Abstract
Vitiligo is an autoimmune skin disease mediated by autoreactive CD8+ T cells that destroy the pigment-producing cells of the epidermis, melanocytes, leading to areas of depigmentation. Patients with vitiligo require life-long treatment to regain and maintain their pigment. Clinical observations uncovered the importance of autoimmune memory in vitiligo, as cessation of treatment frequently led to relapse of disease at the site of previous lesions. A subset of memory T cells known as resident memory CD8+ T cells (TRM) are long lived, non-migratory memory cells that persist in most non-lymphoid tissues, including the skin. Recent reports describe the presence of CD8+ TRM in lesional vitiligo patient skin and suggest their role as active players in disease maintenance. In this review, we will discuss the role of skin CD8+ TRM in maintaining disease in vitiligo, and the opportunity to target this population to induce long-lasting reversal of disease.
Introduction - Vitiligo
Vitiligo is an autoimmune skin disease in which melanocytes, the pigment-producing cells of the skin, are targeted for destruction by autoreactive CD8+ T cells. As a result, patients develop patchy white spots on their skin. Vitiligo affects roughly 1% of the population, has no sex bias, and most patients are diagnosed before the age of thirty. Vitiligo has a significant impact on the patients’ quality of life and self-esteem (1-7). Similar to many other autoimmune diseases, complex interactions among genetic, environmental, and stochastic factors contribute to vitiligo susceptibility (8, 9). While it is still unclear how disease is initiated, intrinsic or extrinsic cellular stress may play a role(10). Melanocytes in healthy human skin are present in both the epidermis and the hair follicle, to which they provide pigment (11). In the majority of vitiligo patients only the epidermal melanocytes are targeted for destruction and melanocytes present in the hair follicles remain unaffected, likely resulting from mechanisms of immune privilege within the hair follicle (12). Because of this, vitiligo can be reversed by both suppressing the immune attack and by stimulating melanocyte precursors that live in the hair follicle to proliferate, migrate, and replenish lost epidermal melanocytes through a process known as perifollicular repigmentation (13, 14).
Conventional treatment uses a combination of topical corticosteroids or calcineurin inhibitors that broadly suppress the local immune response in the skin, together with narrow band ultraviolet light B therapy (nbUVB), which contributes to immunosuppression but also stimulates melanocyte regeneration from the hair follicles. Current therapy can be effective with up to 100% repigmentation possible, but is often unpredictable, time-consuming, and insufficient for many patients (15, 16). Studies reveal that not all vitiligo patients respond to nbUVB treatment, which highlights the need for better targeted therapies. Since hair follicles harbor the melanocyte precursors required for repigmentation, anatomical sites devoid of hair follicles such as the fingertips, knuckles, ventral wrists, and elbows often have poor treatment responses. Also, lesions in which the follicular melanocytes have been destroyed, resulting in white hair, often do not regain pigment following treatment.
Vitiligo is a chronic disease that requires lifelong therapy and approximately 40% of vitiligo patients relapse within 1 year after stopping treatment(16, 17). Clinical observations revealed that depigmented lesions return to the exact same location of previously depigmented spot (17). These insights suggest that the formation of autoimmune memory plays an important role in the recurrence of vitiligo lesions.
CD8+ T cells are sufficient to mediate melanocyte destruction
It is well established that CD8+ T cells are both necessary and sufficient to mediate human vitiligo. Early studies showed that the number of HLA-A2 melanocyte-specific CD8+ T cells in the blood of vitiligo patients correlated with disease severity and expressed high levels of the skin homing receptor, cutaneous lymphocyte-associated antigen (18). Furthermore, isolated melanocyte-specific CD8+ T cells from vitiligo patients were able to lyse HLA-A2 matched peptide pulsed cells and melanoma cells ex vivo whereas non-specific CD8+ T cells had no cytolytic ability. Examination of vitiligo patient skin cells using suction blistering found that the number of CD8+ T cells is significantly increased in active disease compared to stable, non-lesional, and healthy control skin (19). Elegant studies showed that perilesional CD8+ T cells isolated from vitiligo skin could kill melanocytes from normal pigmented skin isolated from the same patient when cultured ex vivo, demonstrating that melanocyte-specific CD8+ T cells are both necessary and sufficient for the destruction of melanocytes (20). A better understanding of the development, formation, and survival of memory CD8+ T cells in vitiligo is important to understand their role in the recurrence of vitiligo lesions.
Memory CD8+ T cells subsets
Much of what we know about the generation of skin memory CD8+ T cells comes from studies in mouse models of viral infections. Naïve T cells circulate between the blood and secondary lymphoid organs because of their expression of CD62L, also known as L-selectin, and the chemokine receptor CCR7, which allow their entry into the lymph nodes through high endothelial venules (21, 22). Naïve T cells are generally not found in peripheral tissues and require recognition of antigen, co-stimulation, and activation by cytokines to enter nonlymphoid tissues (23, 24). Activation of naïve T cells stimulates reprogramming into effector T cells, which includes upregulation of various adhesion molecules and tissue-specific chemokine receptors required to position effector cells at the site of infection(25, 26). Following clearance of a pathogen or resolution of inflammation, a population of antigen-experienced T cells remain in the host as memory T cells to protect against reinfection (27, 28). Peripheral tissues such as the skin are considered restrictive, meaning that effector and memory CD8+ T cells do not enter the tissue in the steady state (29, 30). Thus, the skin becomes accessible to effector CD8+ T cells only after local inflammation and induction of inflammatory chemokines that trigger T cell recruitment.
Memory T cells are divided into different subsets based on their patterns of migration. Circulating memory T cells expressing high levels of CD62L and CCR7 are known as central memory T cells (TCM), trafficking between the blood and secondary lymph organs. Memory T cells that lack expression of these lymphoid homing molecules and also express effector molecules upon stimulation are called effector memory T cells (TEM)(31). TEM cells are recruited to peripheral tissues to fight infection but can return back to the circulation upon upregulation of CCR7, which permits their exit from the tissue(32, 33). Studies suggest that TEM are seemingly in constant transit between the circulation and peripheral tissues, and are responsible for continued immunosurveillance(34, 35). Elegant work originally identified a subset of memory T cells that persist in the peripheral tissue without any replenishment of T cells from the circulation (36). These cells were labeled resident memory T cells (TRM), defined as non-migratory, tissue-resident cells. TRM have been found in most peripheral tissues, including the gut, lung, reproductive tract, and skin, where they are positioned to provide robust protection against reinfection with pathogens (36-38).
Skin TRM
Healthy human skin contains a surprisingly large number of T cells, roughly 20 billion, with 80% of T cells identified as CD45RO+ memory T cells (39). The majority of memory T cells in healthy skin tissue are CD62L− CCR7− TEM, and TCM make up less than 20% of T cells in resting skin. Of the memory T cells present in healthy skin tissue, between 20-60% are TRM (40, 41), revealing that the percentage of TRM is highly variable among healthy individuals. Healthy human skin is populated by both CD4+ and CD8+ TRM populations, which are enriched in the epidermis, compared to the dermal skin compartment(40, 41).
CD8+ TRM are positioned at the basement membrane between basal keratinocytes (41), and are characterized by the expression of CD103, a subunit of the αEβ7 integrin receptor that binds to E-cadherin expressed on cells of the epidermis (36), as well as the activation marker CD69. CD69 associates with the sphingosine 1-phosphate receptor 1, required for lymphocyte egress from peripheral tissues, and promotes its internalization and degradation, thereby maintaining T cell residence in the skin (42). Upregulation of CD103 and CD69 on TRM is important for their development and survival in the skin, as deletion of CD103 or CD69 on virus-specific T cells led to a significant reduction in TRM numbers in the skin following virus infection (43). Additional local signals including TGFβ, which promotes CD103 expression, and IL-15 signaling are also important for their development and survival (44, 45).
Directly targeting TRM in vitiligo is of interest after multiple reports reveal the importance of TRM in promoting allergic responses, inflammation, and autoimmune disease (46-50). Early clues that skin TRM may induce pathogenic immune responses come from clinical observations. For example, fixed drug eruption is a CD8+ T cell mediated allergic reaction of the skin following exposure to a drug, which resolves when the drug is discontinued. However, if the drug is taken again years or even decades later, the inflammation rapidly reappears in the same location. It is thought that the recurrent skin inflammation is a result of a persisting population of drug-reactive CD8+ T cells located at the site of the first encounter with the drug (51). Disease relapse is also seen in patients with the inflammatory skin disease psoriasis (47, 52) and in vitiligo (17). Since vitiligo is a chronic disease, continuous treatment is necessary to regain and maintain pigment. Disease relapse in vitiligo suggests that autoimmune memory forms within lesions, and is involved in their recurrence. A better understanding of the signals driving immune memory formation, maintenance, and activation in vitiligo will provide insights into more durable treatments.
IFNy-CXCR3-CXCL9/10 axis drives vitiligo
Our lab and others have focused on understanding the pathways driving vitiligo pathogenesis as well as the signals responsible for the recruitment, positioning, and survival of melanocyte-specific CD8+ T cells in the skin. Early clinical studies showed that production of the pro-inflammatory cytokines IFNγ and TNFα by CD8+ T cells isolated from perilesional vitiligo skin positively correlated with disease severity and could predict the success of nbUVB therapy (20). Likewise, gene expression analysis of vitiligo lesional skin revealed an IFNγ-specific gene signature and no upregulation of IL-17 transcripts (53-57). These human studies point to IFNγ as the central cytokine in disease, and mechanistic studies in mice support this hypothesis (53, 58). The IFNγ signature in mice parallels that seen in human patient skin. Affected skin in mice induces a significant increase in IFNγ, and melanocyte-specific CD8+ T cells from vitiligo mice produce IFNγ after stimulation with melanocyte antigen. Most importantly, vitiligo progression is dependent on IFNγ, as therapeutic blockade of IFNγ significantly reduced the severity of disease (59).
IFNγ stimulates transcription of the chemokine ligands CXCL9, CXCL10, and CXCL11, which drive the migration of immune cells into tissues in many Type 1 inflammatory diseases and infections. All three chemokine ligands bind to the shared receptor, CXCR3 (60, 61), which is expressed on activated immune cells including effector CD4+ T cells, CD8+ T cells, and NK cells (60). Expression of CXCR3 on melanocyte-specific CD8+ T cells in vitiligo is required for skin tissue homing, as CXCR3-deficient CD8+ T cells are unable to mediate vitiligo in mice (54). This CXCR3-dependent migration is a result of the upregulation of CXCL9 and CXCL10 in the skin of vitiligo mice (62). Treatment of mice with CXCR3-depleting antibodies both prevented and reversed disease (63). In vitiligo patients, the majority of CD8+ T cells in lesional skin express CXCR3 (40, 64, 65) and the chemokine ligands CXCL9 and CXCL10 are enriched within lesional skin compared to non-lesional and healthy control skin (19, 54). These studies establish the IFNγ-CXCR3-CXCL9/10 axis as the central pathway in mediating recruitment of CD8+ T cells in mouse and human vitiligo.
The functional roles of CXCL9 and CXCL10 in vitiligo
Additionally, mice that report the expression of CXCL9 and CXCL10 were used to determine the kinetics and source of CXCR3 ligands in epidermal vitiligo skin(62). Global epidermal expression of CXCL9 followed a bimodal pattern; CXCL9 was maximally upregulated early after disease induction and then again at the peak of disease(62). Epidermal CXCL10 expression gradually increased over the duration of disease with the highest expression at the peak of the immune response in vitiligo(62). Induction of vitiligo in CXCL9-deficient mice led to a significant reduction of melanocyte-specific CD8+ T cells in both the epidermal and dermal skin compartments, suggesting the importance of CXCL9 for early recruitment of CD8+ TEM cells to the skin and the development of new lesions (54). However, CXCL9-deficient mice still developed vitiligo as the few melanocyte-specific CD8+ T cells that did make it into the skin were evidently sufficient to induce disease(54). Interestingly, CXCL10-deficient mice and those treated with CXCL10 antibody did not show significant defects in bulk recruitment of melanocyte-specific CD8+ TEM cells into the dermis, but CD8+ TEM did not efficiently reach the epidermis, which may be partly due to low CD44 expression(54). CD44 is important for memory T cell survival, activation and directed migration through the basement membrane(66). As a result, CXCL10-deficient mice were protected from vitiligo progression. In addition, vitiligo could be reversed in mice by treatment with a CXCL10 neutralizing antibody (54). These results suggest that CXCL10 is critical in both disease progression and maintenance, playing an active role in the directed migration and tethering of CD8+ memory T cells in the epidermis, as well as possibly modulating their effector function.
A recent study by our group used suction blistering of human vitiligo skin to measure IFNγ-induced chemokine expression in situ (19). We found that active vitiligo patient skin contains significantly higher levels of CXCL9 protein than non-lesional and stable skin from vitiligo patients, as well as healthy control skin. In fact, the presence of CXCL9 protein in the skin is sensitive and specific for disease activity and may serve as a biomarker of early treatment responses.
CD8+ TRM in mouse models of vitiligo
In a mouse model of vitiligo(53, 58), transferred naïve melanocyte-specific CD8+ T cells are activated and recruited to the skin through expression of CXCR3 ligands. TEM traffic to the skin and kill epidermal melanocytes, which leads to patchy white depigmentation on the tail, ears, nose, and footpads (53). We found that TRM seed peripheral tissues during the effector phase of the immune response, which peaks at 7 weeks in mice. At this point, about 60-90% of the melanocyte-specific CD8+ T cells express the canonical TRM markers CD69 and CD103(64). TRM in vitiligo mice persist in the epidermis and dermis for over a year, and are enriched within the epidermis (64). It remains unknown exactly how TRM are generated during vitiligo but multiple reports in infection models suggest that KLRG1− precursors give rise to TRM, and that dendritic cell signals during cross priming including IL-2 and IL-15 direct their differentiation into TRM (43, 67, 68). The tissue microenvironment also helps shape TRM development, as hair follicle and keratinocyte derived IL-7 and IL-15 are critical for maintenance of TRM (69) as is TGFβ(44).
Epidermal skin TRM adopt a dendritic shape and have limited mobility allowing for their surveillance of the tissue(70). In contrast to virus-specific CD8+ TRM, which do not encounter viral antigen unless reinfected, self-reactive CD8+ TRM have the potential for frequent exposure to autoantigens. To determine whether CD8+ TRM detect antigen in the skin, vitiligo was induced using melanocyte-specific CD8+ T cells expressing the reporter Nur77-GFP(71, 72). In this system, GFP positivity indicates antigen recognition through activation of the T cell receptor. About 10% of epidermal CD8+ TRM were positive for Nur77-GFP (71). Similar to human disease, melanocytes in our mouse model of vitiligo repigment the epidermis through migration from the hair follicle (58) and we suspect that epidermal CD8+ TRM sense antigen as hair follicle melanocytes migrate to repopulate the epidermis. In the dermis, 30% of melanocyte-specific CD8+ TRM expressed GFP from the Nur77 reporter (71). As melanocytes predominantly reside in the epidermis, this data suggests that either CD8+ TRM sense antigen from an unknown dermal resident melanocyte population or from cross presentation of melanocyte antigen by resident dendritic cells. Interestingly, during stable disease in mice, 60-80% of melanocyte-specific CD8+ TRM in the epidermis appeared to express the effector cytokine IFNγ, measured using the GREAT reporter of IFN-γ expression (71). As the percentage of IFNγ+ CD8+ TRM exceeds the percentage of CD8+ TRM sensing antigen, it suggests that IFNγ production by CD8+ TRM may be induced independent of antigen sensing, or that brief antigen exposure leads to extended production of IFNγ. The expression of IFNγ by CD8+ TRM also suggests that they have the potential to recruit additional T cells via production of effector cytokine and downstream chemokines(71). Together these studies reveal that IFNγ signaling is not only important for disease progression, but expression is chronically maintained in stable disease from CD8+ TRM.
Another recent paper identified CD8+ TRM in the skin of vitiligo mice using a mouse model in which dermal inoculation with B16 melanoma cells, depletion of regulatory T cells, and excision of the melanoma tumor led to autoimmune vitiligo visible by hair depigmentation(73). In this model, melanoma/melanocyte specific CD8+ T cells were highly enriched in lesional skin and expressed the TRM markers CD69 and CD103. These cells were non-migratory and upon ex vivo stimulation produced IFNγ. Likewise, melanoma/melanocyte CD8+ TRM were found located at the epidermal/dermal junction near melanocyte-depleted hair follicles (73).
The authors questioned whether the formation of CD8+ TRM in this model is dependent on autoimmune vitiligo. The authors found significant enrichment of skin CD8+ TRM in mice that developed vitiligo compared to mice without vitiligo (70% to 12% respectively)(73), suggesting that the development of vitiligo drives formation and retention of melanoma/melanocyte TRM in the skin. Melanoma/melanocyte CD8+ TRM formation was dependent on expression of CD103, and CD103 expression was required for protection against melanoma re-challenge. CD103+ TRM were both necessary and sufficient for tumor protection, as treatment of mice with FTY720 did not alter the tumor response. Although development of CD8+ TRM was enhanced by vitiligo, tumor protection after re-challenge was independent of vitiligo and suggests that small pools of seeded melanoma/melanocyte CD8+ TRM are sufficient to mediate protection(73).
An additional report also describes the role of TRM in mediating protection against melanoma(74). Using a mouse model of epicutaneous melanoma, the authors reported that resident memory CD8+ T cells restrained the outgrowth of melanoma cells, and persist in the epidermis for continued tumor surveillance even in the absence of tumor growth. Melanoma TRM alone were able to mediate protection against melanoma challenge in the majority of mice, but complete protection was established when both TRM and recirculating memory T cells were present.
CD8+ TRM in human vitiligo
Recently, we and others identified TRM in human vitiligo patient skin (40, 41, 64). Similar to healthy skin, vitiligo skin is populated by TCM, TEM, and TRM. The majority of CD8+ T cells are CD45RO+ CCR7− TEM with only 10% expressing CCR7+, a marker for circulating memory T cells(40). Of the memory CD8+ T cells in vitiligo skin, many express the canonical TRM markers CD69 and CD103. It is worth noting that CD69+CD103+ TRM are enriched in stable vitiligo skin compared to patients with active disease (42% or 93% respectively) (40, 64), consistent with their role as memory cells that persist after active inflammation clears. CD8+ TRM are located in both the epidermis and dermis but are enriched in the epidermal compartment(40, 41).
Melanocyte-specific CD8+ T cells were identified using HLA-A2*0201 pentamers for MART1, gp100, and tyrosinase in the blood and skin of healthy controls and vitiligo patients. To identify these cells in the skin, we induce a blister through negative pressure or suction to obtain the fluid, which represents interstitial skin fluid and contains cells and proteins involved in local inflammation. This fluid is distinct from intravascular fluid, and thus provides an excellent source for sampling immune components involved in peripheral tissue inflammation. In vitiligo patients, melanocyte-specific CD8+ T cells were highly enriched in the skin fluid in both lesional and non-lesional skin compared to blood(64). The presence of melanocyte-specific CD8+ T cells in non-lesional skin suggests that peripheral tolerance mechanisms might prevent the formation of lesions at these sites. As previously shown by other groups, melanocyte-specific CD8+ T cells were detected in the blood of healthy controls, but no pentamer positive cells were found in the skin fluid (18, 20, 64, 75, 76). Expression of the chemokine receptor CXCR3 is present on the majority of skin CD8+ TRM in both healthy and vitiligo patients. Interestingly, circulating CXCR3+ CD8+ TEM in vitiligo patients have enhanced proliferative capacity compared to CXCR3+ CD8+ TEM in healthy controls and to their CXCR3 negative counterparts(40). Furthermore, CXCR3 expression is enriched on melanocyte-specific CD8+ TRM compared to non-melanocyte reactive TRM(40), highlighting the functional importance of this chemokine receptor in vitiligo.
Further studies investigated the effector and cytolytic functions of CD8+ TRM in vitiligo. Compared to healthy control skin, vitiligo patient skin shows a significant increase in IFNγ+ and IFNγ+TNFα+ producing CD8+ TRM(40, 41, 64). Increases in IFNγ+ CD8+ TRM was not seen in psoriasis patient skin, which is mediated by IL-17 producing effector T cells. Poly-functional IFNγ+TNFα+ CD8+ TRM are enriched in active disease, a state in which there is new lesion formation and high numbers of TEM are recruited to kill melanocytes. Cytotoxic ability of CD8+ TRM measured by granzyme B and perforin was not different between healthy control and vitiligo CD8+ TRM in one study (40), whereas another study found that granzyme B and perforin expressing CD8+ TRM were increased in vitiligo skin(41). These differences may be a result of high variability between the healthy and vitiligo patient cohorts tested. Nevertheless, the results suggest that CD8+ TRM in vitiligo have cytolytic capabilities in the skin tissue.
Phenotypic analyses of CD8+ TRM in healthy human skin revealed heterogeneity within the population (41). Researchers identified a specialized subset of CD8+ TRM that express the integrin alpha subunit, CD49a (41). In healthy human skin CD49a−expressing TRM are enriched within epidermal CD8+ TRM compared to dermal CD8+ TRM. To determine whether CD49a+ TRM were functionally distinct, RNA-sequencing studies were performed. Interestingly, CD49a+ CD8+ TRM were enriched in transcripts encoding cytotoxic components including granzymes and perforin. Of interest, stimulation of epidermal cell suspensions with IL-2 and IL-15, but not other pro-inflammatory cytokines, induced protein expression of granzyme B and perforin. In vitro assays revealed that the cytotoxic ability of CD49a+ CD8+ TRM was dependent on stimulation with IL-15, and that IL-15 augments IFNγ production by CD49a+ TRM. The authors looked for CD49a+ TRM in vitiligo and identified a significant population of CD49a+ TRM in lesional vitiligo skin limited to CD8+ but not CD4+ TRM. Furthermore, CD49a+ CD8+ TRM are significantly increased in the epidermis and dermis of lesional vitiligo skin compared to healthy control skin(41). Collectively, these studies reveal the presence of CD8+ TRM in vitiligo lesions and identify a subset of TRM with enhanced cytolytic and effector cytokine potential.
Sentinel and Alarm Function of TRM in vitiligo
It is clear from recent reports that CD8+ TRM possess cytotoxic ability including those in vitiligo skin, but whether they are actively involved in killing of target cells is still under debate. Elegant studies in viral models report that skin CD8+ TRM alone are sufficient to mediate viral clearance(44, 77, 78), while other studies suggest that CD8+ TRM act as sentinel cells that recruit recirculating memory cells to mediate viral clearance(79-81). In the former studies, mice with vaccinia virus (VACV)-specific CD8+ TRM were able to clear skin re-challenge more efficiently than mice without VACV CD8+TRM, demonstrating that skin CD8+ TRM enhance viral clearance during reinfection (77, 78). Virus was efficiently controlled by VACV TRM even in mice treated with FTY720, an S1P1 inhibitor, which blocks T cell egress from the lymph nodes, suggesting that recruitment of TCM was not required for clearance. However additional studies reported that after sensing viral antigen, local TRM cells produce IFNγ and CXCL9 to promote migration of recirculating memory CD8+ T cells (79). Two additional reports revealed that reactivation of TRM by viral antigen was critical to the amplification of both innate and adaptive antiviral responses including induction of dendritic cell maturation, and NK cell activity. TRM reactivation abruptly reprogrammed the tissue and induced the activation of many inflammatory genes within 3 hours of sensing antigen (80, 81). Thus, some studies report that TRM are effective killers that can control viral infection alone, while others suggest that TRM are not efficient killers, but instead serve to promote the recruitment of appropriate effector cells to the site of infection.
In vitiligo, skin TRM appear to be responsible for maintaining disease, and specifically for relapse of disease after discontinuing treatment(40, 41, 64, 71). During stable disease, melanocyte-specific CD8+ TRM likely sense antigen as melanocytes migrate out of the hair follicles to replenish the skin(71). However, blockade of CXCL10 reversed established disease, suggesting that continued recruitment of T cells was required even for maintenance of disease(54). Therefore, we asked whether vitiligo CD8+ TRM are sufficient to kill the repopulating melanocyte and maintain disease or whether recruitment of additional circulating memory CD8+ T cells are required to kill repigmenting melanocytes (71). We found that FTY720 treatment of mice with vitiligo led to rapid reversal of disease despite persistence of TRM. In addition, depletion of only recirculating autoimmune T cells reversed disease as well, supporting the concept that CD8+ TRM alone are not sufficient for the maintenance of vitiligo, but instead cooperate with recirculating memory CD8+ T cells to maintain disease(71). Since TCM downregulate CD62L and CCR7 to enter the skin tissue and thus resemble TEM, skin migrating melanocyte-specific CD8+ T cells may be newly recruited TCM or recirculating TEM. Active recruitment of recirculating memory CD8+ T cells by TRM may occur through production of IFNγ and downstream chemokines CXCL9 and CXCL10, which CD8+ TRM produce during disease (64).
Collectively, this data supports the model by which vitiligo skin resident CD8+ TRM do not kill melanocytes directly but instead actively recruit melanocyte-specific recirculating memory T cells to the skin to kill repigmenting melanocytes. In this feed forward loop, the cooperation between resident and recirculating memory leads to progression of disease. The local signals inducing CD8+ TRM production of IFNγ and downstream chemokines needs further investigation, but IL-15, which augments IFNγ production by CD8+ T cells (41), may play a role. Because there is significant heterogeneity within mucosal CD8+ TRM, a distinct subgroup of TRM may function in direct cytotoxicity while another may function to recruit circulating cytotoxic cells. Nevertheless, in vitiligo, recirculating memory T cells play an important role in maintaining disease, presumably through destruction of repopulating melanocytes (41, 82).
Checks and balances on TRM
Seeding of CD8+ TRM occurs during the effector phase of the immune response(83) and antigen-specific CD8+ TRM are found not only at the site of infection but throughout the tissue. Vitiligo is a focal disease in which islands of skin are affected and border unaffected areas. As melanocyte-specific CD8+ TRM are found in both lesional and non-lesional skin, it is likely that peripheral tolerance mechanisms prevent the formation of lesions at these sites, and their presence could be a sign of subclinical disease held in check by those mechanisms. Regulatory T cells, which are required to maintain peripheral tolerance to self, have been implicated in suppressing melanocyte-specific CD8+ T cells in vitiligo. Multiple studies report that the ratio of CD4+ to CD8+ T cells in the blood are reduced in vitiligo patients compared to healthy controls (84, 85), and that there is a significant decrease in the ability of Tregs to suppress CD8+ T cell proliferation and cytolytic function in vitiligo patients(84). Interestingly, overexpression of CCL22, a chemokine attractant for Tregs, in the skin of mice led to enhanced Treg numbers in the skin and significantly reduced depigmentation in a mouse model of vitiligo (86). Another study, which adoptively transferred Tregs into host mice and treated with rapamycin, led to lasting remission of vitiligo(87). These studies suggest that an imbalance of Treg number or Treg dysfunction may lead to vitiligo development and that Tregs play a critical role in suppressing melanocyte-specific CD8+ T cells.
In healthy control subjects, although melanocyte-specific CD8 + T cells are present in the blood, no pentamer positive cells are found in the skin tissue(64). It is reported that Tregs control potentially dangerous circulating melanocyte-specific CD8+ T cells by suppressing their proliferation, cytokine production, and by inducing anergy (88). Specifically, natural occurring Tregs induced the expression of CTLA-4 and CCR7 on circulating melanocyte-specific CD8+ T cells. Melanocyte-specific CTLA-4+ CCR7+ CD8+ T cells were detected in larger numbers in healthy individuals compared to vitiligo patients, suggesting that Tregs actively keep autoreactive cells in check within the circulation, and that this tolerance is disrupted in vitiligo patients(88).
A better understanding of the potential functional differences between lesional and non-lesional CD8+ TRM, as well as identification of the local suppressive mechanisms in place will provide insight into how CD8+ TRM are controlled. Further studies will help to identify the breaks in peripheral tolerance in vitiligo patient skin and will determine what signals prompt changes in the reactivation state of CD8+ TRM.
Targeting TRM in vitiligo
Studies in mice show that the cooperation of CD8+ TRM with recirculating memory CD8+ T cells leads to persistence of disease(71). While inhibiting the function of TRM can be an effective approach to treating vitiligo, this alone would not lead to long-lasting treatment responses, since these cells can become active again once the treatment is discontinued. This is presumably why vitiligo patients relapse after stopping conventional treatments(17), as well as newer treatments that inhibit Janus Kinase signaling(89). However, a treatment approach that results in the depletion of TRM from the skin could have long-lasting effects, as the autoimmune memory within the skin would be eliminated.
Multiple tissue-derived CD8+ TRM survival signals have been identified, including TGF-β, IL-7, and IL-15(43, 44, 69). Local IL-15 signaling was reported to be important for skin TRM development, survival and effector function(41, 44). A major source of IL-15 production in the skin is from epidermal basal keratinocytes that constitutively express the cytokine as well as the IL-15 receptor α chain, also known as CD215, which is required for trans-presentation of IL-15 to promote signaling(90). We found that expression of CD215 by keratinocytes in lesional vitiligo patient skin is increased compared to non-lesional skin, suggesting that local IL-15 signaling is dysregulated(64). Interestingly, the majority of melanocyte-specific CD8+ TRM in vitiligo skin express the IL-2/IL-15 receptor beta chain, CD122, and CD122 expression was significantly higher on melanocyte-specific CD8+ TRM compared to non-melanocyte reactive CD8+ TRM. These observations suggest that local IL-15 signaling is important for maintenance of melanocyte-specific CD8+ TRM in vitiligo lesional skin(64).
To determine whether targeting IL-15 signaling would lead to changes in skin CD8+ TRM, we treated mice exhibiting stable vitiligo with an anti-CD122 antibody. Anti-CD122 treatment resulted in significant skin repigmentation and a reduction of melanocyte-specific CD8+ TRM in the epidermis and dermis(64). Not only did long-term anti-CD122 treatment deplete CD8+ TRM in the skin, but even short-term treatment that was insufficient to deplete these cells reduced IFNγ production by TRM (64). This effect on CD8+ TRM was limited to melanocyte-specific cells, and treatment did not reduce host TRM or TCM, presumably because autoreactive CD8+ TRM in both humans and mice express higher amounts of CD122 and may be more dependent on IL-15 signaling(64). Importantly, short-term treatment with anti-CD122 antibody had long-lasting effects on repigmentation (64). This study supports the hypothesis that skin CD8+ TRM are responsible for the maintenance of vitiligo and that targeting this population in vitiligo patients would have durable effects.
Recent studies have identified CD8+ TRM in the female reproductive mucosa that are not dependent on IL-15 for survival or homeostatic proliferation. These studies provide further evidence that CD8+ TRM are heterogeneous and that subpopulations may rely on different signals for survival and maintenance at each tissue site(82). This may explain why a small number of CD8+ TRM remained in the skin after anti-CD122 treatment. Whether IL-15 independent CD8+ TRM populations are also present in vitiligo patient skin and whether these heterogeneous populations cluster in different microanatomical locations is not known. For example, CD8+ TRM located at the hair follicle may rely more on IL-7 than IL-15(69). Identification of these additional local signals will be important for targeting melanocyte-specific CD8+ TRM while sparing other skin resident T cells and would improve the specificity of the therapeutic.
Conclusions
In vitiligo, clinical observations reveal that white spots of depigmentation frequently return in the same exact location after cessation of treatment(16), suggesting that autoimmune memory drives pathology and maintenance of disease. Here we highlight recent studies that identify the signals driving CD8+ T cell memory formation and retention in vitiligo, as well as the potential to develop new therapeutics that target the memory cells responsible for maintaining disease (Figure 1).
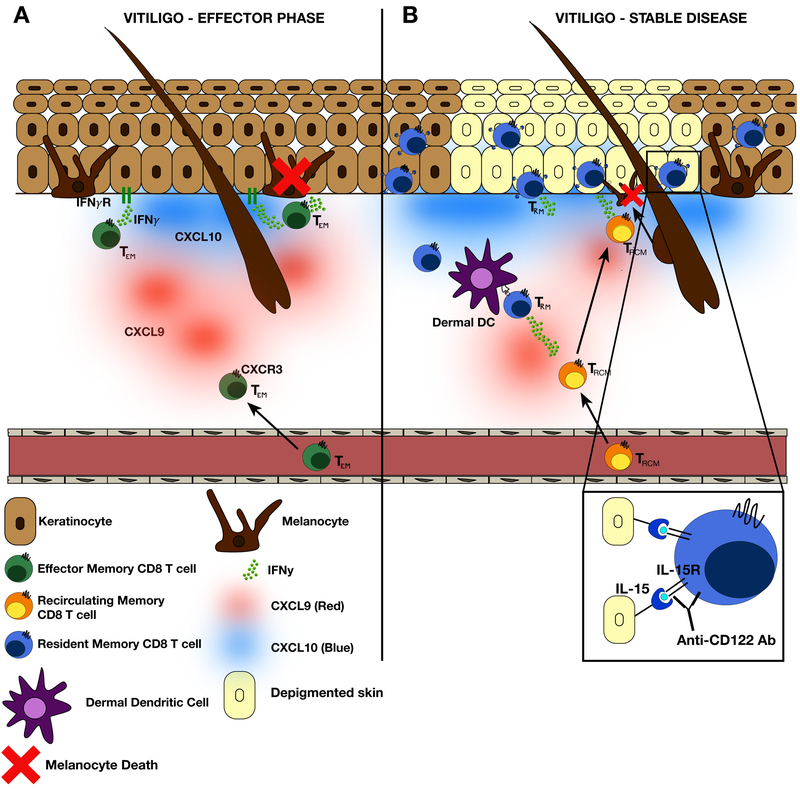
Figure 1: Summary of vitiligo pathogenesis during both the effector phase and stable vitiligo.
(A) In active vitiligo or early lesion development, IFNγ signaling on keratinocytes stimulates the production of the IFNγ-dependent chemokines, CXCL9 and CXCL10. Many epidermal cell types produce CXCL9 and CXCL10, but keratinocytes, which are numerous, produce the greatest total amounts. This chemokine gradient supports continued migration of melanocyte-specific effector memory CD8+ T cells (TEM) through the dermis to the dermal-epidermal junction (DEJ) where melanocytes reside. CXCL9 and CXCL10 both play a role in TEM trafficking; CXCL9 is responsible for bulk recruitment of CD8+ TEM into the skin, whereas CXCL10 is required for TEM tethering and microanatomical positioning at the DEJ, as well as CD8+ TEM function. Chemokine-dependent recruitment and positioning of CD8+ TEM leads to melanocyte death. Activated CD8+ TEM express IFNγ, which induces recruitment of additional CD8+ TEM to the skin and further melanocyte destruction. (B) Over the course of disease, a subset of CD8+ TEM differentiate into TRM resulting from expression of local retention signals and seed the skin tissue, including the depigmented lesion. Melanocyte-specific TRM are retained in the tissue due to trans-presentation of IL-15 by keratinocytes. Within a depigmented lesion, TRM likely sense melanocytes migrating from hair follicles and activation of TRM induces production of the inflammatory cytokines IFNγ and CXCL9 that help to recruit recirculating melanocyte-specific memory T cells (TRCM) to the skin where they can kill the repopulating melanocytes. This feed forward loop and cooperation between TRM and TRCM leads to the persistence of disease. In vitiligo, IL-15 receptor expression is enriched on melanocyte-specific CD8+ TRM, and treatment of mice with an anti-CD122 antibody (IL-15 receptor beta) led to significant reversal of disease characterized by a reduction of TRM numbers and effector function in the skin. Importantly, short-term treatment with anti-CD122 antibody proved to be durable in mice.
Early events in vitiligo pathogenesis are dependent on IFNγ signaling on keratinocytes, which induces expression of the chemokine ligands CXCL9 and CXCL10 by skin resident cells (Figure 1A). This chemokine gradient triggers migration of CXCR3+ melanocyte-specific CD8+ T cells to enter the skin and directs their migration through the dermis to the epidermal/dermal junction where they can identify and destroy melanocytes (Figure 1A). Once T cells enter the epidermis, a subset of melanocyte-specific CD8+ T cells differentiate into TRM due to expression of local retention signals, and these cells persist long-term within the lesional skin (Figure 1B). Melanocyte-specific CD8+ TRM in mice and human vitiligo skin are enriched in the epidermis and located both at the dermal epidermal junction in humans and at the interfollicular epidermis close to hair follicles in mice.
CD8+ TRM retention within the epidermis results from local production of the survival signal IL-15, which is produced by hair follicle and basal keratinocytes (Figure 1B) (91). The hair follicles are also a source of antigen, as melanocytes migrate from their niche at that location to repigment the skin. Detection of antigen and/or local inflammatory cues including IFNγ signaling and IL-15(41) may induce reactivation of quiescent CD8+ TRM to recruit recirculating memory CD8+ T cells to the skin where they can kill repopulating melanocytes. This positive feedback circuit leads to the persistence of disease and recalcitrance to treatment.
Targeting CD8+ TRM by blocking IL-15 signaling using an anti-CD122 antibody led to significant reversal of vitiligo by both reducing CD8+ TRM numbers in the skin and by suppressing their effector function(64). Importantly, short term anti-CD122 treatment led to a durable response in mice. These recent studies provide important mechanistic insight into the role of TRM in the maintenance of vitiligo and supports targeting this population as a new, durable treatment strategy (Figure 1B). We are hopeful that targeting TRM will provide a long-lasting treatment option for vitiligo patients.
Acknowledgments
This work was supported by the National Institutes of Health grants AR09114 and AR07302 to JEH and training grant AI007349 to RLR.
Abbreviations:
- nbUVB
narrow band ultraviolet light B therapy
- HLA
human leukocyte antigen
- TCM
central memory T cells
- TEM
effector memory T cells
- TRM
resident memory T cells
- VACV
vaccinia virus
- CXCL9
chemokine (CXC motif) ligand 9
- CXCL10
chemokine (CXC motif) ligand 10
References
- 1.Taïeb A, and Picardo M. 2009. Vitiligo Clinical practice. New England Journal of Medicine 160–169. [DOI] [PubMed] [Google Scholar]
- 2.Alikhan A, Felsten LM, Daly M, and Petronic-Rosic V. 2011. Vitiligo: A comprehensive overview. Journal of American Dermatology 65: 473–491. [DOI] [PubMed] [Google Scholar]
- 3.Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, Pandya AG, Vitiligo Working Group. 2015. Vitiligo is not a cosmetic disease. Journal of the American Academy of Dermatology 73: 883–885. [DOI] [PubMed] [Google Scholar]
- 4.Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, and Taïeb A. 2015. Vitiligo. Nat Rev Dis Primers 1: 15011. [DOI] [PubMed] [Google Scholar]
- 5.Harris JE 2017. Optimizing Vitiligo Management: Past, Present, and Future. Dermatol Clin 35: xi. [DOI] [PubMed] [Google Scholar]
- 6.Salzes C, Abadie S, Seneschal J, Whitton M, Meurant J-M, Jouary T, Ballanger F, Boralevi F, Taïeb A, Taieb C, and Ezzedine K. 2016. The Vitiligo Impact Patient Scale (VIPs): Development and Validation of a Vitiligo Burden Assessment Tool. J. Invest. Dermatol. 136: 52–58. [DOI] [PubMed] [Google Scholar]
- 7.MD, L. H. MW, Spuls P. Phyllis I MD, de Korte P. John MA, Bos P. Jan D MD, Sprangers P. Mirjam A MA, and Wietze van der Veen PJP MD. 2009. The burden of vitiligo: Patient characteristics associated with quality of life. Journal of American Dermatology 61: 411–420. [DOI] [PubMed] [Google Scholar]
- 8.Spritz RA, and Andersen GHL. 2017. Genetics of Vitiligo. Dermatol Clin 35: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richmond JM, Frisoli ML, and Harris JE. 2013. Innate immune mechanisms in vitiligo: danger from within. Current Opinion in Immunology 25: 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frisoli ML, and Harris JE. 2017. Vitiligo: Mechanistic insights lead to novel treatments. J. Allergy Clin. Immunol. 140: 654–662. [DOI] [PubMed] [Google Scholar]
- 11.Cichorek M, Wachulska M, Stasiewicz A, and Tymińska A. 2013. Skin melanocytes: biology and development. Postepy Dermatol Alergol 30: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falabella R 2009. Vitiligo and the melanocyte reservoir. Indian J Dermatol 54: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birlea SA, Costin G-E, Roop DR, and Norris DA. 2017. Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Med Res Rev 37: 907–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J, Shen LY, and Wang GC. 1991. Role of hair follicles in the repigmentation of vitiligo. J Investig Dermatol 97: 410–416. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. 2017. Current and emerging treatments for vitiligo. Journal of the American Academy of Dermatology 77: 17–29. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaidou E, Antoniou C, Stratigos AJ, Stefanaki C, and Katsambas AD. 2007. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. Journal of the American Academy of Dermatology 56: 274–278. [DOI] [PubMed] [Google Scholar]
- 17.Cavalié M, Ezzedine K, Fontas E, Montaudié H, Castela E, Bahadoran P, Taïeb A, Lacour J-P, and Passeron T. 2015. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J. Invest. Dermatol. 135: 970–974. [DOI] [PubMed] [Google Scholar]
- 18.Ogg GS, Dunbar PR, Romero P, Chen J-L, and Cerundolo V. 1998. High Frequency of Skin-homing Melanocyte-specific Cytotoxic T Lymphocytes in Autoimmune Vitiligo. Journal of Experimental Medicine 188: 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, and Harris JE. 2017. Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity. Journal of American Dermatology 76: 847–855.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Boorn JG, Konijnenberg D, Dellemijn TAM, van der Veen JPW, Bos JD, Melief CJM, Vyth-Dreese FA, and Luiten RM. 2009. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Invest. Dermatol. 129: 2220–2232. [DOI] [PubMed] [Google Scholar]
- 21.Gallatin WM, Weissman IL, and Butcher EC. 1983. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature 304: 30–34. [DOI] [PubMed] [Google Scholar]
- 22.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, Wolf E, and Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. [DOI] [PubMed] [Google Scholar]
- 23.Andrian, von UH, and Mackay CR. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343: 1020–1034. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins MK, Chu HH, McLachlan JB, and Moon JJ. 2010. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu. Rev. Immunol. 28: 275–294. [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt RL, Khoruts A, Merica R, Zell T, and Jenkins MK. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410: 101–105. [DOI] [PubMed] [Google Scholar]
- 26.Masopust D, Vezys V, Marzo AL, and Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. [DOI] [PubMed] [Google Scholar]
- 27.Welsh RM, Selin LK, and Szomolanyi-Tsuda E. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22: 711–743. [DOI] [PubMed] [Google Scholar]
- 28.Kaech SM, and Wherry EJ. 2007. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi Y, Lu B, Gerard C, and Iwasaki A. 2009. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 462: 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H, and Iwasaki A. 2013. Tissue-resident memory T cells. Immunol. Rev. 255: 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, and Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 32.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, and Butcher EC. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromley SK, Thomas SY, and Luster AD. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol 6: 895–901. [DOI] [PubMed] [Google Scholar]
- 34.Mackay CR, Marston WL, Dudler L, Spertini O, Tedder TF, and Hein WR. 1992. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur. J. Immunol. 22: 887–895. [DOI] [PubMed] [Google Scholar]
- 35.Mackay CR, Marston WL, and Dudler L. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. Journal of Experimental Medicine 171: 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. [DOI] [PubMed] [Google Scholar]
- 37.Wakim LM, Woodward-Davis A, and Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. U.S.A. 107: 17872–17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SK, Reed DS, Heath WR, Carbone F, and Lefrancois L. 1997. Activation and migration of CD8 T cells in the intestinal mucosa. The Journal of Immunology 159: 4295–4306. [PubMed] [Google Scholar]
- 39.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, and Kupper TS. 2006. The vast majority of CLA+ T cells are resident in normal skin. The Journal of Immunology 176: 4431–4439. [DOI] [PubMed] [Google Scholar]
- 40.Boniface K, Jacquemin C, Darrigade A-S, Dessarthe B, Martins C, Boukhedouni N, Vernisse C, Grasseau A, Thiolat D, Rambert J, Lucchese F, Bertolotti A, Ezzedine K, Taïeb A, and Seneschal J. 2018. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J. Invest. Dermatol. 138: 355–364. [DOI] [PubMed] [Google Scholar]
- 41.Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, Höög C, Tjernlund A, Michaëlsson J, Folkersen L, Mjösberg J, Blomqvist L, Ehrström M, Ståhle M, Bryceson YT, and Eidsmo L. 2017. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 46: 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, and Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nature Publishing Group 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, and Gebhardt T. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nature Publishing Group 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 44.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, and Carbone FR. 2015. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 45.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, and Hadley GA. 2005. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. Journal of Experimental Medicine 201: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, Peters B, Shen ZT, Vanguri V, Liggitt D, and Huseby ES. 2014. Relapsing-remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J. Immunol. 192: 3029–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matos TR, O’Malley JT, Lowry EL, Hamm D, Kirsch IR, Robins HS, Kupper TS, Krueger JG, and Clark RA. 2017. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J. Clin. Invest. 127: 4031–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pizzolla A, Nguyen TH, Sant S, Jaffar J, Loudovaris T, Mannering SI, Thomas PG, Westall GP, Kedzierska K, and Wakim LM. 2018. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Invest. 128: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuric E, Seiron P, Krogvold L, Edwin B, Buanes T, Hanssen KF, Skog O, Dahl-Jørgensen K, and Korsgren O. 2017. Demonstration of Tissue Resident Memory CD8 T Cells in Insulitic Lesions in Adult Patients with Recent-Onset Type 1 Diabetes. The American Journal of Pathology 187: 581–588. [DOI] [PubMed] [Google Scholar]
- 50.Clark RA 2015. Resident memory T cells in human health and disease. Science Translational Medicine 7: 269rv1–269rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teraki Y, and Shiohara T. 2003. IFN-gamma-producing effector CD8+ T cells and IL-10-producing regulatory CD4+ T cells in fixed drug eruption. J. Allergy Clin. Immunol. 112: 609–615. [DOI] [PubMed] [Google Scholar]
- 52.Cheuk S, Wikén M, Blomqvist L, Nylén S, Talme T, Ståhle M, and Eidsmo L. 2014. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 192: 3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, and Turka LA. 2012. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. J Investig Dermatol 132: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD, and Harris JE. 2014. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science Translational Medicine 6: 223ra23–223ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregg RK, Nichols L, Chen Y, Lu B, and Engelhard VH. 2010. Mechanisms of Spatial and Temporal Development of Autoimmune Vitiligo in Tyrosinase-Specific TCR Transgenic Mice. The Journal of Immunology 184: 1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grimes PE, Morris R, Avaniss-Aghajani E, Soriano T, Meraz M, and Metzger A. 2004. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. Journal of the American Academy of Dermatology 51: 52–61. [DOI] [PubMed] [Google Scholar]
- 57.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, and Restifo NP. 2003. Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8 +T Cells. Journal of Experimental Medicine 198: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riding RL, Richmond JM, and Harris JE. 2018. Mouse Model for Human Vitiligo. Curr Protoc Immunol 135: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, and Turka LA. 2012. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8⁺ T-cell accumulation in the skin. J. Invest. Dermatol. 132: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groom JR, and Luster AD. 2011. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 89: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luster AD, and Ravetch JV. 1987. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). Journal of Experimental Medicine 166: 1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, Youd ME, Luster AD, and Harris JE. 2017. Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. J Investig Dermatol 137: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, and Harris JE. 2017. CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. J. Invest. Dermatol. 137: 982–985. [DOI] [PubMed] [Google Scholar]
- 64.Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, Fan X, Azzolino V, Tovar-Garza A, Tsurushita N, Pandya AG, Tso JY, and Harris JE. 2018. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Science Translational Medicine 10: eaam7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertolotti A, Boniface K, Vergier B, Mossalayi D, Taïeb A, Ezzedine K, and Seneschal J. 2014. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res 27: 398–407. [DOI] [PubMed] [Google Scholar]
- 66.Baaten BJG, Tinoco R, Chen AT, and Bradley LM. 2012. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. frontiers in Immunology 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, Del Fresno C, and Sancho D. 2016. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1+ Dendritic Cells. Immunity 45: 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sheridan BS, Pham Q-M, Lee Y-T, Cauley LS, Puddington L, and Lefrancois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, Saya H, Amagai M, and Nagao K. 2015. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med 21: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AFM, Zal T, de Boer RJ, Haanen JBAG, and Schumacher TN. 2012. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. U.S.A. 109: 19739–19744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, Pell LS, and Harris JE. 2018. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J. Invest. Dermatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, and Hogquist KA. 2011. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. Journal of Experimental Medicine 208: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, Molodtsov AK, Bowers JS, Angeles CV, Paulos CM, Huang YH, and Turk MJ. 2017. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2: eaam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, Wilmott JS, Scolyer RA, Tuting T, Palendira U, Gyorki D, Mueller SN, Huntington ND, Bedoui S, Hölzel M, Mackay LK, Waithman J, and Gebhardt T. 2019. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 565: 366–371. [DOI] [PubMed] [Google Scholar]
- 75.Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Liénard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, and Romero P. 1999. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. Journal of Experimental Medicine 190: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huard B, and Karlsson L. 2000. A subpopulation of CD8+ T cells specific for melanocyte differentiation antigens expresses killer inhibitory receptors (KIR) in healthy donors: evidence for a role of KIR in the control of peripheral tolerance. Eur. J. Immunol. 30: 1665–1675. [DOI] [PubMed] [Google Scholar]
- 77.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, and Gebhardt T. 2012. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. U.S.A. 109: 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schenkel JM, Fraser KA, Vezys V, and Masopust D. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol 14: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, Jacobs H, Haanen JB, and Schumacher TN. 2014. T cell memory. Skin-resident memory CD8⁺ T cells trigger a state of tissue-wide pathogen alert. Science 346: 101–105. [DOI] [PubMed] [Google Scholar]
- 81.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, and Masopust D. 2014. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, and Masopust D. 2016. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J. Immunol. 196: 3920–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, and Ahmed R. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lili Y, Yi W, Ji Y, Yue S, Weimin S, and Ming L. 2012. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS ONE 7: e37513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dwivedi M, Laddha NC, Arora P, Marfatia YS, and Begum R. 2013. Decreased regulatory T-cells and CD4(+) /CD8(+) ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res 26: 586–591. [DOI] [PubMed] [Google Scholar]
- 86.Eby JM, Kang H-K, Tully ST, Bindeman WE, Peiffer DS, Chatterjee S, Mehrotra S, and Le Poole IC. 2015. CCL22 to Activate Treg Migration and Suppress Depigmentation in Vitiligo. J. Invest. Dermatol. 135: 1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang H-K, Kaur N, Naga OS, Murali A, Nishimura MI, Caroline Le Poole I, and Mehrotra S. 2014. A quantitative increase in regulatory T cells controls development of vitiligo. J. Invest. Dermatol. 134: 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, Wing JB, Adeegbe D, Katayama I, and Sakaguchi S. 2014. Detection of self-reactive CD8⁺ T cells with an anergic phenotype in healthy individuals. Science 346: 1536–1540. [DOI] [PubMed] [Google Scholar]
- 89.Harris JE, Rashighi M, Nguyen N, Jabbari A, Ulerio G, Clynes R, Christiano AM, and Mackay-Wiggan J. 2016. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). Journal of the American Academy of Dermatology 74: 370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blauvelt A, Asada H, Klaus-Kovtun V, Altman DJ, Lucey DR, and Katz SI. 1996. Interleukin-15 mRNA is expressed by human keratinocytes Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J Investig Dermatol 106: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 91.Dubois S, Mariner J, Waldmann TA, and Tagaya Y. 2002. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 17: 537–547. [DOI] [PubMed] [Google Scholar]