Abstract
Primary intraosseous non-Hodgkin lymphoma in the mandible is uncommon, representing about 0.6% of all extranodal lymphomas. We present the case of a 51-year-old male with a 4-month complaint of mandibular swelling and paresthesia, which had been previously submitted to an unsuccessful periodontal treatment. The intra-oral evaluation showed an extensive swelling with teeth mobility in the right mandible body. The panoramic radiography and computed tomography images showed an extensive osteolytic lesion. An incisional biopsy was performed and the histopathological and immunohistochemical analysis established the diagnosis of diffuse large B-cell lymphoma. The treatment included six cycles of chemotherapy with complete remission. The patient is under the seventh month of follow-up with no evidence of relapse. Although uncommon in the oral cavity, lymphoma should be considered in the differential diagnosis.
Keywords: Head and Neck Neoplasms; Lymphoma, Large B-Cell, Diffuse; Oral Medicine
INTRODUCTION
Lymphomas are divided into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). Approximately 48% of the NHLs occur in the extranodal sites, and after the gastrointestinal tract, the head and neck is the most common location.1,2
In the head and neck, NHL mostly involves the Waldeyer ring,3 followed by the other soft tissues of this region, such as the tongue and the floor of the mouth. NHLs also sporadically occur in the bone tissue of the head and neck.4 Primary NHL of the mandible is less frequent than the maxillary involvement,5 and it can appear as a solitary lesion or resorption of the margin of the alveolar bone, resembling periodontal disease or cystic lesions.
The clinical features, such as swelling, pain, paresthesia, tooth mobility, or pathologic fracture, are frequent but non-specific, which may lead to misdiagnosis. In the literature, several reports illustrate the challenging diagnosis of oral lymphoma.6-8 Here, we present an uncommon case of primary NHL of the mandible, which was initially misdiagnosed as periodontal disease.
CASE REPORT
A 51-year-old male patient was referred to the Stomatology Department complaining of mandibular swelling and paresthesia in the right body of mandible over the past 4 months. He reported a periodontal treatment followed by a first molar extraction, 1 month prior, at the same site. His medical history revealed prostatic cancer surgically treated 3 years earlier with no evidence of any metastasis during the follow-up.
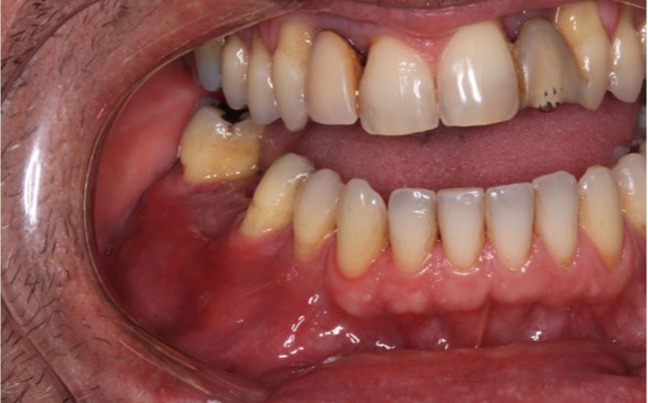
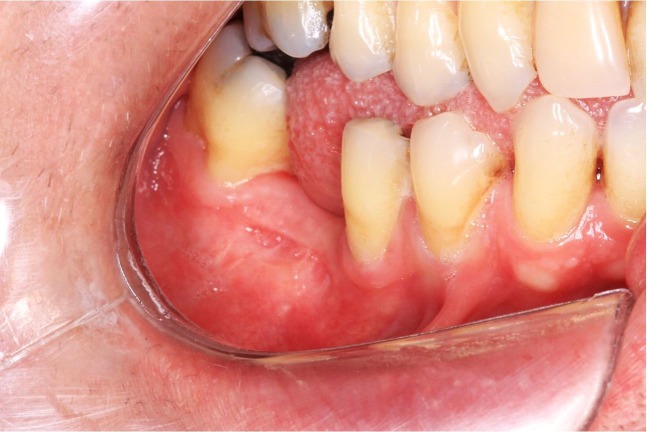
An intraoral examination revealed an extensive mass with fibrous consistency and teeth mobility in the posterior region of the right mandible extending from the canine to the second molar (Figure 1). Extraoral examination showed facial asymmetry due to swelling on the right body of the mandible.
Figure 1. Intraoral examination shows a diffuse swelling in the right vestibular mucosa of the mandible from the first premolar to the second molar.
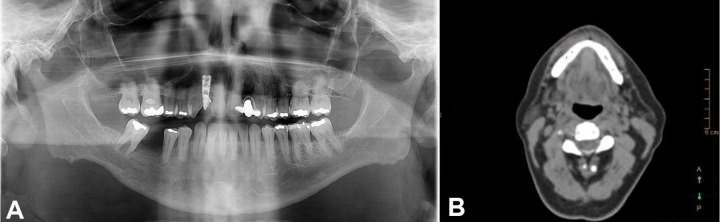
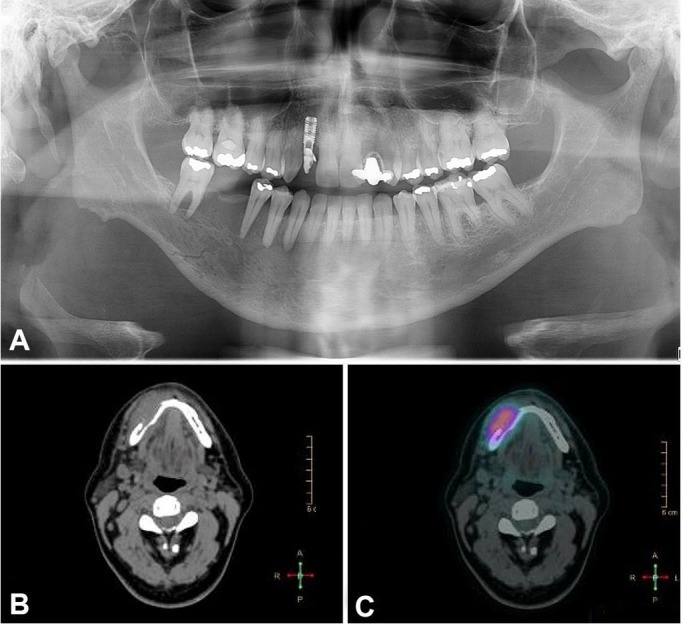
The panoramic radiography showed an extensive radiolucent image, with a line of fracture in the posterior region of the right mandible (Figure 2A). The computed tomography (CT) images showed a 45 × 11 mm osteolytic lesion around the mandibular canal and the mental foramen, which reabsorbed the vestibular cortical bone (Figure 2B).
Figure 2. A – Panoramic radiography showing a radiolucent area in the right side of the mandible from the first premolar to the second molar. Also, it is possible to observe a line of fracture. B – facial CT, axial plane, showing a rupture of the cortical bone. C – PET/CT scan exhibiting an uptake area in the right mandible body.
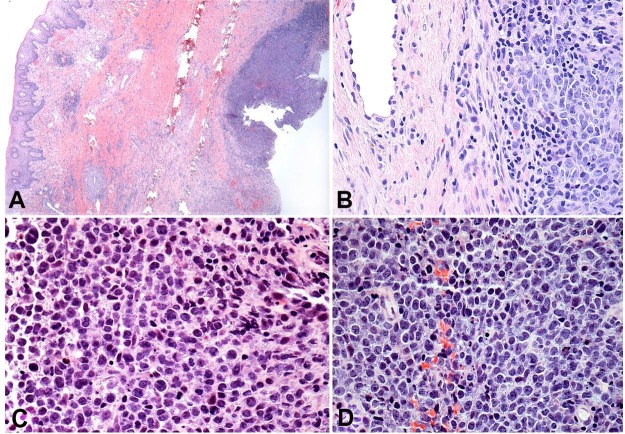
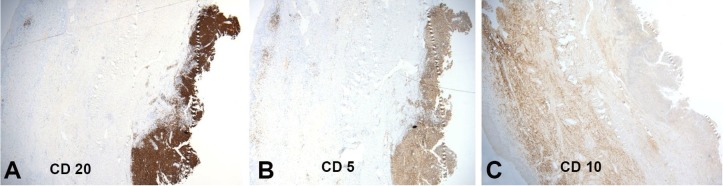
Considering the aggressive clinical signs, the main clinical hypothesis was a malignant tumor. An incisional biopsy was performed under local anesthesia. The histological examination of the biopsy evidenced a dense proliferation of neoplastic cells at the reticular dermis (Figure 3A). At high power, the neoplastic cells showed large pleomorphic cells with nuclear atypia, prominent nucleoli, and the presence of mitoses, consistent with lymphoid neoplasia (Figure 3B). The tumor cells showed immunopositivity for CD20 (Figure 4A), CD5 (Figure 4B), and no expression to CD10 (Figure 4C). According to these findings, the diagnosis of diffuse large B-cell lymphoma (DLBCL) non-germinal center type was established.
Figure 3. Photomicrographs of the biopsy specimen showing in A and B a dense proliferation of neoplastic cells in the submucosa (H&E, original magnification 10X (A), 20X (B). At higher power, it is noticed a sheet of atypical lymphocytic cells showing prominent and hyperchromatic nuclei besides atypical mitosis (C and D) (H&E, original magnification 40X).
Figure 4. Immunohistochemical panel showing the neoplastic cells with a diffuse expression of CD20 (A) and CD5 (B), and no expression of CD10 (C) – (original magnification 20X).
After this diagnosis, the patient was referred to the Hematology Department and underwent a full-body positron emission tomography scan showing a high metabolic activity extra nodal with a standardized uptake value (SUV) of 17.7 in the right mandible and another in the right submandibular area with an SUV of 2.5 (Figure 2C). No tumor activity was noted in other sites. The patient was classified in Stage I Ann Arbor with a localized involvement of a single extra-lymphatic organ site (stage IE).
The patient underwent six cycles of prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (R-CHOP) followed by two cycles of high-dose methotrexate (HD-MTX; 3.5 g/m2). The complete remission was verified in the CT scan after four cycles of R-CHOP (Figure 5A), and the radiographic exams showed a consolidation of fracture (Figure 5B). In the 7-month follow-up, the patient did not present any clinical signs and symptoms of the tumor, and no teeth mobility was found (Figure 6).
Figure 5. Post-treatment image of the panoramic radiography showing fracture healing (A) and computed tomography displaying the new formed cortical bone (B).
Figure 6. Intraoral examination after the treatment showing complete remission of the initial lesion.
DISCUSSION
According to the project of the International Agency for Research on Cancer (IARC)-GLOBOCAN, 509,590 new cases of NHL and 248,724 deaths were estimated to occur in 2018, worldwide.9 The incidence rates for NHL are increasing annually and represent the eleventh most frequent type of cancer in Brazil.8 In the oral cavity, lymphoma can appear anywhere in the mouth but predominates on the hard palate, gingiva, and tongue.1 The mandible is an infrequent localization of primary osseous NHL.
Although rare, these neoplasms are generally diagnosed in the jawbones of the adults in the fourth and fifth decade of life. The oral swelling and pain are the most frequent clinical features, which are often associated with teeth mobility and paresthesia.1 Radiographically, intraosseous involvement is represented by osteolytic lesions, unilocular or multilocular, with poorly defined borders.3 However, these signs may resemblance other diseases, such as benign or malignant tumors, which hampers the correct diagnosis. Based on the aggressive clinical/radiographic findings, the lesion was initially considered to be a malignant tumor.
DLBCL represents the most common type of NHL, accounting for 40% off all cases. DLBCL is a heterogeneous entity with varied clinical features, morphology, immunohistochemistry, and prognosis.10,11 These differences should be carefully analyzed in order to achieve successful management.
As far as the subclassification of DLBCL is concerned, this entity can be characterized in three forms: germinal center B-cell, activated B-cell or does not fit into any classification. In the case presented here, the tumor cells showed negativity for CD10 and BCL6, being classified according to the Hans algorithm as a non-germinal center subtype.12
Regarding the differential diagnosis, it is important to note that Burkitt lymphoma (BL) may share some histopathological features with the DLBCL and it is imperative to differentiate them once the treatment is distinct. In the case reported here, the c-MYC, usually present in BL, was positive in less than 10% of the cells, refuting BL as a possible diagnosis.13
Treatment for DLBCL consists of a standard R-CHOP therapy, which has a cure rate of 60%. Currently, the improved management of the high-risk subgroup of patients with specific histologic, immunophenotypic, and molecular features determines which treatment will achieve the better prognosis.14 The CD5 positivity represents only 5% to 10% of DLBCL cases.11 A recent study of DLBCL patients treated with R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) showed that CD5 expression is an independent prognostic factor for poorer overall survival.12
The CD5 positivity in DLBCL is also associated with a frequent central nervous system (CNS) relapse rate. It is suggested that HD-MTX may be an efficacious treatment to prevent CNS relapse in CD5+ DLBCL patients.15 In this current case, the patient presented positivity for CD5 and received HD-MTX (3.5 g/m2) to CNS prophylaxis.
Due to these various clinical manifestations NHL in the oral cavity, we emphasize that it is crucial for the dentist to be aware of the clinical/radiographic features of intraosseous NHL and refer these patients to a specialized service in order to achieve an early diagnosis.
Footnotes
How to cite: Siqueira JM, Fernandes PM, Oliveira ACF, Vassallo J, Alves FA, Jaguar GC. Primary diffuse large B-cell lymphoma of the mandible. Autops Case Rep [Internet]. 2019 Jul-Sep;9(3):e201910. https://doi.org/10.4322/acr.2019.109
The patient signed an informed consent authorizing the publication of the report as well as the images. The manuscript is by the Institutional Ethics Committee.
Financial support: None
REFERENCES
- 1.Zapater E, Bagán JV, Carbonell F, Basterra J. Malignant lymphoma of the head and neck. Oral Dis. 2010;16(2):119-28. 10.1111/j.1601-0825.2009.01586.x. [DOI] [PubMed] [Google Scholar]
- 2.Thakral B, Zhou J, Medeiros LJ. Extranodal hematopoietic neoplasms and mimics in the head and neck: an update. Hum Pathol. 2015;46(8):1079-100. 10.1016/j.humpath.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005;9(6):340-50. 10.1016/j.anndiagpath.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Pereira DL, Fernandes DT, Santos-Silva AR, Vargas PA, Almeida OP, Lopes MA. Intraosseous non-Hodgkin lymphoma mimicking a periapical lesion. J Endod. 2015;41(10):1738-42. 10.1016/j.joen.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Pazoki A, Jansisyanont P, Ord RA. Primary non-Hodgkin’s lymphoma of the jaws: report of 4 cases and review of the literature. J Oral Maxillofac Surg. 2003;61(1):112-7. 10.1053/joms.2003.50018. [DOI] [PubMed] [Google Scholar]
- 6.Okahata R, Shimamoto H, Marutani K, et al. Diffuse large B-cell lymphoma of the mandible with periosteal reaction: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(2):e228-32. 10.1016/j.oooo.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Jessri M, AbdulMajeed AA, Matias MA, Farah CS. A case of primary diffuse large B-cell non-Hodgkin’s lymphoma misdiagnosed as chronic periapical periodontitis. Aust Dent J. 2013;58(2):250-5. 10.1111/adj.12056. [DOI] [PubMed] [Google Scholar]
- 8.Donaduzzi LC, Reinheimer A, Silva MAR, et al. Primary diffuse large B cell lymphoma mimicking hyperplastic reactive lesion (lymphoma of the oral cavity). Case Rep Pathol. 2018;2018:1- 9. 10.1155/2018/2981689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 10.Jain P, Fayad LE, Rosenwald A, Young KH, O’Brien S. Recent advances in de novo CD5+ diffuse large B cell lymphoma. Am J Hematol. 2013;88(9):798-802. 10.1002/ajh.23467. [DOI] [PubMed] [Google Scholar]
- 11.Harada S, Suzuki R, Uehira K, et al. Molecular and immunological dissection of diffuse large B cell lymphoma: CD5+, and CD5-with CD10+groups may constitute clinically relevant subtypes. Leukemia. 1999;13(9):1441-7. 10.1038/sj.leu.2401487. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74-87. 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Lin P, Dickason TJ, Fayad LE, et al. Prognostic value of MYC rearrangement in cases of B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. Cancer. 2012;118(6):1566-73. 10.1002/cncr.26433. [DOI] [PubMed] [Google Scholar]
- 14.Thakral B, Medeiros LJ, Desai P, et al. Prognostic impact of CD5 expression in diffuse large B-cell lymphoma in patients treated with rituximab-EPOCH. Eur J Haematol. 2017;98(4):415-21. 10.1111/ejh.12847. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki K, Yamaguchi M, Suzuki R, et al. CD5-positive diffuse large B-cell lymphoma: A retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22(7):1601-7. 10.1093/annonc/mdq627. [DOI] [PubMed] [Google Scholar]