Abstract
Adult cancer survivors have increased risk of cardiometabolic conditions, contributing to a “silver tsunami” of aging survivors whose multi-morbidities will greatly challenge healthcare resources. We suggest that partially misaligned common-sense models of health risks held by cancer survivors, oncologists, and primary care providers (PCPs) result in differences in preferred coping strategies and produce inefficiencies and gaps in cancer survivors’ care delivery. Importantly, no entity in the health care system claims major responsibility to address longstanding unhealthy lifestyle behaviors that heighten susceptibility to both cancer and cardiovascular disease (CVD) and whose improvement could enhance quality of life. To address this gap, we propose systems-level changes that integrate health promotion into existing survivorship services by including behavioral risk factor vital signs in the electronic medical record, with default proactive referral to a health promotionist (a paraprofessional coach adept with mobile technologies and supervised by a professional expert in health behavior change). By using the patient’s digital tracking data to coach remotely and periodically report progress to providers, the health promotionist closes a gap, creating a connected care system that supports, reinforces, and maintains accountability for healthy lifestyle improvement. No comparable resource solely dedicated to treatment of chronic disease risk behaviors (smoking, obesity, physical inactivity, treatment nonadherence) exists in current models of integrated care. Integrating health promotionists into care delivery channels would remove burden from overtaxed PCPs and instantiate a comprehensive, actionable systems-level schema of health risks and coping strategies needed to have preventive impact with minimal interference to clinical work flow.
The New Cancer Survivor
Although many adults do still die from cancer, a cancer diagnosis is no longer a certain death sentence. Advances in screening, early detection, and more effective treatments have allowed several cancers to transition from being an inevitably fatal disease to a chronic condition (Hewitt, Greenfield, & Stovall, 2005). A chronic disease is one that persists and cannot be fully cured by medications, but, if controlled, is compatible with long-term survival. Currently, 64% of cancer survivors have lived for 5 years after diagnosis and 40% have lived for 10 years (de Moor et al., 2013; Rowland & Yancik, 2006). Together, two commonly occurring chronic diseases, cancer and cardiometabolic diseases, account for nearly 46% of all deaths in the United States (Centers for Disease Control and Prevention). Cancer survivors can develop cardiac and pulmonary disease as late effects of cancer treatments(Ewer & Ewer, 2015). Additionally, since both cancer and cardiometabolic diseases are associated with aging, many cancer survivors will develop cardiometabolic disease as a result of shared contributory biological mechanisms (e.g., chronic inflammation), and common behavioral risk factors (Koene, Prizment, Blaes, & Konety, 2016; Masoudkabir et al., 2017). Thus, many survivors will have multimorbidity: that is, the co-occurrence of two or more chronic diseases (Suls, Green, & Davidson, 2016). The combination of advanced age, longevity, and multiple health conditions, especially heart disease, that characterize the cancer survivor are believed to presage a “silver tsunami” of survivors, many of whom are baby boomers now straddling the peak 65–69 age period of cancer onset (Smith, Hurria, et al, 2009). The complex needs of this cohort are expected to greatly challenge existing healthcare resources (Bluethmann, Mariotto, & Rowland, 2016).
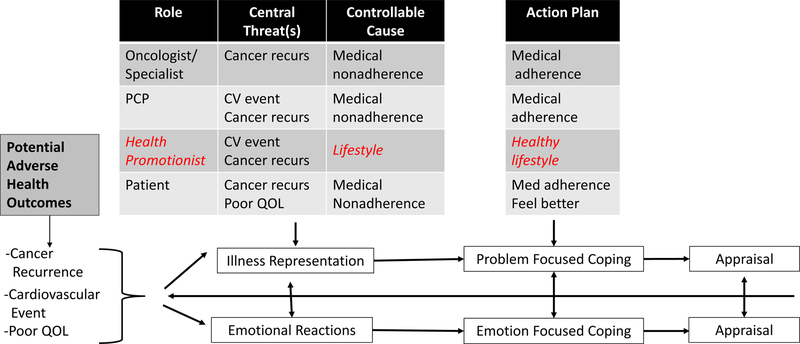
Cancer’s entrance into the chronic disease landscape has introduced new challenges affecting both patients trying to manage life during survivorship and providers trying to curtail patients’ risk of adverse medical events. An entire medical enterprise exists to support the survivor’s efforts, but it is a system that was designed to address one disease at a time. Consider that most cancer survivors with CVD or cardiometabolic risk factors have multiple providers (e.g., the uterine cancer survivor who receives treatment from an oncologist, gynecologist, and primary care provider; PCP). Both patients and providers function in a complex system that entails some ambiguities regarding which medical provider is responsible for which aspects of disease management and health promotion and how cross-specialty communication is expected to occur (Mayer, Nasso, & Earp, 2017; Mitchell, Burridge, Colquist, & Love, 2012). Figure 1 presents a schema, adapted from Leventhal’s Common Sense Model of Illness (Leventhal et al., 1997), depicting how cancer survivors and their multiple providers appear to cognitively represent: the main health threats survivors face, preventable causes of adverse health events, and optimal coping plans.
Figure 1.
Cancer survivors’ and their care providers’ common sense cognitive models of health threat, preventable cause of recurrence, and coping action needed to prevent adverse health outcomes. Red (italics) signifies a systemic gap in role or cognitive representation needed to comprehensively address the threat. Adapted from (Leventhal et al., 1997).
Most individuals who have survived cancer fear a recurrence or a new cancer (Cancer Council Australia, 2018), positioning cancer as their dominant health threat (cf., Figure 1). As the figure also indicates, a majority of survivors would prefer to receive follow-up care from their oncologist, believing that the best way to prevent their most-feared adverse health outcome is to follow the anti-cancer regimen the oncologist prescribes (Costanzo, Lutgendorf & Roeder, 2011; Hudson, Miller, Hemler, Ferrante et al, 2012). Although common cardiometabolic risk factors or co-occurring CVD have become leading causes of death in localized breast cancer (Park et al., 2017) and prostate cancer (Ketchandji, Kuo, Shahinian, & Goodwin, 2009), many cancer survivors view multimorbidities, including type 2 diabetes and CVD, as peripheral to their day-to-day self-management and survival (Shin et al., 2011) (cf., Fig 1’s absence of this perceived threat for survivors). Cancer also trumps CVD and cardiometabolic risk factors as the top-of-mind threat for the patient’s oncologist and medical specialist, perhaps since during active treatment they focused on controlling a cancer to keep the individual alive (Stump et al., 2019). Cardiometabolic risk factors are more likely to be identified as threats and treated pharmacologically by the PCP (cf., Fig. 1), for whom such medications are among the most commonly prescribed (Bean, 2017). Modifications of the patient’s unhealthy lifestyle behaviors that conveyed cancer and cardiovascular disease risks in the first place are also needed to promote long-term health. These unhealthy lifestyle behaviors continue to augment risks of disease progression and new disease onset in the future if left unchanged. Although guidelines advise all physician groups to recommend lifestyle counseling for cancer survivors (NCCN, 2018), the expectation to perform brief health behavior change intervention is greatest for PCPs (Curry, Grossman, Whitlock, & Cantu, 2014), albeit not routinely prioritized (Sabatino et al., 2007).
Ideally, providers’ cognitive representations of patients’ main health threats and needs would align or complement each other, assuring the delivery of comprehensive care. In reality, integrated medical management of multiple conditions has just progressed to the point of recognizing cancer survivors’ need for such services (Sogaard, Thomsen, Bossen, Sorensen, & Norgaard, 2013). Despite evidence that lifestyle interventions could play a critical role in preventing both cancer and CVD (White et al., 2014), healthy lifestyle promotion lacks a champion in current models of integrated care and receives low priority in providers’ and patients’ mental models of prevention tactics (Rabin & Pinto, 2006).
Coping with Survivorship from a Patient Perspective: Managing Complex Medical Regimen Adherence
Ongoing adjuvant treatment is a required part of the treatment regimen for many survivors of prevalent cancers (e.g., breast, prostate), as is regular self-examination to monitor for recurrence (e.g., melanoma, breast). Given the severity of the illness threat, it is common sense for patients to highly prioritize adherence to their anti-cancer regimen. Hormone therapies such as aromatase inhibitors and tamoxifen for breast cancer survivors, and androgen-blocking medications for prostate cancer survivors, reduce the likelihood of cancer recurrence (Schiavon & Smith, 2014; Schulman, Irani, & Aapro, 2012). Even so, among breast cancer survivors, a recent systematic review found that 41–72% of patients were not adherent to the dose or schedule for hormone therapy, and 31–73% of patients discontinued taking these medications after five years (Murphy, Bartholomew, Carpentier, Bluethmann, & Vernon, 2012). For breast cancer survivors prescribed ongoing hormone therapies, nonadherence is associated with being older (or very young), having high out-of-pocket costs, receiving follow-up care through a PCP, experiencing side effects, and having a medication switched (Murphy et al., 2012). For prostate cancer survivors, findings regarding adherence to androgen deprivation therapy are mixed (Aliberti et al., 2017; Franck Lissbrant et al., 2018), but generally among older adults on complex medication regimens, nonadherence can approach 80% (Schlenk, Dunbar-Jacob, & Engberg, 2004).
To anti-cancer hormone therapies, the survivor with multimorbidity adds prescribed pharmacotherapies to manage CVD or cardiometabolic risk factors and prevent disease progression. Given evidence that survivors regard CVD as a less salient threat than cancer (Shin et al., 2011), one might expect poorer adherence to the CVD than the cancer medical regimen. Anecdotally, patients undergoing cancer treatment are thought to perform less daily self-management of chronic diseases such as diabetes and hypertension, although data examining this proposition are scarce. Hypertension (HTN) is the most common comorbid condition in cancer, and antihypertensive medication regimens can exacerbate the burden of cancer-related fatigue. Nevertheless, studies addressing management of antihypertensive regimens in cancer survivors are very limited (Braithwaite et al., 2009). One cross-sectional study found cancer survivors more likely than those without a history of cancer to report optimal medication adherence and blood pressure (BP) monitoring (Shin et al., 2011). On the other hand, studies evaluating long-term adherence to HTN management report less than optimal rates ranging from 50–65%: low enough to compromise treatment efficacy and health-related quality of life (Morgado & Rolo, 2012). In sum, though chronic maintenance medications are the standard of care for managing both cancer and cardiovascular risks, long-term adherence is problematic for either regimen singly and is insufficiently characterized (but probably not better) for the polypharmacy needed to manage both conditions.
Many cancer survivors will have progressed beyond risk factors to have overt CVD or Type 2 diabetes. Up to half of CVD patients are nonadherent to taking medication as directed (Ho, Bryson, & Rumsfeld, 2009). Similarly, less than half of those prescribed medication for the treatment of diabetes, HTN, or hyperlipidemia persist in using the medication through the first year (Brookhart et al., 2007; Vrijens, Vincze, Kristanto, Urquhart, & Burnier, 2008). Nonadherence to CVD is associated with being younger, nonwhite, and depressed. Nonadherence also is more prevalent when a condition is chronic or asymptomatic (Ho et al., 2009). Regimen complexity is an additional predictor of nonadherence, and many patients with cardiometabolic conditions take more than one medication (Grant, Devita, Singer, & Meigs, 2003); yet few studies have assessed adherence for more than one medication at a time. In polypharmacy, as for simpler regimens, patients are less likely to be adherent to taking a particular medication if they do not perceive its immediate benefit or believe it is causing side effects.
Survivors who have Type 2 diabetes (T2DM) face an especially complex added regimen burden, involving not only medication, but also dietary requirements, glucose monitoring and checking feet for irritation or ulcers. Some evidence suggests that providing T2DM education to cancer patients increases patient compliance with multiple HbA1c tests, and results in fewer emergency room visits and hospital admissions (Irizarry et al., 2013). Notably, management of the comorbid cancer and T2DM thrusts survivors and providers into the challenge of communicating across medical fields. Usual “one-disease-at-a-time” approaches to T2DM and to cancer management do very little to address these needs for discourse among health care providers.
Health Promotion: Managing Multiple Healthy Lifestyle Changes
One set of potentially controllable cancer causes - health risk behaviors - has no provider category principally dedicated to its treatment or prevention. Health threats faced by many cancer survivors are partially attributable to unhealthy lifestyle behaviors (e.g., tobacco use, poor-quality diet, excess energy and alcohol intakes, physical inactivity). These behaviors convey risk for both cancer and CVD and, once the patient has acquired chronic disease, often co-occur with medication nonadherence (Fine, Philogene, Gramling, Coups, & Sinha, 2004; Pampel, Krueger, & Denney, 2010; B. Spring, Moller, & Coons, 2012). Risk behaviors tend to cluster, with 52% of adults reporting at least two of four unhealthy lifestyle habits (physical inactivity, overweight, cigarette smoking, risky drinking; (Coups, Gaba, & Orleans, 2004), and 17% reporting three or more (Fine et al., 2004).
One suggestion has been that receiving a cancer diagnosis creates a “teachable moment” that makes mortality feel close at hand, heightening motivation to change habits that could otherwise jeopardize survival (Demark-Wahnefried, Aziz, Rowland, & Pinto, 2005). However, others have questioned the efficacy and even the ethics of uniformly implementing vigorous behavior change intervention at the time of cancer diagnosis, arguing that some vulnerable patients may experience adverse effects as a result of feeling blamed and personally responsible for causing their cancer (Stiefel & Bourquin, 2018). Some evidence suggests that a meaningful subgroup of survivors does make healthy lifestyle changes, such as improving diet quality and quitting smoking after receiving a cancer diagnosis (S. M. Bluethmann et al., 2015; Sprague, Trentham-Dietz, Nichols, Hampton, & Newcomb, 2010). However, other cancer survivors continue to exhibit chronic disease risk behaviors, particularly poor quality diet, obesity, and physical inactivity (Blanchard, Courneya, & Stein, 2008; S. M. Bluethmann et al., 2015; Sprague et al., 2010).
Our model (Figure 1) depicts how health behaviors can fail to appear in survivors’ cognitive maps of health threats. Providers who are primarily focused on addressing the cancer threat may only discuss anti-cancer medications, not pointing the patient’s attention to CVD or lifestyle-related risks. Indeed, many survivors become more overweight and physically inactive as their time since initial diagnosis increases, particularly if emerging functional impairments impede the ability to be physically active. Relatedly, as of 2016, 13% of cancer survivors over the age of 18 still smoke even though it is now known that smoking increases the risk of developing a subsequent cancer and that quitting smoking lowers that risk (Miele et al., 2018). The influences that bind many individuals to unhealthy lifestyle habits are strong. For many, smoking and overeating have become one of few reliable, accessible means to engender immediate, short-term pleasure and distress relief, especially in life contexts that supply many burdens but few rewards (B. Spring, Cook, et al., 2008; B. Spring, Pingitore, & McChargue, 2003; B. Spring, Schneider, et al., 2008). Although brief counseling from PCPs can modestly improve patients’ health risk behaviors (Quinn et al., 2009), it is often crowded out by acute care needs. Health psychologists and other allied health professionals (nutritionists, kinesiologists, health educators) who could bring lifestyle risk factors into the patient’s focus are not routinely on the patient’s health care path.
Managing Life Demands
Managing comorbidities combined with ongoing life demands creates considerable stressful burden for many cancer survivors (Danese, O’Malley, Lindquist, Gleeson, & Griffiths, 2012; Wykle, Kahana, & Kowal, 1992). Cancer treatments can cause chronic side effects including appetite and gastrointestinal disturbance, bruising, sexual dysfunction, and pain (Skolarus et al., 2014). Because cancer occurs primarily in the elderly (age > 65), other stressors such as retirement, isolation, loss of independence, loss of productivity, caregiving burdens and comorbidities can further compromise quality of life (QOL) (Wykle et al., 1992). Negative emotional sequelae add to the residual adverse physical effects of cancer and its treatments. Some studies show more than 70% of cancer survivors report fear of recurrence at a level of intensity warranting clinical intervention (Bell et al., 2017; Costa, Dieng, Cust, Butow, & Kasparian, 2016). Moreover, a symptom cluster of depression, fatigue, sleep disturbances, and cognitive dysfunction is prevalent among cancer patients and survivors, reflecting either adverse mental health consequences of having cancer, or direct neuroendocrine-immunologic effects of the disease and its treatments (Miller, Ancoli-Israel, Bower, Capuron, & Irwin, 2008). Improved QOL is therefore an important clinical outcome for cancer survivors and often a key treatment focus of work with professionals such as health psychologists, when available. Not only is engaging in healthy behaviors associated with better QOL in cancer survivors (Blanchard et al., 2008), but improvements such as increasing physical activity, can produce improvements in QOL (Buffart et al., 2017).
Coping with Survivorship from a Physician Perspective
Managing Health Risks
The physician’s role, like the patient’s, is complex and demanding. Above all else, physicians embody primary responsibility to mitigate health risks (cf., Figure 1). However, that main mission must be accomplished while following practice guidelines (that sometimes conflict), staying up to date on new research findings, managing patient flow, maintaining a positive financial balance sheet for providing care, advocating to insurers to cover the treatments patients need, and coordinating care with other healthcare providers. For oncologists and other specialists treating cancer patients, challenges can be exacerbated by needing to address multimorbidities and provide advice in unfamiliar areas (i.e., health behavior change), while prioritizing specialty-consistent attention to rapidly evolving findings about the therapeutic efficacy and side-effects of immunotherapy and targeted therapies.
Medical training emphasizes the diagnosis and treatment of disease. Clinical guidelines focus on treating one disease at a time and give physicians very little information about how to address several conditions simultaneously. Few guidelines for common conditions provide specific treatment recommendations for managing comorbidities (Lugtenberg, Burgers, Clancy, Westert, & Schneider, 2011). This lack of guidance results from a sparse evidence base concerning clinical co-management of chronic disease, because patients with comorbidities are typically excluded from clinical trials and, even when included, comorbidities are rarely analyzed as potential moderators of treatment effects (Boyd, Vollenweider, & Puhan, 2012). Despite recent calls to update clinical guidelines to reflect the complexities of treating comorbidities (Arnett et al., 2014; Uhlig et al., 2014), the additional data collection needed and translation of research findings to practice probably will require more than a decade to accomplish (Morris, Wooding, & Grant, 2011). Meanwhile, lack of clear guidance regarding care of comorbid conditions, such as cancer and CVD, can create ambiguous or competing clinical foci (cf. Figure 1) that yield insufficient care coordination and delivery (Burgers, Voerman, Grol, Faber, & Schneider, 2010; Francke, Smit, de Veer, & Mistiaen, 2008).
Even though current guidance advises all physicians to provide health promotion counseling (2018 Physical Activity Guidelines Advisory Committee, 2018; Jensen et al., 2013; US Department of Health and Human Services, Office of Disease Prevention and Health Promotion, US Department of Health and Human Services, & Office of Disease Prevention and Health Promotion, 2010). The assignment may be most unfamiliar for oncologists. Uniquely skilled at delivering care aimed at controlling life-threatening cancers, may become the physician that survivors rely upon for all medical care. (Hudson et al., 2012; Wallner et al., 2017).. Uniquely skilled at delivering care aimed at controlling life-threatening cancers, many oncologists were trained to believe that the task of promoting a healthy lifestyle is the responsibility of PCPs, whereas they are unfamiliar and uncomfortable with undertaking healthy lifestyle promotion. Hence, patients who choose oncologists as their sole physician will receive limited healthy lifestyle guidance, potentially augmenting disparities, since this subset of survivors overrepresents minorities and those with lower education (Hudson et al., 2012; Wallner et al., 2017). In actuality, few physicians, particularly oncologists and other specialists, have had sufficient positive clinical experiences to build confidence that they can help patients succeed at behavior change; most report a need for additional training in health promotion counseling (Anderson, Caswell, Wells, & Steele, 2013).
According to cancer survivors, neither oncologists nor PCPs reliably discuss diet (30% of cancer survivors) or exercise (26%), and 70% of melanoma survivors report that their physician did not address their anxiety about cancer recurrence (Sabatino et al., 2007). Most physicians report lack of time, expertise, and/or inclination to counsel about nutrition. Referral to a dietician may be feasible if the patient has insurance coverage and an eligible comorbid disease (e.g., T2DM) or personal resources to pay for the added service. Moreover, although some behavior change programs such as intensive, multi-session behavioral weight loss treatment are now covered by Centers for Medicare and Medicaid (CMS), treatment must be provided by physicians or nurse practitioners and is ineligible for reimbursement if provided by health psychologists, nutritionists, or exercise scientists, who have greater training and interest in providing lifestyle intervention. In other words, health promotion is frequently omitted from any medical visits due to significant system burdens and diffusion of responsibility across providers.
Coordinating Care
PCPs traditionally act as the point person for the patient’s health care team. They coordinate care, maintain a long-term relationship with the patient, and help reconcile conflicting recommendations received from medical specialists focusing primarily on one disease. As such, primary care settings have become a pivotal hub for cancer survivors as they complete their oncologic treatment. At the end of active cancer treatment, the oncologist creates a survivorship plan (i.e., a summary of treatment and recommended follow-up care) and transfers the patient to a PCP for continuing care (Hewitt et al., 2005). This “hand-off” can work well when patients are willing to separate from the oncologist, when PCPs are readily available, and particularly when the PCP has a good working relationship with the treating oncologist (Barnett, Keating, Christakis, O’Malley, & Landon, 2012); (O’Malley, Davis, Crabtree, & Hudson, 2017). Such conditions are not always in place and many PCPs express frustration about informational gaps and misaligned expectations that impede their clinical decision-making (O’Malley et al., 2017). Implementation of the transfer of care is still a work in progress, with different oncologists and PCPs expressing varying opinions about whether and how responsibility for survivorship care can be shared (Hudson et al., 2012; Nekhlyudov, O’Malley D, & Hudson, 2017; Potosky et al., 2011).
For example, even the apparently simple task of reconciling the patient’s medication regimen illustrates a care coordination challenge. Medication lists in the electronic medical record (EMR) are frequently inaccurate: one study found inaccuracies for 71% of patients (DeCarolis, Leraas, & Rowley, 2005). Incompleteness is one problem. Approximately 25% of prescribed drugs being taken by patients are omitted, such that 61% of patients are taking at least one medication not noted in the EMR (Lau, Florax, Porsius, & De Boer, 2000). Another key problem is polypharmacy, such that, particularly for older adults, the number of drugs prescribed by different providers grows longer, the pill-taking regimen more complex, new prescriptions added to address side effects of prior prescriptions, and the possibility of interactions among drugs and nutraceuticals increasingly nontrivial (Rambhade, Chakarborty, Shrivastava, Patil, & Rambhade, 2012).
Managing Work Demands
With the proportion of Americans who are 65 years and older projected to increase from 12% in 2005 to 20% by 2030 (Institute of Medicine, 2008), and the total number of cancer cases projected to increase by 45% in the same time frame (B. D. Smith, G. L. Smith, A. Hurria, G. N. Hortobagyi, & T. A. Buchholz, 2009), more cancer survivors will continue to need post-cancer care, placing a greater burden on PCPs. By 2025, the United States is expected to face a shortage of 25,250 PCPs, and the shortage may be even greater (Dall, West, Ritashree, Reynolds, & Iacobucci, 2018). In the US, PCPs account for about 30% of all physicians; most other nations have a more desirable 50% ratio. Bringing the US into line with other nations would require approximately 150,000 more PCPs (Carrier, Yee, & Stark, 2011).
The average PCP has a patient panel of 2500 patients. The estimated time required per day to meet clinical guideline recommendations for this panel is: 3.7 hrs. for acute needs, 10.6 hrs. for chronic needs and 7.4 hrs. for preventive services (Yarnall et al., 2009), totaling an untenable 21.7 hrs per day. Given the shortening of visits, made necessary to meet the needs of increasing caseloads, it is difficult to discern how the PCP can find time to discuss comorbidity management or healthy lifestyle change.
System Level Considerations and Recommendations
We have argued that optimal care of cancer survivors is impeded by insufficient coordination of care, complicated by ambiguous guidance regarding comorbidity management, made worse by an insufficient PCP workforce with an untenable increase in responsibilities, and a resulting neglect of the need to promote longevity and QOL by modifying habitual unhealthy lifestyle patterns. Further, because health promotion tends to be perceived as non-urgent, the service is likely to be dropped when time runs short in a treatment visit for a survivor with multimorbidities (Tai-Seale, McGuire, & Zhang, 2007).
Although significant, these challenges can be overcome by system re-engineering to redistribute workload (particularly, primary responsibility for health promotion) and make more effective use of digital tools that are already available to the healthcare system. Psychologists are well-advised to gain appointment to care coordination committees that design workflow infrastructure for their health care setting to ensure that provision of behavioral and psychosocial services are represented on care pathways.
Care Coordination and Behavioral Vital Sign Documentation via the EMR
Figure 2 illustrates such a system hypothetically re-engineered to deliver connected care for cancer survivors. Two technological components (the EMR and consumer digital tracking tools) are central to the proposed care management infrastructure. Different providers’ EMRs will soon achieve universal interoperability, enabling oncologists, specialist physicians, and PCPs to access each other’s medical records about a particular patient, greatly facilitating care coordination (“Carequality,” 2016; Well, 2016; “What’s the Difference,” 2018). As the bidirectional arrows in Figure 2 indicate, each category of provider will be able to both enter and access a patient’s health data via the EMR, thus updating the PCP on any cancer-related procedures and reminding oncologists and specialists about the patient’s comorbidities.
Figure 2.
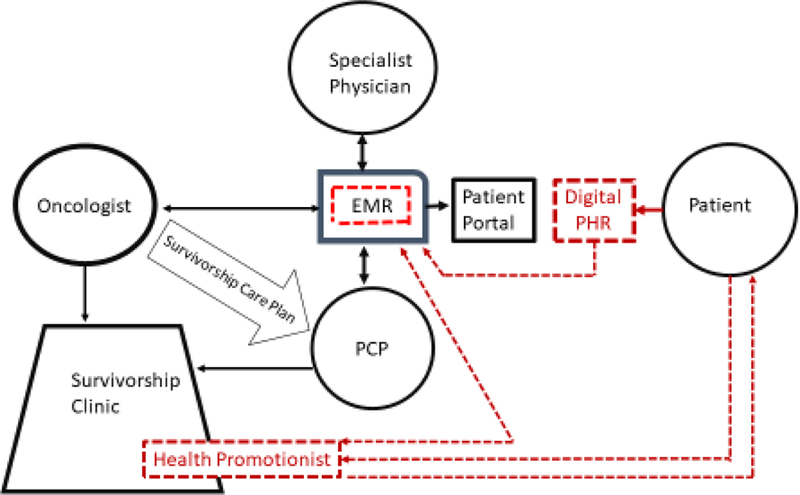
Infrastructure design for a re-engineered workflow that distributes major responsibility for healthy lifestyle change intervention to health promotionists (HP). Black lines signify existing infrastructure paths; red (dotted) lines signify paths that need to be built. Providers enter behavioral vital sign data for smoking, obesity, and physical inactivity into the EMR, where data are analyzed, automatically alerting the HP of patients who display risk behaviors. HP contacts primary provider through EMR asking about contraindications to health promotion intervention and, if none, outreaches to patient. Interested patients use their existing apps and consumer wearables to transmit behavioral self-monitoring data to HP and digital personal health record (PHR). Data summary appears in HP’s dashboard and EMR, enabling HP to coach patient remotely and interested providers to monitor patient’s progress toward behavioral goals. HP submits written progress reports to EMR summarizing patient’s healthy lifestyle progression.
In addition to supporting care coordination among providers, the EMR reduces the physician’s cognitive load by triggering alerts to perform many evidence-based protocols. One set of alerts, triggered at every visit, prompts providers to assess vital signs (e.g., BP, temperature). Even though physicians complain of “alert fatigue” and may ignore or override these prompts (Ancker et al., 2017), alerts have become standard, accepted EMR decision support. We propose that prompting EMR recording of the main chronic disease risk behaviors as vital signs is an essential system modification to support health promotion. The Institute of Medicine has recommended integration of behavioral vital signs (Institute of Medicine, Board on Population Health and Public Health Practice, & Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records, 2015). EMR alerts to document smoking as a vital sign have gotten greatest traction (Fiore & Baker, 2011), but prompts to document physical inactivity, obesity, diet quality, substance use, and distress/depression as vital signs also have seen some uptake (Coleman et al., 2012; Dwinnells, 2015; Steglitz et al., 2015).
In fact, we argue that the medical system’s track record of systematically promoting healthy lifestyle behaviors is so poor, and the curve of continually worsening U.S. population health so ominous, that a more stringent tactic warrants consideration. For the three behavioral vital signs – smoking, obesity, and physical inactivity – that are major preventable causes of cancers and CVD, we recommend insertion of a stop code requiring data entry before the provider can move further in the EMR for a patient visit. Despite physician dislike of stop codes, system administrators have allowed them for items like distress screening as mandated by American College of Surgeons (ACS) hospital accreditation requirements (Lazenby, Tan, Pasacreta, Ercolano, & McCorkle, 2015). We recommend adding the three main behavioral vital signs to be queried during the stop for required distress screening.
Additionally, we propose that vital sign entry indicating the presence of a risk behavior automatically trigger proactive outreach from a health promotionist. The initiating pathway from the EMR to the health promotionist appears in red in Figure 2 to represent a new bidirectional digital connective path that needs to be built in the re-engineered care system. Initial outreach via the EMR to the patient’s physician would be made immediately, requesting any contraindications to engaging the patient in health promotion intervention. Receiving no objections, the health promotionist would contact the patient digitally through the patient portal, asking about interest in receiving health promotion services. By changing the default to automated proactive outreach to patients at risk, rather than reactive outreach to the few patients referred by overburdened physicians, these system changes would take a step toward providing population level health promotion that reaches more cancer survivors. We find mandatory behavioral vital sign entry facilitated by stop codes to be warranted in an effort to finally move our health care system beyond the paradox that commitment to prevention is “celebrated in principle but resisted in practice “ (Fineberg, 2013).
Health Promotionist
The proposed health promotionist role is a new category of health care position dedicated solely to the neglected priority of helping patients improve chronic disease risk behaviors. The training background is similar to that of a health/wellness coach (Bachelors degree in behavioral or social science, communication, health education, exercise science, dietetics or equivalent lived community-based experience), but with added experience in using digital technologies, such as the EMR, mobile and internet apps, and wearable sensors. In particular, health promotionists maintain current knowledge of behavior change tools and devices, giving them an ability to provide tailored recommendations to patients and providers. Their skills and training match those of the coaches deployed in effective technology-supported behavior change interventions (B. Spring et al., 2012; Bonnie Spring et al., 2018). Health promotionists are trained and supervised by one or more licensed masters or doctoral level health professionals (in psychology, social work, kinesiology, nutrition, or public health). This, in turn, enables supervisors and health promotionists to be integrated into clinics with physician oversight, which qualifies them as physician extenders, allowing reimbursement for intensive lifestyle interventions that are covered by CMS, but not feasibly implemented within the care system as now designed.
In the hypothetical system shown in Figure 2, health promotionists are housed in a survivorship clinic led by a physician or psychologist and staffed by at least one nurse, social worker, exercise specialist, and nutritionist. The clinic accepts cancer survivors referred directly by an oncologist’s survivorship care plan, and those referred to primary care but who prove to need added behavioral or psychosocial services. Health promotionists are housed in the survivorship clinic to situate them among other allied health care staff, but their patient referrals come via direct automated vital sign notifications through the EMR. As seen in Figure 2, health promotionists first make digital outreach via the patient portal (patient-facing extension of the EMR) to engage those whose vital signs show behavioral risk factors. Then they coach interested patients remotely by telephone, text, or e-mail on the basis of behavioral self-tracking data the patient collects via a smartphone application with or without digitally connected wearable sensors.
Consumer Digital Tools
To exercise self-determination and autonomous motivation (Ryan & Deci, 2000), the survivor must be at the center of his or her own pursuit of a healthy lifestyle and quality of life. Developments in consumer technology enable the individual to be aided by a suite of readily available technology-supported behavioral intervention programs accessible via the internet or as native apps coupled with wearable sensors whose output is accessible via portable mobile devices. Often using self-administered consumer technologies will be sufficient for patients to accomplish health behavior change (Burke et al., 2015; Leahey et al., 2014). In the connected care system shown in Figure 2, those who need more support and accountability can be aided by a health promotionist who monitors the patient’s digital self-monitoring data, using it to coach in a tailored, personalized manner (B. Spring et al., 2012; Bonnie Spring et al., 2018). Note that Figure 2 depicts patients’ digital data as being uploaded into a personal health record (PHR), a system where patients can enter or upload their own data and control with whom they share it (e.g., providers, family members, coach). The PHR differs from the EMR wherein patients can see but not modify some of their own health data. In the proposed system, the PHR functions similarly to the coach dashboard in hybrid technology- plus coach-supported behavior change interventions, enabling both patient and coach to view a synthesized representation of the digital tracking data that depicts progress toward goals. As Figure 2 indicates, functionality exists to import such data synopses into the EMR. However, given both technical challenges and providers’ fear of being overwhelmed by such data (Genes et al., 2018; Quinn et al., 2009), we expect the more common communication to providers to be pithy EMR progress reports entered by the health promotionist.
Our proposed model differs from current models of integrated care (Anastas et al., 2018; Miller-Matero et al., 2016), which refer to co-location, consultation, or referral relationships between primary care practices and psychologists chiefly to address patients’ mental health needs. No resource comparable to the health promotionist, solely dedicated to treatment of chronic disease risk behaviors (smoking, obesity, physical inactivity, treatment nonadherence) exists in current care models. Integrating health promotionists into care delivery channels would remove burden from overtaxed PCPs, reduce very high loss to follow-up that occurs with referral to community health promotion (Anastas et al., 2018; Leppin et al., 2018), and instantiate a comprehensive, actionable systems-level schema of behavioral strategies needed to have preventive impact with minimal interference to clinical work flow. The proposed system is designed to allow all health professionals, including health psychologists, to function to the top of their licensure. Supervising paraprofessionals expands the health psychologist’s ability to reach more of the population in need of health behavior change, while participating in care system design and still calling upon the psychologist to do direct service intervention with patients who have more complex needs or comorbidities. By using the patient’s digital tracking data to coach remotely and periodically report progress to providers, the health promotionist closes a gap, creating a connected care system that supports, reinforces, and maintains accountability for healthy lifestyle improvement.
Leveraging Technology and Health Promotionists
Such systems re-design would be disruptive in a positive way. The proposed re-engineering would require multiple behavior changes from patients, providers, and system administrators as well as engagement from these multiple stakeholders. These adjustments would be asked in the service of reallocating untenable PCP workloads to reduce overburden. Excess burden would be lowered via workflow efficiencies achieved by introducing a new category of worker (health promotionist) who enables more providers to work at the top of their licensed capabilities. Health promotionists would simultaneously provide as needed support for the patient and maintain connection with concerned providers. Re-engineering would place the cancer survivor squarely at the center of efforts to extend and improve QOL via creative use of consumer-facing technologies and outreach to receive behavioral services.
Conclusion
The Institute of Medicine called for lifestyle recommendations to be included as a standard part of the cancer survivorship care plan to optimize health and well-being (Hewitt et al., 2005; Page & Adler, 2008). The hurdles for optimizing health for cancer survivors are real, but solvable with available technology and adaptation of existing evidence based behavioral interventions. An online, collaborative connected-health delivery model that uses technology to deliver behavioral interventions may further extend life and result in improvements in the QOL of cancer survivors. This strategy will foster cross-specialist communication and deliver behavioral interventions to cancer survivors who want to improve their health, preventing functional decline and new comorbidities (Lustberg, Reinbolt, & Shapiro, 2012; Rock et al., 2012). Patients will get predictable and comprehensive support to achieve a healthier lifestyle, and health care providers will have more time to do what they are most skilled at doing: making a diagnosis, deciding upon treatment with patients and families, and responding to questions that cancer survivors raise about managing multimorbidities. Given the excellent cancer-specific prognoses experienced by survivors of many cancers and the need to co-manage medical regimens to prevent cancer and CVD, redesigning care path infrastructure to include the behavioral services needed to reduce smoking, obesity, and physical inactivity could positively impact survival and QOL in the years after cancer diagnosis.
Acknowledgments
Preparation of this paper was supported in part by NIH P30CA60553 for the Robert Lurie Comprehensive Cancer Center, by T32 CA193193 (PIs Spring and Penedo, providing salary support for TS) and by a Northwestern University I3 support grant to Spring, Robinson, and Penedo. The authors report no financial disclosures or conflicts of interest. They express thanks to Drs. Katrina Champion, Siobhan Phillips, Laura Finch, and Annie Lin for helpful feedback about the manuscript.
References
- 2018 Physical Activity Guidelines Advisory Committee. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report . Retrieved from: https://health.gov/paguidelines/second-edition/report/
- Aliberti A, Bada M, Rapisarda S, Natoli C, Schips L, & Cindolo L (2017). The adherence to hormonal deprivation therapy for prostate cancer in a real life contest: Retrospective, single-centre study. Clinics in Oncology, 2. [DOI] [PubMed] [Google Scholar]
- Anastas T, Waddell EN, Howk S, Remiker M, Horton-Dunbar G, & Fagnan L (2018). Building behavioral health homes: Clinician and staff perspectives on creating integrated care teams. The Journal of Behavioral Health Services & Research, 1–12. doi: 10.1007/s11414-018-9622-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, & Kaushal R (2017). Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak, 17(1), 36. doi: 10.1186/s12911-017-0430-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AS, Caswell S, Wells M, & Steele RJ (2013). Obesity and lifestyle advice in colorectal cancer survivors - How well are clinicians prepared? Colorectal Dis, 15(8), 949–957. doi: 10.1111/codi.12203 [DOI] [PubMed] [Google Scholar]
- Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, & Zoghbi WA (2014). AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions. From the American Heart Association, American College of Cardiology, and US Department of Health and Human Services, 130(18), 1662–1667. doi: 10.1161/cir.0000000000000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ML, Keating NL, Christakis NA, O’Malley AJ, & Landon BE (2012). Reasons for choice of referral physician among primary care and specialist physicians. J Gen Intern Med, 27(5), 506–512. doi: 10.1007/s11606-011-1861-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean M (2017). 10 most popular prescription drugs for 2017 Retrieved from https://www.beckershospitalreview.com/supply-chain/10-most-popular-prescription-drugs-for-2017.html
- Bell KJL, Mehta Y, Turner RM, Morton RL, Dieng M, Saw R, … Webster AC (2017). Fear of new or recurrent melanoma after treatment for localised melanoma. Psychooncology, 26(11), 1784–1791.doi: 10.1002/pon.4366 [DOI] [PubMed] [Google Scholar]
- Blanchard CM, Courneya KS, & Stein K (2008). Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol, 26(13), 2198–2204. doi: 10.1200/jco.2007.14.6217 [DOI] [PubMed] [Google Scholar]
- Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, … Demark-Wahnefried W (2015). Grasping the ‘teachable moment’: time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology doi: 10.1002/pon.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluethmann SM, Mariotto AB, & Rowland JH (2016). Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology Biomarkers & Prevention, 25(7), 1029–1036. doi: 10.1158/1055-9965.epi-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd CM, Vollenweider D, & Puhan MA (2012). Informing evidence-based decision-making for patients with comorbidity: Availability of necessary information in clinical trials for chronic diseases. PLOS ONE, 7(8), e41601. doi: 10.1371/journal.pone.0041601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, … Esserman L (2009). Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer, 124(5), 1213–1219. doi: 10.1002/ijc.24054 [DOI] [PubMed] [Google Scholar]
- Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, … Solomon DH (2007). Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med, 167(8), 847–852. doi: 10.1001/archinte.167.8.847 [DOI] [PubMed] [Google Scholar]
- Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, … Brug J (2017). Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev, 52, 91–104. doi: 10.1016/j.ctrv.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Burgers JS, Voerman GE, Grol R, Faber MJ, & Schneider EC (2010). Quality and coordination of care for patients with multiple conditions: Results from an international survey of patient experience. Eval Health Prof, 33(3), 343–364. doi: 10.1177/0163278710375695 [DOI] [PubMed] [Google Scholar]
- Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, … Quinn CC (2015). Current science on consumer use of mobile health for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation, 132(12), 1157–1213. doi: 10.1161/cir.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Council Australia. (2018). Living well after cancer: Cancer Council Australia
- Carequality and CommonWell Health Alliance Agree on Connectivity and Collaboration to Advance Interoperability. Retrieved from https://www.commonwellalliance.org/news-center/commonwell-news/carequality-commonwell-health-alliance-collaboration.
- Carrier ER, Yee T, & Stark L (2011). Matching supply to demand: Addressing the US primary care workforce shortage. Looking Ahead, 5(4). [Google Scholar]
- Centers for Disease Control and Prevention. Table 19. Leading causes of death and numbers of deaths, by sex, race, and Hispanic origin: United States, 1980 and 2014. In. Health, United States, 2015.
- Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, … Sallis RE (2012). Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc, 44(11), 2071–2076. doi: 10.1249/MSS.0b013e3182630ec1 [DOI] [PubMed] [Google Scholar]
- Costa DS, Dieng M, Cust AE, Butow PN, & Kasparian NA (2016). Psychometric properties of the Fear of Cancer Recurrence Inventory: An item response theory approach. Psychooncology, 25(7), 832–838. doi: 10.1002/pon.4018 [DOI] [PubMed] [Google Scholar]
- Coups EJ, Gaba A, & Orleans CT (2004). Physician screening for multiple behavioral health risk factors. Am J Prev Med, 27(2 Suppl), 34–41. doi: 10.1016/j.amepre.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Curry SJ, Grossman DC, Whitlock EP, & Cantu A (2014). Behavioral counseling research and evidence-based practice recommendations: U.S. Preventive Services Task Force perspectives. Ann Intern Med, 160(6), 407–413. doi: 10.7326/M13-2128 [DOI] [PubMed] [Google Scholar]
- Dall T, West T, Ritashree C, Reynolds R, & Iacobucci W (2018). The complexities of physician supply and demand: Projections from 2016 to 2030 Washington, DC: Association of American Medical Colleges. [Google Scholar]
- Danese MD, O’Malley C, Lindquist K, Gleeson M, & Griffiths RI (2012). An observational study of the prevalence and incidence of comorbid conditions. Ann Oncol, 23(7), 1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, … Rowland JH (2013). Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev, 22(4), 561–570. doi: 10.1158/1055-9965.EPI-12-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis D, Leraas M, & Rowley C (2005). Medication reconciliation upon admit using an electronic medical record. Paper presented at Pharmacotherapy annual conference. [Google Scholar]
- Demark-Wahnefried W, Aziz NM, Rowland JH, & Pinto BM (2005). Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol, 23(24), 5814–5830. doi: 10.1200/jco.2005.01.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinnells R (2015). SBIRT as a vital sign for behavioral health identification, diagnosis, and referral in community health care. Ann Fam Med, 13(3), 261–263. doi: 10.1370/afm.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer MS, & Ewer SM (2015). Cardiotoxicity of anticancer treatments. Nature Reviews Cardiology, 12(9), 547. [DOI] [PubMed] [Google Scholar]
- Fine LJ, Philogene GS, Gramling R, Coups EJ, & Sinha S (2004). Prevalence of multiple chronic disease risk factors. 2001 National Health Interview Survey. Am J Prev Med, 27(2 Suppl), 18–24. doi: 10.1016/j.amepre.2004.04.017 [DOI] [PubMed] [Google Scholar]
- Fineberg HV (2013). The paradox of disease prevention: Celebrated in principle, resisted in practice. JAMA, 310(1), 85–90. doi: 10.1001/jama.2013.7518 [DOI] [PubMed] [Google Scholar]
- Fiore MC, & Baker TB (2011). Treating smokers in the health care setting. The New England journal of medicine, 365(13), 1222–1231. doi: 10.1056/NEJMcp1101512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck Lissbrant I, Ventimiglia E, Robinson D, Törnblom M, Hjälm-Eriksson M, Lambe M, … Stattin P (2018). Nationwide population-based study on the use of novel antiandrogens in men with prostate cancer in Sweden. Scandinavian Journal of Urology, 1–8. doi: 10.1080/21681805.2018.1426039 [DOI] [PubMed] [Google Scholar]
- Francke AL, Smit MC, de Veer AJ, & Mistiaen P (2008). Factors influencing the implementation of clinical guidelines for health care professionals: A systematic meta-review. BMC Med Inform Decis Mak, 8, 38. doi: 10.1186/1472-6947-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genes N, Violante S, Cetrangol C, Rogers L, Schadt EE, & Chan Y-FY (2018). From smartphone to EHR: A case report on integrating patient-generated health data. npj Digital Medicine, 1(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, Devita NG, Singer DE, & Meigs JB (2003). Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care, 26(5), 1408–1412. [DOI] [PubMed] [Google Scholar]
- Hewitt M, Greenfield S, & Stovall E (2005). From cancer patient to survivor: Lost in transition Institute of Medicine and National Research Council; Washington, DC. [Google Scholar]
- Ho PM, Bryson CL, & Rumsfeld JS (2009). Medication adherence: its importance in cardiovascular outcomes. Circulation, 119(23), 3028–3035. doi: 10.1161/circulationaha.108.768986 [DOI] [PubMed] [Google Scholar]
- Hudson SV, Miller SM, Hemler J, Ferrante JM, Lyle J, Oeffinger KC, & Dipaola RS (2012). Adult cancer survivors discuss follow-up in primary care: ‘Not what i want, but maybe what i need’. Ann Fam Med, 10(5), 418–427. doi: 10.1370/afm.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (2008). Retooling for an Aging America: Building the Health Care Workforce Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Institute of Medicine, Board on Population Health and Public Health Practice, & Committee on the Recommended Social and Behavioral Domains and Measures for Electronic Health Records. (2015). Capturing social and behavioral domains and measures in electronic health records: phase 2: National Academies Press. [PubMed] [Google Scholar]
- Irizarry L, Li QE, Duncan I, Thurston AL, Fitzner KA, Edwards BJ, … McKoy JM (2013). Effects of Cancer Comorbidity on Disease Management: Making the Case for Diabetes Education (A Report from the SOAR Program). Population Health Management, 16(1), 53–57. doi: 10.1089/pop.2012.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, … Yanovski SZ (2013). 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PubMed] [Google Scholar]
- Ketchandji M, Kuo YF, Shahinian VB, & Goodwin JS (2009). Cause of death in older men after the diagnosis of prostate cancer. J Am Geriatr Soc, 57(1), 24–30. doi: 10.1111/j.1532-5415.2008.02091.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene RJ, Prizment AE, Blaes A, & Konety SH (2016). Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation, 133(11), 1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HS, Florax C, Porsius AJ, & De Boer A (2000). The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. British Journal of Clinical Pharmacology, 49(6), 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby M, Tan H, Pasacreta N, Ercolano E, & McCorkle R (2015). The five steps of comprehensive psychosocial distress screening. Curr Oncol Rep, 17(5), 447. doi: 10.1007/s11912-015-0447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahey TM, Thomas G, Fava JL, Subak LL, Schembri M, Krupel K, … Wing RR (2014). Adding evidence-based behavioral weight loss strategies to a statewide wellness campaign: A randomized clinical trial. Am J Public Health, 104(7), 1300–1306. doi: 10.2105/ajph.2014.301870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Benyamini Y, Brownlee S, Diefenbach M, Leventhal EA, Patrick-Miller L, & Robitaille C (1997). Illness representations: Theoretical foundations. In Petrie KJ & Weinman J (Eds.), Perceptions of health an illness: Current research and applications (pp. 19–45). Amsterdam, Netherlands: Harwood Academic Publishers. [Google Scholar]
- Lugtenberg M, Burgers JS, Clancy C, Westert GP, & Schneider EC (2011). Current guidelines have limited applicability to patients with comorbid conditions: A systematic analysis of evidence-based guidelines. PLOS ONE, 6(10), e25987. doi: 10.1371/journal.pone.0025987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustberg MB, Reinbolt RE, & Shapiro CL (2012). Bone health in adult cancer survivorship. Journal of Clinical Oncology, 30(30), 3665–3674. doi: 10.1200/jco.2012.42.2097 [DOI] [PubMed] [Google Scholar]
- Masoudkabir F, Sarrafzadegan N, Gotay C, Ignaszewski A, Krahn AD, Davis MK, … Mani A (2017). Cardiovascular disease and cancer: Evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis, 263, 343–351. doi: 10.1016/j.atherosclerosis.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DK, Nasso SF, & Earp JA (2017). Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol, 18(1), e11–e18. doi: 10.1016/S1470-2045(16)30573-3 [DOI] [PubMed] [Google Scholar]
- Miele A, Thompson M, Jao NC, Kalhan R, Leone F, Hogarth L, … Schnoll R (2018). Cancer patients enrolled in a smoking cessation clinical trial: Characteristics and correlates of smoking rate and nicotine dependence. J Addict, 2018, 2438161. doi: 10.1155/2018/2438161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Matero LR, Dykuis KE, Albujoq K, Martens K, Fuller BS, Robinson V, & Willens DE (2016). Benefits of integrated behavioral health services: The physician perspective. Families, Systems, & Health, 34(1), 51. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, & Irwin MR (2008). Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol, 26(6), 971–982. doi: 10.1200/JCO.2007.10.7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GK, Burridge LH, Colquist SP, & Love A (2012). General practitioners’ perceptions of their role in cancer care and factors which influence this role. Health Soc Care Community, 20(6), 607–616. doi: 10.1111/j.1365-2524.2012.01075.x [DOI] [PubMed] [Google Scholar]
- Morgado M, & Rolo S (2012). Factors influencing medication adherence and hypertension management revisited: Recent insights from cancer survivors. Hypertens Res, 35(9), 894–896. doi: 10.1038/hr.2012.100 [DOI] [PubMed] [Google Scholar]
- Morris ZS, Wooding S, & Grant J (2011). The answer is 17 years, what is the question: Understanding time lags in translational research. Journal of the Royal Society of Medicine, 104(12), 510–520. doi: 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, & Vernon SW (2012). Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat, 134(2), 459–478. doi: 10.1007/s10549-012-2114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekhlyudov L, O’Malley D,M, & Hudson SV (2017). Integrating primary care providers in the care of cancer survivors: Gaps in evidence and future opportunities. Lancet Oncol, 18(1), e30–e38. doi: 10.1016/s1470-2045(16)30570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Davis SN, Crabtree BF, & Hudson SV (2017). Primary care physicians experiences of caring for cancer survivors: Toward developing a primary care-responsive cancer survivorship research agenda. In: American Society of Clinical Oncology
- Page AE, & Adler NE (2008). Cancer care for the whole patient: Meeting psychosocial health needs: National Academies Press. [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, & Denney JT (2010). Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol, 36, 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N-J, Chang Y, Bender C, Conley Y, Chlebowski RT, van Londen G, … Kuller LH (2017). Cardiovascular disease and mortality after breast cancer in postmenopausal women: Results from the Women’s Health Initiative. PloS one, 12(9), e0184174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potosky AL, Han PK, Rowland J, Klabunde CN, Smith T, Aziz N, … Stefanek M (2011). Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med, 26(12), 1403–1410. doi: 10.1007/s11606-011-1808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn VP, Hollis JF, Smith KS, Rigotti NA, Solberg LI, Hu W, & Stevens VJ (2009). Effectiveness of the 5-As tobacco cessation treatments in nine HMOs. J Gen Intern Med, 24(2), 149–154. doi: 10.1007/s11606-008-0865-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin C, & Pinto B (2006). Cancer-related beliefs and health behavior change among breast cancer survivors and their first-degree relatives. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer, 15(8), 701–712. [DOI] [PubMed] [Google Scholar]
- Rambhade S, Chakarborty A, Shrivastava A, Patil UK, & Rambhade A (2012). A survey on polypharmacy and use of inappropriate medications. Toxicology international, 19(1), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, … Gansler T (2012). Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin, 62(4), 243–274. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- Rowland JH, & Yancik R (2006). Cancer Survivorship: The Interface of Aging, Comorbidity, and Quality Care. JNCI: Journal of the National Cancer Institute, 98(8), 504–505. doi: 10.1093/jnci/djj154 [DOI] [PubMed] [Google Scholar]
- Ryan RM, & Deci EL (2000). Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American psychologist, 55(1), 68. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, & Zauderer LJ (2007). Provider counseling about health behaviors among cancer survivors in the United States. Journal of Clinical Oncology, 25(15), 2100–2106. doi: 10.1200/jco.2006.06.6340 [DOI] [PubMed] [Google Scholar]
- Schiavon G, & Smith IE (2014). Status of adjuvant endocrine therapy for breast cancer. Breast Cancer Research, 16(2), 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk EA, Dunbar-Jacob J, & Engberg S (2004). Medication non-adherence among older adults: a review of strategies and interventions for improvement. J Gerontol Nurs, 30(7), 33–43. [DOI] [PubMed] [Google Scholar]
- Schulman C, Irani J, & Aapro M (2012). Improving the management of patients with prostate cancer receiving long-term androgen deprivation therapy. BJU international, 109, 13–21. [DOI] [PubMed] [Google Scholar]
- Shin DW, Baik YJ, Kim YW, Oh JH, Chung KW, Kim SW, … Cho J (2011). Knowledge, attitudes, and practice on second primary cancer screening among cancer survivors: a qualitative study. Patient Educ Couns, 85(1), 74–78. doi: 10.1016/j.pec.2010.09.015 [DOI] [PubMed] [Google Scholar]
- Skolarus TA, Wolf AM, Erb NL, Brooks DD, Rivers BM, Underwood W 3rd, … Cowens-Alvarado RL (2014). American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin, 64(4), 225–249. doi: 10.3322/caac.21234 [DOI] [PubMed] [Google Scholar]
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, & Buchholz TA (2009). Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol, 27(17), 2758–2765. doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- Smith BD, Smith GL, Hurria A, Hortobagyi GN, & Buchholz TA (2009). Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol, 27(17), 2758–2765. [DOI] [PubMed] [Google Scholar]
- Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, & Norgaard M (2013). The impact of comorbidity on cancer survival: A review. Clin Epidemiol, 5(Suppl 1), 3–29. doi: 10.2147/clep.s47150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Trentham-Dietz A, Nichols HB, Hampton JM, & Newcomb PA (2010). Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat, 124(2), 487–495. doi: 10.1007/s10549-010-0869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, … Hedeker D (2008). Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl), 196(3), 461–471. doi: 10.1007/s00213-007-0977-7 [DOI] [PubMed] [Google Scholar]
- Spring B, Moller AC, & Coons MJ (2012). Multiple health behaviours: Overview and implications. J Public Health (Oxf), 34 Suppl 1, i3–10. doi: 10.1093/pubmed/fdr111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pellegrini C, McFadden H, Pfammatter AF, Stump TK, Siddique J, … Hedeker D (2018). Multicomponent mHealth Intervention for Large, Sustained Change in Multiple Diet and Activity Risk Behaviors: The Make Better Choices 2 Randomized Controlled Trial. Journal of medical Internet research, 20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pingitore R, & McChargue DE (2003). Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry, 160(2), 316–322. doi: 10.1176/appi.ajp.160.2.316 [DOI] [PubMed] [Google Scholar]
- Spring B, Schneider K, Smith M, Kendzor D, Appelhans B, Hedeker D, & Pagoto S (2008). Abuse potential of carbohydrates for overweight carbohydrate cravers. Psychopharmacology (Berl), 197(4), 637–647. doi: 10.1007/s00213-008-1085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglitz J, Edberg D, Sommers M, Talen MR, Thornton LK, & Spring B (2015). Evaluation of an electronic health record-supported obesity management protocol implemented in a community health center: a cautionary note. Journal of the American Medical Informatics Association, 22(4), 755–763. doi: 10.1093/jamia/ocu034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel F, & Bourquin C (2018). Adverse effects of “teachable moment” interventions in lung cancer: Why prudence matters. J Thorac Oncol, 13(2), 151–153. doi: 10.1016/j.jtho.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Stump TK, Robinson JK, Yanez B, Penedo F, Ezeofor A, & Spring B (2019). Physician practices and beliefs about healthy lifestyle counseling for cancer survivors. Poster presented at the Annual Meeting of Society of Behavioral Medicine, Washington, DC. [Google Scholar]
- Suls J, Green PA, & Davidson KW (2016). A biobehavioral framework to address the emerging challenge of multi-morbidity. Psychosom Med, 78(3), 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai-Seale M, McGuire TG, & Zhang W (2007). Time allocation in primary care office visits. Health services research, 42(5), 1871–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig K, Leff B, Kent D, Dy S, Brunnhuber K, Burgers JS, … Boyd CM (2014). A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med, 29(4), 670–679. doi: 10.1007/s11606-013-2659-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion, US Department of Health and Human Services, & Office of Disease Prevention and Health Promotion. (2010). Healthy People 2020
- Vrijens B, Vincze G, Kristanto P, Urquhart J, & Burnier M (2008). Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. BMJ, 336(7653), 1114–1117. doi: 10.1136/bmj.39553.670231.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner LP, Li Y, Furgal AKC, Friese CR, Hamilton AS, Ward KC, … Hawley ST (2017). Patient Preferences for Primary Care Provider Roles in Breast Cancer Survivorship Care. Journal of Clinical Oncology, 35(25), 2942–2948. doi: 10.1200/jco.2017.73.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Well J How open and interoperable apps can extend the capabilities of health informatics (2018, December 10). Retrieved from https://www.cerner.com/blog/how-open-and-interoperable-apps-can-extend-the-capabilities-of-health-informatics.
- What’s the difference between eHealth Exchange, Carequality and the Sequoia Project: Interoperability Is a Multifaceted Challenge. Retrieved from https://sequoiaproject.org/about-us/whats-difference-ehealth-exchange-carequality-sequoia-project/
- White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, & Henley SJ (2014). Age and cancer risk: A potentially modifiable relationship. American Journal of Preventive Medicine, 46(3), S7–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykle ML, Kahana E, & Kowal J (1992). Stress and health among the elderly: Springer Pub. Co. [Google Scholar]
- Yarnall KS, Ostbye T, Krause KM, Pollak KI, Gradison M, & Michener JL (2009). Family physicians as team leaders: “time” to share the care. Prev Chronic Dis, 6(2), A59. [PMC free article] [PubMed] [Google Scholar]