestier@wanadoo.fr |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random order determined by dice rolls. |
| Allocation concealment (selection bias) | Low risk | Block sizes of 6, 8 or 10 participants was determined by random order. Every block comprised 50% treatment and 50% control. Concealed allocation was performed by the same investigator who was not in contact with the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and spa physician not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Statistician blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions reported with reasons and by study group. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but it was clear that the published reports included all expected outcomes, including those that were prespecified. |
| Other bias | Unclear risk | Funding: Thermes Nationaux d’Aix‐les‐Bains, France provided the financial support for this study. |
Mancini 2003.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 70 participants (50 in balneotherapy group and 20 control group) Start date: no details given Duration of participation: 6 months End date: no details given Method of randomisation: asymmetrically on blocks of 7 participants, 5 to enter group A and 2 group B Blinding: participants – no; treating professional – no; outcome assessors – yes Power calculation: no details given Losses to follow‐up: 5 (all in control group: 4 refused to come back and 1 had an episode of deep vein thrombosis) Unit of allocation: participant Source of funding: no details given |
|
| Participants |
Setting: outpatients (secondary care) Country: Italy Gender: 18 men and 52 women Age (range): 19–78 years Inclusion criteria: aged 18–85 years, both sexes and informed consent, people with primary monolateral or bilateral varices of the reticular or truncular type, symptomatic, with or without oedema, skin changes, or healed ulcer, variously localised in the superficial veins, with or without perforating veins or reflux. Exclusion criteria: people with active venous ulcer, deep vein thrombosis within the previous 12 months or superficial phlebitis within the previous 6 months, or recurrent forms, vein surgery or sclerotherapy within the previous 6 or respectively 3 months, any general condition contraindicating spa treatments. |
|
| Interventions |
Technique: included balneokinesis (passive and active balneotherapy), consisting of assisted walking in 2 basins containing thermal water at 27 °C (passive) and 31 °C (active). Besides walking, the participants were asked to perform lower limb exercises in water assisted by a physiotherapist. The whole balneokinetic exercise lasted 30 minutes. Treatment was performed with sulphurous salso‐bromojodinated carbon dioxide‐rich water from local springs (sulphidimetric grade 7, 25 mg/L) Balneotherapy group: balneotherapy for 12 days and elastic compression Control group: elastic compression alone Duration of follow‐up: 6 months |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
|
|
| Identification |
Authors name: Sergio Coccheri Institution: Cardiovascular Department, Chair and Division of Angiology, University Hospital St Orsola email: coccheri@med.unibo.it |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation. |
| Allocation concealment (selection bias) | Low risk | Allocation performed asymmetrically on blocks of 7 participants, 5 to enter group A and 2 to enter group B. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and spa physician not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants (all in the control group) lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on. |
| Other bias | Unclear risk | No evidence of other bias. |
Stefanini 1996.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 55 participants (20 in melilotus officinalis group, 15 in balneotherapy group and 20 melilotus officinalis plus balneotherapy group) Start date: no details given Duration of participation: 15 days End date: no details given Methods of randomisation: no details given Blinding: mentioned "double‐blind" Power calculation: no details given Losses to follow‐up: no details given Unit of allocation: individuals Source of funding: no details given |
|
| Participants |
Setting: outpatients (second care) Country: Italy Gender: 4 men and 51 women Age (mean): 55 years Inclusion criteria: people with CVI Exclusion criteria: no details provided |
|
| Interventions |
Technique: 1 bath per day with hypertonic alkaline saline‐sulphonated water for 15 days, at a temperature of 36.5 °C. The ozonated water was obtained by bubbling ozonated air under pressure in water. Melilotus officinalis group:melilotus officinalis 200 mg per day Balneotherapy group: bath with hypertonic alkaline saline‐sulphonated water Balneotherapy plus melilotus officinalis group:melilotus officinalis 200 mg per day plus bath with hypertonic alkaline saline‐sulphonated water Duration of follow‐up: 15 days |
|
| Outcomes |
Outcomes relevant for this review (collected)
Reported outcomes
Used a scale with score of 0–3 to quantify results. |
|
| Identification |
Author's name: L Stefanini Institution: Direzione Sanitaria Terme di Montecatini email: no details provided |
|
| Notes | For this study, we used the data from the melilotus officinalis group and balneotherapy group only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and spa physician not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Study sponsored by Sanitary Direction of Terme di Montecatini. |
CEAP: chronic venous insufficiency classification (clinical manifestations (C), aetiological factors (E), anatomical distribution (A) and pathophysiological findings (P)); CIVIQ2: Chronic Venous Disease Quality of life questionnaire; CVI: chronic venous insufficiency; EQ‐5D: Euro quality of life five dimensions; PASS: Patient Acceptable Symptom State; SF‐36: 36‐item Short Form; VAS: visual analogue scale; VCSS : Venous Chronic Severity Score; VFT: venous filling time.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aquino 2016 | Wrong study design – not an RCT. |
| Blain 2016 | Wrong study design – not an RCT. |
| Brock 2001 | Wrong intervention – balneotherapy plus verum gel vs balneotherapy plus placebo gel. |
| Carpentier 2002 | Wrong participant population – not people with chronic venous insufficiency. |
| Coccheri 2002 | Wrong study design – not an RCT. |
| Costantino 2003 | Wrong study design – not an RCT. |
| Hartmann 1991 | Wrong intervention – participants did not receive balneotherapy as treatment. |
| Hartmann 1993 | Wrong participant population – not people with chronic venous insufficiency. |
| Hartmann 1995 | Wrong participant population – not people with chronic venous insufficiency. |
| NCT00348907 | Wrong participant population – not people with chronic venous insufficiency. |
| Roques 2012 | Wrong study design – not an RCT. |
| Schumann 2011 | Wrong intervention – participants did not receive balneotherapy as treatment. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT02553720.
| Trial name or title | Aqua therapy to lower adverse and negative effects of deep vein thrombosis and post thrombotic syndrome (ATLANTIS) |
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Total number: 100 participants Start date: 10 September 2015 Duration of participation: 2 years End date: no details given Method of randomisation: no details given Blinding: participants – no, treating professional – no Power calculation: no details given Unit of allocation: participants Source of funding: Arizona Cardiovascular Consultants |
| Participants |
Setting: outpatient care Country: US |
| Interventions |
Aqua group: conventional management plus aquatic exercise ≥ 15 minutes of walking 3 times per week for 3 months Control group: conventional management without aquatic exercise |
| Outcomes |
|
| Starting date | 2015 |
| Contact information | seyedmohsensharifi@yahoo.com |
| Notes |
CT: computed tomography; SF‐36: 36 item Short Form; V/Q: ventilation‐perfusion.
Differences between protocol and review
We have used odds ratio instead of risk ratio to present the effect estimates.
Contributions of authors
MAMS: drafted protocol, acquired trial reports, trial selection, data extraction, data analysis, data interpretation, drafted review and guarantor of the review.
LCUN: drafted protocol, trial selection, data extraction, data analysis, data interpretation and drafted review.
LLC: drafted protocol, trial selection, data extraction, data analysis, data interpretation and drafted review.
FM: drafted protocol, trial selection, data extraction, data analysis, data interpretation and drafted review.
All authors will contribute to review updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
MAMS: none.
LCUN: none.
LLC: none.
FM: none.
New
References
References to studies included in this review
Carpentier 2009 {published data only}
- Carpentier PH, Satger B. Randomized trial of balneotherapy associated with patient education in patients with advanced chronic venous insufficiency. Journal of Vascular Surgery 2009;49(163):163‐70. [DOI: 10.1016/j.jvs.2008.07.075] [DOI] [PubMed] [Google Scholar]
Carpentier 2014 {published data only}
- Carpentier PH, Blaise S, Satger B, Genty C, Rolland C, Roques C, et al. A multicenter randomized controlled trial evaluating balneotherapy in patients with advanced chronic venous insufficiency. Journal of Vascular Surgery 2014;59(2):447‐54. [DOI: 10.1016/j.jvs.2013.08.002] [DOI] [PubMed] [Google Scholar]
- NCT00838500. Thermes et veines: spa for prevention of leg ulcers. clinicaltrials.gov/ct2/show/NCT00838500 (first received 6 February 2009).
Ernst 1991 {published data only}
- Ernst E, Saradeth T, Resch KL. A single blind randomized, controlled trial of hydrotherapy for varicose veins. VASA 1991;20(2):147‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Ernst 1992 {published data only}
- Ernst E, Saradeth T, Resch KL. Hydrotherapy for varicose veins: a randomized, controlled trial. Phlebology 1992;7(4):154‐7. [DOI: 10.1177/026835559200700407] [DOI] [PubMed] [Google Scholar]
Forestier 2014 {published data only}
- Forestier RJ, Briancon G, Francon A, Erol FB, Mollard JM. Balneohydrotherapy in the treatment of chronic venous insufficiency. VASA 2014;43(5):365‐71. [DOI: 10.1024/0301-1526/a000374] [DOI] [PubMed] [Google Scholar]
- NCT01956318. Crenobalneotherapy in the treatment of chronic venous insufficiency. clinicaltrials.gov/ct2/show/NCT01956318 (first received 8 October 2013).
Mancini 2003 {published data only}
- Mancini S Jr, Piccinetti A, Nappi G, Mancini S, Caniato A, Coccheri S. Clinical, functional and quality of life changes after balneokinesis with sulphurous water in patients with varicose veins. VASA 2003;32(1):26‐30. [DOI: 10.1024/0301-1526.32.1.26] [DOI] [PubMed] [Google Scholar]
Stefanini 1996 {published data only}
- Stefanini L, Gigli P, Galassi A, Pierallini F, Tillieci A, Scalabrino A. Pharmacologic treatment and/or balneotherapy of chronic venous insufficiency [Trattamento farmacologico e/o balneoterapico dell'insufficienza venosa cronica]. Gazzetta Medica Italiana 1996;155(4):179‐85. [Google Scholar]
References to studies excluded from this review
Aquino 2016 {published data only}
- Aquino MA, Paixão LC, Leal FJ, Couto RC. Analysis of the effects of aquatic exercise on the quality of life of people with chronic venous disease [Análise dos efeitos dos exercícios aquáticos na qualidade de vida de indivíduos com doença venosa crônica]. Jornal Vascular Brasileiro 2016;15(1):27‐33. [EMBASE: 10.1590/1677‐5449.005115] [Google Scholar]
Blain 2016 {published data only}
- Blain H, Bernard P, Canovas G, Raffort N, Desfour H, Soriteau L, et al. Combining balneotherapy and health promotion to promote active and healthy ageing: the Balaruc‐MACVIA‐LR approach. Aging Clinical and Experimental Research 2016;28(6):1061‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brock 2001 {published data only}
- Brock FE. Synergystic effect of vein‐typical hydrotherapy according to Kneipp and topical arnica‐treatment in patients with chronic venous insufficiency [Additiver Effekt venentypischer Hydrotherapie nach Kneipp und lokaler Arnika‐Anwendung bei Patienten mit chronisch venöser Insuffizienz – Synergismus naturheilkundlicher Therapien]. Erfahrungsheilkunde 2001;50(6):357‐63. [DOI: 10.1055/s-2001-15774] [DOI] [Google Scholar]
Carpentier 2002 {published data only}
- Carpentier PH, Fechoz C, Poensin D, Satger B. Influence of spray application of La Lechere mineral water on the cutaneous microcirculation in the lower limbs in healthy subjects [Influence de l'application locale d'eau thermale de la léchère en spray sur la microcirculation cutanée des membres inférieurs]. Journal des Maladies Vasculaires 2002;27(4):211‐3. [PubMed] [Google Scholar]
Coccheri 2002 {published data only}
- Coccheri S, Nappi G, Valenti M, Orio F, Altobelli E, Luca S, et al. Changes in the use of health resources by patients with chronic phlebopathies after thermal hydrotherapy. Report from the Naiade project, a nation‐wide survey on thermal therapies in Italy. International Angiology 2002;21(2):196‐200. [PubMed] [Google Scholar]
Costantino 2003 {published data only}
- Costantino M, Nappi G, Granieri MA, Lampa E. Effects of thermal therapy in venous chronic insufficiency: experimental‐clinic study. Medicina Clinica e Termale 2003;15(53‐54):469‐76. [Google Scholar]
Hartmann 1991 {published data only}
- Hartmann B, Drews B, Bassenge E. Effects of hetero‐thermal water administration on leg vein hemodynamics, skin microcirculation and O2 tension in chronic venous insufficiency. VASA. Supplementum 1991;33:226. [PubMed] [Google Scholar]
Hartmann 1993 {published data only}
- Hartmann B, Drews B, Bassenge E. Effects of bathing in CO2‐containing thermal water in the venous hemodynamics of healthy persons and patients with venous diseases. VASA. Supplementum 1993;3(6):153‐7. [Google Scholar]
Hartmann 1995 {published data only}
- Hartmann B, Drews B, Kayser T, Bassenge E. Venous function in patients with venous disease and healthy controls before and after a bathing procedure and subsequent cold stimulus. Journal of Japanese Association of Physical Medicine Balneology and Climatology 1995;58(2):134‐40. [Google Scholar]
- Hartmann BR, Drews B, Bassenge E. Venous function in patients with venous disease and healthy controls before and after a bathing procedure and subsequent cold stimulus. International Journal of Angiology 1998;7(3):252‐4. [PUBMED: 9585462] [DOI] [PubMed] [Google Scholar]
NCT00348907 {published data only}
- NCT00348907. EVENT: hydrotherapy and deep venous thrombosis. clinicaltrials.gov/ct2/show/NCT00348907 (first received 6 July 2006).
Roques 2012 {published data only}
- Roques C, Mancret RC, Tabone W. Beneficial effect of balneotherapy: achievements of the French association of balneotherapy research and methodological difficulties. Annals of Physical and Rehabilitation Medicine 2012;55 (Suppl 1):e349‐51. [DOI: 10.1016/j.rehab.2012.07.891] [DOI] [Google Scholar]
Schumann 2011 {published data only}
- Schumann H, Calow T, Weckesser S, Muller ML, Hoffmann G. Water‐filtered infrared A for the treatment of chronic venous stasis ulcers of the lower legs at home: a randomized controlled blinded study. British Journal of Dermatology 2011;165(3):541‐51. [DOI: 10.1111/j.1365-2133.2011.10410.x] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02553720 {published data only}
- NCT02553720. Aqua therapy in deep venous thrombosis and insufficiency (ATLANTIS). clinicaltrials.gov/ct2/show/NCT02553720 (first received 18 September 2015).
Additional references
Angoules 2014
- Angoules AG. Conservative treatment of chronic venous insufficiency. Journal of Novel Physiotherapies 2014;5(1):1‐2. [DOI: 10.4172/2165-7025.1000e135] [DOI] [Google Scholar]
Becker 2009
- Becker BE. Aquatic therapy: scientific foundations and clinical rehabilitation applications. Physical Medicine and Rehabilitation 2009;1:859‐72. [DOI: 10.1016/j.pmrj.2009.05.017] [DOI] [PubMed] [Google Scholar]
Beebe‐Dimmer 2005
- Beebe‐Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Annals of Epidemiology 2005;15(3):175‐84. [DOI: 10.1016/j.annepidem.2004.05.015] [DOI] [PubMed] [Google Scholar]
Bender 2005
- Bender T, Karagülle Z, Bálint GP, Gutenbrunner C, Bálint PV, Sukenik S. Hydrotherapy, balneotherapy and spa treatment in pain management. Rheumatology International 2005;25(3):220‐4. [DOI: 10.1007/s00296-004-0487-4] [DOI] [PubMed] [Google Scholar]
Bergan 2006
- Bergan JJ, Schmid‐Schönbein GW, Coleridge SP, Nicolaides AN, Boisseau MR, Eklöf B. Chronic venous disease. New England Journal of Medicine 2006;355(5):488‐98. [DOI: 10.1056/NEJMra055289] [DOI] [PubMed] [Google Scholar]
Brooks 1996
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1):53‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Caggiati 2018a
Caggiati 2018b
- Caggiati A, Lattimer C, Kalodiki E, Oberto S, Bergamo G, Kontothanassis D. Underwater sonography of leg veins. European Journal of Vascular and Endovascular Surgery 2018;41:13‐5. [DOI: 10.1016/j.ejvssr.2018.10.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2013
- Carroll C, Hummel S, Leaviss J, Ren S, Stevens JW, Everson‐Hock E, et al. Clinical effectiveness and cost‐effectiveness of minimally invasive techniques to manage varicose veins: a systematic review and economic evaluation. Health Technology Assessment 2013;17(48):1‐141. [DOI: 10.3310/hta17480] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro‐Ferreira 2018
- Castro‐Ferreira R, Cardoso R, Leite‐Moreira A, Mansilha A. The role of endothelial dysfunction and inflammation in chronic venous disease. Annals of Vascular Surgery 2018;46:380‐93. [DOI: 10.1016/j.avsg.2017.06.131] [DOI] [PubMed] [Google Scholar]
Da Silva 2010
- Silva GC, Medeiros RJ, Oliveira LS, Araújo Júnior AT, Aniceto RR, Sousa MS, et al. Strength training does not affect the diameter of the main veins of lower limbs in adult women with mild to moderate chronic venous insufficiency [Treinamento de sobrecarga muscular não afeta o diâmetro das principais veias dos membros inferiores em mulheres adultas com insuficiência venosa]. Revista Brasileira de Medicina do Esporte 2010;16(6):413‐7. [Google Scholar]
Eklöf 2004
- Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of Vascular Surgery 2004;40(6):1248‐52. [DOI: 10.1016/j.jvs.2004.09.027] [DOI] [PubMed] [Google Scholar]
Gloviczki 2011
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. Journal of Vascular Surgery 2011;53(5):2S‐48S. [DOI: 10.1016/j.jvs.2011.01.079] [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI: 10.1136/bmj.328.7454.1490] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gutenbrunner 2010
- Gutenbrunner C, Bender T, Cantista P, Karagülle Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. International Journal of Biometeorology 2010;54(5):495‐507. [DOI: 10.1007/s00484-010-0321-5] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnson 1990
- Johnson RH. Arthur Stanley Wohlmann, the first government balneologist in New Zealand. Medical History 1990;34(S10):114‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klick 2008
- Klick J, Stratmann T. Do spa visits improve health: evidence from German micro data. Eastern Economic Journal 2008;34(3):364‐74. [DOI: 10.1057/palgrave.eej.9050038] [DOI] [Google Scholar]
Konschake 2016
- Konschake W, Riebe H, Pediaditi P, Haase H, Jünger M, Lutze S. Compression in the treatment of chronic venous insufficiency: efficacy depending on the length of the stocking. Clinical Hemorheology and Microcirculation 2016;64:425‐34. [DOI: 10.3233/CH-168122] [DOI] [PubMed] [Google Scholar]
Launois 1996
- Launois R, Reboul‐Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Quality of Life Research 1996;5(6):539‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2016
- Lee BB, Nicolaides AN, Myers K, Meissner M, Kalodiki E, Allegra C, et al. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. International Angiology 2016;35(3):236‐352. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Martinez‐Zapata 2016
- Martinez‐Zapata MJ, Vernooij RW, Uriona Tuma SM, Stein AT, Moreno RM, Vargas E, et al. Phlebotonics for venous insufficiency. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD003229.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Motykie 1999
- Motykie GD, Caprini JA, Arcelus JI, Reyna JJ, Overom E, Mokhtee D. Evaluation of therapeutic compression stockings in the treatment of chronic venous insufficiency. Dermatologic Surgery 1999;25:116‐20. [PUBMED: 10037516] [DOI] [PubMed] [Google Scholar]
Nicolaides 2014
- Nicolaides A, Kakkos S, Eklof B, Perrin M, Nelzen O, Neglen P, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. International Angiology 2014; Vol. 33, issue 2:87‐208. [PubMed]
Padberg 2004
- Padberg FT, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. Journal of Vascular Surgery 2004;39:79‐87. [DOI: 10.1016/j.jvs.2003.09.036] [DOI] [PubMed] [Google Scholar]
Pasek 2010
- Pasek J, Ciesla G. Health resort treatment – a new chance for the treatment of vessel diseases?. Acta Angiologica 2010;16:1‐16. [Google Scholar]
Perrin 2016
- Perrin M, Eklöf B, Rij A, Labropoulos N, Vasquez M, Nicolaides A, et al. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. International Angiology 2016;35(4):374‐98. [PUBMED: 27081866] [PubMed] [Google Scholar]
Pittler 2012
- Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD003230.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plot Digitizer
- Plot Digitizer. Available from plotdigitizer.sourceforge.net/ (accessed 12 February 2019).
Raju 2007
- Raju S, Hollis K, Neglén P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Annals of Vascular Surgery 2007;21(6):790‐5. [DOI: 10.1016/j.avsg.2007.07.014] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossi 2015
- Rossi FH, Volpato MG, Metzger PB, Beteli CB, Almeida BL, Rossi CB, et al. Relationships between severity of signs and symptoms and quality of life in patients with chronic venous disease. Jornal Vascular Brasileiro 2015;14(1):22‐8. [DOI: 10.1590/1677-5449.20140039] [DOI] [Google Scholar]
Rutherford 2000
- Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. Journal of Vascular Surgery 2000;31(6):1307‐12. [PUBMED: 10842165] [DOI] [PubMed] [Google Scholar]
Verhagen 2007
- Verhagen AP, Bierma‐Zeinstra SM, Boers M, Cardoso JR, Lambeck J, Bie RA, et al. Balneotherapy for osteoarthritis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006864] [DOI] [PubMed] [Google Scholar]
Verhagen 2015
- Verhagen AP, Bierma‐Zeinstra SM, Boers M, Cardoso JR, Lambeck J, Bie R, et al. Balneotherapy (or spa therapy) for rheumatoid arthritis. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD000518.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2012
- Wong IK, Man MB, Chan OH, Siu FC, Abel M, Andriessen A. Comparison of the interface pressure and stiffness of four types of compression systems. Journal of Wound Care 2012;21:161‐7. [DOI: 10.12968/jowc.2012.21.4.161] [DOI] [PubMed] [Google Scholar]
Wood‐Dauphinee 1999
- Wood‐Dauphinee S. Assessing quality of life in clinical research: from where have we come and where are we going?. Journal of Clinical Epidemiology 1999;52(4):355‐63. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
de Moraes Silva 2018
- Moraes Silva MA, Nakano LCU, Cisneros LL, Miranda Jr F. Balneotherapy for chronic venous insufficiency. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD013085] [DOI] [Google Scholar]


