Abstract
The acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in critically ill patients and is defined by the acute onset of noncardiogenic pulmonary oedema, hypoxaemia and the need for mechanical ventilation. ARDS occurs most often in the setting of pneumonia, sepsis, aspiration of gastric contents or severe trauma and is present in ~10% of all patients in intensive care units worldwide. Despite some improvements, mortality remains high at 30–40% in most studies. Pathological specimens from patients with ARDS frequently reveal diffuse alveolar damage, and laboratory studies have demonstrated both alveolar epithelial and lung endothelial injury, resulting in accumulation of protein-rich inflammatory oedematous fluid in the alveolar space. Diagnosis is based on consensus syndromic criteria, with modifications for under-resourced settings and in paediatric patients. Treatment focuses on lung-protective ventilation; no specific pharmacotherapies have been identified. Long-term outcomes of patients with ARDS are increasingly recognized as important research targets, as many patients survive ARDS only to have ongoing functional and/or psychological sequelae. Future directions include efforts to facilitate earlier recognition of ARDS, identifying responsive subsets of patients and ongoing efforts to understand fundamental mechanisms of lung injury to design specific treatments.
Subject terms: Respiratory distress syndrome, Sepsis, Respiratory tract diseases, Immunology
Acute respiratory distress syndrome (ARDS) is the rapid onset of noncardiogenic pulmonary oedema, hypoxaemia and the need for mechanical ventilation in hospitalized patients. This Primer describes the risk factors for ARDS, the underlying pulmonary damage and repair in ARDS and the long-term consequences for survivors.
Introduction
The acute respiratory distress syndrome (ARDS) was initially defined in 1967 with a case-based report that described the clinical presentation in critically ill adults and children of acute hypoxaemia, noncardiogenic pulmonary oedema, reduced lung compliance (increased lung stiffness), increased work of breathing and the need for positive-pressure ventilation in association with several clinical disorders including trauma, pneumonia, sepsis and aspiration1. In 1992, an American–European consensus conference established specific diagnostic criteria for the syndrome2; these criteria were updated in 2012 in the so-called Berlin definition3 of ARDS in adults (Box 1). Depending on the level of oxygenation, ‘mild’, ‘moderate’ and ‘severe’ descriptors can be added to the diagnosis of ARDS (Box 1). The diagnosis of ARDS depends on clinical criteria alone because it is not practical to obtain direct measurements of lung injury by pathological samples of lung tissue in most patients; furthermore, neither distal airspace nor blood samples can be used to diagnose ARDS.
ARDS develops most commonly in the setting of pneumonia (bacterial and viral; fungal is less common), nonpulmonary sepsis (with sources that include the peritoneum, urinary tract, soft tissue and skin), aspiration of gastric and/or oral and oesophageal contents (which may be complicated by subsequent infection) and major trauma (such as blunt or penetrating injuries or burns). Several other less common scenarios are also associated with the development of ARDS, including acute pancreatitis; transfusion of fresh frozen plasma, red blood cells and/or platelets (that is, transfusion-associated acute lung injury (TRALI)); drug overdose with various agents; near drowning (inhalation of fresh or salt water); haemorrhagic shock or reperfusion injury (including after cardiopulmonary bypass and lung resection); and smoke inhalation (often associated with cutaneous burn injuries). Other causes of noncardiogenic pulmonary oedema that are often considered as additional aetiologies of ARDS include primary graft dysfunction following lung transplantation, high-altitude pulmonary oedema, neurogenic oedema (following a central nervous system insult or injury) and drug-induced lung injury. The frequency of the clinical disorders associated with ARDS varies depending on the geographical location, the health-care systems that are available and whether they are resource-rich or resource-poor.
In the past 50 years, considerable progress has been made in understanding the epidemiology, pathogenesis and pathophysiology of ARDS. In addition, randomized trials to optimize mechanical ventilation and fluid therapy for ARDS have resulted in improved clinical outcomes. Although much progress has been made to improve supportive care for ARDS, effective pharmacological therapies for ARDS have not yet been identified. However, ARDS is increasingly being recognized as a heterogenous syndrome, generating momentum to identify clinical and biological features to classify patients into subphenotypes that might be more responsive to specific therapies. Furthermore, evidence of important long-term effects in survivors of ARDS is growing, driving the need for research strategies to study how these effects could be mitigated.
This Primer on adult and paediatric ARDS considers the epidemiology of ARDS from a global perspective, mechanisms of lung endothelial and alveolar epithelial injury (including experimental and clinical studies) and optimal approaches to diagnosis with a focus on screening, early recognition and evaluation for infectious and non-infectious causes. We also discuss management strategies, including mechanical ventilation, fluids and rescue therapies for severe ARDS. Finally, we describe quality of life (QOL) after recovery from ARDS. Throughout the Primer, the differences between adult and paediatric ARDS are considered.
Box 1 Definitions of ARDS in adults.
2012 Berlin definition 3
Timing: respiratory failure within 1 week of a known insult or new and/or worsening respiratory symptoms
Origin: respiratory failure not fully explained by cardiac function or volume overload (need objective criterion such as echocardiography to exclude hydrostatic oedema if no risk factor is present)
Imaging: bilateral opacities on chest radiograph or CT not fully explained by effusion, collapse or nodules
- Oxygenation: acute onset of hypoxaemia defined as PaO2/FiO2 <300 mmHg on at least PEEP 5 cmH2Oa
- PaO2/FiO2 of 201–300 mmHg is mild ARDS
- PaO2/FiO2 of 101–200 mmHg is moderate ARDS
- PaO2/FiO2 ≤100 mmHg is severe ARDS
2016 Kigali modificationb (ref.124)
Timing and origin: as in the Berlin definition
Imaging: bilateral opacities on chest radiography or ultrasonography scan not fully explained by effusion, collapse or nodules
Oxygenation: SpO2/FiO2 <315; no PEEP requirement
aPEEP may be delivered non-invasively if the criteria are in the mild category. bThe Kigali definition was not directly compared with the Berlin definition in the original publication; patients in the Kigali study were not receiving mechanical ventilation. ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SpO2, peripheral capillary oxygen saturation.
Epidemiology
Adults
A well-designed, prospective analysis of the incidence of ARDS in the United States was carried out in a population-based cohort study of 21 hospitals in King County, Washington, from April 1999 to July 2000, focused primarily on adults4. The investigators used a validated screening protocol to identify patients with ARDS (defined as a partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (PaO2/FiO2) of <300 mmHg and bilateral chest radiographic opacities without evidence of left-sided heart failure). On the basis of these data, the authors estimated an annual incidence of 190,000 cases of ARDS in the United States with a hospital mortality of 38.5%. Subsequently, the LUNG-SAFE study provided valuable data in a cross-sectional analysis of 29,144 patients in 50 countries during the winter of 2014 (ref.5); in this study, the prevalence of ARDS in patients in intensive care was 10%, and ARDS was identified in 23% of all ventilated patients. A detailed review of ARDS incidence and time-related changes in epidemiological factors has been recently published6.
The LUNG-SAFE study also reported that hospital mortality was 34.9% for patients with mild ARDS, 40% for those with moderate ARDS and 46.1% for those with severe ARDS (Berlin criteria3; Box 1). However, it remains unclear how much of the reported mortality in ARDS can be attributed to ARDS as opposed to underlying comorbidities, such as cancer and immunosuppression, the associated nonpulmonary organ dysfunction (cardiovascular insufficiency as in septic shock, liver dysfunction and renal failure) and/or the older age of patients with the condition. For example, a follow-up analysis of the LUNG-SAFE study determined that 21% of patients with ARDS in the study were immunocompromised, and hospital mortality was much higher in these patients than in non-immunocompromised patients (52% versus 36%; P < 0.0001)7.
Importantly, the LUNG-SAFE study revealed that clinician recognition of ARDS was low — 51% in mild ARDS and only 79% in severe ARDS. Furthermore, fewer than two-thirds of patients with ARDS were treated with an optimal tidal volume (that is, for mechanical ventilation) of <8 ml per kg predicted body weight (PBW). Thus, this study confirmed that ARDS remains common in critically ill patients and that it is under-recognized and under-treated.
Several comorbidities and exposures have been associated with increased susceptibility to ARDS, including alcohol abuse8, cigarette smoking9,10, air pollution11,12 and hypoalbuminaemia13. Diabetes mellitus has been associated with a lower risk of ARDS development for reasons that remain unclear14,15, although data from the LUNG-SAFE study showed no association between diabetes and the diagnosis of ARDS, developing ARDS or outcomes of ARDS16. A two-centre clinical study identified the major recipient risk factors for developing TRALI, including recent liver surgery, chronic alcohol abuse, current cigarette smoking, higher peak airway pressure (that is, the highest airway pressure while being ventilated) and positive fluid balance17. Transfusion risk factors were receipt of whole blood or fresh frozen plasma from a female donor and the quantity of human leukocyte antigen (HLA) class II antibody or the volume concentration of anti-human neutrophil antigen in the transfused blood components. There was no risk associated with the age of the stored blood. Indeed, eliminating fresh frozen plasma or whole-blood transfusion from female donors markedly reduced the incidence of TRALI, implicating a role for allogeneic antibodies in the pathogenesis of TRALI. In blunt and penetrating trauma, the severity and duration of shock, chest injury, the number of blood product transfusions, the presence of traumatic brain injury and the quantity of crystalloid fluids (as a volume expander) can each contribute to the risk of developing ARDS18,19.
Studies of the association between ethnicity and ARDS outcomes have largely but not uniformly reported higher mortality for black and Hispanic patients with ARDS than for white patients20,21. In an analysis of patients who died with a diagnostic code for ARDS in the United States, men had higher average ARDS-related mortality than women, and black patients had higher mortality than white patients, echoing findings from similar analyses from prior decades22,23. The aetiology of these disparities remains unknown.
Studies designed to identify genetic factors that might contribute to the susceptibility for developing ARDS have been reported in the past decade. However, no specific loci with genome-wide significance for associations with ARDS have been identified, probably in part because of the phenotypic complexity of ARDS engendered by the different risk factors24. However, other strategies, including candidate gene and pathway analyses, have revealed potentially important mechanisms of lung injury that seem to be associated with risk of developing ARDS and, in some associations, higher mortality25. For example, plasma angiopoietin 2 (encoded by ANGPT2) is a protein marker and mediator of increased lung vascular permeability; using a quantitative trait loci analysis, five ANGPT2 genetic variants in a population with European ancestry have been associated with increased levels of plasma angiopoietin 2, and two of the five variants were associated with increased ARDS risk. No significant associations were found with this gene in people with African ancestry26.
Children
From a systematic review of 29 paediatric studies27 and the PARDIE cross-sectional study of 145 international paediatric intensive care units (PICUs)28, the estimated population-based incidence of ARDS in children (2 weeks to 17 years of age) is 2.2–5.7 per 100,000 person-years; most of the children in these studies were <5 years of age. ARDS is diagnosed in 2.3–3% of PICU admissions, with an estimated mortality of 17–33%27,28; mortality is lower in highly resourced countries but was not associated with age. Over the past two decades, ARDS mortality in PICUs has been relatively stable. Although the overall number of ARDS-associated deaths is lower in children than adults, more productive life years are lost from ARDS-related paediatric deaths, as most occur in very young patients and ≥40% of these patients were previously healthy28.
The major risk factors and pathophysiology of ARDS are similar in adults and children28, but paediatric and adult ARDS epidemiology have some differences. ARDS is more frequent in boys than girls28,29, for reasons that are unknown. Over 60% of paediatric ARDS (PARDS) is also caused by pneumonia; however, viral infections such as respiratory syncytial virus and influenza virus more frequently cause life-threatening ARDS in young children30. Overall mortality is lowest in children with ARDS triggered by lower respiratory infection and highest in those with indirect lung injury from sepsis and/or shock28. ARDS occurs in only 0.5% of paediatric trauma patients, but its associated mortality is 18%31. The incidence, presentation and outcome of TRALI in children seems similar to that in adults. A history of prematurity, cancer or immune compromise are risk factors for mortality. The severity of hypoxaemia has consistently predicted mortality in paediatric cohorts32. In intubated children in the PARDIE study, severe ARDS (defined as PaO2/FiO2 <100 mmHg) was associated with threefold higher mortality than in children with a PaO2/FiO2 of 100–300 mmHg (ref.28). In addition, a history of cancer or haematopoietic stem cell transplantation in paediatric patients with ARDS resulted in a mortality of 43% versus 11% in children without these risk factors in a prospective multicentre study33.
Mechanisms/pathophysiology
Here, we focus on the normal and injured lung in ARDS, the pathophysiology of ARDS and the mechanisms of injury that lead to ARDS, including the contribution of ventilator-associated lung injury (VALI). Human lung pathology and research on mechanisms of lung injury from studies of patients with ARDS are also included.
The normal lung is structured to facilitate carbon dioxide excretion and oxygen transfer across the distal alveolar–capillary unit (Fig. 1). The selective barrier to fluid and solutes in the uninjured lung is established by a single-layer lining of endothelial cells linked by plasma membrane structures, including adherens and tight junctions34. The vast surface of the alveolar epithelium is lined by flat alveolar type I (ATI) cells along with cuboidal shaped alveolar type II (ATII) cells, forming a very tight barrier that restricts even the passage of small solutes but allows diffusion of carbon dioxide and oxygen. The ATII cells secrete surfactant, the critical factor that reduces surface tension, enabling the alveoli to remain open and facilitating gas exchange. Both ATI and ATII cells have the capacity to absorb excess fluid from the airspaces by vectorial ion transport, primarily by apical sodium channels and basolateral Na+/K+-ATPase pumps35. Thus, when alveolar oedema develops, reabsorption of the oedematous fluid depends on junctions between ATI and ATII cells and intact ion transport channels in the epithelial cells. Once the oedematous fluid is absorbed into the lung interstitium, the fluid can be removed primarily by lymphatics and the lung microcirculation. The cellular makeup of the normal alveolus includes alveolar macrophages but not polymorphonuclear leukocytes (neutrophils), although they can be rapidly recruited from the circulation. Alveolar macrophages, neutrophils and other immune effector cells, including monocytes and platelets, are critical in defence of the normal lung and have key activities in acute lung injury.
Fig. 1. The normal alveolus.
The alveolar epithelium is a continuous monolayer of alveolar type I (ATI) cells (very thin cells that permit gas exchange) and alveolar type II (ATII) cells (which produce surfactant to enable lung expansion with a low surface tension); both cells transport ions and fluid from the alveolus to maintain dry airspaces. The intact alveolar epithelium is linked by intercellular tight junctions. Tight junctions are responsible for barrier function and regulating the movement of fluid and ions across the epithelium and are composed of transmembrane claudins and occludins and cytoplasmic zonula occludens (ZO) proteins that anchor tight junctions to the actin cytoskeleton. Alveolar epithelial cells express plasma membrane E-cadherin and β-catenin. β-Catenin also functions as a transcriptional cofactor and has a role in cell turnover in the subset of ATII cells that function as stem cells during homeostasis80. Endothelial cells serve to regulate the influx of fluid and inflammatory cells into the interstitial space and are connected by intercellular junctions comprising tight junctions and adherens junctions. Adherens junctions contain vascular endothelial cadherin (VE-cadherin), which mediates cell–cell contact through its extracellular domain and has a key role in barrier function. p120–catentin binds to and stabilizes VE-cadherin, which is linked to the actin cytoskeleton via α-catenin (α in the figure) and has multiple additional functional interactions56. TIE2 acts in concert with vascular endothelial-protein tyrosine phosphatase (VE-PTP), which dephosphorylates VE-cadherin, stabilizing it. Normally, transvascular flux of fluid out of the capillary moves water and low-molecular-weight solutes into the interstitial space and then into the lymphatics; in health, this fluid does not cross the epithelial barrier. Resident alveolar macrophages populate the airspaces, providing host defence. Large numbers of polymorphonuclear leukocytes (PMNs) reside in the alveolar capillaries and can be rapidly mobilized to the airspaces in the setting of infection. β, β-catenin; BASC, bronchioalveolar stem cell; ENaC, epithelial sodium channel; JAM, junctional adhesion molecule; RBC, red blood cell.
In ARDS, there is increased permeability to liquid and protein across the lung endothelium, which then leads to oedema in the lung interstitium. Next, the oedematous fluid translocates to the alveoli, often facilitated by injury to the normally tight barrier properties of the alveolar epithelium. Increased alveolar–capillary permeability to fluid, proteins, neutrophils and red blood cells (resulting in their accumulation into the alveolar space) is the hallmark of ARDS36–38. Arterial hypoxaemia in patients with ARDS is caused by ventilation-to-perfusion mismatch as well as right-to-left intrapulmonary shunting. In addition, impaired excretion of carbon dioxide is a major component of respiratory failure, resulting in elevated minute ventilation that is associated with an increase in pulmonary dead space (that is, the volume of a breath that does not participate in carbon dioxide excretion). Elevation of pulmonary dead space and a decrease in respiratory compliance are independent predictors of mortality in ARDS39.
Pathology
Interstitial and alveolar oedema are key features of diffuse alveolar damage (DAD) in the acute ‘exudative’ phase (~7 days) of ARDS (Fig. 2). Eosinophilic depositions termed hyaline membranes are also defining features of DAD, the classic histopathological hallmark of ARDS40–42. The other findings include alveolar haemorrhage, accumulation of white blood cells (usually predominantly neutrophils), fibrin deposition and some areas of alveolar atelectasis (collapse). After the initial exudative phase, ATII cell hyperplasia follows in a ‘proliferative’ phase that can last >3 weeks in survivors; interstitial fibrosis can also occur in this phase. The original description of DAD was based heavily on analyses of lungs of patients dying with oxygen toxicity, although similar histological changes were identified in lungs from patients with a variety of conditions that underlie ARDS40. Recent reports reveal that DAD is present in only a subset of patients with clinical ARDS, and pathological heterogeneity is evident41–44. For example, one study carried out over two decades (1991–2010) on post-mortem samples reported that 45% of patients who met the Berlin criteria for ARDS had DAD, whereas the other 55% had alveolar inflammation consistent with acute pneumonia with infiltration of neutrophils in the alveoli and distal bronchioles44. Importantly, this study also found that the incidence of DAD declined in the decade after lung-protective ventilation was implemented. Recent reports also indicate key temporal features of histological progression, identify the association of DAD with severity of ARDS and provide evidence that the first 7 days after onset represent a critical window for potential therapeutic intervention43,44. In addition, one meta-analysis of open lung biopsy samples in patients with ARDS found that DAD was present in only 48% of the patients and was associated with a higher mortality45. Neither the severity of hypoxaemia nor the sequential organ failure assessment score were different in patients with or without DAD on lung biopsy.
Fig. 2. Microscopic findings in lung tissue in patients with ARDS.
In acute respiratory distress syndrome (ARDS), features of diffuse alveolar damage (DAD), such as in the acute ‘exudative’ phase (~7 days) (panel a), are typically followed by alveolar type II (ATII) cell hyperplasia and interstitial fibrosis in a ‘proliferative’ phase. Eosinophilic depositions termed hyaline membranes are defining features of DAD (pink structure lining the central alveolus, indicated by the arrowhead in panel b) are defining features of DAD. Leukocytes are embedded in the hyaline membranes (arrows in panel b). Electron microscopic analyses (panel c) demonstrate that alterations in endothelial and epithelial cells are critical features of acute alveolar injury in ARDS37,46. Focal epithelial destruction of alveolar type I (ATI) cells and denudation of the alveolar basement membrane occur early in ARDS, whereas endothelial continuity is preserved with modest alterations in most cases. The pattern shown in panel c was identified in the lungs of a patient with indirect acute lung injury resulting from sepsis37,46. A, alveolar space; BM, basement membrane; C, capillary; EC, erythrocyte; EN, endothelial cell; HM, hyaline membrane; LC, leukocyte. Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. Matthay, M. A. & Zimmerman, G. A. (2005) Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 33, 319–327. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Classic electron microscopic analyses demonstrate that alterations in endothelial and epithelial cells are critical features of acute alveolar injury in ARDS37,46. For example, early involvement of ATI cells is frequently dramatic and includes focal epithelial destruction and denudation of the alveolar basement membrane40,46. By contrast, alveolar endothelial cells are usually morphologically preserved and the endothelial lining is continuous, demonstrating that even ultrastructural analyses cannot precisely detect abnormalities in the normal barrier properties that regulate fluid and protein flux across the lung capillaries37. Epithelial cell necrosis is usually described in the exudative phase42,47, although evidence for apoptosis has also been reported48. Early epithelial injury is followed rapidly by ATII cell proliferation37,40,43,47. Injured but intact alveolar epithelial cells seem to drive release of pro-coagulant factors and intra-alveolar fibrin deposition49,50, which is also deposited adjacent to endothelial cells in injured alveoli37,40,47.
Endothelial damage
The nature of endothelial cell alteration in clinical ARDS is incompletely understood. Endothelial ‘damage’ and ‘injury’ are commonly described, and recent evidence suggests that apoptosis36 and alternative cell death pathways such as pyroptosis51 might be involved. Conceptually, an increase in lung vascular permeability can occur because of a functional breakdown in endothelial junctions or by death of endothelial cells. Ultrastructural alterations of alveolar endothelial cells are frequently subtle compared with the dramatic epithelial cell disruption observed in autopsy analysis37 (Fig. 2), suggesting functional barrier impairment. Experimental evidence has shown that endothelial cell activation can occur, induced by inflammatory signals from microorganisms (including lipopolysaccharide and other toxins) and lung white blood cells in response to pathogens (as in pneumonia or nonpulmonary sepsis), injury from aspiration syndromes, ischaemia–reperfusion (as in trauma-induced shock) or blood product transfusions as in TRALI36. Endothelial cell activation may result in mediator generation (such as angiopoietin 2) and leukocyte accumulation (accompanied by upregulation of P-selectin and E-selectin (cell adhesion molecules) in the lung microvessels, especially in the post-capillary venules)36. Platelet and neutrophil deposition characteristically occur, often as neutrophil–platelet aggregates (Fig. 3), as a result of endothelial cell activation. Neutrophils and platelets seem to play a synergistic role in causing an increase in lung vascular permeability to protein (see below). Endothelial disruption can also be caused by pathogens and their toxins; endogenous danger-associated molecular patterns; barrier-destabilizing factors generated by alveolar macrophages, circulating leukocytes and platelets; and pro-inflammatory signalling molecules such as tumour necrosis factor (TNF), the inflammasome product IL-1β, angiopoietin 2, vascular endothelial growth factor, platelet-activating factor and others36. Increased systemic vascular permeability frequently also occurs, often contributing to hypovolaemia and multiple organ failure.
Fig. 3. The injured alveolus.
A variety of insults (such as acid, viruses, ventilator-associated lung injury, hyperoxia or bacteria) can injure the epithelium, either directly or by inducing inflammation, which in turn injures the epithelium. Direct injury is inevitably exacerbated by a secondary wave of inflammatory injury. Activation of Toll-like receptors (not shown) on alveolar type II (ATII) cells and resident macrophages induces the secretion of chemokines, which recruit circulating immune cells into the airspaces. As neutrophils migrate across the epithelium, they release toxic mediators, including proteases, reactive oxygen species (ROS) and neutrophil extracellular traps (NETs), which have an important role in host defence but cause endothelial and epithelial injury. Monocytes also migrate into the lung and can cause injury, including epithelial cell apoptosis via IFNβ-dependent release of tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), which activates death receptors. Activated platelets form aggregates with polymorphonuclear (PMN) leukocytes, which are involved in NET formation, and monocyte–platelet aggregates. Red blood cells (RBCs) release cell-free haemoglobin, which exacerbates injury via oxidant-dependent mechanisms. Angiopoietin 2 inhibits TIE2-stabilization of vascular endothelial cadherin (VE-cadherin); vascular endothelial growth factor and other permeability-promoting agonists also destabilize VE-cadherin via dissociation from p120-catenin, resulting in its internalization and enhanced paracellular permeability. Additionally, loss of cell–cell adhesion in the setting of actomyosin contraction results in the formation of occasional gaps between endothelial cells. Epithelial injury also includes wounding of the plasma membrane, which can be induced by bacterial pore-forming toxins or mechanical stretch, and mitochondrial dysfunction. Together, these effects result in endothelial and epithelial permeability, which further facilitate the transmigration of leukocytes and lead to the influx of oedematous fluid and RBCs. Airspace filling with oedematous fluid causes hypoxaemia, resulting in the need for mechanical ventilation. The vascular injury and alveolar oedema contribute to the decreased ability to excrete CO2 (hypercapnia), accounting for the elevated pulmonary dead space in acute respiratory distress syndrome. In turn, hypoxaemia and hypercapnia impair vectorial sodium transport, reducing alveolar oedema clearance. ATI, alveolar type I cell; BASC, bronchioalveolar stem cell; ENaC, epithelial sodium channel.
Mechanistic examination of disrupted endothelial barriers has required experimental models. An extremely informative large-animal preparation demonstrated that clinically relevant insults, including intravenous bacteria, lipopolysaccharide and microemboli, cause an increase in lung endothelial permeability and filtration and that there are different responses to these insults by the endothelial and epithelial barriers52,53. Although the duration of increased lung endothelial permeability induced by specific insults in clinical ARDS is unknown, this model and more recent studies in mice suggest that it can persist for many hours to weeks52–54. In experimental models of influenza pneumonia, for example, the persistent duration of increased lung vascular permeability is associated with lung injury and slow recovery54. More precise understanding of the duration of barrier disruption may influence the timing of administration of candidate therapeutics aimed at reducing vascular permeability.
VE-cadherin disruption
Studies using cultured endothelium and murine models indicate that homophilic calcium-dependent vascular endothelial cadherin (VE-cadherin) bonds between adjacent endothelial cells are critical for basal lung microvascular integrity and that their ‘loosening’ is central in increased alveolar–capillary permeability in inflammatory acute lung injury36. VE-cadherin and TIE2, an endothelial receptor kinase, act in concert to establish junctional integrity and are regulated by vascular endothelial-protein tyrosine phosphatase (VE-PTP; also known as receptor-type tyrosine-protein phosphatase β). Genetic or pharmacological manipulation of the molecular interactions and activities of VE-cadherin, TIE2 and VE-PTP alters alveolar leak in a complex fashion in mice36,55. VE-cadherin function and adherens junction stability are also regulated by cytoskeletal interactions, small GTPases and other intracellular modulators, multiple molecular interactions (including associations with catenins, plakoglobin and VE-PTP) and phosphorylation and dephosphorylation events36,56. Destabilizing signals from pathogens or inflammatory cells and mediators responding to infectious agents induce phosphorylation of VE-cadherin and its internalization, frequently by altering activity and balance of GTPases56. Dissociation of VE-PTP from VE-cadherin is required for loosening of endothelial cell junctions and inflammatory alveolar protein leak in mice57. Recent observations indicate that inflammation-induced weakening of endothelial junctions is a process involving at least two steps, including modification of VE-cadherin contacts and alterations in the endothelial actomyosin system55. Genetic or pharmacological manipulation of VE-PTP can alter alveolar endothelial junctions via TIE2-dependent influences on the cytoskeleton independently of VE-cadherin55.
Although parallel experiments with cultured human endothelial cells suggest translational relevance55, direct recapitulation of these observations to alveolar endothelial barrier disruption in patients with ARDS has not been established. Nevertheless, administration to mice of an antibody against VE-cadherin resulted in intravascular sequestration of neutrophils and platelets, alveolar neutrophil accumulation and pulmonary oedema58 — mimicking the histological pattern in clinical ARDS37. Of note, substantial alveolar oedema in experimental acute lung injury was not accompanied by widespread overt disruption of endothelial cell junctions detectable by electron microscopy55, consistent with ultrastructural observations of lung tissue from patients with ARDS in which the endothelium was found to be largely continuous and endothelial cell junctions were, for the most part, morphologically intact37. Thus, in animal models and human ARDS, changes in paracellular permeability to protein seem to occur in the absence of dramatic alterations in the morphology of the lung endothelium.
Immune cell recruitment to the lung
Re-establishing endothelial junctional bonds may mitigate both endothelial leak and excessive myeloid leukocyte accumulation in ARDS36. Indeed, genetic replacement of VE-cadherin with a fusion construct that prevented its internalization in response to inflammatory signals greatly reduced alveolar neutrophil accumulation in lipopolysaccharide-challenged mice and reduced vascular permeability59. Recent analysis of samples from patients and lipopolysaccharide-challenged volunteers indicates that synergistic activity of chemokines contributes to neutrophil recruitment60. Other signalling molecules are also likely to be involved36. Degranulation of neutrophils with release of intracellular enzymes such as neutrophil elastase and oxidant products contributes to the lung injury36.
Neutrophils in the intravascular and extravascular compartments in acute lung injury are often associated with platelets (Fig. 3), which have intricate thrombo-inflammatory activities including the ability to trigger deployment of neutrophil extracellular traps (NETs)36. NETs correlate with alveolar–capillary and epithelial barrier disruption in ARDS and experimental models61. NETs are filamentous chromatin fibres complexed with neutrophil-derived antimicrobial proteins, generated by a process that is not completely defined. NETs probably evolved as an innate mechanism for pathogen containment and clearance but are also involved in inflammatory insults to the lung and other organs, as illustrated in a recent experimental and clinical study of acute lung injury and ARDS61.
A recent observation suggests that early intravascular interactions of platelets with monocytes — which, similar to neutrophils, accumulate and have complex activities in acute lung injury — drive development of ARDS in individuals at risk62. Intra-alveolar macrophages play an important part in releasing chemotactic factors such as IL-8 and chemokines such as CC-chemokine ligand 2 (also known as MCP1) that enhance the recruitment of neutrophils and monocytes into the lung, particularly in response to acute pulmonary infections.
Epithelial injury and repair
In the early phase of experimental acute lung injury, the epithelium is more resistant to injury than the endothelium53, but as described above, some degree of epithelial injury is characteristic of ARDS. The extent of epithelial injury is also an important determinant of the severity of ARDS. The epithelium can be injured directly, for example, by bacterial products, viruses, acid, oxygen toxicity (hyperoxia), hypoxia and mechanical forces, or by inflammatory cells or their products, as in sepsis, TRALI and pancreatitis (Fig. 3).
As with endothelial injury36,57–59, epithelial injury includes dissociation of intercellular junctions63,64. Release of cell-free haemoglobin from red blood cells contributes to paracellular permeability by oxidant-dependent mechanisms. On the basis of experimental studies, the cyclo-oxygenase inhibitor acetaminophen reduces the tyrosine radical that results from oxidation of cell-free haemoglobin (Fe4+ oxidation state to Fe3+ oxidation state), thereby reducing the potential for lipid peroxidation65. In addition, epithelial cell death37 (apoptotic or necrotic48,66,67) is a key feature of alveolar injury in ARDS and can be directly caused by lytic viral infections, bacterial toxins, acid, hypoxia, hyperoxia and mechanical stretch68,69. Neutrophil-derived mediators also induce epithelial cell death via multiple mechanisms, including oxidation of soluble TNF ligand superfamily member 6 (FasL)70 and NETs71, whereas inflammatory macrophages can induce cell death via mechanisms including secretion of TNF-related apoptosis-inducing ligand (TRAIL)67. Notably, endogenous mechanisms (such as syndecan-1-dependent MET–AKT signalling) can limit cell death72.
Additionally, plasma membrane wounding without cell death (that is, sublethal injury) may result from bacterial pore-forming toxins and/or overdistention from positive-pressure ventilation with high tidal volumes. A recent study demonstrated that after membrane wounding by Staphylococcus aureus toxin, calcium waves spread through gap junctions to neighbouring epithelial cells, inducing widespread mitochondrial dysfunction and loss of barrier integrity without cell death73. Indeed, mitochondrial dysfunction is common in lung injury and may be induced by various mechanisms, including hypercapnia74.
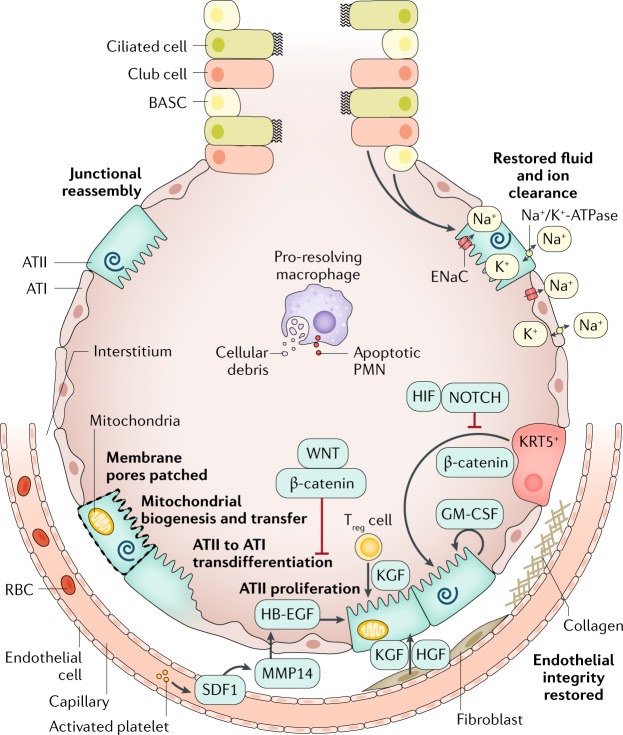
Repair of the injured epithelium is critical for clinical recovery75. The time frame for epithelial repair may be 2–3 days or several weeks. Because ATI cells provide >95% of the normal surface area of the alveolar epithelium and facilitate gas exchange, the process of generating new ATI cells is critical to the complete repair process. However, initially the proliferation of ATII cells can provide a provisional epithelial barrier before they transdifferentiate into ATI cells (Fig. 4). Many growth factors contribute to ATII cell proliferation and, although ATII cells are the default progenitors responsible for creating new alveolar epithelial cells through proliferation, in severe injury, alternate progenitor cells may be mobilized. These alternate progenitor cells include club cells76 (secretory cells that normally line the airways), bronchoalveolar stem cells and keratin-5-expressing (KRT5+) cells68,77. Expansion of KRT5+ epithelial progenitors is driven by HIF–NOTCH and fibrocyte growth factor receptor 2 signalling78, with ATII cell fate induced by WNT–β-catenin and impeded by NOTCH and HIF79 (Fig. 5). The mechanisms underlying ATII-to-ATI transdifferentiation are less well understood, but recent studies have suggested that deactivation of WNT–β-catenin is necessary80. Our knowledge of the regenerative role of the alternative progenitors is mainly based on mouse models of lung injury, although there is evidence that some of these progenitors exist in humans as well79.
Fig. 4. Epithelial cell regeneration in ARDS.
Mice in which the alveolar type II (ATII) epithelial cells and all their progeny express green fluorescent protein (GFP) (SftpcCreERT2;mTmG mice) were treated with intratracheal lipopolysaccharide to induce lung injury. Mice were euthanized 27 days later and lung sections were stained for GFP (green), the alveolar type I (ATI) cell marker T1α (purple) and 4′,6-diamidino-2-phenylindole (DAPI; for nuclear staining (blue)). Some ATII cell-derived cells (GFP-staining cells in panels a (×40) and c (×40)) expressed ATI markers (T1α-staining cells in panels b (×40) and c), as shown by dual GFP-staining and T1α-staining cells (panel c) — indicating transdifferentiation during repair after lung injury. Arrowheads indicate nascent ATI cells that transdifferentiated from ATII cells during repair after injury (dual GFP-staining and T1α-staining cells). Arrows indicate ATI cells that withstood the initial injury (GFP-negative but T1a-staining cells). These experimental data support the notion that ATI cells are damaged during acute lung injury and are then replaced by ATII cells that transdifferentiate into ATI cells. Reprinted with permission of the American Thoracic Society. Copyright © 2019 American Thoracic Society. Jansing, N. L. et al. (2017) Unbiased quantitation of alveolar type II to alveolar type I cell transdifferentiation during repair after lung injury in mice. Am. J. Respir. Cell Mol. Biol. 57, 519–526. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Fig. 5. The repaired alveolus.
Several mechanisms promote endothelial cell junctional reassembly. Slit binds to its receptor, ROBO4, stabilizing the adherens junctions by promoting the association between p120–catenin and vascular endothelial cadherin (VE-cadherin) (not shown). Activated platelets release the lipid mediator sphingosine 1-phosphate (S1P), which activates Rho/Rac signalling to induce cytoskeletal reorganization that promotes endothelial barrier integrity (not shown). The receptor tyrosine kinase TIE2 is bound by its activating ligand, angiopoietin 1, which results in actin cytoskeletal reorganization and stability of VE-cadherin at the adherens junctions (not shown). To repair the damaged epithelium, surviving alveolar type II (ATII) cells replace lost epithelial cells via proliferation and differentiation into alveolar type I (ATI) cells. Many growth factors promote ATII proliferation, including keratinocyte growth factor (KGF), epidermal growth factor (EGF)80, hepatocyte growth factor (HGF) and granulocyte–macrophage colony-stimulating factor (GM-CSF); similarly, transcriptional pathways also promote ATII proliferation, including the WNT–β-catenin pathway80,82,239 and the forkhead box protein M1 (FOXM1) pathway240. Toll-like receptor 4 and hyaluronan signalling also contribute241. In severe injury, alternate progenitors (keratin 5-expressing epithelial progenitors (KRT5+) and club cells) are mobilized to proliferate and differentiate into ATII cells. Withdrawal of β-catenin signalling induces ATII cells to transdifferentiate into ATI cells80. Fibroblasts and endothelial cells (and epithelial cells) secrete epithelial growth factors80,242; for example, platelet-derived stromal cell-derived factor 1 (SDF1) stimulates endothelial cells to secrete matrix metalloproteinase 14 (MMP14), which cleaves heparin-bound EGF (HB-EGF), enabling it to ligate the EGF receptor and stimulate ATII cell proliferation242. Membrane pores can be patched and damaged mitochondria can be removed by mitophagy. Once the alveolar epithelium is regenerated, pro-resolving macrophages clear dead cells and debris and ATII and ATI epithelial cells reabsorb oedematous fluid. BASC, bronchioalveolar stem cell; ENaC, epithelial sodium channel; PMN, polymorphonuclear; RBC, red blood cell; Treg cell, regulatory T cell.
Repair of the alveolar epithelium is regulated by crosstalk between multiple alveolar cell types and the extracellular matrix. Although injury-inducing, immune cells and their mediators may also promote epithelial repair81,82. Fibroblasts secrete epithelial growth factors and deposit collagen, which, if excessive, can lead to fibrosis. Sublethal epithelial cell injury can also be repaired. For example, plasma membrane pores can be excised by endocytosis or exocytosis and patched by fusion with lipid endomembrane vesicles83. Additionally, damaged mitochondria are degraded via mitophagy and replaced via biogenesis or mitochondrial transfer84. Finally, reassembly of intercellular junctions is regulated by multiple mechanisms, including beneficial effects from angiopoietin 1 (ref.85) and signals from the basement membrane86. The timing of endothelial and epithelial repair in various causes of acute lung injury has not been systematically worked out. Once epithelial barrier integrity is restored, oedematous fluid can be reabsorbed to the interstitium either by paracellular pathways or by diffusion through water channels driven by an osmotic gradient that is established by active apical sodium uptake, in part by the epithelial sodium channels and sodium transport through the Na+/K+-ATPase pumps (Fig. 5).
Unfortunately, many endogenous reparative mechanisms are specifically inhibited during ARDS. For example, influenza virus infects KRT5+ progenitors78. Influenza infection, hypoxaemia, hypercapnia and other factors downregulate sodium channel and/or Na+/K+-ATPase function, resulting in impaired alveolar fluid clearance in patients with ARDS35,75,87,88. Hypercapnia impairs alveolar epithelial cell proliferation74. Keratinocyte growth factor, while stimulating proliferation, increases the susceptibility of ATII cells to influenza virus infection and mortality in mice89. In addition, the many biological changes resulting from both endothelial and epithelial injury, and culminating in protein-rich oedematous fluid, contribute to surfactant dysfunction90. Surfactant dysfunction can then increase atelectasis, which in turn can increase the risk of biophysical injury.
Mechanical VALI
All the mechanisms that injure the lung endothelium and epithelium lead to pulmonary oedema with acute respiratory failure owing to reduced oxygenation, impaired carbon dioxide excretion and decreased lung compliance. As described in the original report of ARDS in 1967 (ref.1), the use of mechanical ventilation with supplemental oxygen and positive end-expiratory pressure (PEEP) was life-saving in this context. For many years, the standard therapy with mechanical ventilation support included high tidal volumes (12–15 ml per kg PBW). Nevertheless, a potential contribution of high tidal volumes and elevated airway pressures to worsening acute lung injury was suggested by preclinical studies beginning in 1974 (refs91–93). The clinical importance of VALI was provided by the ARMA trial in 2000 (see below, Management), in which lower tidal volume and limited airway pressure markedly reduced mortality in patients with ARDS94. The mechanisms for VALI have been established in both experimental and clinical studies. High tidal volume and elevated airway pressure induce biomechanical inflammatory injury and necrosis of the lung endothelium and alveolar epithelium that are associated with release of neutrophil products, including proteases, oxidants and pro-inflammatory cytokines, and a reduction in the capacity of the alveolar epithelium to remove oedematous fluid36,95,96. Clinical studies focused on biology and clinical factors have also confirmed the injurious effects of high tidal volume in patients with ARDS (see below). It should be emphasized that it has not been possible to eliminate VALI as it can still occur from patient–ventilator dyssynchrony and elevated plateau airway pressure (that is, the elevated pressures generated by the mechanical ventilator to the alveoli), especially in patients with severe lung injury and extensive pulmonary oedema.
Insights from clinical studies
With the exception of supportive care therapies such as lung-protective ventilation and fluid-conservative therapy (see Management), translating insights from experimental studies in animal models into effective therapies for human ARDS has proved challenging. Indeed, the search for specific pharmacotherapies that effectively treat ARDS has been fruitless, despite decades of promising preclinical research97. In an attempt to overcome this obstacle, investigators have sought to further understand the biology of human ARDS through studies of human samples obtained from observational and interventional clinical studies. These studies from patients with ARDS have validated several pathophysiological observations from experimental studies, led to the identification of promising prognostic biomarkers in humans (Box 2) and identified molecular subphenotypes of human ARDS that may have therapeutic implications.
Initial studies of ARDS pathogenesis using human samples confirmed that the biological mechanisms identified in many models are relevant. Important initial observations included confirmation in humans of the fundamental hallmark of ARDS, disruption of the alveolar–capillary barrier, by documentation of elevated protein levels in pulmonary oedematous fluid38,98. Subsequent studies of biological markers from bronchoalveolar lavage, pulmonary oedematous fluid or (most commonly) plasma (which is more readily available in most cases) demonstrated that endothelial injury99–101 and an exaggerated inflammatory response102,103 are consistent features of human ARDS. Endothelial activation in particular seems to occur early in the development of human ARDS, in some cases predating frank respiratory failure104. Lung epithelial injury in human ARDS has been identified by measurement of impaired alveolar fluid clearance75 and more recently by measurement of specific markers of alveolar epithelial cell injury, including surfactant protein D105 and the receptor for advanced glycation end products (RAGE), a marker of lung epithelial injury and innate immune activation106. Importantly, many of these markers of injury have also been linked to poor clinical outcomes in ARDS (Box 2), suggesting that the severity of the endothelial and epithelial injury in humans is a major determinant of clinical outcome107.
Biological samples from interventional clinical trials in ARDS have proved to be particularly useful in furthering understanding of ARDS pathogenesis. As one example, analysis of plasma inflammatory markers from patients enrolled in the seminal trial of low tidal volume ventilation for ARDS94 demonstrated that a lung-protective ventilation strategy decreased circulating markers of inflammation (IL-6 and IL-8) compared with a traditional high tidal volume strategy108. Analyses of plasma levels of RAGE from the same trial confirmed that lung-protective ventilation also decreased alveolar epithelial cell injury compared with traditional tidal volumes109. Another study reported a reduction in distal airspace IL-6, TNF and neutrophils in patients treated with a lung-protective ventilation strategy110.
Most recently, analyses of biological samples from large clinical trials have enabled studies focused on heterogeneity in ARDS, specifically on the identification of molecular subphenotypes of ARDS that may respond differently to therapies. In many other medical disciplines, most notably in oncology, the identification of discrete molecular subtypes within broader clinical syndromes has led to remarkable therapeutic progress and a revision of diagnostic paradigms — consider, for instance, hormone receptor status in breast cancer. In ARDS, biological heterogeneity has long been suspected as a potential obstacle to successful development of therapeutics; early descriptions of the syndrome speculated whether direct (that is, pulmonary) and indirect (that is, nonpulmonary) causes of ARDS, for example, might be pathologically distinct2. Supporting this hypothesis, a recent analysis of direct and indirect ARDS in two clinical cohorts showed that patients with direct lung injury had higher plasma levels of biomarkers of lung epithelial injury (such as RAGE and surfactant protein D), whereas patients with indirect lung injury had higher plasma levels of biomarkers of endothelial injury and inflammation (including angiopoietin 2)111.
An alternative approach to deconvoluting ARDS heterogeneity has been the use of unsupervised analytical approaches, most prominently latent class analysis, to identify subphenotypes within ARDS. This approach has now been replicated in analyses of five clinical trials, finding strong evidence for two distinct subphenotypes of ARDS112–115. Approximately 30% of patients with ARDS consistently fall into the so-called hyperinflammatory subphenotype, characterized by high plasma levels of inflammatory biomarkers (IL-6, IL-8, soluble TNF receptor 1), low protein C and high prevalence of shock and metabolic acidosis. By contrast, the so-called hypo-inflammatory subphenotype comprises ~70% of patients who have lower levels of the inflammatory biomarkers, less acidosis and less vasopressor-dependent shock. Mortality was higher in the hyperinflammatory subphenotype than in the hypo-inflammatory subphenotype in all of the five clinical trials.
In secondary analyses of the completed randomized controlled trials, these two ARDS subphenotypes responded differently to PEEP, fluid management and, of most interest, simvastatin therapy114. Statins have been proposed as a treatment for ARDS because of their potential anti-inflammatory properties. In a randomized placebo-controlled clinical trial of simvastatin in 540 patients with ARDS, there was no effect on mortality116. However, a secondary analysis of the trial data revealed that the patients who were classified in the hyperinflammatory subphenotype and were treated with simvastatin had a significantly higher 28-day survival than the hyperinflammatory patients who were treated with placebo. There was no difference in 28-day survival in the hypo-inflammatory patients treated with simvastatin or placebo114. If validated in prospective clinical studies, this approach suggests that future clinical trials targeting patients on the basis of distinct biological subphenotype may be more successful than trials based on a clinical syndromic definition. The definitive mechanistic differences between these two subphenotypes, including whether they are driven by environmental or genetic factors, remain yet to be identified. The goal of integrating the biological and clinical variables of greatest value for predictive and prognostic enrichment in clinical trials will require more research.
In addition, research to study transcriptomics in both observational and clinical trials of ARDS will be needed to identify patterns that may help segregate pathways of lung and systemic injury. One recent study of an observational cohort of 210 patients with sepsis-related ARDS used microarray analysis to compare whole-blood gene expression in patients stratified into ‘reactive’ (61% of the cohort) and ‘uninflamed’ subphenotypes of ARDS117. In those with a reactive subphenotype, genes associated with neutrophils were more expressed than in those with the uninflamed subphenotype. Pathway analysis in the reactive patient group showed enrichment of oxidative phosphorylation, mitochondrial dysfunction and cholesterol metabolism whereas the uninflamed group showed patterns associated with cell proliferation. Future studies that include RNA-sequencing will reduce bias compared with the microarray approach, and studies that include samples from both the blood and distal airspaces should provide insight into the pulmonary versus systemic pathways that contribute to lung injury118. Studies that link gene expression to protein expression will be helpful, as illustrated in one prior study of sepsis-related ARDS119.
Box 2 Selected biomarkers associated with human ARDS.
Epithelial markers (principal source)
Receptor for advanced glycation end products (alveolar epithelial type 1 cells)
Surfactant protein D (alveolar epithelial type 2 cells)
Club cell 16 (airway epithelial cells)
Endothelial markers (principal source)
von Willebrand factor (endothelium and platelets)
Angiopoietin 2 (endothelium and platelets)
Intercellular adhesion molecule 1 (endothelium, epithelium and macrophages)
Syndecan (endothelial glycocalyx)
Endocan (endothelium)
Inflammatory markers (principal source)
IL-6 (monocytes, macrophages, neutrophils and alveolar epithelium)
IL-8 (monocytes, macrophages, endothelium and alveolar epithelium)
Soluble tumour necrosis factor receptor 1 (alveolar epithelial type 1 and type 2 cells and macrophages)
IL-1β, IL-1 R antagonist (monocytes, macrophages and alveolar epithelium)
Neutrophil extracellular traps (neutrophils)
Coagulation and fibrinolysis markers (principal source)
Protein C (plasma)
Plasminogen activator inhibitor 1 (endothelium and macrophages)
Apoptosis markers (principal source)
FAS and FasL (endothelium, alveolar epithelium and inflammatory cells)
Selected on the basis of several clinical studies that were focused on the pathogenesis and prognosis of ARDS. ARDS, acute respiratory distress syndrome; FAS, tumour necrosis factor receptor superfamily member 6; FasL, tumour necrosis factor ligand superfamily member 6.
Diagnosis, screening and prevention
Most patients who present with the early phase of acute lung injury complain of feeling short of breath. If pneumonia is the cause, they will often have a cough that produces purulent sputum. On physical examination, they appear to be in moderate or severe respiratory distress with an elevated respiratory rate and tachycardia and they usually are working harder than normal to breathe. Hypoxaemia may manifest with evidence of cyanosis in their fingernail beds. Oxygen saturation on room air will be decreased. Respiratory deterioration in ARDS may be hyper-acute (for example, in the case of TRALI, in which respiratory failure is often fulminant) or may develop slowly over a period of hours to days.
Diagnosis
Adults
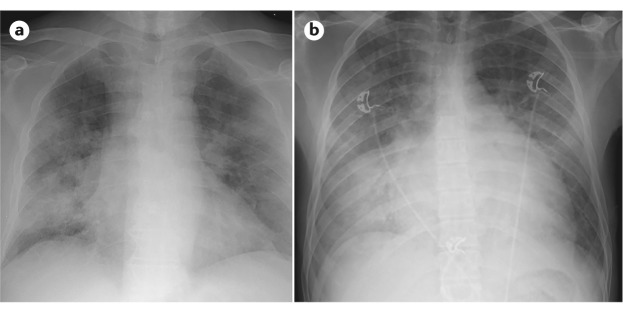
Even after the development and refinement of the Berlin diagnostic criteria (Box 1), several aspects of diagnosing ARDS remain challenging. Chest radiograph interpretation is inherently (at present) subjective, and investigators studying chest radiograph interpretation in ARDS in particular have demonstrated suboptimal inter-rater reliability120. Similarly, differentiation of ARDS from hydrostatic pulmonary oedema due to heart failure and/or volume overload can be difficult in critically ill patients (Fig. 6). The 1992 consensus definition of ARDS included a requirement that the pulmonary arterial wedge pressure be <18 mmHg if measured; however, pulmonary arterial catheters are now rarely used in this setting, primarily because a randomized trial showed no clinical benefit of their use compared with central venous catheters in 1,000 patients with ARDS121. The Berlin definition of ARDS recommends the use of echocardiography to assess cardiac function if no clear ARDS risk factor is identified. Other tests that may be useful in this distinction include measurement of brain natriuretic peptide (BNP; which, if elevated, suggests cardiac insufficiency), assessment of the vascular pedicle width on chest radiograph (which suggests intravascular volume overload) and/or measurement of the ratio of protein in pulmonary oedema fluid to plasma (which, if <0.65, suggests elevated lung vascular pressures as in cardiogenic pulmonary oedema versus a ratio >0.65, which suggests increased alveolar–capillary permeability as in ARDS), although this test is less widely available122,123. These complexities may contribute to persistent under-recognition of ARDS by clinicians. In under-resourced settings without ready access to advanced diagnostic modalities and therapies, diagnosis of ARDS can be further hindered. Thus, in 2016, investigators proposed alternative criteria for ARDS diagnosis in the absence of chest radiography, mechanical ventilation and/or blood gas measurements — the so-called Kigali modification of the Berlin criteria124 (Box 1).
Fig. 6. Distinguishing ARDS on radiography.
Similarities in the chest radiographs from a patient with acute respiratory distress syndrome (ARDS) from influenza pneumonia (panel a) and a patient with pulmonary oedema due to cardiac failure (panel b) reflect the difficulty in identifying ARDS. In both cases, diffuse bilateral parenchymal opacities are consistent with alveolar filling. The cardiac silhouette (panel b) is slightly more globular, consistent with heart failure; however, this feature is not reliable for distinguishing ARDS from cardiogenic pulmonary oedema.
Another important aspect of diagnosing ARDS depends on excluding mimics of the syndrome that may require specific treatment. Such mimics include acute eosinophilic pneumonia, diffuse alveolar haemorrhage, acute interstitial pneumonia, cryptogenic organizing pneumonia, acute exacerbations of idiopathic pulmonary fibrosis and acute heart failure (Fig. 6). ARDS occurs in the setting of an underlying risk factor (for example, sepsis, pneumonia or severe trauma); identification of this risk factor is required to ensure that the patient actually has ARDS and not an ARDS mimic and because treatment of the underlying risk factor is vitally important for patient care. If no ARDS risk factor is readily apparent, suspicion for an ARDS mimic should increase. In this case, bronchoscopy with bronchoalveolar lavage and cell counts and differential can be helpful diagnostically. If the aetiology remains unclear after bronchoscopy, lung biopsy (thoracoscopic or, less commonly, open) may be considered if results would change patient management. Studies on the diagnostic yield of lung biopsy samples in these settings report varying diagnostic yields (60–84%) and substantial morbidity and mortality; a recent review provides a thoughtful algorithm for when to consider lung biopsy in ARDS125.
Children
Given the differences in ARDS between adults and children, the international Pediatric Acute Lung Injury Consensus Conference (PALICC) defined criteria for PARDS126 (Box 3). There are major differences between the PALICC PARDS definition and the adult American–European Consensus Conference or Berlin definitions. In PARDS, as in the Kigali modification of the Berlin definition, the peripheral capillary oxygen saturation (SpO2) to FiO2 ratio is used when arterial PaO2 is not available; however, lung ultrasonography is not incorporated into the diagnostic criteria for PARDS126. The presence of bilateral radiographic opacities, used to distinguish diffuse lung disease but difficult to detect with accuracy, is not required for PARDS126. Hypoxia assessment for intubated children requires adjustment for level of respiratory support using mean airway pressure (MAP) by calculating an oxygenation index126 (Box 3).
Neonates outside of the perinatal period were included in the PARDS definition, and additional criteria for diagnosing PARDS in children with paediatric cyanotic congenital heart disease and chronic lung disease were proposed126. The PARDS definition was more inclusive, aiming to diagnose ARDS in the early stages. Children diagnosed with PARDS using the PALICC criteria had 24% mortality if they also met the Berlin criteria, versus <8.5% if they did not28. If the patients were treated with noninvasive ventilation and using the oxygen saturation (SaO2) to FiO2 ratio for diagnosis with unilateral infiltrates, the mortality was 7%. Neonatal lung disease experts have proposed a consensus definition for neonatal ARDS called the Montreaux definition127, which is proposed for neonates from birth to 44 weeks post-menstrual age (4 weeks if born at term). Children with respiratory distress syndrome from prematurity and surfactant deficiency and those with transient tachypnoea of the newborn or congenital anomalies causing the respiratory condition are excluded. The Montreaux definition is similar to the PALICC PARDS definition (Box 3) in terms of timing and uses PALICC cut-offs for oxygenation index but requires arterial or transcutaneous oxygen tension values rather than SpO2. Furthermore, determining origin of oedema requires use of echocardiography to verify absence of congenital heart disease as a contributor, and imaging must show diffuse bilateral infiltrates or complete lung opacification. Validation of these definitions is needed in large prospective cohorts with autopsy and pathophysiological correlates to differentiate ARDS from other causes of hypoxaemia.
Box 3 PALICC criteria for PARDS.
Age: exclude patients with perinatal-related lung disease
Timing: respiratory failure within 1 week of known insult
Origin: respiratory failure not fully explained by cardiac function or fluid overload
Imaging: new unilateral or bilateral infiltrate or infiltrates consistent with acute pulmonary parenchymal disease
- Oxygenation: invasive mechanical ventilation severity stratification is as follows:
- OI ≥4 to <8 or OSI ≥5 to <7.5 is mild PARDS
- OI ≥8 to <16 or OSI ≥7.5 to <12.3 is moderate PARDS
- OI ≥16 or OSI ≥12.3 is severe PARDS
- Oxygenation: noninvasive mechanical ventilation severity (not stratified) is as follows:
- Full face-mask bi-level ventilation or CPAP ≥5 cmH2O and
- PaO2/FiO2 ≤300 or SaO2/FiO2 ≤264
CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; MAP, mean airway pressure; OI, oxygenation index (which is (MAP × FiO2 × 100)/PaO2); OSI, oxygen saturation index (which is (MAP × FiO2 × 100)/SpO2, with SpO2 ≤97% required for assessment); PALICC, Pediatric Acute Lung Injury Consensus Conference; PARDS, paediatric acute respiratory distress syndrome; PaO2, partial pressure of arterial oxygen; SaO2, oxygen saturation; SpO2, peripheral capillary oxygen saturation.
Diagnostic work-up
A comprehensive history and physical examination, radiographic imaging (including a chest radiograph and sometimes abdominal imaging) and laboratory tests are required to search for a clinical risk factor that is associated with ARDS. Because bacterial and viral respiratory infections of the lung (including secondary bacterial infections after an initial viral infection) are the most common risk factors associated with ARDS, this section focuses on pulmonary infections as part of the diagnostic work-up. The typical diagnostic approach includes blood cultures and microscopic examination and culture of respiratory specimens.
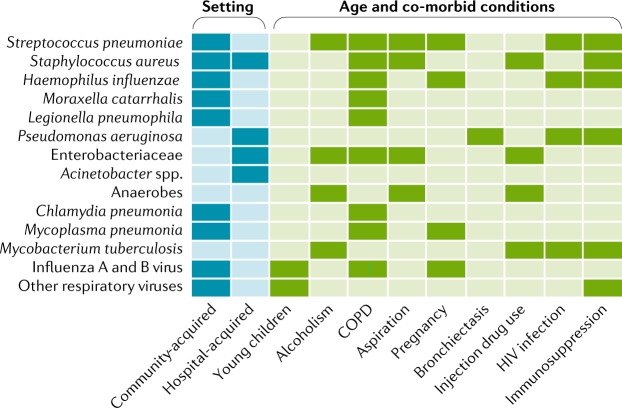
When evaluating a patient with suspected ARDS for the likely causative pathogen or pathogens, several factors should be taken into consideration. Whether infection is community-acquired or healthcare-associated is an important determinant of the potential pathogens (Fig. 7). Other important factors include age and the presence of comorbid conditions, exposure history and vaccination status. In addition to commonly isolated organisms, physicians should consider other uncommon but important pathogens that are linked to travel or residence in certain geographic distributions or to specific exposures. Examples include the Middle East respiratory syndrome coronavirus (MERS-CoV), avian influenza H5N1 and H7N9 viruses, hantavirus, human adenovirus subtype-55 (HAdV-55), Plasmodium species, Blastomyces dermatitidis, Coxiella burnetti, coccidioidomycosis, histoplasmosis, Mycobacterium tuberculosis and Yersinia pestis (Fig. 7). For instance, patients who have travelled to Saudi Arabia and have been exposed to camels have a higher risk of developing MERS-CoV; patients who live in the central valley of California have a higher risk of developing coccidioidomycosis.
Fig. 7. Common respiratory pathogens in ARDS and associated demographic features and comorbidities.
Common community-acquired and hospital-acquired pathogens that cause pneumonia should always be considered in patients with suspected acute respiratory distress syndrome (ARDS). Some organisms such as Streptococcus pneumoniae are more common as community-acquired infections whereas Pseudomonas aeruginosa is more common as a hospital-acquired infection in ARDS. Enterobacteriaceae include Klebsiella pneumoniae, Escherichia coli and Enterobacter species. The group ‘other respiratory viruses’ includes parainfluenza virus, human metapneumovirus virus, respiratory syncytial virus, rhinovirus, coronaviruses and adenovirus. A detailed, expanded version of this figure can be found in Supplementary Fig. 1. COPD, chronic obstructive pulmonary disease.
In patients without an identified pathogen using traditional microbiological tests of tracheal aspirate or sputum examination, the use of bronchoalveolar lavage leads to identification of pathogens in some but not all patients. The percentage of patients with ARDS with no identified organisms, even with bronchoalveolar lavage examination, remains high (>50–60%)128. Pathogens, or their markers (such as DNA), can also enter the bloodstream, which may in the future enable pathogen identification without the need for bronchoalveolar lavage129.
Nucleic acid detection tests, such as PCR, are available to detect multiple common viruses and bacteria such as Mycoplasma spp., Chlamydia pneumoniae and Bordetella pertussis. PCR panels for identifying and quantifying common bacteria such as S. aureus and Streptococcus pneumoniae are also becoming available. However, establishing causation remains a challenge because these tests do not differentiate pathogens from colonizers, nor do they distinguish primary pathogens that trigger ARDS from pathogens that may have invaded secondarily. In addition, the variable availability of PCR testing, local testing practices and specimen timing, quality and type (sputum versus bronchoalveolar lavage) all influence pathogen identification, further influencing reported epidemiology.
Next-generation sequencing is likely to become more common to detect pathogens and may change our understanding of ARDS epidemiology130. Increased test sensitivity will identify pathogens that frequently colonize the respiratory tract in patients with pneumonia and ARDS128,131. However, physicians need to consider pre-test probability in interpreting these results; for example, viruses such as respiratory syncytial virus and human metapneumovirus are more likely to cause ARDS in young children, elderly individuals and immunosuppressed individuals than in those who are immunocompetent30,131,132. Finally, it is always important to consider nonpulmonary causes of ARDS, including intra-abdominal infections.
Screening for and predicting ARDS
At present, there is neither strong evidence nor consensus regarding whether or how patients should be screened for ARDS. ARDS occurs only in a minority of patients with a risk factor, making screening challenging133. Furthermore, ARDS development often occurs quite quickly, with the majority of patients who go on to develop ARDS doing so in the first 12–48 hours of hospitalization104,133–135.
Clinical scores have been developed to predict ARDS in at-risk patients, most prominently the Lung Injury Prediction Score (LIPS)136. The LIPS synthesizes available clinical data that include predisposing risk factors, comorbidities and acute physiological variables to generate a risk score; higher scores indicate greater risk of developing ARDS. The negative predictive value of the LIPS is high, but the positive predictive value even in the original description was low (18% for a LIPS ≥4); a clinical trial using this LIPS cut-off as an enrolment criterion found an even lower positive predictive value of 10%137. An alternative approach is the Early Acute Lung Injury (EALI) score, which aims to identify patients with incipient lung injury before frank ARDS138,139. This simpler score was developed from analysis of patients with acute bilateral radiographic opacities and is comprised of three variables: level of supplemental oxygen required; tachypnoea; and presence of immune suppression. In comparison with LIPS, the negative predictive value of the EALI score was again high, but the positive predictive value was better (53%). Although the LIPS and EALI scores have not been rigorously tested in children, the PARDIE study showed that many children with mild disease will progress to meeting the Berlin criteria within 3 days of being diagnosed with PARDS.
Biomarkers have also been evaluated as potential ARDS predictive instruments. For example, levels of von Willebrand Factor, a marker of endothelial injury, were predictive of development of ARDS in a small study of patients with nonpulmonary sepsis100 and in a more recent, larger study in patients with severe trauma140. Angiopoietin 2 and IL-8 were also found to be elevated in critically ill patients before ARDS development104. In this analysis, angiopoietin 2 improved the positive predictive value of the LIPS. The soluble form of RAGE has been reported to be predictive of ARDS development in children undergoing cardiac surgery141 and more recently in critically ill patients at risk of ARDS142, although other reports have not found soluble RAGE to be predictive in this setting104. These data provide important evidence that endothelial and lung epithelial injury are well underway before patients fulfil ARDS diagnostic criteria; however, as the biomarkers in question are not clinically available, their use is restricted to research settings.
Prevention and early treatment
For decades, clinicians and researchers have wondered whether preventive therapies implemented early in the progression of acute lung injury, before patients meet ARDS diagnostic criteria, could improve clinical outcomes. Unfortunately, most trials focused on prevention using pharmacotherapies have met disappointing results. In the earliest trials to test this approach, corticosteroids were evaluated for ARDS prevention in at-risk patients, with no evidence of benefit143,144. More recently, a phase IIb clinical trial testing aspirin as a preventive therapy in at-risk patients was negative137, although a subsequent re-analysis of the data raised interesting issues62. A smaller phase IIa trial of aerosolized budesonide (a corticosteroid) and formoterol (a long-acting β2-agonist that may improve alveolar fluid clearance) demonstrated improved oxygenation and a decreased rate of progression to ARDS in the treatment group, although baseline randomization imbalances may have affected this result145. In contrast to these results with pharmacotherapy trials, studies evaluating the use of low tidal volume ventilation in mechanically ventilated patients without ARDS have provided more evidence of benefit146,147, but a recent large clinical trial comparing low with intermediate tidal volumes in patients without ARDS was negative148. Several studies have noted reductions in nosocomial ARDS incidence thought to be associated with improvements in supportive care149,150.
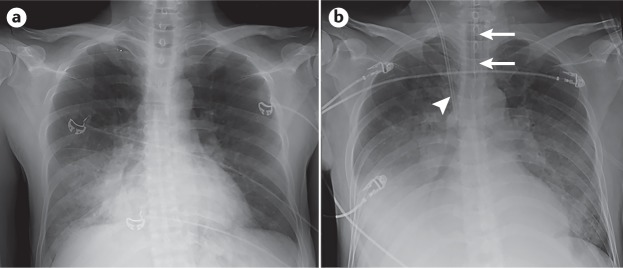
Although true prevention trials have been challenging to conduct because of the rapid development and low incidence of ARDS, an alternative approach has been to test the value of early treatment in patients with incipient acute hypoxaemic respiratory failure, which in many cases progresses to ARDS. A French trial illustrated the potential value of this approach, comparing high-flow nasal cannula to noninvasive ventilation and face-mask oxygen in 310 non-intubated patients with a PaO2/FiO2 <300 mmHg (ref.151). The majority of patients (64%) had pneumonia, and 79% had bilateral radiographic opacities on chest radiography, indicating that they likely had early ARDS. High-flow nasal cannula did not affect the primary outcome of endotracheal intubation but did lead to statistically significantly lower mortality than both noninvasive ventilation (P = 0.006) and face-mask oxygenation (P = 0.046). This trial may serve as a useful paradigm for future early treatment trials in selected patient populations, especially emphasizing the opportunity to identify patients in the early phase of acute lung injury before endotracheal intubation (Fig. 8). Of note, the potential benefit of high-flow nasal cannula may not be generalizable across all patients in intensive care. A recent randomized controlled study showed that high-flow oxygen therapy did not significantly decrease 28-day mortality compared with standard oxygen therapy among critically ill immunocompromised patients with acute respiratory failure152.
Fig. 8. Identifying patients with early acute lung injury before progression to ARDS by the Berlin criteria.
Anterior–posterior chest radiographs in a critically ill 48-year-old man who presented to the emergency department with worsening dyspnoea, hypoxaemia (oxygen saturation of 70% on room air) and a 3-day history of fever, chills and a productive cough. He also had acute kidney failure with severe oliguria and a serum creatinine of 6.2 mg per dl. His systemic blood pressure was low, at 105/50 mmHg. He was diagnosed with acute pneumonia, acute renal failure and sepsis. a | Chest radiograph showing right lower lobe consolidation consistent with pneumonia. At this time, the patient was breathing spontaneously with 6 litres nasal oxygen that increased his oxygen saturation to 91%. b | Chest radiograph taken 24 hours later showing an endotracheal tube in place (arrows) for positive-pressure ventilation with bilateral opacities, consistent with the Berlin radiographic criteria. At this time, the patient had a partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio of 125 mmHg on positive-pressure ventilation with a tidal volume of 6 ml per kg predicted body weight and a positive end-expiratory airway pressure of 15 cmH2O. The patient also had a central line (arrowhead) inserted for administration of fluids and vasopressors as he progressed to developing septic shock. Time elapsed between the images demonstrates the potential window for early acute respiratory distress syndrome (ARDS) detection and early administration of therapies designed to prevent progression.
Management
Management of ARDS focuses on the diagnosis and treatment of infections, respiratory support (including oxygen supplementation and positive-pressure ventilation), careful fluid management (which is especially important if the patient is in shock) and general supportive measures such as nutritional supplementation. The details of appropriate antimicrobial therapy for the many pulmonary infections that can cause ARDS are beyond the scope of this Primer. However, as general principles, when prescribing antimicrobial therapies targeting lung infections, clinicians should consider antibiotic potency (low minimal inhibitory concentration (MIC) values against the organism), likelihood of antimicrobial resistance, ability of the antibiotic to penetrate lung tissue and speed of lung penetration153. Continuous infusions can increase the amount of time antibiotics exceed the MIC and have been shown to improve clinical outcomes with certain antibiotics154. In addition, some patients will have infections that require surgical intervention, including localized or diffuse peritonitis155, soft tissue infections (including necrotizing fasciitis) and empyema, which requires chest tube drainage of the pleural space.
Respiratory support
Historically, the focus of mechanical ventilation in acute respiratory failure has been to maintain adequate oxygenation and carbon dioxide elimination. Several preclinical studies indicated that the common clinical practice of using relatively high tidal volumes and elevated airway pressures for ARDS patients might exacerbate the degree of lung injury36. As mentioned above, in 2000, investigators supported by the US National Heart Lung and Blood Institute ARDS Network completed a randomized phase III clinical trial in which a tidal volume of 6 ml per kg PBW, compared with the more common higher tidal volume of 12 ml per kg PBW, improved survival, shortened duration of mechanical ventilation, attenuated systemic inflammation and accelerated recovery of extra-pulmonary organ failures94, and the biologic findings were reported in other studies105,108–110. Thus, with the discovery of the major role that mechanical forces play in the pathogenesis of lung injury, optimizing ventilator support to minimize VALI has become central to clinical management of ARDS, leading to the concept of lung-protective ventilation.
Tidal volume
The ARDS lung is non-uniformly aerated, with nonaerated areas predominantly in gravity-dependent regions, owing to the superimposed weight of inflammatory pulmonary oedematous fluid156. Aerated lung volume is much smaller than normal, a phenomenon termed baby lung, identified and illustrated first with CT scans; this concept of the baby lung accounts for the low compliance of the respiratory system because it identifies the areas of the lung that are consolidated with oedema and inflammation and associated atelectasis157. Thus, lower tidal volumes are needed in ARDS to prevent regional overdistension, as described above. However, scaling tidal volume to PBW targets estimated healthy lung size, although aerated baby lung volume can differ substantially between patients. Although the crucial importance of the use of a low tidal volume is now universally accepted, the best method for scaling tidal volume to patient-specific surrogates of stress or strain is still debated158,159 and warrants further investigation. Targeting airway driving pressure (plateau pressure minus PEEP) is one strategy for tailoring tidal volume to patient-specific mechanics that has garnered considerable attention159, but a universally safe threshold has yet to be validated in a prospective trial. For patients with mild lung injury at lower risk of biophysical injury, the benefit conferred by lowering tidal volume should be weighed against risks of more aggressive sedation or use of paralytics if needed to achieve the intended tidal volumes. Real-time bedside imaging techniques such as electrical impedance tomography hold some potential to identify overdistension or tidal recruitment with each breath, which could be useful for monitoring protective ventilation and to individualize ventilator strategy.
PEEP
PEEP (5–20 cmH2O) is a key element of protective ventilation91 and is routinely applied in all patients with ARDS to facilitate adequate oxygenation and maintain alveolar recruitment. The ideal PEEP might be sufficiently high to prevent cyclic opening and collapse of distal airspaces during tidal ventilation yet low enough to avoid tidal overdistension. Unfortunately, there is still not a reliable method to assess at the bedside the risk-to-benefit ratio of different PEEP levels in individual patients. Titrating PEEP to offset oesophageal pressure, a surrogate of pleural pressure, showed promise in a single-centre study160, but the results from a follow-up multicentre trial indicate no benefit for clinical outcomes for titrating PEEP by an oesophageal-guided strategy, compared with empirical high PEEP in patients with moderate or severe ARDS161. No multicentre clinical trials to date have definitively shown that any one PEEP titration strategy provides superior patient-centred outcomes162–164. However, in accordance with data from studies assessing lung recruitability with CT scan156, a meta-analysis of these trials suggests that higher levels of PEEP might be preferable in moderate or severe ARDS but not in patients with mild ARDS165. Recruitability is a term used to identify distal airspaces of the lung that may be collapsed or oedematous that could be inflated with higher levels of PEEP, therefore, participating in gas exchange. However, a recent study reported worse outcomes with a strategy of aggressive recruitment manoeuvres (to open the collapsed lung) and very high PEEP in patients with moderate or severe ARDS receiving 6 ml per kg PBW tidal volume166. To maximize benefit and limit the risk of overdistension, further reduction in tidal volume may be necessary when using high PEEP. The role of recruitment manoeuvres in managing ARDS is uncertain at this time167; whereas a brief recruitment manoeuvre (for example, 30 seconds of continuous airway pressure applied at 30 cmH2O) may transiently improve oxygenation in some patients, the impact of repeated manoeuvres with higher airway pressures and for a longer duration on clinical outcomes remains unclear168.
Prone positioning
By modifying the regional distribution of transpulmonary pressure, prone positioning decreases regional heterogeneity of lung aeration, leading to an improvement of gas exchange and a decreased risk of mechanical lung injury169. In the multicentre PROSEVA trial170, prone positioning improved survival and shortened the duration of mechanical ventilation compared with supine positioning. Unlike prior trials that yielded mixed results, PROSEVA enrolled only patients with moderate or severe ARDS (PaO2/FiO2 <150 mmHg) (Box 1), used prone positioning early in the patient’s course, prescribed the prone position for at least 16 hours per day, tailored treatment course to patient recovery and used concomitant low tidal volume ventilation — all potential requisites for therapeutic efficacy171. Long sessions of the prone position are now recommended in most patients with severe ARDS172.
Neuromuscular blockade
In spontaneously breathing patients with acute lung injury, it is possible that elevated transpulmonary pressures may exacerbate the degree of lung injury, thereby raising the question of whether intubation and ventilation with lower tidal volumes and reduced transpulmonary pressure might be beneficial173. In addition, owing to a high respiratory drive, ventilated patients with ARDS frequently exhibit strong respiratory effort even when receiving high doses of sedatives. This respiratory effort may result in severe patient–ventilator dyssynchronies and increased mechanical lung injury owing to high transpulmonary pressures and/or cyclic atelectasis173,174. Paralysis can prevent these effects; thus, neuromuscular blockade may decrease mechanical lung injury. In a multicentre trial in patients with severe ARDS, infusion with the neuromuscular-blocking drug cisatracurium for 48 hours improved adjusted survival and ventilator-free days compared with deep sedation without cisatracurium175. Results are expected soon for a follow-up multicentre trial comparing cisatracurium to protocolized sedation managed according to usual care176.
Noninvasive respiratory support
For patients with mild ARDS (Box 1), avoiding invasive mechanical ventilation altogether may be beneficial. Invasive positive-pressure ventilation and the related co-interventions carry their own risks: sedative infusions that predispose to delirium, decreased mobility that predisposes to neuromuscular weakness and risk of ventilator-associated pneumonia, among other complications177,178. Noninvasive positive-pressure ventilation improves oxygenation179 and is used often in patients with mild ARDS but without a clear benefit on outcome180; device interface may influence patient tolerance and efficacy181,182. High-flow oxygen (for example, up to 60 l per min) via large-bore nasal cannula is safe, well tolerated and effective in supporting patients with mild ARDS, in part by providing low level PEEP and modestly increasing carbon dioxide excretion151,183. As mentioned earlier, one recent study found that high-flow nasal cannula led to lower mortality than noninvasive ventilation or usual care151, and additional studies are ongoing. The optimal threshold to proceed to intubation and the drawbacks of delaying invasive support in patients who are progressing towards such have not been well defined.
Fluid management
Dating back to 1978, several preclinical studies indicated that elevated lung vascular hydrostatic pressure would increase the quantity of pulmonary oedema in animal models of ARDS184. In 2006, a randomized clinical trial in 1,000 patients with ARDS demonstrated that adopting a fluid-conservative approach after vasopressor-dependent shock had resolved led to an increase in ventilator-free days and improved oxygenation index185. In the trial, the fluid-conservative arm was guided by a detailed algorithm that required measurement of central venous or pulmonary arterial wedge pressure measurements every 4 hours to determine the use of diuretics to achieve lower vascular filling pressures. Since that trial, a simplified fluid-conservative approach has been recommended to reduce overall fluid balance by 500–1,000 ml per day in patients with ARDS who no longer have shock by reducing intravenous fluids and using diuretics. In the presence of haemodynamic instability, transpulmonary thermodilution estimation of extravascular lung water could help identify risk of exacerbating pulmonary oedema with a volume challenge, potentially helping inform resuscitation. Some have cautioned against overly conservative fluid management, citing a small study that suggested that a fluid-conservative strategy might be associated with long-term cognitive impairment; however, methodological issues, potential survivorship bias and underpowering limit definitive conclusions185,186. This trial also demonstrated no value in using a pulmonary arterial catheter compared with a central venous fluid catheter to guide fluid management, as mentioned above121. No randomized controlled trial has assessed conservative fluid therapy in children with ARDS, but one observational study indicated that elevated cumulative fluid balance on day 3 of PARDS was associated with higher mortality, especially in patients with concomitant acute kidney injury187.
Rescue therapies
To maximize efficacy, therapies for ARDS should be instituted early in the patient’s course. Some patients experience continued clinical deterioration despite optimized standard therapies; in this scenario, clinicians may consider use of so-called rescue therapies. Although no rescue therapy has been definitively proved to be beneficial, several interventions described below may have value188.
Veno-venous extracorporeal membrane oxygenation
Veno-venous extracorporeal membrane oxygenation (ECMO) is a treatment in which blood is circulated outside of the body for oxygenation on a gas-permeable membrane. ECMO has been proposed as a rescue therapy for patients with established very severe ARDS; for these trials, patients are typically those with severe ARDS (Box 1) in whom sufficient correction of gas exchange is inconsistent with lung-protective ventilation189. Observational data from the 2009 influenza A H1N1 virus epidemic suggested that ECMO rescue may have a role for patients with refractory hypoxaemia due to isolated pulmonary failure190. One trial in 249 patients with very severe ARDS, refractory hypoxaemia and/or hypercapnia randomly assigned participants to ECMO or continued conventional treatment. The trial reported an 11% absolute reduction in 60-day mortality with ECMO with ventilator settings targeting very low tidal volumes to achieve a plateau airway pressure less than 24 cmH2O, compared with the now conventional strategy of 6 ml per kg PBW tidal volumes and plateau airway pressures up to 30 cmH2O191. However, this clinically substantial effect for survival did not achieve statistical significance (P = 0.09), leaving the role of ECMO in very severe ARDS open for debate192. The best potential candidates for ECMO are patients with very severe ARDS within the first week of mechanical ventilation and without multiple organ failure189,191. To optimize the risk-to-benefit ratio, ECMO should be provided only in centres experienced in both the care of severe ARDS and the use of extracorporeal support189.
Extracorporeal CO2 removal
Extracorporeal CO2 removal partially removes CO2 from the venous blood using a moderate (0.5-1 l per min) extracorporeal blood flow. This method permits the use of very low tidal volume (3–4 ml per kg PBW) without causing severe respiratory acidosis193. This so-called ultra-protective strategy has been shown to attenuate biomarkers of inflammation in bronchoalveolar lavage and blood in pilot trials in humans194,195, indicating that some patients experience ongoing VALI with conventional protective ventilation. As with all extracorporeal methods, extracorporeal CO2 removal carries its own risks, especially bleeding. Pending the results of ongoing studies, the benefit of ultra-protective ventilation associated with extracorporeal CO2 removal on outcomes in patients with ARDS remains unknown.
Glucocorticoids
Methylprednisolone was among the first therapies tested in trials for preventing acute lung injury143,144, intuitively appealing for its anti-inflammatory properties. Up to one-fifth of patients with ARDS receive systemic steroids5, although their efficacy for attenuating lung injury remains unclear. Although some have reported a positive effect of steroids on survival196, a multicentre trial of methylprednisolone versus placebo for persistent moderate to severe ARDS observed no survival benefit (7–28 days duration)197. Steroids accelerated resolution of respiratory failure and circulatory shock but also increased risk of neuromuscular weakness; patients initiating steroids >14 days after ARDS onset experienced increased mortality. Thus, steroids should probably not be initiated 2 weeks after ARDS onset and have an uncertain risk-to-benefit ratio even when initiated earlier, unless the specific cause of ARDS is Pneumocystis carinii pneumonia198. The effectiveness of steroids as adjunct therapy in P. carinii pneumonia may be related to anti-inflammatory effects, although the benefit has not been demonstrated convincingly in other pathogenic causes of ARDS. On balance, the role for corticosteroids in early ARDS remains controversial owing to mixed results from existing literature and no definitive large-scale trial in the era of lung-protective ventilation.
Inhaled pulmonary vasodilators
Inhaled nitric oxide and prostaglandin achieve selective vasodilation of the pulmonary circulation, improving ventilation-perfusion matching and, transiently, oxygenation in patients with ARDS199. However, a benefit in patient-centred outcomes such as mortality has not been demonstrated200. Pulmonary vasodilation could benefit patients with ARDS in whom associated acute cor pulmonale (right heart failure) is contributing to circulatory failure201.
Alternative ventilator modes
High-frequency oscillatory ventilation (HFOV) is a mode of ventilation support that uses very rapid respiratory rates and very low tidal volumes in an attempt to maximize lung recruitment and avoid cyclic alveolar collapse. In two recent trials in adults with ARDS202,203, HFOV conferred no benefit compared with conventional ventilation, and in one trial mortality was increased. Thus, HFOV should be used with extreme caution, if at all, in adult ARDS. HFOV is more commonly used in children with ARDS204, and the PROSPECT study is currently evaluating its effectiveness using a factorial design. Ventilation modes that combine controlled breaths and unassisted spontaneous breaths, such as airway pressure release ventilation, may improve oxygenation and haemodynamics while decreasing the need for sedation205,206. However, concern for VALI risk warrants its limited use until studied further207.
Other pharmacological or adjunct therapies
Multiple pharmacological therapies to improve clinical outcomes in ARDS have been studied in phase II and phase III trials, but none have shown efficacy. The list of unsuccessful therapies includes surfactant replacement, aerosolized and intravenous β2-adrenergic agonists, prostaglandin E1, activated protein C, antioxidants, omega-3 supplementation, ketoconazole (an anti-fungal), lisofylline (an anti-inflammatory), recombinant human factor VIIa, IFNβ1α, granulocyte–macrophage colony-stimulating factor and statins208. However, it is certainly possible that one or more of these treatments might have been effective if a predictive enrichment strategy had been incorporated in the trial design, as illustrated in a recent study114. In addition, optimal targets for arterial oxygenation and arterial CO2 concentration have not been defined in ARDS or other critical care conditions; once the targets are established, it may be possible to test more specific oxygenation targets in clinical trials.
Quality of life
The longitudinal evaluation of survivors of ARDS helped spark a research agenda tracking long-term morbidity after critical illness; different assessments of QOL and function have been used in ARDS survivors. Through the mid-1990s, long-term outcomes in ARDS focused primarily on the pulmonary system209–211. Over the past two decades, the assessment of longer-term outcomes has expanded to include several new methods for clinical assessments. These methods have included QOL measures focused on limitations in functional domains, including muscle weakness and quantitative walking distance, as well as neurocognitive measures that include attention, memory, concentration and mood disorders. In addition, data have been collected on caregiver burden, medical complexity and increased health-care costs. Here, we consider the evolution of outcome measures in survivors of ARDS.
Early reports observed that some survivors of ARDS experienced obstructive and restrictive ventilatory deficits that persisted for weeks to months after recovery from acute illness209–211. Variability in outcome among survivors may be related to differences in study sample selection, clinical management during the acute illness, duration and completeness of follow-up, heterogeneity in baseline pulmonary disease and variable contributions from long-term respiratory muscle and diaphragmatic muscle weakness. Nevertheless, most patients recover near-normal pulmonary function within 6–12 months after ARDS209–211.
QOL studies have consistently demonstrated a decrement in physical function domains among survivors of ARDS. Early reports postulated these decrements might be due to exercise limitation from residual pulmonary function abnormalities212–214. Later studies combined pulmonary and functional outcome measures with QOL assessments, observing that both pulmonary function and self-perceived overall health gradually improve through 6 months after the acute illness. However, the severity of disability was not wholly explained by changes in pulmonary function or respiratory symptoms215. The aetiology of this reported dysfunction was uncertain. A later study combined measures of pulmonary function with an evaluation of quality-adjusted life years using the Quality of Well-Being Scale (a general health questionnaire that measures well-being over the previous 3 days in terms of physical activities, social activities, mobility and symptoms) and found that ARDS survivors had poor quality-adjusted survival associated with an important illness burden. Again, the specific contributors to this dysfunction remained unclear216.
A subsequent study combined serial QOL, pulmonary function and 6-minute walk test (a commonly used test to assess cardiopulmonary capacity) data with in-person history and physical examination and an evaluation of pattern and cost of health-care utilization217. With more detailed follow-up evaluations, these investigators observed that survivors of ARDS had significant exercise limitation and poor physical QOL at 1-year follow-up related to marked muscle wasting and weakness; myriad physical disabilities including heterotopic ossification, joint contractures and tracheal stenosis; and cosmetic concerns related to scarring at tracheostomy, central line or chest tube sites. This study focused attention on muscle dysfunction as a major morbidity after ARDS and served to highlight the prevalence and persistence of medical complexity after critical illness217. This same group of investigators continued follow-up to 5 years after ARDS and noted persistence of disability and compromised QOL, which were associated with increased cost and health-care use218. These findings are robust and have been replicated by many other investigators219–221.
Hopkins and her group were the first to describe neurocognitive and mood disorder outcomes in survivors of ARDS. They noted important decrements in attention, concentration, processing speed, memory and executive function that persisted up to 2 years after discharge from intensive care222,223. Post-traumatic stress disorder in patients with ARDS has also been described and is associated with decrements in the mental health domains of the 36-Item Short Form Survey (SF-36) QOL measure213. These observations have led the way for other investigators to evaluate neurocognitive dysfunction and its risk factors in ARDS and other survivors of critical illness, including hypoglycaemia during the intensive care stay224, hypoxaemia and fluid resuscitation186, duration of delirium225 and premorbid functional status226.
The observation of persistent muscle wasting and weakness in ARDS survivors has spawned intense interest in an entity described as intensive care unit-acquired weakness (ICUAW), which is the consequence of a myosin depletion myopathy and axonopathy occurring in isolation or together227. Injury to the diaphragm and muscles of the axial skeleton is due at least in part to upregulation of the ubiquitin-proteasome pathway and marked proteolysis that begins within hours of the critical illness228 and may continue throughout the first week of the illness229. Recent seminal observations show that muscle repair after an episode of critical illness may be differential and the recovery of contractile force and muscle mass may be discordant at long-term follow-up230.
One challenge for studies of long-term outcomes is knowing the baseline status of the patients with ARDS. Thus, the extent to which the deficits manifested before developing ARDS is not always clear. In addition, the deficits may be a function of prolonged and severe critical illness rather than specifically from ARDS.
Outlook
In the past two decades, outcomes from ARDS have improved considerably from the first reports of the syndrome, in which the majority of patients did not survive. However, mortality remains a serious threat, and long-term complications remain common and problematic in survivors. Additional research on several fronts may help to improve prognosis. From a mechanistic perspective, there is a need for strategies that can mitigate lung endothelial and epithelial injury and novel approaches that promote lung endothelial and epithelial repair. In addition, there is a need to optimize preclinical models for testing novel therapies in ARDS to improve the pipeline from drug discovery to improved patient outcomes.
In the clinical setting, increased recognition of ARDS in all regions of the world is important to better identify patients earlier in their clinical course so that supportive care with lung-protective ventilation and a conservative fluid approach can be implemented. Prognostic and/or predictive enrichment approaches that include biological and clinical variables in randomized trials may improve the chances of identifying responsive subsets of patients with ARDS. Some have questioned whether the syndromic definition of ARDS will continue to be useful given its lack of specificity, overlap with other distinct syndromes (that is, ARDS mimics) and the relentless failure of pharmacotherapeutics in this condition; however, there is not yet a consensus within the field on a suitable alternative approach.
There is new evidence that the degree of pulmonary oedema can be quantified on the chest radiograph231, a method that can applied rapidly for assessing all four quadrants of the standard anterior–posterior radiograph in the intensive care unit. In addition, a clinically practical method (the ventilatory ratio) for estimating pulmonary dead space has been validated232. Thus, a modified lung injury score that incorporates oxygenation abnormalities (PaO2/FiO2), the level of PEEP, the pulmonary dead space and the degree of pulmonary oedema may prove useful to combine with the promising biological variables for risk stratification in clinical trials. Novel diagnostic approaches may improve our ability to identify and treat causative pathogens in patients with ARDS, thereby improving patient outcomes233. Extracorporeal therapies may provide life-sustaining support for severe ARDS234. Multi-pathway targeted cell-based therapies such as mesenchymal stromal cells show promise as a potential therapeutic option because they have the capacity to reduce lung vascular injury, restore alveolar epithelial fluid transport properties to remove alveolar oedematous fluid and switch the pro-inflammatory responses to a pro-resolving paradigm235,236. Finally, increased understanding of the importance of long-term patient-centred outcomes may lead to both improved mechanistic understanding of the causes of these sequelae and improved efforts to design trials that target these symptoms237.
Supplementary information
Acknowledgements
The authors declare the following grant support: M.A.M. by the US National Health Lung and Blood Institute (NHLBI; HL140026 and HL134828); C.S.C. by NHLBI (HL140026); G.A.Z. by NHLBI (HL044525, HL077671 and HL130541); Y.M.A. by the Miracle Trial; J.R.B. by NHBLI (HL133489); R.L.Z. by NHLBI (HL131608); A.G.R. by the US National Institute of Child Health and Disease (HD095228); and M.H. by the Canadian Institute of Health. The authors thank S. Ke, University of California–San Francisco, for assistance with organizing the reference list for this article.
Reviewer information
Nature Reviews Disease Primers thanks O. Gajic, Y. Odeyemi, R. Rossaint, W. Seeger, J.-L. Vincent and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author contributions
Introduction (M.A.M. and C.S.C.); Epidemiology (M.A.M., A.G.R. and C.S.C.); Mechanisms/pathophysiology (M.A.M., R.L.Z., G.A.Z. and C.S.C.); Diagnosis, screening and prevention (M.A.M., Y.M.A., A.G.R. and C.S.C.); Management (M.A.M., J.R.B., A.M. and C.S.C.); Quality of life (M.H.); Outlook (M.A.M. and C.S.C.); Overview of the Primer (M.A.M. and C.S.C.)
Competing interests
M.A.M. declares grant support from Bayer (current), GlaxoSmithKline (prior) and Amgen (prior); has served as Data Safety and Monitoring Board chair for Roche-Genentech and has served as a consultant for GlaxoSmithKline, Bayer, Boehringer, CSL Berhring, Navigen, Quark and Cerus. G.A.Z. has served as a consultant for Navigen. Y.M.A. has served as a consultant for Gilead Sciences (past), Regeneron (past) and SAB Therapeutics (current). A.M. received fees for serving on a steering committee for Faron Pharmaceuticals, consulting fees from Air Liquide Medical Systems, grant support for research and lecture fees from Fisher & Paykel and Covidien, and lecture fees from Drager, Pfizer and ResMed. A.G.R. declares grant support from Roche-Genentech (current) and has served as a consultant for La Jolla Pharma and Bristol Meyer Squibb. C.S.C. declares grant support from Bayer (current) and GlaxoSmithKline (prior) and has served as a consultant for GlaxoSmithKline, Bayer, Boehringer, Prometic, Roche-Genentech, CSL Behring and Quark. The remaining authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
PROSPECT study: http://www.prospect-network.org/
Supplementary information
Supplementary information is available for this paper at 10.1038/s41572-019-0069-0.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Bellani G, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 6.Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am. J. Respir. Crit. Care Med. 2017;195:860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 7.Cortegiani A, et al. Immunocompromised patients with acute respiratory distress syndrome: secondary analysis of the LUNG SAFE database. Crit. Care. 2018;22:157. doi: 10.1186/s13054-018-2079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. doi: 10.1001/jama.1996.03530250054027. [DOI] [PubMed] [Google Scholar]
- 9.Calfee CS, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am. J. Respir. Crit. Care Med. 2011;183:1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calfee CS, et al. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit. Care Med. 2015;43:1790–1797. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, et al. Long-term ozone exposure increases the risk of developing the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2016;193:1143–1150. doi: 10.1164/rccm.201507-1418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reilly JP, et al. Low to moderate air pollutant exposure and acute respiratory distress syndrome after severe trauma. Am. J. Respir. Crit. Care Med. 2018;199:62–70. doi: 10.1164/rccm.201803-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangialardi RJ, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen Sepsis Study Group. Crit. Care Med. 2000;28:3137–3145. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Moss M, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit. Care Med. 2000;28:2187–2192. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit. Care Med. 2009;37:2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle AJ, et al. Identifying associations between diabetes and acute respiratory distress syndrome in patients with acute hypoxemic respiratory failure: an analysis of the LUNG SAFE database. Crit. Care. 2018;22:268. doi: 10.1186/s13054-018-2158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toy P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson BR, et al. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. J. Trauma Acute Care Surg. 2013;75:S61–S67. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard BM, et al. Differences in degree, differences in kind: characterizing lung injury in trauma. J. Trauma Acute Care Surg. 2015;78:735–741. doi: 10.1097/TA.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson SE, et al. Racial and ethnic disparities in mortality from acute lung injury. Crit. Care Med. 2009;37:1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryb GE, Cooper C. Race/ethnicity and acute respiratory distress syndrome: a National Trauma Data Bank study. J. Natl Med. Assoc. 2010;102:865–869. doi: 10.1016/S0027-9684(15)30700-8. [DOI] [PubMed] [Google Scholar]
- 22.Cochi SE, Kempker JA, Annangi S, Kramer MR, Martin GS. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann. Am. Thorac Soc. 2016;13:1742–1751. doi: 10.1513/AnnalsATS.201512-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996) Crit. Care Med. 2002;30:1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Reilly JP, Christie JD, Meyer NJ. Fifty years of research in ARDS. Genomic contributions and opportunities. Am. J. Respir. Crit. Care Med. 2017;196:1113–1121. doi: 10.1164/rccm.201702-0405CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer NJ, Calfee CS. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir. Med. 2017;5:512–523. doi: 10.1016/S2213-2600(17)30187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reilly JP, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44:1849–1858. doi: 10.1007/s00134-018-5328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schouten LR, et al. Incidence and mortality of acute respiratory distress syndrome in children: a systematic review and meta-analysis. Crit. Care Med. 2016;44:819–829. doi: 10.1097/CCM.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 28.Khemani RG, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir. Med. 2018;7:115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bindl L, et al. Gender-based differences in children with sepsis and ARDS: the ESPNIC ARDS Database Group. Intensive Care Med. 2003;29:1770–1773. doi: 10.1007/s00134-003-1948-z. [DOI] [PubMed] [Google Scholar]
- 30.Nye S, Whitley RJ, Kong M. Viral infection in the development and progression of pediatric acute respiratory distress syndrome. Front. Pediatr. 2016;4:128. doi: 10.3389/fped.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Roulet A, et al. Pediatric trauma-associated acute respiratory distress syndrome: incidence, risk factors, and outcomes. J. Pediatr. Surg. 2018 doi: 10.1016/j.jpedsurg.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit. Care Med. 2009;37:2448–2454. doi: 10.1097/CCM.0b013e3181aee5dd. [DOI] [PubMed] [Google Scholar]
- 33.Spicer AC, et al. A simple and robust bedside model for mortality risk in pediatric patients with acute respiratory distress syndrome. Pediatr. Crit. Care Med. 2016;17:907–916. doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu. Rev. Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 35.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am. J. Respir. Crit. Care Med. 2014;189:1301–1308. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 38.Fein A, et al. The value of edema fluid protein measurement in patients with pulmonary edema. Am. J. Med. 1979;67:32–38. doi: 10.1016/0002-9343(79)90066-4. [DOI] [PubMed] [Google Scholar]
- 39.Nuckton TJ, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N. Engl. J. Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 40.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage—the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976;85:209–228. [PMC free article] [PubMed] [Google Scholar]
- 41.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr. Opin. Crit. Care. 2005;11:29–36. doi: 10.1097/00075198-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Cardinal-Fernandez P, Lorente JA, Ballen-Barragan A, Matute-Bello G. Acute respiratory distress syndrome and diffuse alveolar damage. New insights on a complex relationship. Ann. Am. Thorac Soc. 2017;14:844–850. doi: 10.1513/AnnalsATS.201609-728PS. [DOI] [PubMed] [Google Scholar]
- 43.Thille AW, et al. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir. Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- 44.Thille AW, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am. J. Respir. Crit. Care Med. 2013;187:761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 45.Cardinal-Fernandez P, et al. The presence of diffuse alveolar damage on open lung biopsy is associated with mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Chest. 2016;149:1155–1164. doi: 10.1016/j.chest.2016.02.635. [DOI] [PubMed] [Google Scholar]
- 46.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am. Rev. Respir. Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 47.Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000;21:435–466. doi: 10.1016/S0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 48.Albertine KH, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, et al. Novel role of the human alveolar epithelium in regulating intra-alveolar coagulation. Am. J. Respir. Cell Mol. Biol. 2007;36:497–503. doi: 10.1165/rcmb.2005-0425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1035–L1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng KT, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Invest. 2017;127:4124–4135. doi: 10.1172/JCI94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brigham KL, Woolverton WC, Blake LH, Staub NC. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J. Clin. Invest. 1974;54:792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J. Clin. Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frye M, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J. Exp. Med. 2015;212:2267–2287. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev. Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Broermann A, et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J. Exp. Med. 2011;208:2393–2401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corada M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl Acad. Sci. USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulte D, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams AE, et al. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax. 2017;72:66–73. doi: 10.1136/thoraxjnl-2016-208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lefrancais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3:98178. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdulnour RE, et al. Early intravascular events are associated with development of acute respiratory distress syndrome. A substudy of the LIPS-A clinical trial. Am. J. Respir. Crit. Care Med. 2018;197:1575–1585. doi: 10.1164/rccm.201712-2530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Short KR, et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur. Respir. J. 2016;47:954–966. doi: 10.1183/13993003.01282-2015. [DOI] [PubMed] [Google Scholar]
- 64.Schlingmann B, et al. Regulation of claudin/zonula occludens-1 complexes by hetero-claudin interactions. Nat. Commun. 2016;7:12276. doi: 10.1038/ncomms12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaver CM, et al. Cell-free hemoglobin promotes primary graft dysfunction through oxidative lung endothelial injury. JCI Insight. 2018;3:98546. doi: 10.1172/jci.insight.98546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Budinger GR, et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am. J. Respir. Crit. Care Med. 2011;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hogner K, et al. Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLOS Pathog. 2013;9:e1003188. doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imai Y, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 70.Herrero R, et al. The biological activity of FasL in human and mouse lungs is determined by the structure of its stalk region. J. Clin. Invest. 2011;121:1174–1190. doi: 10.1172/JCI43004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saffarzadeh M, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLOS ONE. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brauer R, et al. Syndecan-1 attenuates lung injury during influenza infection by potentiating c-Met signaling to suppress epithelial apoptosis. Am. J. Respir. Crit. Care Med. 2016;194:333–344. doi: 10.1164/rccm.201509-1878OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hook JL, et al. Disruption of staphylococcal aggregation protects against lethal lung injury. J. Clin. Invest. 2018;128:1074–1086. doi: 10.1172/JCI95823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vohwinkel CU, et al. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011;286:37067. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 76.Hogan BL, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ray S, et al. Rare SOX2(+) airway progenitor cells generate KRT5(+) cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Rep. 2016;7:817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quantius J, et al. Influenza virus infects epithelial stem/progenitor cells of the distal lung: impact on Fgfr2b-driven epithelial repair. PLOS Pathog. 2016;12:e1005544. doi: 10.1371/journal.ppat.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xi Y, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dial CF, Tune MK, Doerschuk CM, Mock JR. Foxp3+regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am. J. Respir. Cell Mol. Biol. 2017;57:162–173. doi: 10.1165/rcmb.2017-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zemans RL, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc. Natl Acad. Sci. USA. 2011;108:15990–15995. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cong X, Hubmayr RD, Li C, Zhao X. Plasma membrane wounding and repair in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L371–L391. doi: 10.1152/ajplung.00486.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schumacker PT, et al. Mitochondria in lung biology and pathology: more than just a powerhouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L962–L974. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koval M, et al. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am. J. Respir. Cell Mol. Biol. 2010;42:172–180. doi: 10.1165/rcmb.2008-0270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gwozdzinska P, et al. Hypercapnia impairs ENaC cell surface stability by promoting phosphorylation, polyubiquitination and endocytosis of beta-ENaC in a human alveolar epithelial cell line. Front. Immunol. 2017;8:591. doi: 10.3389/fimmu.2017.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vadasz I, Sznajder JI. Gas exchange disturbances regulate alveolar fluid clearance during acute lung injury. Front. Immunol. 2017;8:757. doi: 10.3389/fimmu.2017.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nikolaidis NM, et al. Mitogenic stimulation accelerates influenza-induced mortality by increasing susceptibility of alveolar type II cells to infection. Proc. Natl Acad. Sci. USA. 2017;114:E6613–E6622. doi: 10.1073/pnas.1621172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2012;185:702–708. doi: 10.1164/rccm.201109-1667PP. [DOI] [PubMed] [Google Scholar]
- 91.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Respir. Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 92.Parker JC, Townsley MI, Rippe B, Taylor AE, Thigpen J. Increased microvascular permeability in dog lungs due to high peak airway pressures. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984;57:1809–1816. doi: 10.1152/jappl.1984.57.6.1809. [DOI] [PubMed] [Google Scholar]
- 93.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am. Rev. Respir. Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 94.Brower RG, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 95.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frank JA, et al. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am. J. Respir. Crit. Care Med. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 97.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 98.Sprung CL, Rackow EC, Fein IA, Jacob AI, Isikoff SK. The spectrum of pulmonary edema: differentiation of cardiogenic, intermediate, and noncardiogenic forms of pulmonary edema. Am. Rev. Respir. Dis. 1981;124:718–722. doi: 10.1164/arrd.1981.124.6.718. [DOI] [PubMed] [Google Scholar]
- 99.Idell S, et al. Angiotensin converting enzyme in bronchoalveolar lavage in ARDS. Chest. 1987;91:52–56. doi: 10.1378/chest.91.1.52. [DOI] [PubMed] [Google Scholar]
- 100.Rubin DB, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J. Clin. Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donnelly SC, et al. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994;344:215–219. doi: 10.1016/S0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- 102.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 103.Meduri GU, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 104.Agrawal A, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am. J. Respir. Crit. Care Med. 2013;187:736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eisner MD, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uchida T, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am. J. Respir. Crit. Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ware LB, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parsons PE, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med. 2005;33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC. [DOI] [PubMed] [Google Scholar]
- 109.Calfee CS, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ranieri VM, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 111.Calfee CS, et al. Distinct molecular phenotypes of direct versus indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calfee CS, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Famous KR, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calfee CS, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sinha P, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McAuley DF, et al. Simvastatin in the acute respiratory distress syndrome. N. Engl. J. Med. 2014;371:1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 117.Bos LD, et al. Understanding heterogeneity in biological phenotypes of ARDS by leukocyte expression profiles. Am. J. Respir. Crit. Care Med. 2019 doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 118.Morrell ED, et al. Peripheral and alveolar cell transcriptional programs are distinct in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2018;197:528–532. doi: 10.1164/rccm.201703-0614LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kangelaris KN, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116:1347–1353. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 121.Wheeler AP, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N. Engl. J. Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 122.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur. Respir. J. 2010;35:331–337. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rice TW, et al. Vascular pedicle width in acute lung injury: correlation with intravascular pressures and ability to discriminate fluid status. Crit. Care. 2011;15:R86. doi: 10.1186/cc10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Riviello ED, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali Modification of the Berlin Definition. Am. J. Respir. Crit. Care Med. 2016;193:52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]
- 125.Palakshappa JA, Meyer NJ. Which patients with ARDS benefit from lung biopsy? Chest. 2015;148:1073–1082. doi: 10.1378/chest.15-0076. [DOI] [PubMed] [Google Scholar]
- 126.Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr. Crit. Care Med. 2015;16:S23–S40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 127.De Luca D, et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir. Med. 2017;5:657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]
- 128.Kao KC, et al. Coinfection and mortality in pneumonia-related acute respiratory distress syndrome patients with bronchoalveolar lavage: a prospective observational study. Shock. 2017;47:615–620. doi: 10.1097/SHK.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hong DK, et al. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn. Microbiol. Infect. Dis. 2018;92:210–213. doi: 10.1016/j.diagmicrobio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 130.Fischer N, et al. Rapid metagenomic diagnostics for suspected outbreak of severe pneumonia. Emerg. Infect. Dis. 2014;20:1072–1075. doi: 10.3201/eid2006.131526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hasvold J, Sjoding M, Pohl K, Cooke C, Hyzy RC. The role of human metapneumovirus in the critically ill adult patient. J. Crit. Care. 2016;31:233–237. doi: 10.1016/j.jcrc.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robert D, et al. A series of five adult cases of respiratory syncytial virus-related acute respiratory distress syndrome. Anaesth. Intensive Care. 2008;36:230–234. doi: 10.1177/0310057X0803600214. [DOI] [PubMed] [Google Scholar]
- 133.Ferguson ND, et al. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Crit. Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matthay MA. Challenges in predicting which patients will develop ARDS. Lancet Respir. Med. 2016;4:847–848. doi: 10.1016/S2213-2600(16)30306-X. [DOI] [PubMed] [Google Scholar]
- 135.Levitt JE, Calfee CS, Goldstein BA, Vojnik R, Matthay MA. Early acute lung injury: criteria for identifying lung injury prior to the need for positive pressure ventilation*. Crit. Care Med. 2013;41:1929–1937. doi: 10.1097/CCM.0b013e31828a3d99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gajic O, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am. J. Respir. Crit. Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kor DJ, et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-A randomized clinical trial. JAMA. 2016;315:2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135:936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Levitt JE, Matthay MA. Clinical review: early treatment of acute lung injury—paradigm shift toward prevention and treatment prior to respiratory failure. Crit. Care. 2012;16:223. doi: 10.1186/cc11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Afshar M, et al. Injury characteristics and von Willebrand Factor for the prediction of acute respiratory distress syndrome in patients with burn injury: development and internal validation. Ann. Surg. 2018 doi: 10.1097/SLA.0000000000002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu X, et al. Plasma sRAGE enables prediction of acute lung injury after cardiac surgery in children. Crit. Care. 2012;16:R91. doi: 10.1186/cc11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jabaudon M, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci. Rep. 2018;8:2603. doi: 10.1038/s41598-018-20994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luce JM, et al. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am. Rev. Respir. Dis. 1988;138:62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 144.Weigelt JA, Norcross JF, Borman KR, Snyder WH., 3rd Early steroid therapy for respiratory failure. Arch. Surg. 1985;120:536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 145.Festic E, et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit. Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Determann RM, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit. Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neto AS, Jaber S. What’s new in mechanical ventilation in patients without ARDS: lessons from the ARDS literature. Intensive Care Med. 2016;42:787–789. doi: 10.1007/s00134-016-4309-4. [DOI] [PubMed] [Google Scholar]
- 148.Writing Group for the, P. I. et al. Effect of a low versus intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018;320:1872–1880. doi: 10.1001/jama.2018.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li G, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am. J. Respir. Crit. Care Med. 2011;183:59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahmed AH, et al. The role of potentially preventable hospital exposures in the development of acute respiratory distress syndrome: a population-based study. Crit. Care Med. 2014;42:31–39. doi: 10.1097/CCM.0b013e318298a6db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frat JP, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N. Engl. J. Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 152.Azoulay E, et al. Effect of high-flow nasal oxygen versus standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320:2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Drusano GL. What are the properties that make an antibiotic acceptable for therapy of community-acquired pneumonia? J. Antimicrob. Chemother. 2011;66(Suppl. 3):61–67. doi: 10.1093/jac/dkr100. [DOI] [PubMed] [Google Scholar]
- 154.Roberts JA, et al. Continuous versus intermittent beta-lactam infusion in severe sepsis. A meta-analysis of individual patient data from randomized trials. Am. J. Respir. Crit. Care Med. 2016;194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 155.Ross JT, Matthay MA, Harris HW. Secondary peritonitis: principles of diagnosis and intervention. BMJ. 2018;361:k1407. doi: 10.1136/bmj.k1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gattinoni L, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 157.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 158.Beitler JR, et al. Volume delivered during recruitment maneuver predicts lung stress in acute respiratory distress syndrome. Crit. Care Med. 2016;44:91–99. doi: 10.1097/CCM.0000000000001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Amato MB, et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 160.Talmor D, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N. Engl. J. Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beitler, J. R. et al. EPVent-2 Study Group. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy versus an empiric high PEEP-FiO2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA (in the press). [DOI] [PMC free article] [PubMed]
- 162.Brower RG, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 163.Meade MO, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 164.Mercat A, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 165.Briel M, et al. Higher versus lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 166.Cavalcanti AB, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sahetya SK, Brower RG. Lung recruitment and titrated PEEP in moderate to severe ARDS: is the door closing on the open lung? JAMA. 2017;318:1327–1329. doi: 10.1001/jama.2017.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goligher EC, et al. Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann. Am. Thorac Soc. 2017;14:S304–S311. doi: 10.1513/AnnalsATS.201704-340OT. [DOI] [PubMed] [Google Scholar]
- 169.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am. J. Respir. Crit. Care Med. 2013;188:1286–1293. doi: 10.1164/rccm.201308-1532CI. [DOI] [PubMed] [Google Scholar]
- 170.Guerin C, et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 171.Beitler JR, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014;40:332–341. doi: 10.1007/s00134-013-3194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fan E, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 173.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 174.Beitler JR, et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med. 2016;42:1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Papazian L, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 176.Huang DT, et al. Design and rationale of the reevaluation of systemic early neuromuscular blockade trial for acute respiratory distress syndrome. Ann. Am. Thorac Soc. 2017;14:124–133. doi: 10.1513/AnnalsATS.201608-629OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ely EW, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 178.Schweickert WD, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Antonelli M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N. Engl. J. Med. 1998;339:429–435. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 180.Bellani G, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 181.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet versus face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Beitler JR, Owens RL, Malhotra A. Unmasking a role for noninvasive ventilation in early acute respiratory distress syndrome. JAMA. 2016;315:2401–2403. doi: 10.1001/jama.2016.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matthay MA. Saving lives with high-flow nasal oxygen. N. Engl. J. Med. 2015;372:2225–2226. doi: 10.1056/NEJMe1504852. [DOI] [PubMed] [Google Scholar]
- 184.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 186.Mikkelsen ME, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am. J. Respir. Crit. Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zinter MS, et al. Positive cumulative fluid balance is associated with mortality in pediatric acute respiratory distress syndrome in the setting of acute kidney injury. Pediatr. Crit. Care Med. 2019 doi: 10.1097/PCC.0000000000001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fielding-Singh V, Matthay MA, Calfee CS. Beyond low tidal volume ventilation: treatment adjuncts for severe respiratory failure in acute respiratory distress syndrome. Crit. Care Med. 2018;46:1820–1831. doi: 10.1097/CCM.0000000000003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Combes A, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am. J. Respir. Crit. Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 190.Davies A, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 191.Combes A, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 192.Mi MY, Matthay MA, Morris AH. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018;379:884–887. doi: 10.1056/NEJMclde1804601. [DOI] [PubMed] [Google Scholar]
- 193.Fanelli V, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit. Care. 2016;20:36. doi: 10.1186/s13054-016-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Terragni PP, et al. Tidal volume lower than 6ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111:826–835. doi: 10.1097/ALN.0b013e3181b764d2. [DOI] [PubMed] [Google Scholar]
- 195.Bein T, et al. Lower tidal volume strategy (approximately 3ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meduri GU, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42:829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 197.Steinberg KP, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 198.Ewald H, et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst. Rev. 2015;4:CD006150. doi: 10.1002/14651858.CD006150.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N. Engl. J. Med. 2005;353:2683–2695. doi: 10.1056/NEJMra051884. [DOI] [PubMed] [Google Scholar]
- 200.Taylor RW, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 201.Repesse X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147:259–265. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 202.Young D, et al. High-frequency oscillation for acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 203.Ferguson ND, et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 204.Bateman ST, et al. Early high-frequency oscillatory ventilation in pediatric acute respiratory failure. A propensity score analysis. Am. J. Respir. Crit. Care Med. 2016;193:495–503. doi: 10.1164/rccm.201507-1381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Putensen C, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2001;164:43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 206.Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999;159:1241–1248. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- 207.Lalgudi Ganesan S, Jayashree M, Singhi SC, Bansal A. Airway pressure release ventilation in pediatric acute respiratory distress syndrome: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 2018;198:1199–1207. doi: 10.1164/rccm.201705-0989OC. [DOI] [PubMed] [Google Scholar]
- 208.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir. Med. 2017;5:524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 209.Downs JB, Olsen GN. Pulmonary function following adult respiratory distress syndrome. Chest. 1974;65:92–93. doi: 10.1378/chest.65.1.92. [DOI] [PubMed] [Google Scholar]
- 210.Lakshminarayan S, Stanford RE, Petty TL. Prognosis after recovery from adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1976;113:7–16. doi: 10.1164/arrd.1976.113.1.7. [DOI] [PubMed] [Google Scholar]
- 211.Klein JJ, van Haeringen JR, Sluiter HJ, Holloway R, Peset R. Pulmonary function after recovery from the adult respiratory distress syndrome. Chest. 1976;69:350–355. doi: 10.1378/chest.69.3.350. [DOI] [PubMed] [Google Scholar]
- 212.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am. J. Respir. Crit. Care Med. 1997;156:1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 213.Schelling G, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit. Care Med. 1998;26:651–659. doi: 10.1097/00003246-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 214.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–360. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 215.McHugh LG, et al. Recovery of function in survivors of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- 216.Angus DC, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;163:1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 217.Herridge MS, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 218.Herridge MS, et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 219.Fan E, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit. Care Med. 2014;42:849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Needham DM, et al. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am. J. Respir. Crit. Care Med. 2014;189:1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pfoh ER, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–1566. doi: 10.1007/s00134-016-4530-1. [DOI] [PubMed] [Google Scholar]
- 222.Hopkins RO, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 223.Hopkins RO, et al. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 224.Dowdy DW, et al. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit. Care Med. 2008;36:2726–2733. doi: 10.1097/CCM.0b013e31818781f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pandharipande PP, et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ferrante LE, et al. Functional trajectories among older persons before and after critical illness. JAMA Intern. Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014;371:287–288. doi: 10.1056/NEJMc1406274. [DOI] [PubMed] [Google Scholar]
- 228.Levine S, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 229.Puthucheary ZA, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 230.Dos Santos C, et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am. J. Respir. Crit. Care Med. 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 231.Warren MA, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73:840–846. doi: 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sinha P, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2019;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Langelier C, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc. Natl Acad. Sci. USA. 2018;115:E12353–E12362. doi: 10.1073/pnas.1809700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Boyle AJ, et al. Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation: research questions and clinical potential for the future. Lancet Respir. Med. 2018;6:874–884. doi: 10.1016/S2213-2600(18)30326-6. [DOI] [PubMed] [Google Scholar]
- 235.Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Matthay MA, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 2018;7:P154–P162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am. J. Respir. Cell Mol. Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jansing NL, et al. Unbiased quantitation of alveolar type II to alveolar type I cell transdifferentiation during repair after lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2017;57:519–526. doi: 10.1165/rcmb.2017-0037MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zacharias WJ, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu Y, et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J. Exp. Med. 2011;208:1473–1484. doi: 10.1084/jem.20102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liang J, et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat. Med. 2016;22:1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rafii S, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat. Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.